Abstract
A 26-year-old woman was sent to the emergency room by her primary care physician for a new petechial rash and thrombocytopenia 2 weeks after receiving the Moderna mRNA-1273 SARS-CoV-2 vaccine. Her hospital course was complicated by transaminitis. Her platelet count improved to normal on hospital day 5 after receiving intravenous steroids and intravenous immunoglobulin to treat her suspected diagnosis of immune thrombocytopenic purpura. Extensive workup for her thrombocytopenia and transaminitis was unremarkable including ruling out infectious, autoimmune and toxic causes. A liver biopsy was unrevealing and her transaminitis was improved on discharge. Although not proven, the temporal relationship of her vaccination with thrombocytopenia and abnormal liver enzymes points towards the Moderna mRNA-1273 SARS-CoV-2 vaccine as the most likely inciting factor.
Keywords: immunological products and vaccines, hepatitis other, haematology (incl blood transfusion), immunology, public health
Background
During a worldwide pandemic with widespread vaccination programmes, it is important to report and document any potential serious vaccine-related events.
To date, there has only been one published case of suspected immune thrombocytopenic purpura (ITP) related to the Pfizer‐BioNTech BNT16B2b2 mRNA vaccine but none regarding the Moderna mRNA-1273 SARS-CoV-2 vaccine.
There have been no documented or published cases of acute liver injury related to the Moderna mRNA-1273 SARS-CoV-2 vaccine.
Both thrombocytopenia and acute liver injury were not reported in clinical trials evaluating the safety and efficacy of the Moderna mRNA-1273 SARS-CoV-2 vaccine.
Case presentation
A 26-year-old woman with a previous medical history of irregular menses on oral contraceptives was sent to the emergency room by her primary care physician for a petechial rash of 6 days and abnormal platelet count of 19×109/L (reference range: 150–400×109/L) (figure 1). The patient had been in her usual state of health and received her first dose of Moderna mRNA-1273 SARS-CoV-2 vaccine 2 weeks prior to hospitalisation. The only initial side effect was arm soreness at the injection site. Approximately 1 week later, she developed a petechial rash on her neck and chest associated with increased bruising, particularly on her lower extremities. She went to an urgent care facility where she was diagnosed with an idiopathic allergic reaction and was prescribed prednisone 40 mg/day to treat the urticaria. After taking three doses of prednisone, her rash improved and a repeat complete blood count (CBC) showed a platelet count of 19×109/L. On hospital day (HD) 1, the patient’s CBC revealed a platelet count of 28×109/L with normal haemoglobin and white blood cell (WBC) levels. She was afebrile and haemodynamically stable without any signs of overt bleeding. She was not actively menstruating; however, she had unusual heavy bleeding with clots during menstruation 3 days prior to hospitalisation. Coagulation studies, D-dimer, fibrinogen and creatinine levels were normal. SARS-CoV-2 PCR returned negative. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were both elevated at 284 U/L (10–40 U/L) and 707 U/L (10–45 U/L), respectively. Alkaline phosphatase (82 U/L) and total bilirubin (1.0 mg/dL) levels were normal, as were her vitamin B12, folate and thyroid hormone levels. The peripheral blood smear (PBS) showed rare schistocytes, giant platelets, no platelet clumping, toxic neutrophils and atypical lymphocytes without immature WBCs or blasts. A clinical diagnosis of ITP was made and the patient received four doses of dexamethasone 40 mg intravenously daily with two doses of intravenous immunoglobulin (IVIg) 1 g/kg/day. Her platelet count appropriately responded and was normal at 213×109/L by HD 5. Given her isolated thrombocytopenia, unremarkable PBS and improvement in platelet count with ITP-directed treatment, a bone marrow biopsy was not performed or deemed necessary for diagnosis. Her hospital course was complicated by worsening transaminitis with her AST and ALT levels peaking on HD 3 at 446 U/L and 1257 U/L, respectively. The hepatitis panel was positive for hepatitis B virus (HBV) total core antibody; however, her IgM antibody was negative; HBV surface antibody was positive at 1387.7 mIU/mL indicating immunity; HBV surface antigen and HBV e-antigen/antibody were negative with an undetectable viral load; hepatitis C antibody and viral load were undetectable. HIV antigen, antibody and viral load were undetectable. Epstein-Bar virus (EBV), parvovirus (PV), herpes simplex virus (HSV) 1/2 and cytomegalovirus (CMV) were not detected by means of serologic antibody and PCR testing. Antinuclear antibody (ANA), antismooth muscle antibody and antimitochondrial antibody were negative. IgG subsets, specifically IgG4 measuring 100 mg/dL (2–96 mg/dL), were not indicative of an autoimmune process. The remaining IgG subsets were elevated due to IVIg infusion. Other pertinent laboratory results included a negative serum pregnancy test and serum lactate that was transiently elevated on HD 3 to 3.3 mmol/L (0.7–2.0 mmol/L) but normalised to 1.9 mmol/L by HD 5. An abdominal ultrasound with Doppler showed normal liver and spleen morphology with no identifiable thrombus. On HD 4, a liver biopsy was performed showing no granulomas, steatosis or evidence of necroinflammatory injury. The patient was started on N-acetylcysteine (NAC), though acetaminophen and salicylate levels were unremarkable. Her total bilirubin peaked at 2.1 mg/dL (0.2–1.2 mg/dL) on HD 3, with mixed component of direct and indirect bilirubin. On HD 5, her total bilirubin was normal at 1.1 mg/dL, AST and ALT improved to 175 U/L and 763 U/L, respectively. Given her normal platelet count and improving transaminitis, the patient was discharged with outpatient follow-up on HD 5.
Figure 1.
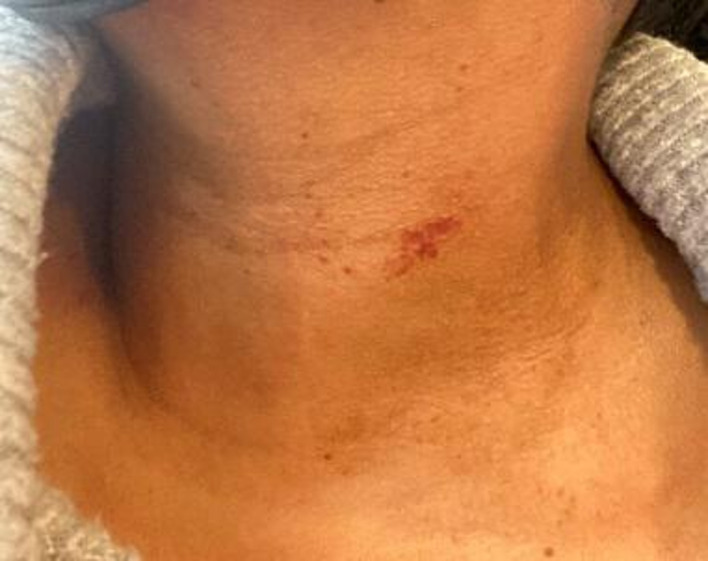
Petechial rash.
Investigations
PBS showed rare schistocytes, giant platelets, no platelet clumping, toxic neutrophils and atypical lymphocytes without immature WBCs or blasts.
Hepatitis panel, HIV, EBV, PV, CMV and HSV were all negative or indicative of past infection. Hepatitis panel included antibody testing. HCV and HBV RNA by PCR were not detectable. EBV antibodies were consistent with past infection and confirmed with a negative PCR. HIV1 and 2 antibodies were negative along with an undetectable transcription mediated amplification of RNA (viral load). PV and CMV PCR both were undetectable.
Antismooth muscle antibody, antimitochondrial antibody and IgG subsets were negative.
Acetaminophen and salicylate serum levels were negative.
Ultrasound abdomen and Doppler to evaluate liver, spleen and assess for any portal/hepatic vein thrombus were negative and unremarkable.
Liver biopsy showed no evidence of necroinflammatory injury.
D-dimer, fibrinogen and coagulation studies were not consistent with disseminated intravascular coagulation.
Differential diagnosis
Autoimmune hepatitis: serology including microsomal antibody, antismooth muscle antibody, antimitochondrial antibody, ANA were negative.
Viral hepatitis: CMV, EBV, HSV serologies were negative or consistent with past infection. Hepatitis B total core antibody was positive, however IgM antibody was nonreactive and surface antibody was positive. HBV e-antigen and antibody were nonreactive. HBV PCR was negative.
Drug-induced liver injury: the patient reported taking acetaminophen infrequently for menstrual cramps, last ingested a week prior to hospitalisation. Acetaminophen level and salicylate level were negative.
Vaccine-induced liver injury: timing of the vaccine administered correlates with subsequent elevation in her transaminases, initially measuring in the hundreds and increased to greater than one thousand, 2 weeks later. All other aetiologies of acute liver injury were excluded and liver biopsy was unremarkable, suggesting that the vaccine is the most likely cause of her transient acute liver injury.
Thrombotic thrombocytopenic purpura/haemolytic uremic syndrome: PBS showed rare schistocytes. The patient was afebrile with normal haemoglobin level, mental status and kidney function, which rules out a microangiopathic haemolytic process.
ITP: viral studies were negative. The patient was not on any medications that could cause thrombocytopenia. Her thyroid hormone tests were normal. PBS showed giant platelets, which is more consistent with ITP.
Treatment
Oral prednisone 40 mg/day for 3 days.
Dexamethasone 40 mg IVP for 4 days.
IVIg 1 g/kg for 2 days.
NAC protocol for 4 days.
Outcome and follow-up
On HD 5, the patient’s platelet count was normal at 213×109/L. Her liver biopsy and other workup for acute liver injury were unremarkable. The PBS was significant for giant platelets, which supports a diagnosis of ITP. She was discharged with outpatient follow-up after receiving high-dose dexamethasone, IVIg and NAC.
Discussion
We present a young woman who developed a petechial rash associated with thrombocytopenia and marked elevation of her liver function tests 2 weeks after receiving the Moderna mRNA-1273 SARS-CoV-2 vaccine. No new medications nor her oral contraceptive could explain the abnormal laboratory findings. An exhaustive workup was unremarkable, which included ruling out infectious, autoimmune and toxic causes. The patient underwent a liver biopsy, the results of which also could not explain her transient transaminitis. Of note, she was taking dexamethasone at the time of the biopsy, which could have altered the integrity of the biopsy sample. Clinically, the patient’s only symptoms were easy bruising and petechial rash, which improved after steroids and IVIg treatment. It appears that the only inciting event that she encountered prior to hospitalisation was receiving the Moderna mRNA-1273 SARS-CoV-2 vaccine. In general, ITP pathogenesis is not completely understood, especially in the setting of the new COVID-19 vaccine. Typically, ITP occurs when specific autoantibodies are directed at platelet membrane glycoproteins.1 The immunopathogenic mechanism of vaccine-induced ITP is challenging to study as it is rather difficult to distinguish between cases of de novo ITP from potential vaccine-induced exacerbation of pre-existing ITP. Furthermore, specific testing to confirm the presence of autoantibodies is lacking. Pishko et al propose that perhaps through molecular mimicry, mRNA-based vaccines against SARS-CoV-2 generate cross-reactive immunity or alter a host protein, thereby influencing the emergence of pre-existing autoreactive antibodies into the immune system.2
Currently, in the literature, there has only been one reported case of a young man who developed ITP after receiving the Pfizer‐BioNTech BNT16B2b2 mRNA vaccine.3 To our knowledge, this would be the first reported case of ITP and acute liver injury secondary to the Moderna mRNA-1273 SARS-CoV-2 vaccine. There is one other reported case of a 56-year-old man who developed thrombocytopenia and an intracranial haemorrhage 16 days after receiving the Pfizer vaccine.4 Although urticarial rash was reported as a potential side effect in the Moderna SARS-CoV-2 trial, our patient’s rash appeared to be petechial given its appearance and absence of pruritus.
It is possible our patient was coincidentally diagnosed with ITP after her vaccination; however, she reportedly always had normal blood work in the past and denied any signs or symptoms of autoimmune disease. It is important to note that the phase 3 Moderna mRNA-1273 SARS-CoV-2 vaccine trial, which enrolled 30 420 participants, had no reported cases of ITP, thrombocytopenia, bleeding, petechial rash or acute liver injury.5 It is difficult to discern the cause of her transient transaminitis, though it appears to be related to the same aetiology as her thrombocytopenia.
The case was reported to the Center for Disease Control Vaccine Adverse Event Reporting System.6 The Moderna mRNA-1273 SARS-CoV-2 vaccine has a well-documented safety profile overall, and while it is important to acknowledge and report serious vaccine-related adverse events, the need for this preventative strategy during an ongoing worldwide pandemic should not be diminished.
Learning points.
There have been no published or known case reports of immune thrombocytopenic purpura (ITP) and acute liver injury secondary to the Moderna mRNA-1273 SARS-CoV-2 vaccine.
Clinicians should be cognizant of ITP and acute liver injury complications following SARS-CoV-2 vaccination and remain hypervigilant even if young patients with no comorbidities present with new onset bruising and/or petechiae.
Although it is essential to report and acknowledge potential serious vaccine-related adverse events, the utility of both Pfizer and Moderna mRNA vaccines cannot be emphasised enough during a worldwide pandemic. Both vaccines have proven to have a comprehensive safety profile.
Footnotes
Contributors: AH and JCS conceived the idea. AH, JCS and CO wrote the manuscript. SS approved, edited and provided expert opinion of the manuscript. SS, AH, JCS and CO also were involved with editing and finalising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med 2002;346:995–1008. 10.1056/NEJMra010501 [DOI] [PubMed] [Google Scholar]
- 2.Pishko AM, Bussel JB, Cines DB. COVID-19 vaccination and immune thrombocytopenia. Nat Med 2021. 10.1038/s41591-021-01419-1. [Epub ahead of print: 09 Jun 2021]. [DOI] [PubMed] [Google Scholar]
- 3.Tarawneh O, Tarawneh H. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am J Hematol 2021;96:E133–4. 10.1002/ajh.26106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grady D, Mazzei P. Doctor’s Death after Covid Vaccine Is Being Investigated. The New York Times, 2021. https://www.nytimes.com/2021/01/12/health/covid-vaccine-death.html [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC . COVID‐19 vaccines, 2021. Available: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html


