Abstract
Background & Aims:
Restorative proctocolectomy with ileal pouch-anal anastomosis is a surgical procedure in patients with ulcerative colitis refractory to medical therapies. Pouchitis, the most common complication, is inflammation of the pouch of unknown etiology. To define how the intestinal immune system is distinctly organized during pouchitis, we analyzed tissues from patients with and without pouchitis and from patients with ulcerative colitis using single-cell RNA sequencing (scRNA-seq).
Methods:
We examined pouch lamina propria CD45+ hematopoietic cells from intestinal tissues of ulcerative colitis patients with (n=15) and without an ileal pouch-anal anastomosis (n=11). Further in silico meta-analysis was performed to generate transcriptional interaction networks and identify biomarkers for patients with inflamed pouches.
Results:
In addition to tissue-specific signatures, we identified a population of IL1B/LYZ+ myeloid cells and FOXP3/BATF+ T cells that distinguish inflamed tissues which we further validated in other single cell RNA-seq datasets from IBD patients. Cell type specific transcriptional markers obtained from single-cell RNA-sequencing was used to infer representation from bulk RNA sequencing datasets, which further implicated myeloid cells expressing IL1B and S100A8/A9 calprotectin as interacting with stromal cells, and Bacteroidiales and Clostridiales bacterial taxa. We found that non-responsiveness to anti-integrin biologic therapies in ulcerative colitis patients was associated with the signature of IL1B+/LYZ+ myeloid cells in a subset of patients.
Conclusions:
Features of intestinal inflammation during pouchitis and ulcerative colitis are similar, which may have clinical implications for the management of pouchitis. scRNA-seq enables meta-analysis of multiple studies, which may facilitate the identification of biomarkers to personalize therapy for IBD patients.
Keywords: Inflammatory bowel disease, mucosal immunology, single cell RNA-Seq, inflammation
Lay Summary
Single cell analysis of immune cells in the colon and pouch of ulcerative colitis patients uncovers similar gene programs which contribute to inflammation and disease severity.
Introduction
The intestinal immune system is organized distinctly between anatomically defined segments with different physiological functions. While immune cells in the small intestine protect the epithelium from infection to enable nutrient absorption while maintaining tolerance to dietary antigens, the colon must maintain a detente with a large number of bacteria without triggering overt inflammation. This fine balance breaks down in the context of inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD), whereby inflammatory damage to the epithelium results in mucosal ulceration causing diarrhea, bleeding, and abdominal pain. Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is a common surgical procedure in UC patients with medically refractory disease1. The ileal pouch reservoir, or J-pouch, is a novel organ created from small intestine formed into a J-shaped pouch that restores intestinal continuity. Unfortunately, nearly 50% of UC patients who undergo IPAA develop de novo intestinal inflammation in the pouch, or pouchitis, with symptoms similar to IBD2. The etiology of pouchitis is unknown and there are no FDA approved therapies. Treatment usually entails chronic antibiotics and often results in disease relapses. Increased resolution of pouchitis may improve our understanding of these conditions.
Single-cell RNA sequencing (scRNA-seq) has enabled high resolution characterization of tissue to better understand human diseases. Surveys of intestinal tissues have identified unknown subtypes of intestinal epithelial cells and cellular inflammation modules that predict treatment responsiveness3–6. In the field of IBD, this approach is providing new insights and opportunities to define drug targets and personalized treatment options7. Recently, high dimensional profiling of ileal CD lesions identified a network incorporating lymphoid and myeloid subsets that predicts lack of a durable response to TNFα blockade8. Therefore, investigating immune cell subsets across IBD manifestations involving different regions of the gut may facilitate therapeutic strategies.
In mice, peripheral regulatory T cells (pTregs) and Th17 cell numbers are differentially regulated in the small intestine versus colon in response to region-specific exposure or sampling of food antigens, metabolites, and microbes9–12. Homing signals, structural features inherent to the organ, and compartmentalized draining by lymph nodes can also contribute to the localization and function of immune cell subsets13,14. It is unclear whether the inflamed pouch harbors immune infiltrates similar to the inflamed colon or retains immune characteristics of the small intestine. A previous study using bulk RNA-Seq showed that, in addition to acquiring colonic markers, the pouch is enriched for transcripts related to IL-17 signaling and dendritic cell (DC) maturation when compared with the small intestine15. Therefore, a detailed survey of immune cells may provide novel insight into pouchitis. Here, we utilized scRNA-seq to examine immune cells and their activation states from biopsies collected from the J-pouch and colon of UC patients, with the goal of identifying shared and distinctive features of inflammation in each site.
Materials and Methods
Study Design and Patient metadata
15 patients with J-pouch and 13 patients with UC (Table S2) were recruited and consented at the IBD Center under an NYU Langone Health IRB-approved study (S12-01137). Endoscopic appearance determined inflammatory activity. All UC patients (n=11) had a Mayo endoscopic subscore of ≥216. J-pouch patients were stratified into an endoscopic pouchitis cohort (n=10), with a pouchitis disease activity index (PDAI) endoscopic subscore ≥2 or a normal J-pouch cohort (n=5) for those with a PDAI endoscopic subscore <217 (Table S2). Potential participants were excluded if clinical presentation was consistent with “Crohn’s disease of the pouch”, defined by the presence of fistula/fistulae, stricture involving the pouch or prepouch ileum, and the presence of long-segment prepouch ileitis, or if they did not provide tissue.
Biopsies
Typically, 6 pinch biopsies were obtained from each patient, however fewer biopsies were obtained from severely inflamed patients to reduce risk of excessive bleeding and perforation, which may have contributed to fewer recovered cells in inflamed samples. For UC patients, all biopsies were obtained from the rectum. For J-pouch patients, all biopsies were obtained from the pouch body or inlet. If active endoscopic inflammation was present, this area was targeted. For each location sampled, one biopsy was collected for standard histopathology assessment and read by two expert pathologists for the PDAI histology subscore and histologic pouch activity score (PAS)17,18. Batch testing FBS lots from multiple vendors for the freezing media was extremely important for optimizing recovery of cells. Cryopreserved biopsies were gently thawed at 37°C and enzymatically digested in collagenase VIII (Sigma) and DNase (Sigma) for 1h to obtain single cell suspensions for sorting CD45+ cells on a Sony SY3200 cell sorter (live/dead, CD45 PE-Cy7, CD3 PerCP-Cy5.5, CD19 PE, CD14 FITC, and CD16 Pacific Blue (BioLegend) for scRNA-seq.
Single cell library and sequencing
Approximately 10,000 CD45+ cells were loaded per channel on a 10x Genomics Chromium instrument to generate single-cell gel beads in emulsion (GEMs). scRNA-Seq libraries were prepared using the following Single Cell 3’ Reagent Kits v2: Chromium™ Single Cell 3’ Library & Gel Bead Kit v2, Single Cell 3’ Chip Kit v2, and i7 Multiplex Kit (catalog# PN-120237, PN-120236, # PN-120262, 10x Genomics)19 and following the Single Cell 3’ Reagent Kits v2 User Guide (Manual Part # CG00052), Rev A. Libraries were run on Illumina HiSeq 4000 as 2×150 paired-end reads, one full lane per sample, for approximately >90% sequencing saturation. There were some differences in downstream cell recovery, especially for inflamed samples for which fewer biopsies were obtained. Samples that failed to meet a minimum of 1,000 sequenced cells were deemed poor quality and removed from subsequent analysis. Quantitative analysis was performed in R and discussed in detail in the supplementary methods. The processed single cell count tables are provided in Gene Expression Omnibus; GSE162335. Raw sequence data are not public and are protected by controlled-access for patient privacy.
Immunohistochemistry staining for FOXP3+ cells
FOXP3 nuclear expression, stained with 3,3-Diaminobenzidine (DAB) was digitally quantified by QuPath20 using hematoxylin as background staining. Images were thresholded using binary categorization of positive (DAB, brown stain) and negative (blue stain). To detect and count FOXP3 nuclei, the cell analysis mode including “cell detection command” was used as previously published21. Values were exported and significance was determined by nested ANOVA correcting for multiple data points per patient.
All authors had access to study data and reviewed and approved the final manuscript.
Results
Immune cell transcriptional landscape of J-pouch and colon biopsies from UC patients.
To minimize batch effects and streamline processing, we established a cryopreservation pipeline to store and analyze leukocytes from intestinal biopsies (Figure S1). Optimizing freezing medium (Methods) enabled a robust yield of live immune cells that could be sorted for high quality transcriptome analysis. Cells were sorted for CD45+ surface expression and sequencing on the 10X platform. Nearly 56,000 CD45+ cells were obtained from scRNA-seq of 26 frozen biopsies from inflamed UC (18,375 cells, n=11 samples, median 2,000 cells per sample), pouchitis (20,678 cells, n=10 samples, median 1,500 cells per sample) and uninflamed pouch (16,678 cells, n=5 samples, median 3,500 cells per sample). Single cell transcriptomes from all samples were normalized and merged with Seurat version 322 (Figure S2A). Over half of the profiled cells, 30,863 cells, were from T cell subsets, 21,324 cells from B cell subsets and the remaining 3,747 cells were myeloid subsets (Fig. 1A). Specifically, we identified T cells, germinal center/follicular cells, plasma cells, cycling B cells, mast cells and monocyte/macrophages using indicated markers (Fig. 1B,D, Supplementary Table 3). Relative percentages of these 6 major populations did not separate inflamed versus uninflamed or pouch versus UC samples by principle component analysis (PCA) (Fig. S2B–C), indicating there is not a discernible difference in the profiles of major immune cells between UC colon and pouch. When examining inter-individual variation in the 6 major populations, there was a significantly greater proportion of monocyte/macrophages in pouchitis and inflamed UC patient samples compared to uninflamed pouch by a Wilcox test (Fig. 1C, Supplementary Table 3), indicating the inflammatory condition was most consistently driven by differences in myeloid cells.
Figure 1. Immune cell landscape of pouch and colon biopsies from UC patients.
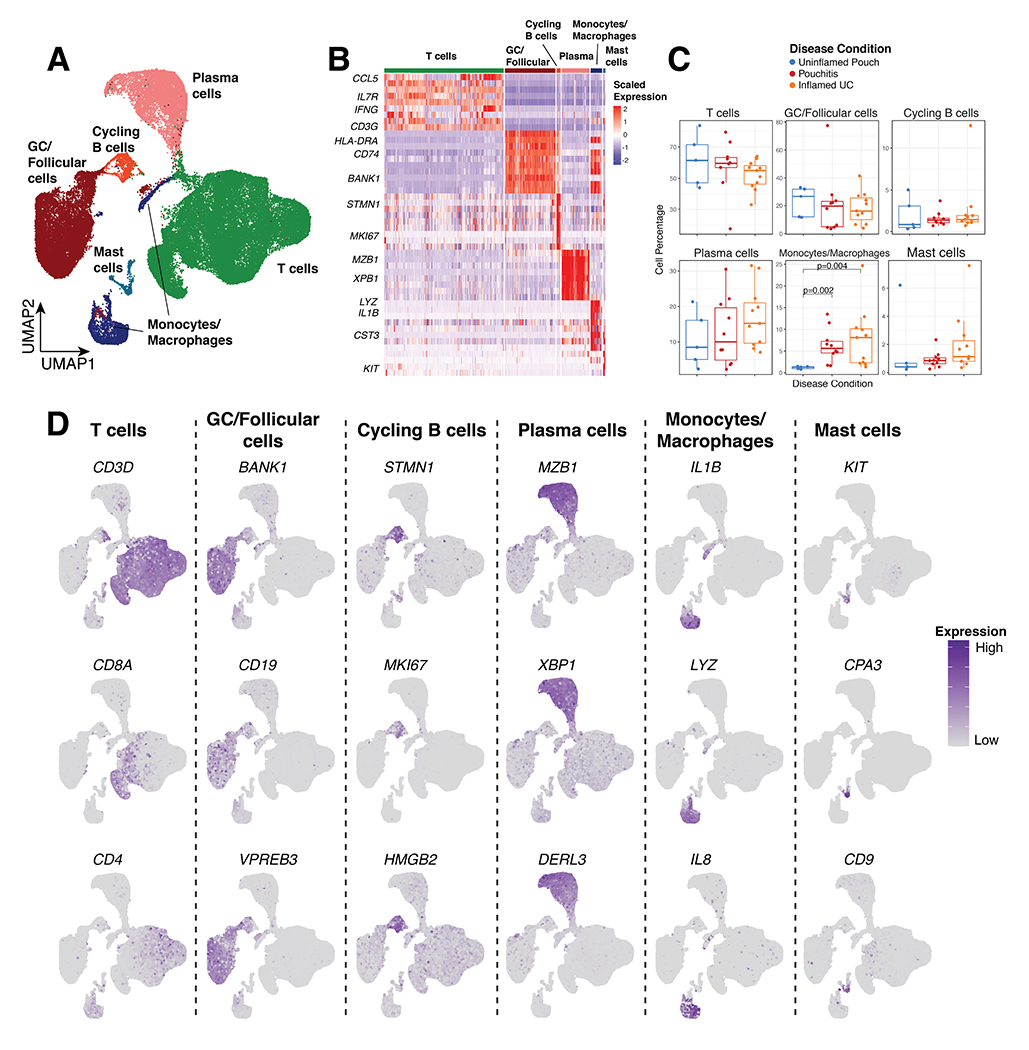
(A) UMAP of major immune cell clusters and (B) gene expression of significant marker genes. (C) Relative frequency of clusters as a percentage of total cells per patient. (D) UMAP of marker genes enriched in major cell clusters. P-values indicate significance testing for Wilcoxon ranked test.
Percentages of monocyte/macrophage cells are increased in inflamed patient samples
At least 6 minor myeloid clusters including three distinct types of monocyte/macrophages, mast cells, dendritic cells (DC) and a small population of plasmacytoid DCs (pDCs) were identified (Fig. 2A). Differential expression (DE) analysis identified specific markers for each of the monocyte/macrophage populations (Fig. 2B, Supplementary Table 3), leading us to refer to them as SOX4+/MAFA+, IL1B+/LYZ+ and APOE+/C1QC+ from here on. SOX4+/MAFA+ and IL1B+/LYZ+ monocyte/macrophage populations were more abundant in patients with inflamed UC and pouchitis compared to uninflamed pouches (Fig. 2C, Supplementary Table 3). DCs differed between pouchitis and uninflamed pouches while APOE+/C1QC+ and mast cells had no significant differences (Supplementary Table 3). pDCs were not detected in uninflamed pouches (Fig. 2C).
Figure 2. Increased accumulation of myeloid cell populations in inflamed tissue.
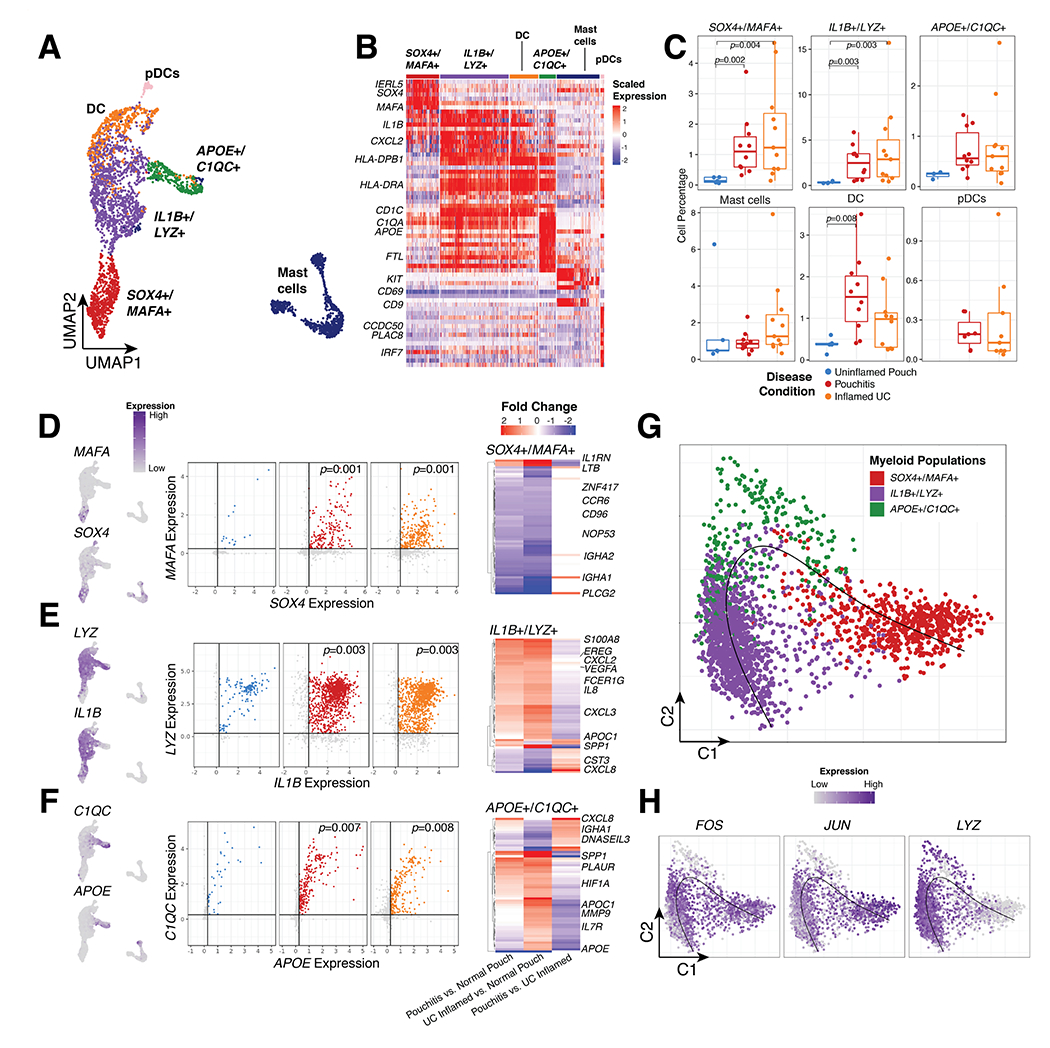
(A) UMAP of myeloid cell types and (B) gene expression of significant markers genes. (C) Relative frequency of major myeloid cell types as a percentage of total cells per patient. (D-F) UMAP of marker genes pairs (left), gene by gene expression plots (center) and differentially expressed genes between Uninflamed Pouch, Pouchitis and UC inflamed samples in 3 major monocyte/macrophage populations. Significantly expressed genes are determined by Log2 fold change >0.75 and adjusted p-value <0.05. Gene by gene expression plots indicate in silico gating at a scaled expression value of 0.25, black lines. gating (G) Diffusion map of monocyte/macrophages clusters (top) with pseudotime projection (black line). (H) Diffusion map of normalized FOS, JUN and LYZ expression. P-values indicate significance testing for Wilcoxon ranked test.
In SOX4+/MAFA+ monocyte/macrophages, SOX4 and MAFA were used as markers for in silico gating to identify cells with enriched expression for both of these genes (Fig. 2D–F). Within individual cells, the relative percentage of SOX4 and MAFA expressing cells are significantly increased in pouchitis and inflamed UC compared to uninflamed pouches (Fig. 2D, Supplementary Table 3). DE between pouch and UC patients in these cells indicated an increase in IL1RN and LTB in inflamed samples compared to uninflamed pouches (Fig. 2D, Supplementary Table 3). LYZ and IL1B expressing cells were also increased in inflamed samples, and DE genes between pouch and UC included increases in EREG, S100A8, CXCL2, VEGFA, IL8 and APOC1 in inflamed samples (Fig. 2E, Supplementary Table 3). Additionally, SPP1, an important macrophage marker for colorectal cancer23, expression was increased in inflamed UC samples compared to uninflamed pouches, although not significantly different between inflamed and uninflamed pouches. APOE and C1QC expressing cells were also associated with inflammation and HIF1A, PLAUR, APOC1, MMP9 and IL7 all increased in inflamed samples (Fig. 2F, Supplementary Table 3). Interestingly, CXCL8 and DNASEIL3 increased in pouchitis compared to UC inflamed samples (Fig 2F, Supplementary Table 3).
Pseudotime analysis restricted to monocyte/macrophages maintained the difference observed in the UMAP (Fig. 2A) between SOX4+/MAFA+ and IL1B+/LYZ+ cells, but suggested APOE+/C1QC+ cells as an intermediary population (Fig. 2G, black line). Specifically, LYZ is strongly associated with pseudotime as a marker of IL1B+/LYZ+ cells (Fig. 2H). FOS and JUN are also associated with pseudotime but were not expressed in APOE+/C1QC+ cells (Fig. 2H). Hence, we identify 3 types of related monocyte/macrophage cell states, all increased with inflammation independent of large or small intestine origin.
T cell subsets differ according to inflammation status
12 T cell clusters were identified and characterized by expression of IFNG, CD8, FOXP3, IL2, ATF3, IL7, TNF, CCL5, GZMB, TOX2 (Fig. 3A, Figure S3A–B). From these subsets we find a cluster of FOXP3+ T cells (Tregs) increased in inflamed UC and pouchitis samples compared to uninflamed pouches (Fig. 3B, Supplementary Table 3). Naive and CD8+ cells were increased in relative percentage in uninflamed pouches and pouchitis samples compared to inflamed UC samples while CD8+ NR4A2+ cells were generally higher in uninflamed pouches compared to pouchitis and UC samples (Fig. 3B, Supplementary Table 3). The relative percentages were not significantly different between patient groups for other T cell subsets (Figure S3C, Supplementary Table 3).
Figure 3. Dysregulated T cell compartment in inflamed tissue.
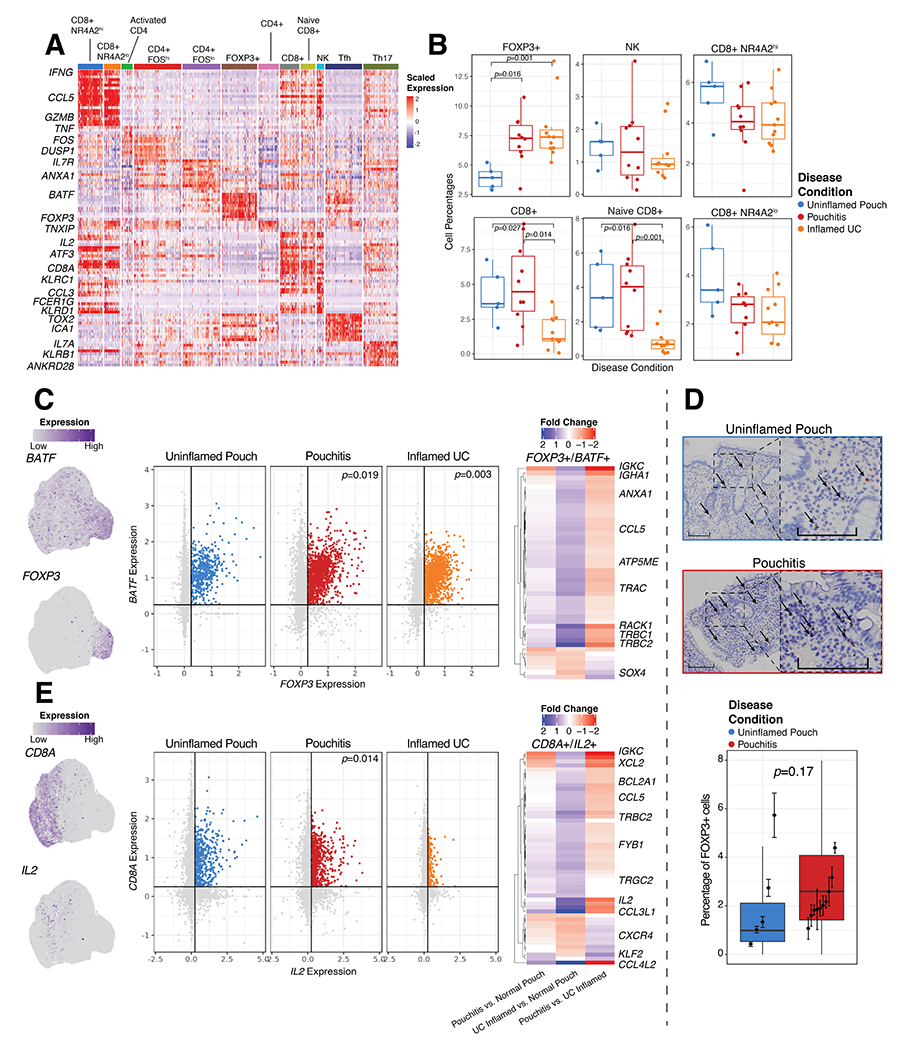
(A) Gene expression of significant markers genes in 12 T cell types. (B) Relative frequency of selected T cell clusters as a percentage of total cells per patient. (C) UMAP (left) and expression plots (center) of FOXP3 and BATF expression in T cells, and DE heat maps of genes with log2 fold change between Uninflamed Pouch, Pouchitis and UC inflamed samples (right) in FOXP3+/BATF+ T cells. (D) Representative FOXP3 stained pouch tissue sections (top) and quantification (bottom), where dark brown colored cells indicate FOXP3 nuclear expression and quantification of percent FOXP3+ cells in 5 uninflamed patient samples and 10 pouchitis patient samples. Significance was determined by. (E) UMAP (left) and expression plots (center) of CD8A and IL2 expression in T cells (left), and DE heat maps of log2 fold change between Uninflamed Pouch, Pouchitis and UC inflamed samples (right) in CD8A+/IL2+ T cells. Significantly differentially expressed genes are determined by Log2 fold change >0.5 and adjusted p-value <0.05. FOXP3 by BATF and CD8A by IL2 expression plot indicate in silico gating at a scaled expression value of 0.25, black lines. P-values indicate significance testing for Wilcoxon ranked test. Scale bars indicate 200μm imaged at 10X magnification.
The marked expansion of FOXP3+ T cells in inflamed samples was consistent with a previous report whereby FOXP3+ regulatory T cells also expressed TNF3. Here, we find that expression of BATF is also an important feature of these cells. In silico gating of FOXP3+/BATF+ expression in T cells clearly shows an increase in pouchitis and inflamed UC samples compared to uninflamed pouches (Fig. 3C, Supplementary Table 3). DE analysis of FOXP3+/BATF+ cells indicated a number of differences between sample groups including an increase in TRBC1, TRBC2, RACK1, ANXA1 and CCL5 in pouchitis compared to UC. We stained for Foxp3 nuclear expression and found increased Foxp3 (p=0.17) in tissue sections from pouchitis (n=10) compared to uninflamed pouch (n=5) (Fig. 3D), consistent with increased Foxp3+ T cells found in actively inflamed UC patients by CYTOF analysis4.
In contrast, both CD8+ and naive CD8+ T cells were less represented in the colon samples from inflamed UC patients compared to pouch samples, regardless of inflammation status (Fig. 3B). Within these CD8+ populations in silico gating of CD8+/IL2+ expression in T cells identified differences between inflamed UC and pouch patients regardless of inflammation (Fig. 3E). IL2 expression was identified as a marker for CD8+ and naive CD8+ T cell subsets (Fig. 3A). Additionally, DE analysis of the CD8+/IL2+ cells indicated IL2, CCL4L2, CCL5, TRBC2, XCL2 and IGKC are all DE genes in both pouchitis and uninflamed pouch compared to inflamed UC (Fig. 3E, Supplementary Table 3). This difference between pouch and colon may reflect the increased proportion of CD8+ T cells observed in the ileum compared with colon24,25. Hence, FOXP3+BATF+ T cells are the most increased population in inflamed samples, whereas CD8+ cells in general are more abundant in the pouch than colon independent of inflammation state.
Tregs may be induced or maintained by intestinal antigen presenting cells such as macrophages and DCs26. While all three monocyte/macrophages subsets were significantly associated with FOPX3+/BATF+ T cells by linear regression (Fig. S3D), SOX4+/MAFA+ cells were most strongly associated. TREM1 is expressed by SOX4+/MAFA+ monocyte/macrophage cells and can be induced by retinoic acid27 which also induces FOXP3+ Treg differentiation. However, apart from CXCL10 expression, these cells do not exhibit many other features of activation (Figure S4).
B cell subsets are similar across disease types
Despite their high frequency in many of the samples, B cells clustered into only 5 clusters (Figure S5A). These subsets include cycling B cells, follicular cells, germinal center cells and two types of plasma cells differing in NFKBIA expression. Traditional B cell markers and differentially expressed transcripts were used to identify these subtypes including STMN1, MKI67, BANK1, LMO2, LY9, MZB1 and XPB1 (Figure S5B, Supplementary Table 3). While there is a slight increase in plasma cell percentages in inflamed UC compared to inflamed and uninflamed pouches, there were no other significant differences in B cell states between groups (Figure S5C, Supplementary Table 3).
Validation of inflammation associated immune cells in public datasets.
Smillie3 et al. and Martin8 et al. recently published single cell datasets for UC and CD, which we used to compare the immune cell populations identified in this study. We integrated over 350,000 immune cells from Smillie3, Martin8 and our scRNA-seq study and observed a conservation of major immune cell types including T cells, GC/follicular cells, plasma cells, mast cells, myeloid cells and a small number of epithelial cells included from Martin et al8. (Fig. 4A). Importantly, we identified 13 T cells clusters including a FOXP3+/BATF+ regulatory T cells (Fig. 4B) expressing IL32 and IL2RA (Fig. 4D) in all 3 studies which were found to be increased strongly in inflamed UC (p=0.017), CD (p=0.041) and Pouch (p=0.028) patient samples when compared to their uninflamed counterparts (Figure 4B–D, Supplementary Table 3). We also observed a marked increase in innate lymphoid cells (ILCs) in CD samples independent of inflammation state (Figure 4C, Supplementary Table 3). These ILCs were marked by DE of ID2 and CXCL2 (Figure 4D, Supplementary Table 3) compared to the other T cell populations. Additionally, we confirmed the presence of IL1B+/LYZ+ inflammatory cells expressing IL8 and CCL4 in all 3 studies which significantly increased in inflamed conditions of CD (p=0.003) and Pouch (p=0.024), but less so in UC (p=0.096), when compared to their uninflamed counterparts (Figure 4F–G, Supplementary Table 3). This inflammatory population, denoted “inflammatory macrophages” in Martin et al8, importantly expresses oncostatin M (OSM)-signature genes previously associated with histological disease severity28 such as IL1A, CXCL2, CXCL3, IL1B, CCL3, CCL4 and IL8 (Fig. 4G). DCs were also identified in all 3 studies but markedly increased in CD. However, inflamed pouches were the only samples to differ from their uninflamed counterpart in DC abundance (p=0.013) (Figure 4F, Supplementary Table 3). DCs were identified by expression of CLEC10A and HLA-DRB1 (Figure 4G). Interestingly, the SOX4+/MAFA+ monocyte population was increased in pouchitis compared to uninflamed pouches, (p=0.012) (Fig. SF6B, Supplementary Table 3) but did not differ significantly in any other comparison. In CD these cells were not frequently detected and reflect less than 1% of the total immune cells captured. Additional populations in the T, B and myeloid cell compartments were investigated (Fig. S6, Supplementary Table 3), but were not found to differ substantially either between disease conditions (UC, CD and pouch) or between inflamed and uninflamed status and are therefore not discussed further.
Figure 4. Defining cell states related in active inflammation in pouch, UC and CD.
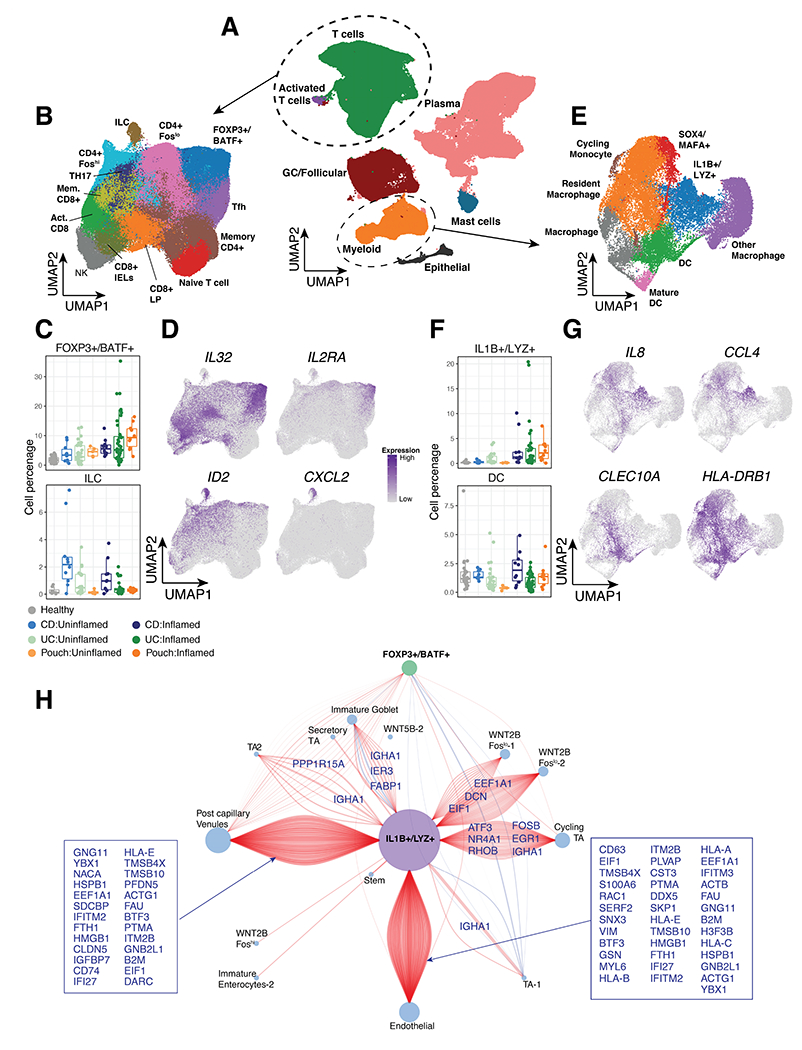
(A) Integrated UMAP of ~350,000 immune cells from inflamed and uninflamed UC, CD and Pouch patient samples and healthy controls. (B) UMAP of T cell clusters. (C) Relative frequency of FOXP3+/BATF+ Tregs (top) and ILC (bottom) cells as a percentage of total cells per patient and (D) UMAP of representative gene expression markers. (E) UMAP of Myeloid cell clusters. (F) Relative frequency of IL1B+/LYZ+ myeloid cells (top) and DC (bottom) cluster as a percentage of total cells per patient and (G) UMAP of representative gene expression markers. (H) Correlation network of IL1B, LYZ, FOXP3 and BATF expression to non-immune cells from Smillie et al. Positive correlations are shown in red and negative correlations in blue. The size of each node is proportional to the number of connections and connections are annotated by the gene correlation in the non-immune cell.
To identify associations between IL1B+/LYZ+ and FOXP3+/BATF+ populations and non-immune cells, we first used linear regression. Using non-immune cell data from Smillie et al. we observed significant associations between the relative percentage of IL1B+/LYZ+ cells with WNT2B+ fibroblasts as well as 6 other non-immune cell types (Fig. SF7). FOXP3+/BATF+ cells were significantly associated with 14 different non-immune cells including WNT2B Foslo cells, Inflammatory Fibroblasts, Enterocytes and Pericytes (Fig. SF7). Next, we assessed gene-by-gene correlations between IL1B, LYZ, FOXP3 and BATF with marker genes in named epithelial cell, fibroblast and endothelial cell clusters from Smillie et al. (Fig. 4H). This analysis associated IL1B/LYZ with WNT2B fibroblasts, post-capillary venules and endothelial cells through correlation with DCN, IFITM2 and CD63 respectively. FOXP3/BATF expression was primarily associated with transcripts from post-capillary venules and expression correlated less frequently with other non-immune cell type markers (Fig. 4H). All together, these findings confirm the presence of IL1B+/LYZ+ and FOXP3+/BATF+ cells in independently generated datasets and suggest their role as key immune cells that interact with other potentially inflammatory populations, including non-immune cells.
Prioritizing inflammatory markers in a large IPAA cohort
We extracted 706 non-overlapping signature genes by DE and outlier analysis29 (Fig. 5A, Table S1, Supplementary Methods) as a signature matrix for the 12 T cell, 5 B cell and 6 myeloid subsets. We also extracted expression data for GWAS IBD risk genes3 (Fig. 5A, S8). 26 of the IBD risk genes were associated with specific immune cell populations, including IFNG in CD8+ T cells, CCL20 and IL23R in Th17 cells, IRF8 in NK cells, HLA-DQB1 in DCs and LY9 in follicular B cells. 38 other IBD-associated genes were found in the scRNA-seq dataset but were not significant markers, indicating that expression of these genes was not specific to a particular immune cell.
Figure 5. Identification of cell type-specific transcripts and analysis of an independent dataset of patients with IPAA.
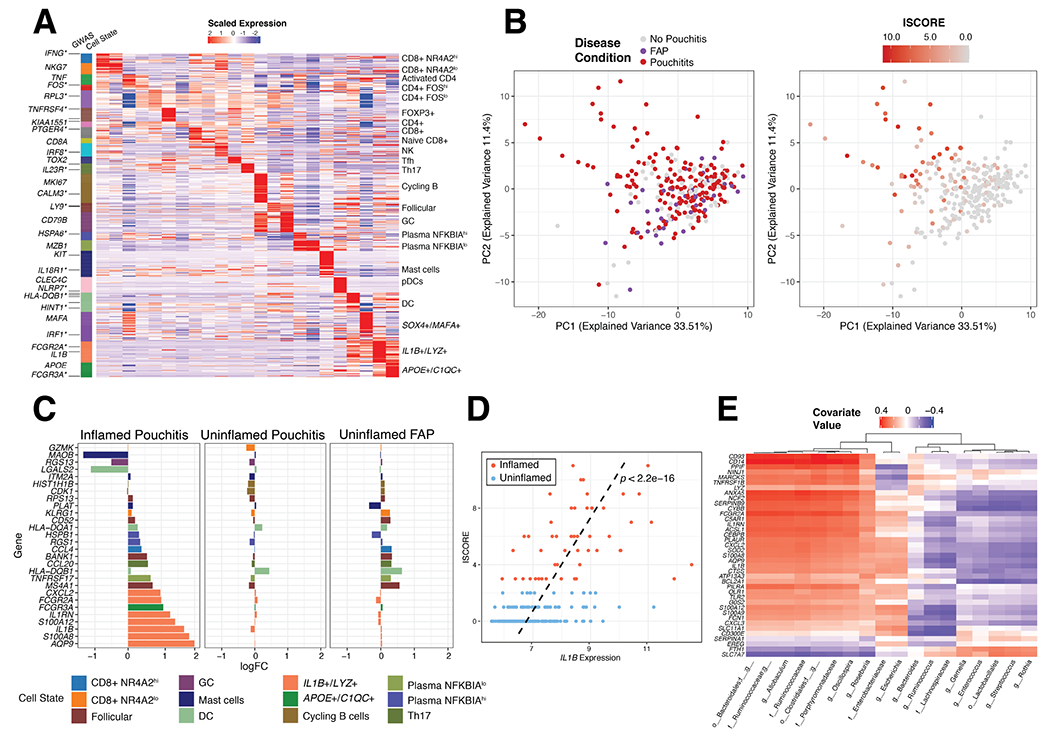
(A) Expression of 706 marker genes for 23 identifiable T, B and Myeloid cell states from scRNA-seq. Asterisks indicate GWAS genes related to IBD. (B) Principal component analysis of 250 IPAA patient samples with familial adenomatous polyposis (FAP), active pouchitis (PI) or no pouchitis (NP) based on the 706-gene signature and colored by disease condition (left) and inflammation score (ISCORE) (right). (C) Top 10, by log fold change, immune-associated marker genes in inflamed (ISCORE>2) Pouchitis, uninflamed (ISCORE<2) Pouchitis and uninflamed FAP patient samples compared to no pouchitis. Fold changes were determined with limma64 version 3.38.3 (D) Linear relationship between Inflammation Score (ISCORE) and IL1B expression was assessed by a linear model with covariates gender and antibiotic treatment within 30 days of visit (yes/no) and the line of best fit from this model was plotted as a dashed black line along with the p-value, p<2.2e-16. (E) sPLS analysis of gene signature for IL1B+/LYZ+ monocyte/macrophages compared to microbial taxa measured by 16S rRNA sequencing of the 250 patient IPAA samples from Morgan et al. Significant genes determined by log2 Fold Change
After generating an immune cell signature matrix, we examined expression of these genes from a study of IPAA patients (n=250) with active pouchitis (PI), no pouchitis (NP) and pouches from familial adenomatous polyposis (FAP) patients that included 16S rRNA microbial profiling from paired biopsies30. FAP pouches rarely develop inflammation and serve as an additional uninflamed control30. Based on the 706-gene signature matrix, there were no appreciable differences between groups of IPAA and FAP patient samples by PCA (Fig. 5B). However, we found greater separation of patient samples in relation to the composite inflammation score (ISCORE)30. Patients with the highest ISCORE contributed most to the separation in PCA space (Fig. 5B). We therefore split the patient samples according to the ISCORE values into uninflamed (ISCORE <2, n=223) and inflamed (ISCORE >2, n=50) and performed DE analysis by inflammation status and disease type against no pouchitis. The most differentially expressed transcripts in inflamed pouchitis samples were related to IL1B+/LYZ+ monocyte/macrophages, marked by IL1B, S100A8, IL1RN and CXCL2 (Fig. 5C). Uninflamed FAP pouch samples included increased expression of HLA-DQB1 (DC) CCL20 (Th17 cells), CD52, BANK1 and MS4A1 (Follicular cells), suggesting that FAP pouches may have a distinct immunological signature despite the lack of active inflammation (Fig. 5C). Expression of the inflammatory marker IL1B alone was significantly related to ISCORE (Fig. 5D) in all patients. With paired samples of 16S rRNA we used sPLS regression of the operational taxonomic unit (OTU) table against IL1B+/LYZ+ signature genes to identify positive associations with the order Clostridiales and Bacteroidiales and negative associations with the genera Streptococcus and Enterococcus (Fig. 5E). Hence, these inflammatory macrophages are associated with specific microbial communities in patients with inflamed pouchitis.
Response to therapies and inflammation status in UC
We next investigated receptor-ligand networks31 between the 23 immune cell subsets using a curated database from cellPhoneDB32, which identified 856 significant interactions in our 706 gene signature matrix (Fig. 6A). IL1B+/LYZ+, DC and APOE+/C1QC+ monocyte/macrophages had the most interacting pairs and are the largest nodes of this network by number of connections, with many significant interactions between the three cell types. Th17 cells represented another major node displaying interactions with DC, IL1B+/LYZ+ monocyte/macrophages and APOE+/C1QC+ monocyte/macrophages, and included activity involving the chemokines CCL3, CCL4, CCL5, HLA-DPB1, and CCL20 with receptors CCR1, CCR4, CCR5, SF13B and CCR6 (Fig. 6B). Costimulatory molecule interactions with their ligands CTLA-4, ICOS, PD-1 and CD40 were also significant between Th17 and FOXP3+ T cells with the IL1B+/LYZ+ and APOE+/C1QC+ monocyte/macrophages populations (Fig. 6B). Other notable interactions relate to cytokines important in disease pathogenesis, such as TNF and CSF33. In summary, this approach enabled identification of IL1B+/LYZ+ monocyte/macrophages interactions with Th17 cells that could be an important component of the immune response during inflammation for these UC patients.
Figure 6. Response to clinical therapies and inflammation status in UC.
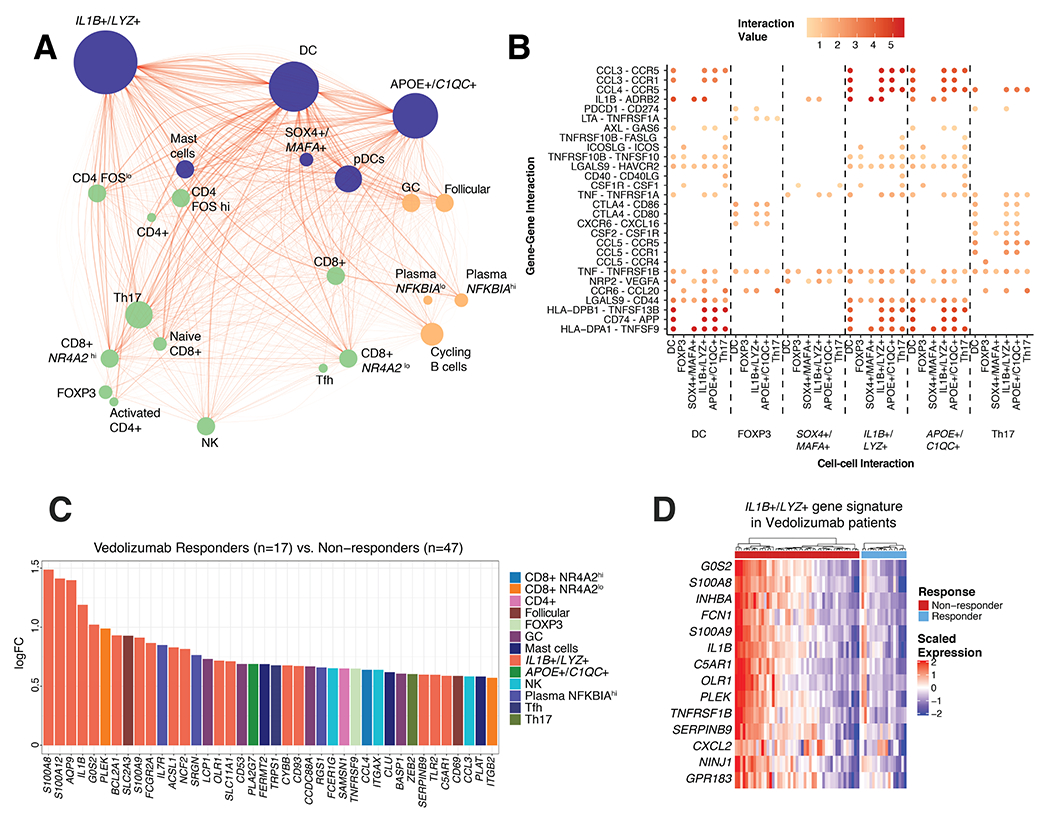
(A) Receptor-ligand network connections between 23 major cell populations identified in scRNA-seq. Nodes represent cell populations and edges significant receptor-ligand association according to curated database, cellPhoneDB. Size of each node is proportional to the number of connected edges and thickness of the edges is proportional to the significance value of the connection. (B) Selected ligand-receptor interactions specifically enriched between DC, FOXP3+ Tregs, SOX4+/MAFA+, IL1B+/LYZ+, APOE+/C1QC+ monocyte/macrophages and Th17 cells. (C) Log fold change of differentially expressed genes in Vedolizumab responders (n=17) versus non-responders (n=47). (D) Expression of IL1B+/LYZ+ monocyte/macrophage marker genes in responders and non-responders to Vedolizumab treatment.
Several new therapeutic agents developed for treating UC are designed to block immune cell interactions. We assessed if immune cell subset-specific signatures distinguish responders and non-responders for different therapeutic targets in UC: Vedolizumab34 (anti-α4β7 integrin; GSE73661), Etrolizumab35 (ant²7 subunit of α4β7 and αEβ7 integrins; not FDA approved; GSE72819), and Golimumab36 (anti- TNFα; GSE92415). Within our 706-gene signature matrix transcripts associated with IL1B+/LYZ+ monocyte/macrophages were the most significantly different between responders and non-responders for Vedolizumab. Non-responders were most enriched in transcripts for the proinflammatory IL1B+/LYZ+ monocyte/macrophages (Fig. 6C,D), indicating that the presence of these macrophages could be an indicator of resistance to integrin blockade. In addition to IL1B, calprotectin components S100A8/A9 were more highly expressed in non-responders. However, there is considerable heterogeneity. Approximately 50% of non-responders have high expression of IL1B+/LYZ+ monocyte/macrophages transcripts (Fig. 6D). Non-responders to Etrolizumab, although not FDA approved for UC, also exhibited higher expression of proinflammatory genes like IL1B, IL1RN and S100A9 compared to responders (Figure S9). Golimumab nonresponders, while still slightly enriched for expression of these transcripts, are less strikingly associated with IL1B+/LYZ+ monocyte/macrophages transcripts (Figure S9), perhaps reflecting different mechanism of action. Hence, a proportion of non-responders to α4β7 integrin blockade in UC patients are associated with increased transcripts for an inflammatory monocyte/macrophage population.
Discussion
We found that inflammation in the J pouch, a novel organ created from ileal tissue, has an inflammatory landscape similar to the colon of UC patients. We identified FOXP3+/BATF+ T cells and 3 different monocyte/macrophage populations associated with inflamed tissues in both UC and pouchitis. Of these cells, IL1B+/LYZ+ myeloid cells were the most highly connected cell type in the inflammatory network and associated with lack of responsiveness to α4β7 integrin blockade and an increased abundance of Bacteroidiales and Clostridiales taxa. We hypothesize that the increased activation of these IL1B+/LYZ+ myeloid cells may represent a biomarker of intestinal inflammation in non-responders to α4β7 integrin blockade.
This analysis concludes that the inflammatory response for pouchitis and UC is remarkably similar despite the different origin tissues. While the pouch has more CD8+ T cells of different phenotypes this is not significantly altered by the inflammatory response. This data supports previous studies indicating that pouchitis and UC are driven by similar inflammatory mechanisms15 and may therefore respond to similar therapeutics37–41. This has direct implications for clinical practice as current treatments rely heavily on long-term antimicrobials, such as fluoroquinolone antibiotics, which may pose a greater risk of long-term adverse effects than biologic or small molecule therapies. Notably, we did not characterize the CD45+ immune cells FAP pouch patients, who develop pouchitis less frequently. However, meta-analysis of transcriptional data from FAP patients indicate that their immunological signature is distinct from pouchitis and UC samples. Recently, secondary bile acids and associated microbes were found to distinguish UC and FAP pouches, and may result in the pro-inflammatory conditions preceding pouchitis in UC patients42. Efforts are underway in determining the distinct roles of secondary bile acids and butyrate, both of which are byproducts of similar bacterial taxa that mediate Th17 and Treg polarization, and discerning the action of these metabolites on antigen presenting cells versus T cells remains an important area of research11,42–46. Thus, it is important to identify any association between the IL1B+/LYZ+ monocyte/macrophages and other metabolites such as bile acids beyond butyrate47.
The relationship between microbes and antimicrobial macrophages is particularly relevant to the pathogenesis of IBD. Here we provide evidence that IL1B+LYZ+ macrophages, with a similar signature to antimicrobial macrophages47, can directly be identified from intestinal biopsies of UC patients. This observation may reflect the recruitment of monocytes to the gut where they differentiate into macrophages to counteract a breach in the epithelial barrier, a characteristic of IBD patients who are colonized by pro-inflammatory bacteria related to Bacteroidiales and Clostridiales taxa48–50. In contrast to healthy individuals, local cytokine responses and inefficient autophagy may prevent macrophages from resolving the breach in the barrier, leading to a detrimental pro-inflammatory effect of macrophages51. Consistent with this possibility, our previous work indicates that antimicrobial monocytes and macrophages recruited to the gut are beneficial when damage to the colon is temporary, even with an inflammatory cytokine signature exacerbated by the absence of autophagy52–54.
A consistent finding between our scRNA-seq data and the meta-analysis of public datasets is the expansion of FOXP3+/BATF+ Tregs in actively inflamed IBD. In the visceral adipose tissue, FOXP3+ Tregs require BATF for differentiation downstream of ST2 and PPARG activity55. In the intestine, BATF regulates expression of CCR9 and a4b7 and BATF deficient mice have reduced effector as well as FOXP3+ T cells in the intestine56. Hence, BATF is likely an important transcription factor for the differentiation and recruitment of FOXP3+ Tregs to the intestinal tissues during inflammation, which has recently been noted in additional studies4. Notably, a recent report on scRNA-seq analysis of immune cell populations in immune checkpoint inhibitor-induced colitis also reveals the persistence and expansion of Tregs57. The increased accumulation of FOXP3+ Tregs in the inflamed colon is likely driven by the need to restrain inflammation, but why these Tregs are not successful in controlling inflammation requires further study. One possibility may be that the presence of inflammatory monocyte/macrophage populations somehow inhibit the appropriate function of these regulatory cells.
We previously found increases in Th17 cells in inflamed UC biopsies58,59 linked by expression of SAA1 with Bacteroides abundance, but this was by flow cytometry and intracellular cytokine staining. Serum amyloid A (SAA) proteins produced by intestinal epithelial cells can drive differentiation of inflammatory Th17 cells according to the tissue environment60, however expression of SAA proteins was not detected in the present study because we selected for immune cells. We also do not observe significantly more Th17 cells by transcriptional signature (Figure S3C). Nonetheless, this population of cells is highly connected with the IL1B+/LYZ+ and APOE+/C1QC+ populations expanded in inflamed samples and hence their activity could be highly dependent on the interaction with these macrophages. Causal relationships between these populations may be discerned in the future through in vitro co-culture assays utilizing surgical specimens to improve cellular yield rather than the mucosal pinch biopsies. In addition, future studies with a larger sample size from multiple pouch locations in various clinical pouchitis cohorts may assist in the further molecular differentiation of pouchitis.
IL-1B release can be triggered by activating NLRP3 and other inflammasomes in macrophages exposed to invasive microbes. Elegant experiments examining very early onset IBD (VEOIBD) patients and mouse models genetically deficient in IL-10 signaling indicate that inflammasome-triggered IL-1B production by macrophages polarizes CD4+ T cells that mediate colitis61,62. Blocking IL-1B signaling was effective in two IL10R-deficient patients with treatment-refractory disease62, suggesting macrophage-T cell interactions drive disease in the absence of IL-10 and other immuno-suppressive Treg effectors, consistent with predictions from the receptor-ligand analysis performed in this study (Fig. 6). If the presence of IL1B+ macrophages is indicative of unresponsiveness to α4β7 integrin blockade as suggested by our results, therapies that target IL1B itself or JAK/STAT inhibitors that broadly target signaling downstream of IL-1B-induced cytokines63 may be promising alternatives for UC patients with this signature. Detailed analysis of tissue from patients receiving JAK/STAT inhibitors will be highly informative.
In conclusion, this work utilizes scRNA-seq to identify unique features of pouchitis and a specific population of IL1B+ macrophages that could potentially be targeted in a subset of UC patients who are not responding to treatment with α4β7 integrin antagonists. Hence, this study provides an example of how utilizing precision medicine to identify changes in cell proportions, gene expression and cell-cell signaling by scRNA-seq, followed by further analyses of publicly available datasets, could be used to improve our understanding of individual patient responsiveness to IBD therapies and provide a hypothesis for alternative treatment options.
Supplementary Material
What You Need To Know.
Background and Context
Pouchitis is a common complication following restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA, J-pouch) to treat ulcerative colitis (UC). The immune landscape of pouchitis is poorly understood treatment often results in disease relapses.
New Findings
Using single cell-RNA sequencing (scRNA-seq) of immune cells from intestinal tissues of IBD patients with a pouch or UC, we find similar inflamed features including IL1B+/LYZ+ myeloid cells and FOXP3+/BATF+ T cells.
Limitations:
This study is observational. Pouchitis patients were not distinguished by clinical characteristics.
Impact:
The mucosal immune response of the pouch and UC is remarkably similar despite the different tissue origins, which may have direct clinical implications for the management of pouchitis. scRNAseq enables meta-analysis of multiple studies, which may facilitate the identification of biomarkers to personalize therapy for IBD patients.
Grant Support:
This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and NIH grants DK103788 (K.C. and P.L.), AI121244 (K.C.), HL123340 (K.C.), DK093668 (K.C.), AI130945 (P.L. and K.C.), R01 HL125816 (K.C.), R01 AI140754 (K.C.), HL084312, AI133977 (P.L.). Pilot award from the NYU CTSA grant UL1TR001445 from the National Center for Advancing Translational Sciences (NCATS) (K.C., P.L.), pilot award from the NYU Cancer Center grant P30CA016087 (K.C.). This work was also supported by the Faculty Scholar grant from the Howard Hughes Medical Institute (K.C.), Crohn’s & Colitis Foundation (K.C.), Merieux Institute (K.C.), Kenneth Rainin Foundation (K.C.). K.C. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
Disclosures:
K.C. receives research funding from Pfizer and Abbvie; P.L. receives research funding from Pfizer; J.A. receives research funding from BioFire Diagnostics. K.C. has consulted for or received an honorarium from Puretech Health, Genentech, and Abbvie; P.L. consults for and has equity in Toilabs. K.C. has provisional patents, U.S. Patent Appln. No. 15/625,934 and 62/935,035. P.L. is a federal employee. J.A. has consulted for or received an honorarium from BioFire Diagnostics and Janssen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Transcript Profiling: The processed single cell count tables are provided in gene expression omnibus; GSE162335. Raw sequence data are not public and are protected by controlled-access for patient privacy.
Preprint DOI: https://doi.org/10.1101/2020.07.31.231308
References
- 1.Acute Shen B. and chronic pouchitis—pathogenesis, diagnosis and treatment. Nature Reviews Gastroenterology & Hepatology 2012;9:323–333. [DOI] [PubMed] [Google Scholar]
- 2.Dalal RL, Shen B, Schwartz DA. Management of Pouchitis and Other Common Complications of the Pouch. Inflamm Bowel Dis 2018;24:989–996. [DOI] [PubMed] [Google Scholar]
- 3.Smillie CS, Biton M, Ordovas-Montanes J, et al. Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis. Cell 2019;178:714–730.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitsialis V, Wall S, Liu P, et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology 2020;159:591–608.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haber AL, Biton M, Rogel N, et al. A single-cell survey of the small intestinal epithelium. Nature 2017;551:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh K, Antanaviciute A, Fawkner-Corbett D, et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019;567:49–55. [DOI] [PubMed] [Google Scholar]
- 7.Corridoni D, Chapman T, Antanaviciute A, et al. Inflammatory Bowel Disease Through the Lens of Single-cell RNA-seq Technologies. Inflamm Bowel Dis. Available at: 10.1093/ibd/izaa089/5834990 [Accessed July 13, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin JC, Chang C, Boschetti G, et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell 2019;178:1493–1508.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu M, Pokrovskii M, Ding Y, et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 2018;554:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sano T, Huang W, Hall JA, et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 2015;163:381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–450. [DOI] [PubMed] [Google Scholar]
- 12.Kim KS, Hong S-W, Han D, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016;351:858–863. [DOI] [PubMed] [Google Scholar]
- 13.Esterházy D, Canesso MCC, Mesin L, et al. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 2019;569:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habtezion A, Nguyen LP, Hadeiba H, et al. Leukocyte Trafficking to the Small Intestine and Colon. Gastroenterology 2016;150:340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Dalal S, Antonopoulos D, et al. Early Transcriptomic Changes in the Ileal Pouch Provide Insight into the Molecular Pathogenesis of Pouchitis and Ulcerative Colitis. Inflamm Bowel Dis 2017;23:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. New England Journal of Medicine 1987;317:1625–1629. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Tremaine WJ, Batts KP, et al. Pouchitis After Ileal Pouch-Anal Anastomosis: A Pouchitis Disease Activity Index. Mayo Clinic Proceedings 1994;69:409–415. [DOI] [PubMed] [Google Scholar]
- 18.Heuschen UA, Allemeyer EH, Hinz U, et al. Diagnosing Pouchitis. Dis Colon Rectum 2002;45:776–786. [DOI] [PubMed] [Google Scholar]
- 19.Zheng GXY, Terry JM, Belgrader P, et al. Massively parallel digital transcriptional profiling of single cells. Nature Communications 2017;8:14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: Open source software for digital pathology image analysis. Scientific Reports 2017;7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 2011;103:1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuart T, Butler A, Hoffman P, et al. Comprehensive Integration of Single-Cell Data. Cell 2019;177:1888–1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H-O, Hong Y, Etlioglu HE, et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nature Genetics 2020;52:594–603. [DOI] [PubMed] [Google Scholar]
- 24.Senda T, Dogra P, Granot T, et al. Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol 2019;12:378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nature Reviews Immunology 2014;14:667–685. [DOI] [PubMed] [Google Scholar]
- 26.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunological Reviews 2014;260:102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontana R, Raccosta L, Rovati L, et al. Nuclear receptor ligands induce TREM-1 expression on dendritic cells: analysis of their role in tumors. OncoImmunology 2019;8:1554967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West NR, Hegazy AN, Owens BMJ, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nature Medicine 2017;23:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertins P, Mani DR, Ruggles KV, et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 2016;534:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan XC, Kabakchiev B, Waldron L, et al. Associations between host gene expression, the mucosal microbiome, and clinical outcome in the pelvic pouch of patients with inflammatory bowel disease. Genome Biology 2015;16:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramilowski JA, Goldberg T, Harshbarger J, et al. A draft network of ligand–receptor-mediated multicellular signalling in human. Nature Communications 2015;6:7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Efremova M, Vento-Tormo M, Teichmann SA, et al. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nature Protocols 2020;15:1484–1506. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich M, Pohin M, Powrie F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019;50:992–1006. [DOI] [PubMed] [Google Scholar]
- 34.Arijs I, Hertogh GD, Lemmens B, et al. Effect of vedolizumab (anti-α4β7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut 2018;67:43–52. [DOI] [PubMed] [Google Scholar]
- 35.Tew GW, Hackney JA, Gibbons D, et al. Association Between Response to Etrolizumab and Expression of Integrin αE and Granzyme A in Colon Biopsies of Patients With Ulcerative Colitis. Gastroenterology 2016;150:477–487.e9. [DOI] [PubMed] [Google Scholar]
- 36.Telesco SE, Brodmerkel C, Zhang H, et al. Gene Expression Signature for Prediction of Golimumab Response in a Phase 2a Open-Label Trial of Patients With Ulcerative Colitis. Gastroenterology 2018;155:1008–1011.e8. [DOI] [PubMed] [Google Scholar]
- 37.Ferrante M, D’Haens G, Dewit O, et al. Efficacy of infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: a Belgian case series. Inflamm Bowel Dis 2010;16:243–249. [DOI] [PubMed] [Google Scholar]
- 38.Barreiro-de Acosta M, García-Bosch O, Souto R, et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm Bowel Dis 2012;18:812–817. [DOI] [PubMed] [Google Scholar]
- 39.Barreiro-de Acosta M, García-Bosch O, Gordillo J, et al. Efficacy of adalimumab rescue therapy in patients with chronic refractory pouchitis previously treated with infliximab: a case series. Eur J Gastroenterol Hepatol 2012;24:756–758. [DOI] [PubMed] [Google Scholar]
- 40.Bär F, Kühbacher T, Dietrich NA, et al. Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment Pharmacol Ther 2018;47:581–587. [DOI] [PubMed] [Google Scholar]
- 41.Ollech JE, Rubin DT, Glick L, et al. Ustekinumab Is Effective for the Treatment of Chronic Antibiotic-Refractory Pouchitis. Dig Dis Sci 2019;64:3596–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha SR, Haileselassie Y, Nguyen LP, et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host & Microbe 2020;27:659–670.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell C, McKenney PT, Konstantinovsky D, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020;581:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hang S, Paik D, Yao L, et al. Bile acid metabolites control T H 17 and T reg cell differentiation. Nature 2019;576:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X, Sun X, Oh SF, et al. Microbial bile acid metabolites modulate gut RORγ + regulatory T cell homeostasis. Nature 2020;577:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013;504:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulthess J, Pandey S, Capitani M, et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019;50:432–445.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y-G, Kamada N, Shaw MH, et al. The Nod2 Sensor Promotes Intestinal Pathogen Eradication via the Chemokine CCL2-Dependent Recruitment of Inflammatory Monocytes. Immunity 2011;34:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramanan D, Tang MS, Bowcutt R, et al. Bacterial sensor Nod2 prevents small intestinal inflammation by restricting the expansion of the commensal Bacteroides vulgatus. Immunity 2014;41:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schirmer M, Garner A, Vlamakis H, et al. Microbial genes and pathways in inflammatory bowel disease. Nature Reviews Microbiology 2019;17:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuzawa-Ishimoto Y, Hwang S, Cadwell K. Autophagy and Inflammation. Annual Review of Immunology 2018;36:73–101. [DOI] [PubMed] [Google Scholar]
- 52.Marchiando AM, Ramanan D, Ding Y, et al. A Deficiency in the Autophagy Gene Atg16L1 Enhances Resistance to Enteric Bacterial Infection. Cell Host & Microbe 2013;14:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin PK, Marchiando A, Xu R, et al. Autophagy proteins suppress protective type I interferon signalling in response to the murine gut microbiota. Nature Microbiology 2018;3:1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neil JA, Matsuzawa-Ishimoto Y, Kernbauer-Hölzl E, et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nature Microbiology 2019;4:1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasanthakumar A, Moro K, Xin A, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nature Immunology 2015;16:276–285. [DOI] [PubMed] [Google Scholar]
- 56.Wang C, Thangamani S, Kim M, et al. BATF is required for normal expression of gut-homing receptors by T helper cells in response to retinoic acid. J Exp Med 2013;210:475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luoma AM, Suo S, Williams HL, et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020;0. Available at: https://www.cell.com/cell/abstract/S0092-8674(20)30688-7 [Accessed July 20, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung JM, Davenport M, Wolff MJ, et al. IL-22-producing CD4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunology 2014;7:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang MS, Bowcutt R, Leung JM, et al. Integrated Analysis of Biopsies from Inflammatory Bowel Disease Patients Identifies SAA1 as a Link Between Mucosal Microbes with TH17 and TH22 Cells. Inflamm Bowel Dis 2017;23:1544–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J-Y, Hall JA, Kroehling L, et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell 2020;180:79–91.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ip WKE, Hoshi N, Shouval DS, et al. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017;356:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shouval DS, Biswas A, Kang YH, et al. Interleukin 1β Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 2016;151:1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salas A, Hernandez-Rocha C, Duijvestein M, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology 2020;17:323–337. [DOI] [PubMed] [Google Scholar]
- 64.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


