Abstract
Immunological memory is a hallmark of adaptive immunity that confers long-lasting protection from reinfections. Memory CD8+ T cells provide protection by actively scanning for their cognate antigen and migrating into inflamed tissues. Trafficking patterns of CD8+ T cells are also a major determinant of cell fate outcomes during differentiation into effector and memory cell states. CD8+ T-cell trafficking must therefore be dynamically and tightly regulated to ensure that CD8+ T cells arrive at the correct locations and differentiate to acquire appropriate effector functions. This review aims to discuss the importance of CD8+ T-cell trafficking patterns in regulating effector and memory differentiation, maintenance, and reactivation.
IMPORTANCE OF TRAFFICKING IN DISTINGUISHING T-CELL SUBSETS
A hallmark of adaptive immunity is defined by the ability of memory T and B lymphocytes to “remember” an initial pathogen encounter, which underlies the success of vaccines to provide long-lived protection against recurrent infections. This review will focus on “cytotoxic” memory CD8+ T cells that provide immune surveillance by actively scanning for and killing cells infected with viruses or intracellular bacteria within tissues and the blood, which depends on tissue-specific homing molecules and cell migration. In short, we will discuss how CD8+ T-cell priming, function, and cell fates are determined in a spatiotemporal manner during acute infection.
Following pathogen encounter, CD8+ T cells expand and acquire cytotoxic effector functions to facilitate pathogen clearance. Upon elimination of infection, the majority of the effector cells die via apoptosis, while some remain to generate various subsets of long-lasting CD8+ T memory cells. Foundational work on elucidating the different memory T-cell subsets came from Sallusto et al. (1999), where they subdivided peripheral blood human T cells according to their homing and chemokine receptor expression patterns. Cells that circulate mainly through lymph nodes (LNs) and express CD62L and CCR7, two key molecules necessary for LN homing, were referred to as central memory T (Tcm) cells (Sallusto et al. 1999). Instead, cells lacking these receptors with higher effector functions that circulate in blood and express inflammatory chemokine receptors were termed effector memory (Tem) cells. Over time, it has become clear that there is further heterogeneity within the memory T-cell populations; for example, it was later appreciated that a portion of Tem cells actually do not circulate, but rather persist long term in peripheral tissues. These cells became known as tissue-resident memory (Trm) cells (Gebhardt et al. 2009) and express tissue-specific residency markers. Trm cells are found in nearly every tissue and often provide a local first line of defense to reinfection, especially those situated in barrier tissues such as the lungs, skin, and intestine. More recently, another subpopulation of cells referred to as peripheral memory (Tpm) cells was characterized by intermediate levels of CX3CR1int and their unique ability to enter and survey peripheral tissues and then recirculate back to the blood (Gerlach et al. 2016). Tpm cells are distinguished from longer-lived terminal effector (TE) cells that dwell exclusively in the blood and express the highest amounts of CX3CR1 and KLRG1. Collectively, these outline unique trafficking patterns between the different subsets of memory T cells that help the cells “divide and conquer” to maximize immune surveillance across a wide range of tissue landscapes.
Herein we will discuss the importance of spatiotemporal regulation of effector and memory CD8+ T-cell fate determination, maintenance, and reactivation. We will follow the cascade of events proceeding CD8+ T-cell activation and review how their trafficking patterns influence early and late stages of effector and memory cell fate differentiation and key transcriptional changes that drive the functional and anatomical diversification of memory T cells. Finally, we will discuss how tissue-specific environmental factors orchestrate effector and memory CD8+ T-cell homing to and maintenance in peripheral tissues.
SPATIOTEMPORAL REGULATION OF CD8+ T-CELL PRIMING AND EFFECTOR DIFFERENTIATION
Upon infection, activated CD8+ T cells give rise to a heterogeneous pool of effector cells with distinct long-term fates to form the various types of memory CD8+ T cells (Tcm, Tem, Trm, Tpm) described above. We often conceptualize this process as effector CD8+ T cells differentiating into multiple cell states along a spectrum. On one end of the spectrum exists “less” differentiated or more stem-like cells that have more memory cell potential, and on the other end cells that progress to more “terminally” differentiated effector states and have lost memory cell potential. Cells in between the two ends tend to have mixtures of effector- and memory-like properties and display dynamic developmental plasticity (i.e., they can interconvert between differentiation states over the course of an immune response) (Kaech et al. 2003; Cui et al. 2009; Rutishauser et al. 2009; Gerlach et al. 2016; Youngblood et al. 2017; Herndler-Brandstetter et al. 2018).
For the purposes of communication and clarity, we will refer to particular subsets of effector and memory CD8+ T cells, the differentiation state(s) for which have been well characterized. In several acute viral and intracellular bacterial infections, the majority of activated CD8+ T cells acquire a terminally differentiated effector state and are referred to as TE cells, which are characterized by high levels of KLRG1 and CX3CR1 surface expression (Fig. 1; Joshi et al. 2007; Gerlach et al. 2016). The majority of TE cells die via apoptosis after infection (Kaech et al. 2003; Sanjabi et al. 2009), whereas a minority of effector cells differentiate into memory precursor (MP) cells that express IL-7R and encompass the greatest potential to seed the memory pool (Tem, Tcm, Tpm, and Trm) (Kaech et al. 2003; Mackay et al. 2013; Gerlach et al. 2016). Some MP cells also coexpress KLRG1, but then lose KLRG1 and differentiate into multiple memory cell subsets (Herndler-Brandstetter et al. 2018). One of the most dominant factors that influences CD8+ T-cell activation and their stratification into various effector differentiation states is their migration patterns as it dictates the types of cell–cell interactions as well as inflammatory and antigenic signals received. In this section, we will dissect the roles of certain chemokine receptors in regulating effector and memory CD8+ T-cell fates during many types of acute viral or bacterial infections.
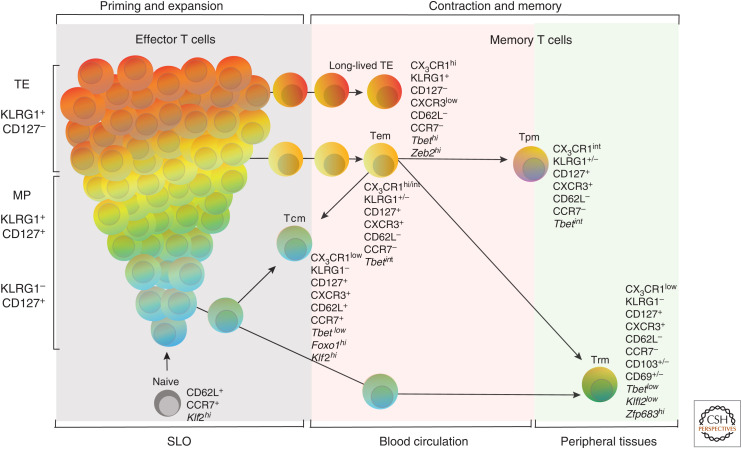
Figure 1.
CD8+ T-cell effector and memory differentiation. Effector and memory differentiation of CD8+ T cells. During many acute infections, activated CD8+ T cells form a heterogeneous effector population with distinct developmental trajectories. The majority of activated CD8+ T cells acquire a terminally differentiated effector state known as terminal effector (TE) cells. After clearance of infection, most of the KLRG1+ cells die, while a small subset survives as long-lived TE cells. Additionally, surviving effector cells that also express IL-7Rα (CD127) seed the effector memory T-cell (Tem) pool, and some of these cells even lose KLRG1 expression and develop into various memory T-cell subsets (Herndler-Brandstetter et al. 2018). In contrast, a smaller fraction of effector cells differentiates into memory precursor (MP) cells, which encompass the greatest potential to develop into long-lived memory cells and are characterized by increased amounts of CD127 (Kaech et al. 2003). After resolution of infection, the MP cells seed most of the memory pool, giving rise to the various memory T-cell subsets (Tem, Tcm, Tpm, and Trm).
Trafficking Patterns that Influence CD8+ T-Cell Priming and Effector Cell Formation
Secondary lymphoid organs ([SLOs], i.e., spleen, LNs, and Peyer's patches) are specialized organs that are highly compartmentalized into distinct regions (Fig. 2) to enable efficient priming and activation of CD8+ T cells (von Andrian and Mempel 2003; Bajénoff et al. 2007). Naive T cells express CD62L and CCR7, which recognize endothelial peripheral node addressin and the chemokines CCL19 and CCL21, respectively, to facilitate their entry into the T-cell zones within SLOs (Luther et al. 2000; Stein et al. 2000). While naive CD8+ T cells in mice that lack CCR7 ligands (plt/plt mice) have defective T-cell zone trafficking, surprisingly, following infection or immunization, CD8+ T cells in these mice only show delayed kinetics in activation and effector cell development, a result that was also supported later by CCR7 knockout (KO) CD8+ T cells (Mori et al. 2001; Junt et al. 2002; Jung et al. 2016). These experiments highlight that CD8+ T–dendritic cell (DC) encounters can occur outside of the T-cell zones of LNs. In fact, several studies using immunization or infection with vaccinia virus (VV), vesicular stomatitis virus (VSV), and Toxoplasma gondii showed that optimal CD8+ T-cell priming occurs via multiple interactions with distinct DC subsets in diverse regions of LNs (Hickman et al. 2008; John et al. 2009; Gerner et al. 2012, 2015; Eickhoff et al. 2015). For instance, during VV infection, early priming of CD8+ T cells occurred in the interfollicular area (IFA) and subcapsular sinus (SCS) of the LN by VV-infected CD11b+ classical DC (cDC), and subsequent contacts occurred in the paracortex with cross-presenting XCR1+ DCs (Eickhoff et al. 2015). These intranodal interactions are guided by diverse chemokine receptors such as CXCR3 (Hickman et al. 2008; Ozga et al. 2016), CCR5, and CCR4 (Semmling et al. 2010). Notably, CCR5 and CCR4 facilitate CD8+ T-cell migration toward CCL3- and CCL4-producing cDCs that have been “helped” by CD4+ T cells, which not only enhances priming and expansion of effector CD8+ T cells but also aids in their memory cell development (Castellino et al. 2006).
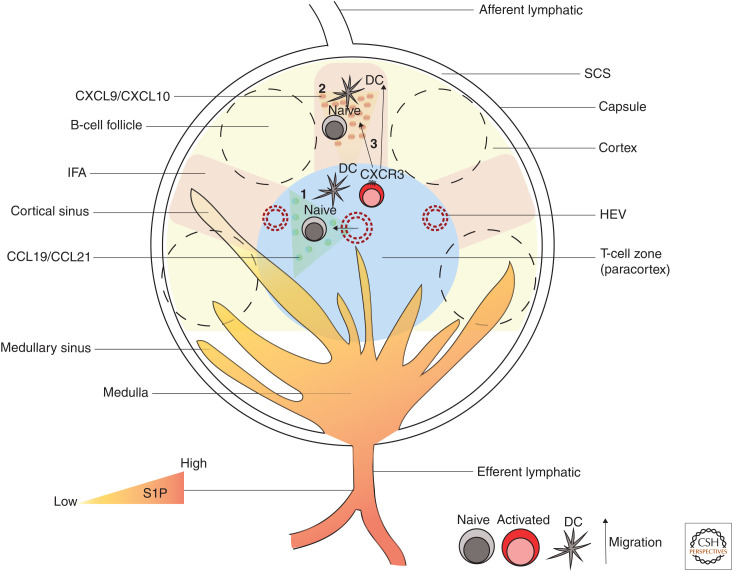
Figure 2.
Lymph node (LN) architecture and CD8+ T-cell priming. A schematic representation of the major regions of an LN and CD8+ T-cell priming sites. The main regions of LNs consist of the subcapsular sinus (SCS), B-cell follicles, interfollicular areas (IFAs), T-cell zone (paracortex), and the medulla. Lymph enters LN from afferent lymphatics and flows through the SCS. IFAs lie beneath the SCS and between B-cell follicles and are considered the inflammatory hotspots of LN as they contain innate immune cells that secrete various inflammatory molecules. Naive T cells enter LNs via high endothelial venules (HEVs) and locate to T-cell zones by responding to chemokines CCL19 and CCL21, where they get primed and activated by DCs (1). Alternatively, naive CD8+ T cells can also get activated in the IFA and SCS of LNs (2). Activated CD8+ T cells express CXCR3, which enables their migration toward the IFA, SCS guided by CXCL9 and CXCL10 gradients (3). Effector CD8+ T cells use sphingosine-1-phosphate (S1P) gradients in cortical and medullary sinuses to leave LNs via the efferent lymphatics.
One of the best-studied chemokine receptors involved in effector CD8+ T-cell development is CXCR3, which permits homing to CXCL9, CXCL10, and CXCL11 (Groom and Luster 2011a). Cxcr3 is rapidly induced upon T-cell receptor (TCR) activation and its expression is influenced by TCR–peptide major histocompatibility complex (pMHC) affinity and inflammatory cytokines such as IL-12 and type I and II interferons (IFNs) (Groom and Luster 2011a,b; Ozga et al. 2016). High-affinity interactions allow prolonged CD8+ T–DC contacts and augment Cxcr3 and Il2ra (CD25) expression, enhancing CD8+ T-cell expansion (Zehn et al. 2009; Ozga et al. 2016). Temporally, CXCR3 has two main roles on CD8+ T-cell trafficking: (1) early on, it promotes migration of antigen (Ag)-specific CD8+ T cells to inflamed regions such as the IFA and SCS in LNs and marginal zones of spleens within SLOs, which promotes T-cell activation, expansion, and effector differentiation. (2) Later, it functions to steer effector cells in the circulation toward infected peripheral tissues to help clear infection. Indeed, in multiple infections, it has been shown that Cxcr3-deficient effector CD8+ T cells that are unable to localize to such areas are impaired in generating TE-like CD8+ T cells and preferentially form more MP-like cells (Hu et al. 2011; Kurachi et al. 2011). Moreover, when CD8+ T cells are forcefully sequestered in T-cell zones by overexpression of CCR7, MP fates are favored over TE fates (Unsoeld et al. 2004; Hu et al. 2011). These studies demonstrate that CXCR3 permits CD8+ T cells to access environments in infected tissues that promote TE cell development, and cells that are shielded from these environments are better able to maintain memory cell potential.
Interestingly, while CXCR3 is critical for acquiring TE cell states, TE cells actually down-regulate CXCR3 and up-regulate CX3CR1 and sphingosine-1-phosphate receptor 5 (S1PR5) as they form (Jung et al. 2010; Hu et al. 2011). This change in trafficking receptors on TE cells presumably enables their egress from the inflamed tissues back to the blood, where sphingosine-1-phosphate (S1P) and CX3CL1 is enriched (Cyster and Schwab 2012). During resolution, the vast majority of TE cells undergo apoptosis as they are exposed and sensitized to molecules that may induce cell death such as TGF-β (Sanjabi et al. 2009) and certain resolvins (i.e., resolvin E1) (Vassiliou et al. 2008; El Kebir et al. 2012) that are highly concentrated at sites of tissue repair as infection resolves, while those that egress back into the blood may circumvent cell death. Therefore, it would be interesting to speculate whether overexpression of CXCR3 would enhance tissue retention and apoptosis of TE cells during contraction. In contrast to TE cells, MP cells maintain high levels of CXCR3 and some express intermediate amounts of CX3CR1 (Kaech and Wherry 2007; Gerlach et al. 2016). CXCR3 likely aids in MP cell retention in tissues, facilitating their exposure to tissue-specific signals and tailoring the type of memory T cells they will become. MP cells are also intrinsically wired in such a way that exposure to environmental factors like TGF-β can enable vastly different outcomes than TE cells. For example, CXCR3-dependent exposure to TGF-β drives CD103+ Trm cell formation in the skin epidermis (Mackay et al. 2013) and in the intestinal epithelium (Sheridan et al. 2014).
Whereas CXCR3 critically influences the formation of effector cells, other chemokine receptors also impact CD8+ T-cell trafficking and differentiation patterns to varying degrees. Among them, CXCR6 is also expressed by activated CD8+ T cells and responds to CXCL16, which is constitutively expressed and up-regulated upon an inflammatory insult in the splenic red pulp (RP), liver, lung, and various other tissues (Matloubian et al. 2000; Kim et al. 2001; Sato et al. 2005; Wein et al. 2019). Interestingly, while a study using graft versus host disease observed reduction of CXCR6 KO CD8+ T-cell infiltration into inflamed liver (Sato et al. 2005), other studies using malaria and VV infections did not observe migration differences between CXCR6 KO and wild-type (WT) effector CD8+ T cells to livers. These differences may indicate that the requirement for CXCR6 in liver homing may be dependent on the inflammation status of the liver or possibly the differentiation status of the T cells (and other chemokine receptors they express) (Tse et al. 2014). In support of this, as effector cells transition to memory cells following infection, CXCR6 becomes important for the formation and/or maintenance of memory cells in liver, skin, and lung (Tse et al. 2014; Zaid et al. 2017; Wein et al. 2019).
Tissue Is the Issue: How Migratory DCs from Draining Tissues Influence CD8+ T-Cell Differentiation and Trafficking Patterns
To locate infected tissues and clear infection, CD8+ T cells need to exit LNs and join the circulation. Initially, early activated CD8+ T cells up-regulate CD69 expression, which down-regulates S1PR1 allowing tissue retention (Shiow et al. 2006; Bankovich et al. 2010; Mackay et al. 2015a). Following successful priming and activation, CD8+ T cells become temporarily unresponsive to pMHC (Bohineust et al. 2018) and down-regulate CD69, which results in up-regulation of S1PR1, leading to CD8+ T-cell egress from LNs via efferent lymphatics (Cyster and Schwab 2012). Interestingly, low-affinity pMHC interactions cause accelerated up-regulation of S1PR1 expression on CD8+ T cells that results in early egress from the LNs (Ozga et al. 2016). Once in circulation, guided by their newly expressed chemokine receptors, effector CD8+ T cells traffic into inflamed tissues, where they locate infected cells through chemotactic gradients and perform effector functions to suppress intracellular pathogen spread.
How do T cells know which tissues to migrate to after leaving the SLOs and enter circulation? Evidence indicates that T cells receive these instructions (or zipcodes) from the migratory DCs emigrating from the infected tissues at the time of DC priming. For example, in skin-draining LNs, vitamin D derivatives are metabolized and presented to CD8+ T cells by Ag-presenting DCs. This results in the up-regulation of CCR4 or CCR10 together with P- and E-selectin ligands on CD8+ T cells, which bias their migration toward skin (Reiss et al. 2001; Sigmundsdottir et al. 2007). Similarly, in gut-draining mesenteric LNs and Peyer's patches, the vitamin A derivative retinoic acid enables DCs to imprint gut homing molecules α4β7 and CCR9 on effector CD8+ T cells (Stagg et al. 2002; Mora et al. 2003; Iwata et al. 2004), demonstrating that draining LNs of specific tissues can influence the trafficking patterns of effector CD8+ T cells toward where these cells are primed. Interestingly, a recent study showed that activation of TGF-β by skin-draining migratory DCs imprints an epidermal Trm cell fate on naive CD8+ T cells by epigenetically programming Itgae (CD103) expression (Mani et al. 2019), indicating that imprinting of trafficking and residency molecules on CD8+ T cells can occur at multiple stages throughout the life span of the cell. Interestingly, imprinting of naive cells only occurred in draining LNs and not in the spleen, signifying that organ-specific differences exist even within functionally related tissues (Mani et al. 2019).
Whereas it is clear that DCs can stamp a zipcode onto CD8+ T cells and influence tissue-trafficking, distinct DC subsets can also influence the types of memory CD8+ T cells that form. For example, in the lung-draining mediastinal LN, interactions with migratory CD103+ DCs induce lung-homing effector CD8+ T cells that eventually differentiate into Tem cells, whereas interactions with migratory CD11bhi DCs preferentially induce Tcm cell formation (Kim et al. 2014). Similarly, priming by DNGR-1+ migratory DCs in skin-draining LN is essential for skin-resident Trm cell differentiation, but dispensable for the formation of circulating memory CD8+ T cells following VV infection (Iborra et al. 2016). Non-DC cell types have also found to play roles in Trm cell development; for instance, pulmonary monocytes, intestinal macrophages, and vaginal tissue macrophages can help to establish Trm cells in the lungs, lamina propria, and vagina, respectively (Iijima and Iwasaki 2014; Bergsbaken et al. 2017; Dunbar et al. 2020).
In addition to environmental differences between distinct organs that affect CD8+ T-cell fate, there also exists macroanatomical heterogeneity within a given organ creating more inflammatory or tolerogenic zones that produce different types of T-cell responses. For example, Esterházy and colleagues demonstrated that LNs draining upper parts of the intestines are tolerogenic in nature, while colon-draining LNs show more of an inflammatory signature (Esterházy et al. 2019). Similar observations have been made in the liver-draining LNs, in which the portal LN results in more tolerogenic immune responses than those in the celiac LN (Yu et al. 2017); however, the signals that induce such qualitatively distinct types of immune responses are yet to be discovered.
SPATIOTEMPORAL REGULATION OF CD8+ T-CELL MEMORY CELL MAINTENANCE AND REACTIVATION
As mentioned earlier, following the resolution of infection, the remaining effector cells undergo further differentiation into memory subsets with diverse migratory patterns. Here we will discuss how the trafficking patterns of memory cells facilitate their maintenance at steady state and reactivation upon subsequent exposure to antigen.
Involvement of Trafficking Patterns on Maintenance of CD8+ T-Cell Memory Subsets
If the goal of adaptive immunity is to provide protection against future infection, then memory cells per se must be equipped with the capacity to survive for long periods of time while remaining poised to differentiate and reacquire effector functions. Memory T-cell survival is largely mediated via IL-7 and IL-15 signaling that support the bioenergetic needs for survival and self-renewal (Schluns et al. 2000; Becker et al. 2002; Goldrath et al. 2002). IL-7 is mainly secreted by stromal cells of the SLOs and bone marrow (BM) (Hara et al. 2012; Onder et al. 2012; Miller et al. 2013), whereas IL-15 is predominantly produced and trans-presented by hematopoietic cells via IL-15Rα to T cells (Schluns and Lefrançois 2003). Interestingly, IL-15 is important for CD62L up-regulation on CD8+ T cells (Obar and Lefrançois 2010) and its trans-presentation by DCs is essential for Tcm cell maintenance, whereas trans-presentation of IL-15 by macrophages supports both Tem and Tcm cells (Mortier et al. 2009; Obar and Lefrançois 2010).
Studies in mice and humans demonstrated that Tcm cells undergo a remarkably slow homeostatic turnover rate with an estimated mitotic event every 50 and 450 d (Choo et al. 2010; Akondy et al. 2017), respectively. Indeed, migration of Tcm cells into lymphoid organs is critical for the accession of prosurvival signals that promote longevity and capacity to self-renew, and the BM appears to be the predominant tissue-supporting memory T-cell homeostatic proliferation (Becker et al. 2005; Chaix et al. 2014). Memory T-cell migration into the BM is dependent on CXCR4 as CXCR4-deficient Tcm cells display reduced BM migration and rates of homeostatic proliferation (Becker et al. 2005; Mazo et al. 2005; Chaix et al. 2014). In contrast, CCR7-deficient memory CD8+ T cells have impaired trafficking into SLOs but display enhanced migration into the BM and homeostatic turnover rates (Jung et al. 2016). Likely, the CCR7-deficient memory CD8+ T cells compensate for reduced access to IL-7-rich stromal niches within the SLOs by enhancing their homing to IL-15-rich niches (such as in the BM) for their survival, which induces more proliferation. Niches like the BM may also provide unique organ-specific cues that support the metabolic needs of memory cells. Along this line, a recent study demonstrated that under conditions of caloric restriction in mice, memory CD8+ T cells are depleted in circulation, but accumulate in the BM in a CXCR4-CXCL12- and S1P-S1PR-dependent fashion (Collins et al. 2019). The BM acts like a metabolic reservoir by providing lipids as a key energy source for memory CD8+ T-cell fatty acid oxidation and survival (Collins et al. 2019). Perhaps caloric restriction dampens mTORC2 signaling in CD8+ T cells, which leads to increased CXCR4 expression and BM trafficking (Arojo et al. 2018).
Whereas comparatively less is understood about how Tem cell trafficking influences access to prosurvival signals and growth factors, a recent study showed that GPR18, a chemokine receptor that recognizes N-arachidonyl glycine and resolvin D2, was critical for the formation and maintenance of KLRG1-expressing Tem cells (Sumida and Cyster 2018). Although the conversion of Tem to Tcm cells in mice is generally considered a unidirectional lineage relationship (Wherry et al. 2003), Tcm cells that traffic through the liver, but not the lung, can down-regulate CD62L and adopt Tem-like qualities. This highlights the importance of tissue-derived signals in influencing cell fates.
Whereas the inherent motile nature of circulating memory cells allows for access to diverse homeostatic cues in various tissues, Trm cells by contrast have a limited range of motility and thus require unique trafficking capabilities to access tissue-specific prosurvival signals and maximize their potential for long-term survival while continuously scanning for antigen (Ariotti et al. 2012). One seemingly universal feature of CD8+ Trm cells is the loss of surface S1PR1 and the gain of CD69, which abrogates tissue egress and enables residency within the parenchyma of solid tissues (Skon et al. 2013). The αE integrin subunit (CD103) is another defining feature of some Trm cells, particularly those that localize to the E-cadherin-expressing epithelium, and is critical for promoting tissue retention; fewer Trm cells are found and the cells display faster migration within in barrier tissues in Itgae (CD103) KO mice (Wakim et al. 2010; Casey et al. 2012; Mackay et al. 2013; Zaid et al. 2017). CD103 expression is regulated by TGF-β and Notch signaling in the lungs (Lee et al. 2011; Hombrink et al. 2016) and more generally by TGF-β in diverse epithelial sites like the epidermis, salivary glands, and small intestine (Casey et al. 2012; Mackay et al. 2013; Zhang and Bevan 2013; Hirai et al. 2019). However, in Trm cells in many tissues like the gut lamina propria (Bergsbaken et al. 2017) and liver (McNamara et al. 2017), the Trm cells largely lack CD103 expression. Instead, CD103– Trm cells in liver and lungs use other integrins such as LFA-1 or α1β1 (VLA-1) to help with retention (Ray et al. 2004; McNamara et al. 2017). In addition to adhesion, CD103 may also help the Trm cells sense local survival factors, like IL-15, which is important for Trm cell survival in some tissues like the skin, intestinal epithelium, and liver (Mackay et al. 2013, 2015b; Zhang and Bevan 2013; Holz et al. 2018). However, Trm cell survival in other organs such as the pancreas, small intestine, female reproductive tract, and thymus is IL-15 independent (Schenkel et al. 2016). Additionally, IL-15-deficient mice appear to have an accumulation of Trm cells in LNs, suggesting that mechanisms of Trm cell maintenance in SLOs are distinct from Tem and Tcm cells (Schenkel et al. 2014a). More work is needed to better understand the manner by which Trm cells adapt to the tissue-specific cues to persist and inhabit distinct anatomical niches.
Anatomical Control of Memory CD8 T-Cell Reactivation
Similar to the priming events, homing molecules and chemokine receptors are also critical for memory CD8+ T-cell recall responses. Both Tem and Tcm cells play critical roles in protection against reinfection, but they differ qualitatively by how they functionally respond. For example, Tem cells show immediate cytotoxicity following reinfection (Olson et al. 2013; Ruiz et al. 2014), while Tcm cells display enhanced proliferative capacity and greater IL-2 production. In LNs, Tcm cells are localized within the paracortex, while CX3CR1+ Tem cells are located in the IFA and SCS region of the LNs close to SCS macrophages (Böttcher et al. 2015; Nikolova et al. 2020); but following reinfection, however, Tcm cells rapidly localize in a CXCR3-dependent manner to SCS and IFA where infected cells are first emerging and CXCL9 and CXCL10 are produced (Sung et al. 2012; Kastenmüller et al. 2013).
Because memory T cells are rather mobile and viruses often infect multiple cell types (Sung et al. 2012), one may predict that memory T cells could be reactivated by a variety of cell types; but actually, many studies have shown that following secondary challenges with multiple viruses or Listeria monocytogenes, memory T-cell reactivation depends on CD11c+ or XCR1+ DCs (Zammit et al. 2005; Dorner et al. 2009; Alexandre et al. 2016). However, there do appear to be exceptions to this because XCR1+ DCs are dispensable for memory T-cell recall responses to murine cytomegalovirus (MCMV) infection (Alexandre et al. 2016). Our laboratory recently showed that following secondary influenza infection the CD8+ memory T cells in the draining LNs were, as predicted, dependent on CD11c+ XCR1+ DCs for reactivation; however, the Trm cells in the lungs were not (Low et al. 2021). Rather, lung Trm cells were efficiently reactivated in infected lungs by various types of antigen-presenting cells (APCs) in situ. These findings indicate that the mechanisms of reactivation of memory T cells are distinct in different tissues and reshape the paradigm of how memory T-cell responses are triggered upon reinfection. Moreover, this greater promiscuity in APC partners offers an explanation for how Trm cells may be able to protect more rapidly at the site of infection in peripheral tissues (Ariotti et al. 2012) by not only deploying antiviral functions, but also facilitating the activation of innate immune cells and infiltration of circulating immune cells into the infected tissue. For example, local Trm cell IFN-γ production induces more rapid expression of integrins ICAM-1 and VCAM-1 and chemokines CXCL9 and CXCL10 and extravasation of circulating memory CD8+ T cells into the infected tissues (Parr and Parr 1999; Schenkel et al. 2013, 2014b; Ariotti et al. 2014). The anatomical distribution, tissue-specific adaptations, and specialized functions of each memory T-cell subset synergize with the others to provide systemic protection against invading pathogens.
TRANSCRIPTIONAL REGULATION OF CD8+ T-CELL EFFECTOR AND MEMORY DIFFERENTIATION
Transcription Factors Governing Circulating Memory T-Cell Trafficking and Function
Functional diversity and trafficking patterns are key distinguishing features of various effector and memory T-cell subtypes, but how are those features regulated transcriptionally and how does a single CD8+ T cell give rise to functionally diverse progeny with distinct migratory dispositions? Extracellular signaling inputs are major drivers of transcription factor (TF) expression and the establishment of complex transcriptional networks, and there has been extensive work in the field to describe how certain TFs both guide effector differentiation patterns and preserve the survival and function of long-lived memory cells. Herein, we describe how this transcriptional response controls the spatiotemporal programming of effector and memory CD8+ T-cell differentiation.
As discussed above, Tcm cells bear a remarkably similar trafficking pattern to naive cells, and perhaps not unsurprisingly express a similar set of TFs to regulate this process, including Klf2 (Carlson et al. 2006; Sebzda et al. 2008; Weinreich et al. 2009), Foxo1 (Kerdiles et al. 2009; Lou et al. 2012; Utzschneider et al. 2018), Eomes (Pearce et al. 2003; Intlekofer et al. 2005, 2008), Tcf7 (Zhou et al. 2010), and Zeb1 (Guan et al. 2018). Klf2 maintains the expression of S1PR1 and Sell (CD62L) in naive and Tcm cells (Carlson et al. 2006) while repressing expression of “inflammatory” chemokine receptors found in effector T cells such as CXCR3 and CCR5 (Sebzda et al. 2008; Weinreich et al. 2009). Foxo1 lies upstream of Klf2 and T-cell deficiency of Foxo1 results in reduced expression of Klf2 and Sell (Kerdiles et al. 2009), which consequently impairs the capacity of naive and Tcm cells to home to LNs (Kerdiles et al. 2009; Utzschneider et al. 2018). FOXO1 also programs naive and Tcm cell responsiveness to prosurvival signals through the coordinated expression of IL-7Rα and CCR7 (Kerdiles et al. 2009; Utzschneider et al. 2018), which will drive the cells toward IL-7- and CCL19/21-producing fibroblastic reticular cells (FRCs) (Luther et al. 2000; Hara et al. 2012; Onder et al. 2012). Indeed, deletion of Foxo1 results in the rapid loss of Il7r, Sell, and Ccr7 expression in these cells in vivo, and late inducible deletion impairs the capacity of Tcm cells to access LNs, undergo homeostatic turnover, and mount a productive secondary recall response (Kerdiles et al. 2009; Utzschneider et al. 2018). FOXO1 is typically inactivated by phosphoinositide-3-kinase (PI3K) and protein kinase B ([PKB], also known as AKT) signaling and thus, cytokines like IL-2, IL-12, and IL-15 inactivate FOXO1 to enhance effector differentiation (Kelly et al. 2002; Kim et al. 2012; Rao et al. 2012). In contrast, IL-7 is a relatively weak activator of PI3K and AKT signaling (Wofford et al. 2008), and perhaps this permits IL-7 to sustain memory CD8+ T-cell survival without overtly inducing more effector-like differentiation states.
During the initial expansion phase in response to an acute infection, the transition from naive→effector→TE states is coupled to microanatomical sites in tissues. Synergistic exposure to IL-2 and type I IFNs or IL-12 during priming induces Tbx21 (T-bet) and Prdm1 (Blimp-1) (Rutishauser et al. 2009; Jung et al. 2010; Xin et al. 2016) expression, which, in addition to Zeb2 (Dominguez et al. 2015; Omilusik et al. 2015) and Id2 (Omilusik et al. 2018), program various overlapping and distinct aspects of the cytotoxic and trafficking programs for encountering and combatting infected cells (Jung et al. 2010; Schenkel et al. 2014a) in addition to promoting TE differentiation. T-bet, which is transcriptionally induced by inflammatory signals like IL-12, IFN-γ, and type I IFNs, is a predominant TF involved in determining the ultimate fate of differentiation effector cells (Joshi et al. 2007). Indeed, high T-bet levels specify TE fates, while low T-bet levels are needed by MP cells to program optimal IL-15 responsiveness and ensure memory CD8 T-cell longevity (Joshi et al. 2007). High levels of T-bet and Blimp-1 suppress FOXO1-dependent genes Ccr7 and Il7ra while inducing CXCR3 expression; this simultaneously restricts effector cell access to FRC-derived IL-7 in the splenic white pulp (Lord et al. 2005; Joshi et al. 2007; Jung et al. 2010; Zhu et al. 2010; Hara et al. 2012) and guides them to sites of infection and inflammation like the IL-15-rich RP (Cui et al. 2014). Intrinsic rewiring of IL-7 signaling also contributes to the short-lived nature of TE cells, as forced Il7ra (IL-7R) expression could not rescue them from terminal differentiation and death in vivo despite intact immediate downstream STAT5 signaling (Hand et al. 2007). Thus, by regulating both cell-intrinsic and -extrinsic aspects of effector cell responsiveness to environmental signals, T-bet and Blimp-1 constitute a transcriptional rheostat that fine-tunes effector and memory cell potential in differentiating cytotoxic T lymphocytes (CTLs), ultimately resulting in an effector cell pool with heterogeneous cytotoxic capacity and long-lived potential for optimized host protection.
Graded TF expression, as a mechanism to maintain functional diversity, also exists among circulating memory cells, where the relative level of T-bet controls trafficking potential in Tem cells by tuning chemokine receptor expression. Circulating Tem cells with high T-bet levels express high levels of CX3CR1 but lose expression of CXCR3 and circulate exclusively in blood (Slütter et al. 2013; Laidlaw et al. 2014; Gerlach et al. 2016), while intermediate T-bet expression identifies a subset of Tem that maintains low CX3CR1 but high expression of CXCR3, and broadly traffics throughout blood, lymph, and tissues. How T cells “sense” the relative level of T-bet is not entirely clear, but evidence suggests that Zeb2 may serve as an “interpreter” that modifies the activity of T-bet to fine-tune effector and KLRG1+ Tem cell differentiation. In fact, T-bet overexpression in Zeb2-deficient cells was unable to induce expression of Cx3cr1 or suppress Cxcr3, suggesting that the cooperative action of T-bet and Zeb2 are required for appropriate specification of effector fates (Dominguez et al. 2015). A close cousin of T-bet, Eomes also plays an equally fundamental and often complementary role to T-bet in shaping circulating and tissue-resident memory T-cell identity (Intlekofer et al. 2008) and programming responsiveness to IL-15 (Pearce et al. 2003; Intlekofer et al. 2005). However, there are certain states where T-bet functions reciprocally with Eomes, for example, when T-bet expression increases to its highest levels in TE cells it begins to repress Eomes and CXCR3 expression (Dominguez et al. 2015; Gerlach et al. 2016). Together these results suggest that in addition to the functional redundancy of T-bet and Eomes in promoting core effector and memory cell processes, the relative ratio of the two TFs in Tem cells is a critical determinant of differentiation states and trafficking potential.
Tissue-Specific Regulation of Memory Cell Formation and Function
Trm cells across multiple tissues express a “core” set of TFs that optimize their ability to survive and persist in tissues. Notably, Zfp683 (Hobit) and Runx3 are universally necessary for the establishment of residency in diverse tissues (Mackay et al. 2016; Milner et al. 2017). The Klf2 locus becomes less accessible and is down-regulated early upon entrance into tissues, resulting in the loss of S1pr1 expression (Milner et al. 2017). Blimp-1 also plays an important role in instructing Trm cell identity, possibly by coordinating with Hobit to repress Klf2 (Mackay et al. 2016). Both Eomes and Tbx21 are down-regulated by Trm in the skin and gut, and the residual expression of T-bet releases its transcriptional repression of Itgae (encoding CD103), which in turn is promoted through concurrent exposure to epithelial tissue-derived TGF-β (Laidlaw et al. 2014). On the other hand, low levels of T-bet are also needed for Trm cell survival by maintaining Il2rb expression and IL-15R signaling (Mackay et al. 2015b).
The BM is a major reservoir for both Tem and Tcm cells likely due to its preponderance of IL-7 and IL-15 that support survival and homeostatic proliferation (Becker et al. 2005). Migration into the BM from the circulation depends on a combination of Eomes-dependent CXCR4 (Banerjee et al. 2010) and other Gɑi-dependent receptors for homing and extravasation into the tissue (Mazo et al. 2005). CXCR4 likewise plays a critical role in positioning cells within the BM in proximity to stromal cells, which are major producers of both IL-7 and IL-15 (Cui et al. 2014).
In addition to the core set of genes that define Trm cells broadly, unique environmental cues facilitate tissue-specific transcriptional programs regulating metabolism, effector functions, and tissue-remodeling and healing responses. In the lung, Notch signaling promotes the maintenance of Trm cells, both by regulating expression of Itgae as well as metabolite transporters like Aqp3 and other solute carrier proteins (Hombrink et al. 2016). Recent work has revealed tissue-specific expression of unique fatty acid–binding protein (FABP) isoforms in Trm cells (Frizzell et al. 2020); and given the specificity of FABPs for unique lipid ligands, these expression patterns likely represent metabolic adaptations to distinct anatomical niches regulated by tissue-specific cues. However, the transcriptional regulators governing these particular tissue-specific programs remain to be discovered.
SUMMARY
Immunological memory is a hallmark of the adaptive immune system and the foundation of vaccines, which is entirely dependent upon the formation of a large pool of functionally diverse memory cells. However, designing vaccines is clearly not a “one-size-fits-all” approach, as there are still human endemic diseases for which traditional antibody-based vaccines have not been successful, as with HIV (Burton 2019) and malaria (Wilson et al. 2019). Ideally, memory T cells protect the host at portals of entry like mucosal surfaces, but also systemically in the blood and internal organs—a feat that is currently only achieved through natural infection. Therefore, a greater understanding of the spatial and temporal dynamics of memory cell formation is needed if protective-induced immunity is to be achieved.
Although this review has largely focused on the trafficking patterns of CD8+ T cells that influence their responses to acute pathogens, the concepts described certainly apply to other disease settings like autoimmunity and cancer. In fact, CD8+ T-cell infiltration into tumors has been identified as a major prognostic for favorable outcomes in multiple tumor types (Galon et al. 2006; Gooden et al. 2011; Mahmoud et al. 2011; Tumeh et al. 2014). However, a major challenge in cancer immunotherapy is understanding the mechanisms by which antitumor T cells traffic into tumors and the methods that immunologically “cold” tumors use to prevent proper immune cell trafficking. Several studies in mice have demonstrated the utmost importance of CXCR3 in driving chemotactic migration of CD8+ T cells into tumors (Mikucki et al. 2015) and enabling the antitumor response after PD-1 blockade in models of colon carcinoma (Chow et al. 2019). Similarly, pre- and posttreatment levels of the CXCR3 ligands in melanoma patient plasma and tumor biopsies correlated with response to immune checkpoint blockade (Ji et al. 2012; Chow et al. 2019).
Developments in single-cell omics technologies have significantly advanced our understanding of immune cell functional diversity. Combining these methodologies with spatial omics will be instrumental for further elaborating upon the spatiotemporal dynamics of CD8+ T-cell differentiation, trafficking, and memory cell maintenance in situ. Understanding how these dynamics are transcriptionally regulated by environmental signals will certainly aid in the design of vaccines that induce protective immunity against emerging pathogens in the form of both tissue and circulating memory T cells.
Footnotes
Editors: David Masopust and Rafi Ahmed
Additional Perspectives on T-Cell Memory available at www.cshperspectives.org
REFERENCES
- Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, Youngblood BA, Abdelsamed HA, McGuire DJ, Cohen KW, et al. 2017. Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552: 362–367. 10.1038/nature24633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre YO, Ghilas S, Sanchez C, Le Bon A, Crozat K, Dalod M. 2016. XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses. J Exp Med 213: 75–92. 10.1084/jem.20142350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Marée AFM, Zal T, et al. 2012. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci 109: 19739–19744. 10.1073/pnas.1208927109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. 2014. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346: 101–105. 10.1126/science.1254803 [DOI] [PubMed] [Google Scholar]
- Arojo OA, Ouyang X, Liu D, Meng T, Kaech SM, Pereira JP, Su B. 2018. Active mTORC2 signaling in naive T cells suppresses bone marrow homing by inhibiting CXCR4 expression. J Immunol 201: 908–915. 10.4049/jimmunol.1800529 [DOI] [PubMed] [Google Scholar]
- Bajénoff M, Egen JG, Qi H, Huang AYC, Castellino F, Germain RN. 2007. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol 28: 346–352. 10.1016/j.it.2007.06.005 [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. 2010. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol 185: 4988–4992. 10.4049/jimmunol.1002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankovich AJ, Shiow LR, Cyster JG. 2010. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem 285: 22328–22337. 10.1074/jbc.M110.123299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195: 1541–1548. 10.1084/jem.20020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TC, Coley SM, Wherry EJ, Ahmed R. 2005. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol 174: 1269–1273. 10.4049/jimmunol.174.3.1269 [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Bevan MJ, Fink PJ. 2017. Local inflammatory cues regulate differentiation and persistence of CD8+ tissue-resident memory T cells. Cell Rep 19: 114–124. 10.1016/j.celrep.2017.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohineust A, Garcia Z, Beuneu H, Lemaître F, Bousso P. 2018. Termination of T cell priming relies on a phase of unresponsiveness promoting disengagement from APCs and T cell division. J Exp Med 215: 1481–1492. 10.1084/jem.20171708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Höchst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, et al. 2015. Functional classification of memory CD8+ T cells by CX3CR1 expression. Nat Commun 6: 1–17. 10.1038/ncomms9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR. 2019. Advancing an HIV vaccine; advancing vaccinology. Nat Rev Immunol 19: 77–78. 10.1038/s41577-018-0103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. 2006. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442: 299–302. 10.1038/nature04882 [DOI] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. 2012. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 188: 4866–4875. 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. 2006. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell–dendritic cell interaction. Nature 440: 890–895. 10.1038/nature04651 [DOI] [PubMed] [Google Scholar]
- Chaix J, Nish SA, Lin WHW, Rothman NJ, Ding L, Wherry EJ, Reiner SL. 2014. Cutting edge: CXCR4 is critical for CD8+ memory T cell homeostatic self-renewal but not rechallenge self-renewal. J Immunol 193: 1013–1016. 10.4049/jimmunol.1400488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo DK, Murali-Krishna K, Anita R, Ahmed R. 2010. Homeostatic turnover of virus-specific memory CD8 T cells occurs stochastically and is independent of CD4 T cell help. J Immunol 185: 3436–3444. 10.4049/jimmunol.1001421 [DOI] [PubMed] [Google Scholar]
- Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, Freeman GJ, Boland GM, Luster AD. 2019. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity 50: 1498–1512.e5. 10.1016/j.immuni.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N, Han SJ, Enamorado M, Link VM, Huang B, Moseman EA, Kishton RJ, Shannon JP, Dixit D, Schwab SR, et al. 2019. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178: 1088–1101.e15. 10.1016/j.cell.2019.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Joshi NS, Jiang A, Kaech SM. 2009. Effects of Signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 27: 2177–2187. 10.1016/j.vaccine.2009.01.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, Kitano S, Ishii M, Tani-ichi S, Ikuta K. 2014. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc Natl Acad Sci 111: 1915–1920. 10.1073/pnas.1318281111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. 2012. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Ann Rev Immunol 30: 69–94. 10.1146/annurev-immunol-020711-075011 [DOI] [PubMed] [Google Scholar]
- Dominguez CX, Amezquita RA, Guan T, Marshall HD, Joshi NS, Kleinstein SH, Kaech SM. 2015. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J Exp Med 212: 2041–2056. 10.1084/jem.20150186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Güttler S, Hutloff A, Mages HW, Ranke K, Schaefer M, et al. 2009. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity 31: 823–833. 10.1016/j.immuni.2009.08.027 [DOI] [PubMed] [Google Scholar]
- Dunbar PR, Cartwright EK, Wein AN, Tsukamoto T, Li ZRT, Kumar N, Uddbäck IE, Hayward SL, Ueha S, Takamura S, et al. 2020. Pulmonary monocytes interact with effector T cells in the lung tissue to drive TRM differentiation following viral infection. Mucosal Immunol 13: 161–171. 10.1038/s41385-019-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, Garbi N, Kaisho T, Germain RN, Kastenmüller W. 2015. Robust anti-viral immunity requires multiple distinct T cell–dendritic cell interactions. Cell 162: 1322–1337. 10.1016/j.cell.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir DE, Gjorstrup P, Filep JG. 2012. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci 109: 14983–14988. 10.1073/pnas.1206641109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterházy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, Mucida D. 2019. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569: 126–130. 10.1038/s41586-019-1125-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell H, Fronseca R, Christo SN, Evrard M, Cruz-Gomez S, Zanluqui NG, von Scheidt B, Freestone D, Park SL, McWilliam HEG, et al. 2020. Organ-specific isoform selection of fatty acid-binding proteins in tissue-resident lymphocytes. Sci Immunol 5: eaay9283. 10.1126/sciimmunol.aay9283 [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960–1964. 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, de la Torre JC, von Andrian UH. 2016. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 45: 1270–1284. 10.1016/j.immuni.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. 2012. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37: 364–376. 10.1016/j.immuni.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Torabi-Parizi P, Germain RN. 2015. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42: 172–185. 10.1016/j.immuni.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. 2002. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med 195: 1515–1522. 10.1084/jem.20020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. 2011. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105: 93–103. 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR, Luster AD. 2011a. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89: 207–215. 10.1038/icb.2010.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR, Luster AD. 2011b. CXCR3 in T cell function. Exp Cell Res 317: 620–631. 10.1016/j.yexcr.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T, Dominguez CX, Amezquita RA, Laidlaw BJ, Cheng J, Henao-Mejia J, Williams A, Flavell RA, Lu J, Kaech SM. 2018. ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8+ T cell fates. J Exp Med 215: 1153–1168. 10.1084/jem.20171352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. 2007. Expression of IL-7 receptor α is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci 104: 11730–11735. 10.1073/pnas.0705007104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Shitara S, Imai K, Miyachi H, Kitano S, Yao H, Tani-ichi S, Ikuta K. 2012. Identification of IL-7–producing cells in primary and secondary lymphoid organs using IL-7–GFP knock-in mice. J Immunol 189: 1577–1584. 10.4049/jimmunol.1200586 [DOI] [PubMed] [Google Scholar]
- Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, Lietzenmayer M, Kroehling L, Takumi A, Kometani K, et al. 2018. KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity 48: 716–729.e8. 10.1016/j.immuni.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. 2008. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol 9: 155–165. 10.1038/ni1557 [DOI] [PubMed] [Google Scholar]
- Hirai T, Zenke Y, Yang Y, Bartholin L, Beura LK, Masopust D, Kaplan DH. 2019. Keratinocyte-mediated activation of the cytokine TGF-β maintains skin recirculating memory CD8+ T cells. Immunity 50: 1249–1261.e5. 10.1016/j.immuni.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz LE, Prier JE, Freestone D, Steiner TM, English K, Johnson DN, Mollard V, Cozijnsen A, Davey GM, Godfrey DI, et al. 2018. CD8+ T cell activation leads to constitutive formation of liver tissue-resident memory T cells that seed a large and flexible niche in the liver. Cell Rep 25: 68–79.e4. 10.1016/j.celrep.2018.08.094 [DOI] [PubMed] [Google Scholar]
- Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, Brasser G, Jongejan A, Jonkers RE, Nota B, et al. 2016. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol 17: 1467–1478. 10.1038/ni.3589 [DOI] [PubMed] [Google Scholar]
- Hu JK, Kagari T, Clingan JM, Matloubian M. 2011. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc Natl Acad Sci 108: E118–E127. 10.1073/pnas.1101881108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S, Martínez-López M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, Del Fresno C, Sancho D. 2016. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1+ dendritic cells. Immunity 45: 847–860. 10.1016/j.immuni.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Iwasaki A. 2014. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346: 93–98. 10.1126/science.1257530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6: 1236–1244. 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. 2008. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321: 408–411. 10.1126/science.1159806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21: 527–538. 10.1016/j.immuni.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, et al. 2012. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 61: 1019–1031. 10.1007/s00262-011-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B, Harris TH, Tait ED, Wilson EH, Gregg B, Ng LG, Mrass P, Roos DS, Dzierszinski F, Weninger W, et al. 2009. Dynamic imaging of CD8+ T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog 5: e1000505. 10.1371/journal.ppat.1000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27: 281–295. 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. 2010. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol 185: 5315–5325. 10.4049/jimmunol.1001948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YW, Kim HG, Perry CJ, Kaech SM. 2016. CCR7 expression alters memory CD8 T-cell homeostasis by regulating occupancy in IL-7–and IL-15–dependent niches. Proc Natl Acad Sci 113: 8278–8283. 10.1073/pnas.1602899113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Nakano H, Dumrese T, Kakiuchi T, Odermatt B, Zinkernagel RM, Hengartner H, Ludewig B. 2002. Antiviral immune responses in the absence of organized lymphoid T cell zones in plt/plt mice. J Immunol 168: 6032–6040. 10.4049/jimmunol.168.12.6032 [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. 2007. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27: 393–405. 10.1016/j.immuni.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4: 1191–1198. 10.1038/ni1009 [DOI] [PubMed] [Google Scholar]
- Kastenmüller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. 2013. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity 38: 502–513. 10.1016/j.immuni.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Won A, Refaeli Y, Van Parijs L. 2002. IL-2 and related cytokines can promote T cell survival by activating AKT. J Immunol 168: 597–603. 10.4049/jimmunol.168.2.597 [DOI] [PubMed] [Google Scholar]
- Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. 2009. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol 10: 176–184. 10.1038/ni.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kunkel EJ, Boisvert J, Johnston B, Campbell JJ, Genovese MC, Greenberg HB, Butcher EC. 2001. Bonzo/CXCR6 expression defines type 1–polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest 107: 595–601. 10.1172/JCI11902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. 2012. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol 188: 4305–4314. 10.4049/jimmunol.1103568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Gorski SA, Hahn S, Murphy KM, Braciale TJ. 2014. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8+ T cell differentiation by a CD24-dependent mechanism. Immunity 40: 400–413. 10.1016/j.immuni.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi M, Kurachi J, Suenaga F, Tsukui T, Abe J, Ueha S, Tomura M, Sugihara K, Takamura S, Kakimi K, et al. 2011. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med 208: 1605–1620. 10.1084/jem.20102101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM. 2014. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41: 633–645. 10.1016/j.immuni.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. 2011. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol 85: 4085–4094. 10.1128/JVI.02493-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. 2005. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood 106: 3432–3439. 10.1182/blood-2005-04-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Lu X, Dang X. 2012. FOXO1 up-regulates human l-selectin expression through binding to a consensus FOXO1 motif. Gene Regul Syst Bio 6: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low JS, Farsakoglu Y, Amezcua Vesely MC, Sefik E, Kelly JB, Harman CCD, Jackson R, Shyer JA, Jiang X, Cauley LS, et al. 2000. Tissue-resident memory T cell reactivation by diverse antigen-presenting cells imparts distinct functional responses. J Exp Med 217: e20192291. 10.1084/jem.20192291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. 2000. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci 97: 12694–12699. 10.1073/pnas.97.23.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Asolina Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T. 2015a. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 194: 2059–2063. 10.4049/jimmunol.1402256 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz Gabrielle T, et al. 2015b. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43: 1101–1111. 10.1016/j.immuni.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Minnich M, Kragten NAM, Liao Y, Nota B, Seillet CY, Zaid A, Man K, Preston S, Freestone D, et al. 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352: 459–463. 10.1126/science.aad2035 [DOI] [PubMed] [Google Scholar]
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. 2011. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29: 1949–1955. 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- Mani V, Bromley SK, Äijö T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. 2019. Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 366: eaav5728. 10.1126/science.aav5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. 2000. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol 1: 298–304. 10.1038/79738 [DOI] [PubMed] [Google Scholar]
- Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, et al. 2005. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity 22: 259–270. 10.1016/j.immuni.2005.01.008 [DOI] [PubMed] [Google Scholar]
- McNamara H, Cai Y, Wagle MV, Sontani Y, Roots CM, Miosge LA, O'Connor JH, Sutton HJ, Ganusov VV, Heath WR, et al. 2017. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci Immunol 2: eaaj1996. 10.1126/sciimmunol.aaj1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikucki M, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Felinger JG, Odunsi K, Gajweski TF, et al. 2015. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CN, Hartigan-O'Connor DJ, Lee MS, Laidlaw G, Cornelissen IP, Matloubian M, Coughlin SR, McDonald DM, McCune JM. 2013. IL-7 production in murine lymphatic endothelial cells and induction in the setting of peripheral lymphopenia. Int Immunol 25: 471–483. 10.1093/intimm/dxt012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, et al. 2017. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552: 253–257. 10.1038/nature24993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424: 88–93. 10.1038/nature01726 [DOI] [PubMed] [Google Scholar]
- Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. 2001. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J Exp Med 193: 207–218. 10.1084/jem.193.2.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, Malynn BA, Ma A. 2009. Macrophage- and dendritic-cell-derived interleukin-15 receptor α supports homeostasis of distinct CD8+ T cell subsets. Immunity 31: 811–822. 10.1016/j.immuni.2009.09.017 [DOI] [PubMed] [Google Scholar]
- Nikolova G, Weiss S, Bosnjak B, Förster R. 2020. Differential retention of lymph-borne CD8 memory T cell subsets in the subcapsular sinus of resting and inflamed lymph nodes. Cell Mol Immunol 10.1038/s41423-020-0451-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Lefrançois L. 2010. Early signals during CD8+ T cell priming regulate the generation of central memory cells. J Immunol 185: 263–272. 10.4049/jimmunol.1000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. 2013. Effector-like CD8+ T cells in the memory population mediate potent protective immunity. Immunity 38: 1250–1260. 10.1016/j.immuni.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omilusik KD, Best JA, Yu B, Goossens S, Weidemann A, Nguyen JV, Seuntjens E, Stryjewska A, Zweier C, Roychoudhuri R, et al. 2015. Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J Exp Med 212: 2027–2039. 10.1084/jem.20150194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omilusik KD, Nadjsombati MS, Shaw LA, Yu B, Milner JJ, Goldrath AW. 2018. Sustained Id2 regulation of E proteins is required for terminal differentiation of effector CD8+ T cells. J Exp Med 215: 773–783. 10.1084/jem.20171584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, et al. 2012. IL-7–producing stromal cells are critical for lymph node remodeling. Blood 120: 4675–4683. 10.1182/blood-2012-03-416859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozga AJ, Moalli F, Abe J, Swoger J, Sharpe J, Zehn D, Kreutzfeldt M, Merkler D, Ripoll J, Stein JV. 2016. pMHC affinity controls duration of CD8+ T cell–DC interactions and imprints timing of effector differentiation versus expansion. J Exp Med 213: 2811–2829. 10.1084/jem.20160206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr MB, Parr EL. 1999. The role of γ interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology 258: 282–294. 10.1006/viro.1999.9739 [DOI] [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. 2003. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302: 1041–1043. 10.1126/science.1090148 [DOI] [PubMed] [Google Scholar]
- Rao RR, Li Q, Bupp MRG, Shrikant PA. 2012. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity 36: 374–387. 10.1016/j.immuni.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. 2004. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell–mediated immune protection against heterologous influenza infection. Immunity 20: 167–179. 10.1016/S1074-7613(04)00021-4 [DOI] [PubMed] [Google Scholar]
- Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. 2001. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell–attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med 194: 1541–1547. 10.1084/jem.194.10.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz AL, Soudja SMH, Deceneux C, Lauvau G, Marie JC. 2014. NK1.1+ CD8+ T cells escape TGF-β control and contribute to early microbial pathogen response. Nat Commun 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. 2009. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31: 296–308. 10.1016/j.immuni.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- Sanjabi S, Mosaheb MM, Flavell RA. 2009. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity 31: 131–144. 10.1016/j.immuni.2009.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, Butcher EC. 2005. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol 174: 277–283. 10.4049/jimmunol.174.1.277 [DOI] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. 2013. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol 14: 509–513. 10.1038/ni.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Masopust D. 2014a. Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol 192: 2961–2964. 10.4049/jimmunol.1400003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. 2014b. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346: 98–101. 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, Masopust D. 2016. IL-15–independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J Immunol 196: 3920–3926. 10.4049/jimmunol.1502337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Lefrançois L. 2003. Cytokine control of memory T-cell development and survival. Nat Rev Immunol 3: 269–279. 10.1038/nri1052 [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrançois L. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1: 426–432. 10.1038/80868 [DOI] [PubMed] [Google Scholar]
- Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. 2008. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol 9: 292–300. 10.1038/ni1565 [DOI] [PubMed] [Google Scholar]
- Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, Rossjohn J, Perlmutter P, Cao J, Godfrey DI, et al. 2010. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell–licensed DCs. Nat Immunol 11: 313–320. 10.1038/ni.1848 [DOI] [PubMed] [Google Scholar]
- Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrançois L. 2014. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity 40: 747–757. 10.1016/j.immuni.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdičková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. 2006. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440: 540–544. 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. 2007. DCs metabolize sunlight-induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8: 285–293. 10.1038/ni1433 [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slütter B, Pewe LL, Kaech SM, Harty JT. 2013. Lung airway-surveilling CXCR3hi memory CD8+ T cells are critical for protection against influenza A virus. Immunity 39: 939–948. 10.1016/j.immuni.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg AJ, Kamm MA, Knight SC. 2002. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur J Immunol 32: 1445–1454. [DOI] [PubMed] [Google Scholar]
- Stein JV, Rot A, Luo Y, Narasimhaswamy M, Nakano H, Gunn MD, Matsuzawa A, Quackenbush EJ, Dorf ME, von Andrian UH. 2000. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function–associated antigen 1–mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J Exp Med 191: 61–76. 10.1084/jem.191.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida H, Cyster JG. 2018. G-protein coupled receptor 18 contributes to establishment of the CD8 effector T cell compartment. Front Immunol 9: 660. 10.3389/fimmu.2018.00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. 2012. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell 150: 1249–1263. 10.1016/j.cell.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse SW, Radtke AJ, Espinosa DA, Cockburn IA, Zavala F. 2014. The chemokine receptor CXCR6 is required for the maintenance of liver memory CD8+ T cells specific for infectious pathogens. J Infect Dis 210: 1508–1516. 10.1093/infdis/jiu281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515: 568–571. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsoeld H, Voehringer D, Krautwald S, Pircher H. 2004. Constitutive expression of CCR7 directs effector CD8 T cells into the splenic white pulp and impairs functional activity. J Immunol 173: 3013–3019. 10.4049/jimmunol.173.5.3013 [DOI] [PubMed] [Google Scholar]
- Utzschneider DT, Delpoux A, Wieland D, Huang X, Lai CY, Hofmann M, Thimme R, Hedrick SM. 2018. Active maintenance of T cell memory in acute and chronic viral infection depends on continuous expression of FOXO1. Cell Rep 22: 3454–3467. 10.1016/j.celrep.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliou EK, Kesler OM, Tadros JH, Ganea D. 2008. Bone marrow–derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. J Immunol 181: 4534–4544. 10.4049/jimmunol.181.7.4534 [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. 2003. Homing and cellular traffic in lymph nodes. Nat Rev Immunol 3: 867–878. 10.1038/nri1222 [DOI] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Bevan MJ. 2010. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci 107: 17872–17879. 10.1073/pnas.1010201107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wein AN, McMaster SR, Takamura S, Dunbar PR, Cartwright EK, Hayward SL, McManus DT, Shimaoka T, Ueha S, Tsukui T, et al. 2019. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med 216: 2748–2762. 10.1084/jem.20181308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. 2009. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity 31: 122–130. 10.1016/j.immuni.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 4: 225–234. 10.1038/ni889 [DOI] [PubMed] [Google Scholar]
- Wilson K, Flanagan K, Prakash M, Plebanski M. 2019. Malaria vaccines in the eradication era: current status and future perspectives. Exp Rev Vaccines 18: 133–151. 10.1080/14760584.2019.1561289 [DOI] [PubMed] [Google Scholar]
- Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. 2008. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111: 2101–2111. 10.1182/blood-2007-06-096297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin A, Masson F, Liao Y, Preston S, Guan T, Gloury R, Olshansky M, Lin J-X, Li P, Speed TP, et al. 2016. Molecular threshold for effector CD8+ T cell differentiation controlled by transcription factors Blimp-1 and T-bet. Nat Immunol 17: 422–432. 10.1038/ni.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Hale JS, Kissick HT, Ahn E, Xu X, Wieland A, Araki K, West EE, Ghoneim HE, Fan Y, et al. 2017. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552: 404–409. 10.1038/nature25144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chen Y, Wu Y, Ye L, Lian Z, Wei H, Sun R, Tian Z. 2017. The differential organogenesis and functionality of two liver-draining lymph nodes in mice. J Autoimmun 84: 109–121. 10.1016/j.jaut.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Zaid A, Hor JL, Christo SN, Groom JR, Heath WR, Mackay LK, Mueller SN. 2017. Chemokine receptor–dependent control of skin tissue–resident memory T cell formation. J Immunol 199: 2451–2459. 10.4049/jimmunol.1700571 [DOI] [PubMed] [Google Scholar]
- Zammit DJ, Cauley LS, Pham QM, Lefrançois L. 2005. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity 22: 561–570. 10.1016/j.immuni.2005.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Lee SY, Bevan MJ. 2009. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458: 211–214. 10.1038/nature07657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. 2013. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39: 687–696. 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao D-M, Harty JT, Badovinac VP, Xue H-H. 2010. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33: 229–240. 10.1016/j.immuni.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. 2010. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol 185: 3174–3183. 10.4049/jimmunol.1000749 [DOI] [PubMed] [Google Scholar]