Abstract
Background
Soft-tissue sarcomas (STS) in the extremities and trunk treated with standard-of-care preoperative external beam radiation therapy (EBRT) followed by surgical resection are associated with local and distant relapses. In preclinical studies, oncolytic virotherapy in sarcoma has demonstrated antitumor effects via direct intratumoral oncolysis and cytotoxic T-cell–mediated immune responses. Talimogene laherparepvec (TVEC) is a replication-competent, immune-enhanced, oncolytic herpes simplex virus type 1 engineered for intratumoral injection; it has been approved by the FDA for the treatment of locally advanced and metastatic melanoma.
Methods
We explored a novel combination of TVEC with standard-of-care EBRT administered preoperatively in patients with locally advanced STS of the extremities and trunk in a phase IB/II clinical trial. Thirty patients with primary STS >5 cm for which EBRT was indicated to achieve negative margins were enrolled. FDA-approved TVEC doses were used. Immune correlative studies in peripheral blood, biopsy and resected tumor tissues were performed.
Results
No dose-limiting toxicity was observed. Adverse events were similar to those reported in prior studies with TVEC. One patient with myxoid liposarcoma exhibited a partial response. Seven of the 29 (24%) evaluable patients achieved 95% pathological necrosis. None of the patients developed a herpes infection due to the treatment. Eight of the 29 (27%) patients developed postoperative wound complications, which is consistent with previous studies. None of the patients developed local recurrence after surgical resection of the primary sarcoma. 2-year progression-free and overall survival were 57% and 88%, respectively. Caspase-3 demonstrated increased expression of both in TVEC-treated tissue samples as compared with control samples treated with radiation alone.
Conclusion
Preoperative intratumoral TVEC with concurrent EBRT for locally advanced STS is safe and well-tolerated. This combination treatment may enhance immune responses in some cases but did not increase the proposed rate of pathological necrosis. The Caspase-3 biomarker may be associated with a positive effect of TVEC in sarcoma tumor tissue and should be explored in future studies.
Trial registration number
Keywords: Oncolytic Virotherapy, Radiotherapy, Sarcoma, Tumor Biomarkers, Clinical Trials, Phase II as Topic
Introduction
Soft-tissue sarcomas (STS) account for 1% of all solid tumors, with an annual incidence of 5–6 cases per 100,000 persons.1 Surgical resection of the primary STS with negative margins remains the primary treatment. The addition of neoadjuvant external beam radiation therapy (EBRT) improves the rate of negative surgical margins and results in higher local control rates; hence, it is currently accepted as a standard of care.2 3 Neoadjuvant and adjuvant cytotoxic chemotherapy, though an emerging treatment option in specific high risk STS subtypes, is associated with significant toxicities.4 Despite these aggressive standard-of-care treatments, approximately 50% of patients with localized STS of the extremities or trunk develop metastatic disease with associated mortality.5 Hence, novel treatments are needed that can effectively synergize with and augment the current standard of care treatment to improve survival.
Several preclinical studies evaluated the role of oncolytic viruses (OVs) in sarcomas.6–9 OVs mediate their antitumor effects through multiple mechanisms. They can directly infect tumor cells following intratumoral injection. Once in the cell, the virus replicates, leading to cell lysis and virus progeny release into the tumor microenvironment where they can infect other neighboring tumor cells, leading to further lysis. Second, the virus can mediate an indirect effect by enhancing the activation of innate and adaptive immune responses specific to cancer cell antigens, resulting in augmented systemic antitumor immunity. Ionizing radiation induces direct cellular DNA damage and is routinely used in management of STS. Multiple preclinical and clinical studies have shown a synergistic therapeutic effect when OVs are combined with radiation to treat cancer.10 11 Radiation treated tumors can increase viral uptake and lead to gene expression and replication and resulting in cell death by way of apoptosis and or necrosis; and in turn the viruses may act as radio sensitizing agents. Therefore, the combination of OV and radiation may help with local tumor control and help with enhancing systemic antitumor response in the form of abscopal effect.12
Talimogene laherparepvec (TVEC) is replication-competent, immune-enhanced, oncolytic herpes simplex virus type 1 (HSV-1) engineered for intratumoral injection. It contains the coding sequence for human granulocyte-macrophage colony-stimulating factor, which in addition to the above two mechanisms enhances antitumor responses by inducing the production of proinflammatory cytokines. TVEC has been shown to improve melanoma response rates and has been approved by the FDA for the management of locally advanced unresectable and metastatic melanoma.13 14 It is being studied in several other solid cancers including head and neck cancer.15 16 Radiation therapy in combination with immunotherapies has shown synergistic activity;10 11 therefore, we explored a novel combination of neoadjuvant TVEC and standard-of-care EBRT in locally advanced STS of the extremities and trunk.
Patients and methods
This was a phase IB/II, open-label, non-randomized, single-center trial conducted in patients with a histological diagnosis of STS of the extremities and trunk who received intratumoral injections of TVEC in the primary sarcoma tumor together with standard-of-care neoadjuvant EBRT followed by definitive surgery. The phase IB primary objective was to examine the safety and tolerability of TVEC combined with EBRT based on the incidence of dose-limiting toxicities (DLTs). The phase II primary objective was to determine the efficacy of this combination based on near pathological complete response (pCR) defined as ≥95% tumor necrosis of locally advanced STS that were initially unresectable with clear, wide margins and for which neoadjuvant or preoperative radiotherapy was considered appropriate.
Patients aged ≥18 years with localized, histologically confirmed, STS >5 cm, amenable to direct or ultrasound-guided injection, not suitable for surgical resection alone due to an inability to achieve acceptably wide margins, and for which preoperative EBRT was indicated were included. Patients with metastatic STS as defined by lung nodules>1 cm on staging CT scan were included only if radiation and resection of the primary tumor were indicated. Diagnostic pathology tests were performed using core-needle or incisional biopsy. Surgery was performed with limb-sparing intent and diligence was taken to ensure negative margins. A multidisciplinary team comprising medical oncologists, surgical/orthopedic oncologists, plastic surgeons, and radiation oncologists evaluated the patients before they consented to participation. The eligibility criteria also included an Eastern Cooperative Oncology Group performance status of 0 or 1 and adequate laboratory test values. Patients with retroperitoneal and visceral sarcomas; on anticoagulation therapy; with autoimmune diseases; and prior exposure to TVEC, tumor vaccines, or radiation to the same tumor bed were excluded. Those with histologies including extraosseous Ewing’s sarcoma, primitive neuroectodermal tumors, osteosarcoma or chondrosarcoma, Kaposi’s sarcoma, or angiosarcoma of the scalp/face were also ineligible. The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. All patients signed an informed consent form.
The intratumoral TVEC injection sites were marked with ink by the orthopedic surgeon with the intention of including those sites in the resection specimen. TVEC was administered intratumorally directly or under ultrasound guidance at an initial dose of 106 PFU/mL up to a volume of 4 mL on day 1 of week 1, followed by the administration of a target dose of 108 PFU/mL up to a volume of 4 mL 3 weeks later (on day 1 of week 4). Weekly TVEC injections were continued until surgery. Tumors were injected using a single-entry point at the inked site, but TVEC was delivered along multiple tracts within the lesion to achieve maximum dispersion. Acetaminophen 650 mg and indomethacin 50 mg were administered orally 1 hour before intratumoral injection. Dose delays or reductions were followed per protocol. Safety evaluations were done weekly, with adverse events (AEs) defined and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, V.4.017 (CTCAE 4.0).
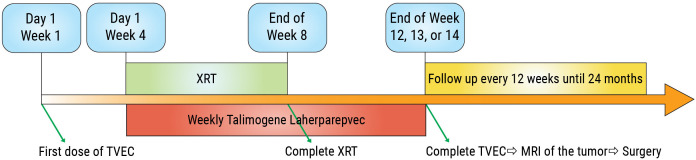
Neoadjuvant EBRT doses of 50 Gy delivered over 25 fractions were administered by either 3D conformal or intensity-modulated radiation therapy as per sarcoma NCCN guidelines.18 Weekly TVEC injections were continued during radiation and until surgery for all patients. Surgery was performed 4–6 weeks from EBRT completion to allow for adequate tissue healing and resolution of EBRT-induced acute toxicities (figure 1).
Figure 1.
Study schema.TVEC, Talimogene laherparepvec; XRT, radiation therapy; MRI, magnetic resonance imaging.
Sarcoma histological subtypes (as defined by the WHO) were determined following examination by a single pathologist with expertise in sarcomas. Tumors were graded based on features such as mitosis rate, necrosis, cellularity, pleomorphism, and differentiation, and tumor size (or the greatest dimension) and the margin description (including centimeters or millimeters to margin) were reported. Tumors were sampled with at least one section per 1 cm of the greatest tumor dimension. Absence of ink on the tumor was accepted as a negative margin. Near-pCR was assessed according to the percentage of viable tumor remaining and percentage necrosis in the post-treatment resected specimen.19 Necrosis was calculated for each block of full-face section and then averaged. Clinical response was assessed using tumor measurements performed at screening and preoperative MRI or CT. Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1 was used for target tumor categorization as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).
Serum samples were collected at day 1 of week 1 (w1d1) as baseline prior to the first injection, day 1 of week 4 (w4d1)—prior to second injection, day 1 of week 8 (w8d1)—after completion of EBRT, day 1 of week 12 (w12d1)—preoperatively, and day 1 of week 18 (w18d1)—after surgery. Luminex Human Magnetic Assay (LXSAHM-36, R&D Systems, online supplemental table S1 summarized all 36 cytokines) was used to determine cytokine concentrations as per the company’s instructions for all serum samples and analyzed using the Luminex MAGPIX Instrument (Luminex).
jitc-2021-003119supp001.pdf (183.8KB, pdf)
Immunoperoxidase stains were performed on 4-μm-thick sections from the archived paraffin-embedded tissues. Caspase-3 (Cell Signaling #9661 rabbit polyclonal 1:100; retrieval: Citrate Buffer, pH 6.0, in Decloaker (Biocare) 110*C 15 min; Secondary: Dako Rabbit Envision) and Granzyme-B (Leica clone 11F1, dilution 1:40, using High pH HIER, in DAKO Autostainer Link48, using DAKO’s detection system) stains and TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay were used to highlight apoptotic tumor cells. Cytoplasmic and nuclear expression were considered positive and the apoptotic index was calculated using the following formula: number of immunoreactive cells present in a section as a fraction of the total number of tumor cells.
Statistical considerations
The phase IB primary objective was to confirm that the current standard dose of TVEC combined with EBRT was well-tolerated. A standard 3+3 design was used with the current standard as the starting dose (4.0 mL of 108 PFU/mL weekly beginning at week 3) and one additional de-escalated dose (4.0 mL of 108 PFU/mL every 2 weeks) according to the investigator brochure that was included should de-escalation be warranted. The initial dose administered to all patients was up to 4.0 mL of 106 PFU/mL. The recommended phase II dose was defined as the highest dose at which at least one out of six patients showed a DLT, which was defined as grade 3 immune-mediated AEs, allergic reactions, or plasmacytoma of any grade. Any unexpected grade 3 or greater hematological or non-hematological toxicity (except for alopecia of any grade, EBRT-related skin toxicity, grade 3 or higher myalgia or arthralgia, fatigue, fever, or diarrhea and vomiting responding to supportive care) was also considered a DLT. The incidence of treatment-emergent AEs attributable (possibly, probable, or definite) to TVEC was descriptively summarized by type and severity based on the maximum grade noted for each patient. Phase IB results were reported for the DLT-evaluable safety population and a full safety profile included all patients (n=30) who received at least one TVEC dose.
The phase II primary objective was to evaluate the preliminary evidence of antitumor activity. The primary endpoint was the proportion of patients with near-pCR. Previous literature suggests that treatment with neoadjuvant EBRT alone results in pathological tumor necrosis ≥95% in approximately 8%–10%20 21 of patients with extremity sarcomas. Thus, a near-pCR rate of ≤12% was defined as essentially being no different than that associated with EBRT alone whereas a rate of at least 35% may warrant further investigation. Sample size requirements were based on an optimal Simon two-stage design with 80% power and a significance level of 5%. Nine patients were to be enrolled in the first stage in phase 2, and the study would have been terminated if one or fewer had pathological tumor necrosis ≥95%. Otherwise, an additional 14 evaluable patients were to be enrolled in the second stage. If 6 or more of the total 23 patients had pathological tumor necrosis ≥95%, the treatment would be deemed worthy of further investigation.
Survival probabilities were estimated and plotted using the Kaplan-Meier method. Estimates along with 95% pointwise CIs were reported. Progression-free survival (PFS) was defined as the time from treatment initiation to the date of the first documented disease progression or death due to any cause. Otherwise, subjects were censored for progression at their last radiographic assessment. Overall survival (OS) was defined as the time from treatment initiation to death due to any cause. Patients alive at the time of submission were censored on the date at which they were last known to be alive. Mixed effects regression models were used to assess the change in Caspase-3 Granzyme-B, CD3, CD4, CD56, CD8, and Foxp3 from pretreatment to post-treatment and to compare the change in TUNEL positive cells between treatment groups. Wilcoxon rank sum tests were used to compare Caspase-3 and Granzyme-B between treatment groups.
Results
Six patients were enrolled in phase IB; 24 patients (23 in whom pathological necrosis was evaluable and 1 who opted against surgery) were enrolled in phase II from July 2015 to June 2018 at the Holden Comprehensive Cancer Center. At the time of data analysis (January 2021), 13 patients have had disease progression of which 12 have died. Two out of these 13 patients had known metastasis to the lungs at study enrollment. The median patient age was 65 years (range 28–80) (table 1).
Table 1.
Patient demographics, histological subtypes, and percentage necrosis in different phases of the trial
| Covariate | Level | Phase | Total N=30 | |
| I N=6 | II N=24 | |||
| Sex | F | 1 (16.7) | 8 (33.3) | 9 (30.0) |
| M | 5 (83.3) | 16 (66.7) | 21 (70.0) | |
| Age (median) | 60 (36–79) | 66 (28–80) | 65 (28–80) | |
| Pathology | Dedifferentiated liposarcoma | 0 (0) | 2 (8.3) | 2 (6.7) |
| Epithelioid sarcoma | 0 (0) | 1 (4.2) | 1 (3.3) | |
| Myxofibrosarcoma | 1 (16.7) | 1 (4.2) | 2 (6.7) | |
| Myxoid liposarcoma | 0 (0) | 5 (20.8) | 5 (16.7) | |
| Pleomorphic liposarcoma | 0 (0) | 1 (4.2) | 1 (3.3) | |
| Spindle cell rhabdomyosarcoma | 1 (16.7) | 0 (0) | 1 (3.3) | |
| Synovial sarcoma | 0 (0) | 2 (8.3) | 2 (6.7) | |
| Undifferentiated pleomorphic sarcoma | 4 (66.7) | 9 (37.5) | 13 (43.3) | |
| Undifferentiated sarcoma with myxoid stroma | 0 (0) | 1 (4.2) | 1 (3.3) | |
| Undifferentiated spindle cell sarcoma | 0 (0) | 2 (8.3) | 2 (6.7) | |
| Grade* | 1 | 0 (0) | 5 (20.8) | 5 (16.7) |
| 2 | 4 (66.7) | 10 (41.7) | 14 (46.7) | |
| 3 | 2 (33.3) | 9 (37.5) | 11 (36.7) | |
| HSV serology—pretreatment | Negative | 1 (16) | 8 (33) | 9 (30) |
| Positive | 5 (83) | 16(66) | 21(70) | |
| Overall best response | PR | 0 (0) | 1 (4.2) | 1 (3.3) |
| SD | 4 (66.7) | 16 (66.7) | 20 (66.7) | |
| PD | 2 (33.3) | 7 (29.2) | 9 (30.0) | |
| ≥95% necrosis | No | 4 (66.7) | 18 (78.3) | 22 (75.9) |
| Yes | 2 (33.3) | 5 (21.7) | 7 (24.1) | |
| Progression | No | 4 (66.7) | 13 (54.2) | 17 (56.7) |
| Yes | 2 (33.3) | 11 (50.0) | 13 (43.3) | |
| Deceased | No | 4 (66.7) | 14 (58.3) | 18 (60.0) |
| Yes | 2 (33.3) | 10 (41.7) | 12 (40.0) | |
| # TVEC injections† (median) | 11 (9–11) | 10 (5–11) | 10 (5–11) | |
| Necrosis (median)† | 75% (11%–99%) | 78% (8%–100%) | 78% (8%–100%) | |
| Median follow-up (months)† | 48.9 (2.9–57.7) | 22.2 (1.5–50.7) | 23.7 (1.5–57.7) | |
*Grading of sarcomas according to the French Federation of Cancer Centers Sarcoma Group.
†Numbers within parentheses indicate the range.
HSV, herpes simplex virus; PD, progressive disease; PR, partial response; SD, stable disease; TVEC, talimogene laherparepvec.
All patients had a measurable tumor >5 cm in size (range 5–22 cm) of the primary site to which neoadjuvant EBRT was delivered following a multidisciplinary group discussion. One patient with undifferentiated pleomorphic sarcoma refused surgery and was excluded from the efficacy analysis but included in the safety analysis. Per the preoperative radiographic evaluation, the majority of subjects (66.7%) had SD according to RECIST V.1.122 and none showed CR. One patient with myxoid liposarcoma had PR. Two patient deaths occurred during the study due to PD. The treatment-related toxicities noted were similar to previous studies using TVEC.23 24 Headaches (3%), chills and rigors (19%), and fatigue (22%) were manageable with single repeat doses of acetaminophen, indomethacin, and meperidine 4–6 hours before each intratumoral injection and with supportive care. Ten per cent of patients developed significant lymphopenia which eventually returned to baseline. None of the patients developed a herpes viral infection. All toxicities attributed to intratumoral TVEC injections are outlined in table 2.
Table 2.
Maximum grade adverse event per patient (Grade 2–4 only as per CTCAE V.4.0) attributed as possibly, probably, or definitely related to talimogene laherparepvec
| Toxicity | Grade | ||
| 2 | 3 | 4 | |
| Anemia | 3 | 2 | 0 |
| Diarrhea | 2 | 0 | 0 |
| Nausea | 6 | 0 | 0 |
| Vomiting | 4 | 2 | 0 |
| Chills | 18 | 1 | 0 |
| Leg edema | 1 | 0 | 0 |
| Fatigue | 19 | 3 | 0 |
| Fever | 4 | 0 | 0 |
| Influenza like symptoms | 6 | 1 | 0 |
| Injection site reaction | 5 | 0 | 0 |
| Malaise | 2 | 0 | 0 |
| Pain | 5 | 1 | 0 |
| Skin infection | 1 | 0 | 0 |
| Lymphopenia | 6 | 2 | 2 |
| Weight loss | 3 | 1 | 0 |
| Anorexia | 1 | 1 | 0 |
| Back pain | 1 | 0 | 0 |
| Generalized muscle weakness | 2 | 1 | 0 |
| Pain in extremity | 3 | 3 | 0 |
| Dizziness | 1 | 0 | 0 |
| Dysgeusia | 1 | 0 | 0 |
| Headache | 2 | 1 | 0 |
| Neuralgia | 1 | 0 | 0 |
| Depression | 2 | 0 | 0 |
| Proteinuria | 0 | 1 | 0 |
| Renal and urinary disorders | 1 | 0 | 0 |
| Hiccups | 1 | 0 | 0 |
| Hypertension | 1 | 1 | 0 |
| Hypotension | 1 | 0 | 0 |
| Total | 65 | 17 | 2 |
CTCAE, Common Terminology Criteria for Adverse Events.
No DLTs were noted in the six subjects enrolled in Phase1b. Two of the first six patient sarcomas showed ≥95% tumor necrosis. Hence, the preliminary safety of the standard TVEC dose of 4 mL of 106 PFU as the initial dose followed by 4 mL of 108 PFU weekly was established and the study proceeded to phase II. Twenty-four subjects were enrolled in phase II. One subject refused surgery. Three patients who showed no evidence of metastatic disease at enrollment developed metastatic disease at the preoperative disease assessment time point. They underwent primary tumor resection as planned and then received systemic therapies. One patient developed regional lymph node progression before surgery. One patient developed local progression during treatment and underwent amputation. All other patients underwent limb sparing surgery of their primary tumor. None of the patients who underwent limb sparing surgery developed local recurrence. A total 8 out of 29 patients (27%) who underwent surgery developed wound healing complications. Six of them had acute complications needing incision and debridement and two (6%) patients had long-term open wounds.
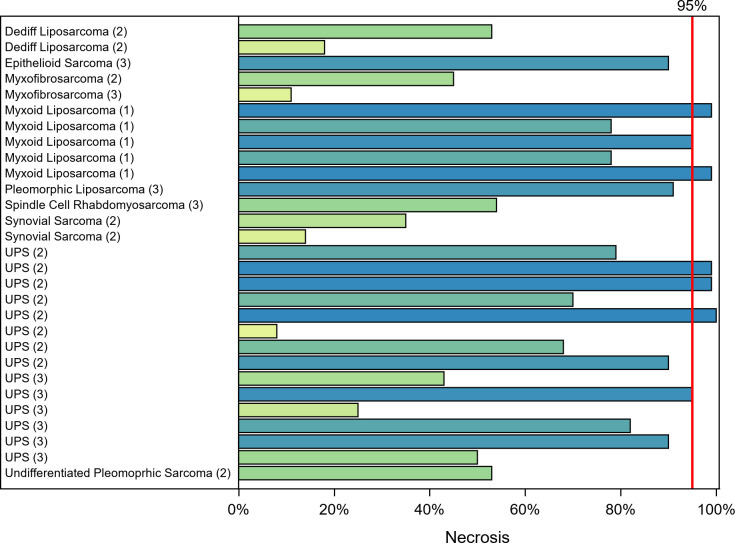
Per the study design, nine patients were enrolled in the first stage and three showed ≥95% tumor necrosis. The study proceeded to the second stage, in which an additional 14 subjects were enrolled. Only 5 of the 23 evaluable patients in phase II achieved ≥95% tumor necrosis; thus, the study did not show a statistical improvement in the near-pCR rate. Sarcoma tumors in 7 out of 29 (24%) evaluable patients from phases I and II who completed treatment and underwent surgery achieved near-pCR as indicated by the swimmer plot (figure 2).
Figure 2.
STS histologic subtypes and corresponding percentage tumor necrosis. Tumor grade is shown within parentheses. UPS, Undifferentiated pleomorphic sarcoma; STS, soft-tissue sarcomas.
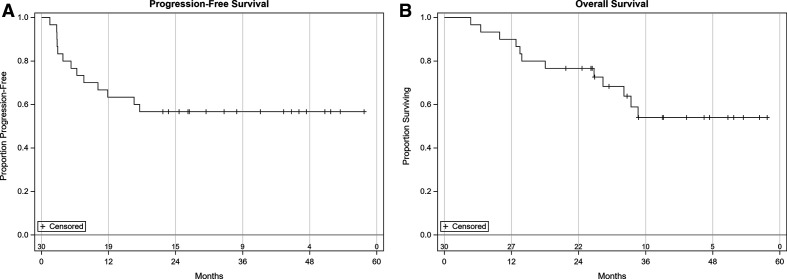
Additional efficacy endpoints including the overall response rate, tumor necrosis, PFS, and OS have been summarized for all patients enrolled in phases IB and II (table 1). As of January 2021, the 2-year PFS is 57% (95% CI 37% to 72%) and 2-year OS is 77% (95% CI 57% to 88%) (figure 3A, B). Median PFS and OS has not been reached. Pretreatment HSV serology did not affect pathological necrosis or radiographic responses. No significant association was noted between AEs and response.
Figure 3.
Overall survival and progression-free survival of 30 patients enrolled in the trial.
Correlative analysis
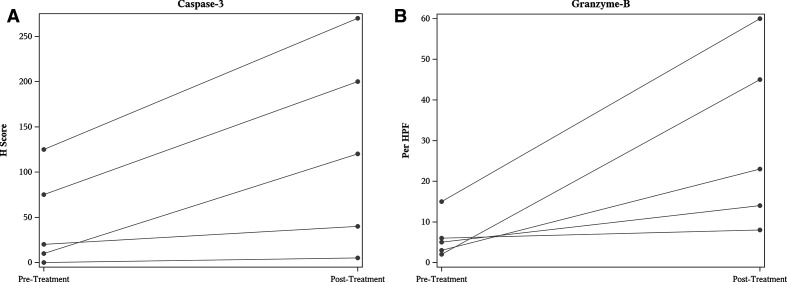
Caspase-3 staining was performed on the diagnostic biopsy tissue specimens and a H score value was calculated (signal intensity (weak, moderate, or strong) * percentage of positive cells). The H scores were compared between diagnostic biopsy specimens with the corresponding TVEC plus EBRT treated resected specimens. A significant increase in the of H score was noted between pre and post treatment tissue specimens (p=0.05) figure 4A. Similarly, Granzyme-B was also noted to be significantly increased between corresponding pretreatment and post-treatment tissue specimens (p=0.05) (figure 4B).
Figure 4.
Caspase -3 (p=0.05) stain on tumor cells as determined by H score and Granzyme B staining (p=0.05) on immune cells determined by number of cells positive per high power field (HPF) in pre-treatment biopsy specimens and TVEC plus EBRT post-treatment resection tissue specimens.
A similar tissue staining for both Caspase-3 and Granzyme-B was performed on the histology matched radiation only treated historical control primary resected tumor specimens (online supplemental tables S2 and S3). The H-score for Caspase-3 staining was noted to be significantly higher for the TVEC plus EBRT treated tumor specimens as compared with radiation alone specimens (p=0.01) (online supplemental figure S1A) but the difference did not reach significance for the Granzyme-B testing results (p=0.10) (online supplemental figure S1B).
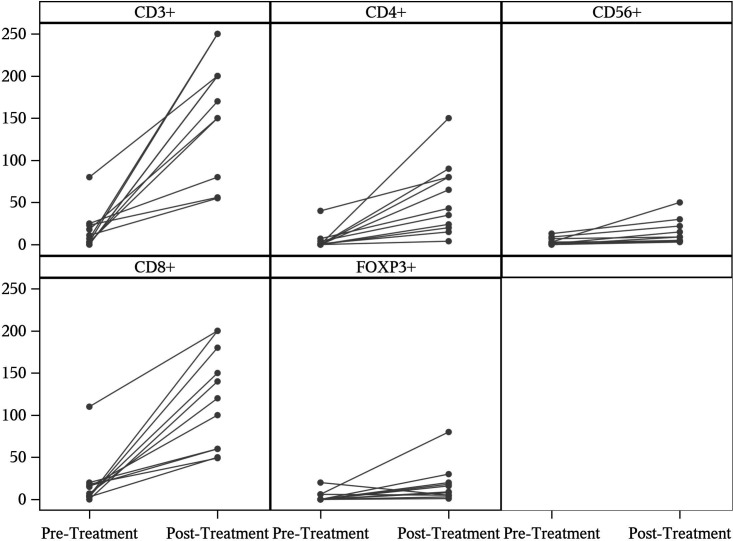
Staining was also performed for T cells using markers against CD3, CD4, CD8, and Foxp3. CD56 staining was performed to identify Natural Killer (NK) cells. Expression of CD3, which is present on all T cells, was significantly increased between pretreatment and post-treatment tissue specimens (p<0.01). Tissue staining for CD4 (p<0.01), CD8 (p<0.01) and CD56 (p<0.03) were also significantly increased in the post-treatment specimens. Foxp3, a transcription factor expressed in regulatory T cells, did not show a significant difference between time points (figure 5).
Figure 5.
Immune cells with respective tissue staining comparison between pre treatment biopsy tissue specimen and post treatment resection tissue specimen. Significant increases were noted for CD3+ (p<0.01), CD4+ (p<0.01), CD8+ (p<0.01) and CD56+ (p<0.03) cells.
TUNEL staining performed on resected sarcoma specimens treated with the TVEC plus EBRT combination and compared with historical tumor specimens treated with radiation therapy alone (online supplemental figure s2) showed no statistical significance between the two groups. Multiple cytokine markers were evaluated but no significant differences were found between serum specimens and did not correlate with survival or necrosis (online supplemental table S1).
Discussion
Preoperative radiation is generally favored as it has better toxicity profile in the long run and is determined to be more cost effective, although it does come at a risk of higher wound complicated rates as compared with postoperative radiation.25 26 Neoadjuvant chemotherapy with ifosfamide and doxorubicin is a commonly used regimen which is associated with radiographic responses, significant toxicities and only marginal survival benefit.27 28 Recently, a study with neoadjuvant and adjuvant chemotherapy with combination of epirubicin and ifosfamide did not show any benefit as compared with histology tailored chemotherapy regimen in common histologies of localized STS.4 A study in children and young adults with chemotherapy sensitive localized STS showed that targeted therapy with pazopanib in addition to neoadjuvant chemoradiotherapy had improved 90% pathological responses as compared with chemoradiotherapy alone. With the availability of newer immunotherapy drugs, their safety and efficacy with intratumoral injections warrants exploration in STS which have limited treatment options.
The first application of immunotherapy in sarcoma was reported by Sir William Coley in the 18th century. He injected streptococcal toxins in malignant sarcomas intratumorally.29 Since then, researchers have explored the efficacy of several immunotherapeutic agents in the treatment of STS. EBRT either preoperatively or postoperatively remains a standard of care for high-risk deep STS with the intent to improve local disease control and metastasis-free survival. A recent literature review indicates that 10% of patients with STS will achieve ≥95% tumor necrosis and approximately 25% will achieve ≥80% tumor necrosis following preoperative EBRT alone. Patients who achieve ≥95% tumor necrosis with preoperative radiation may have improved local and distant control.20 21 Tumor necrosis has not yet been shown to be a surrogate of OS; however, it is an acceptable end point in various sarcoma studies evaluating novel therapeutics in the neoadjuvant therapeutic space. Furthermore, in the era of immunotherapy and OVs with the potential for an abscopal effect,12 13 23 24 30 31 the use of near-pCR as a study endpoint remains to be fully explored.
We found the combination of intratumoral TVEC and preoperative EBRT to be safe and well-tolerated in patients with STS of the extremities and trunk. The toxicity profile of TVEC was similar to prior experiences reported in advanced melanoma and head and neck cancers.13 16 32 There was no treatment-related death. Postoperative wound complications were noted in 27% of the patients, which was consistent with other studies.26 33 34 The TVEC 106 and 108 PFU in 4 mL dose used in this study, with its well-studied safety profile, was similar to the dose used in previous melanoma clinical trials. The larger size of the sarcoma tumors perhaps warranted a higher injection dose for better virus distribution, which may translate into better efficacy.
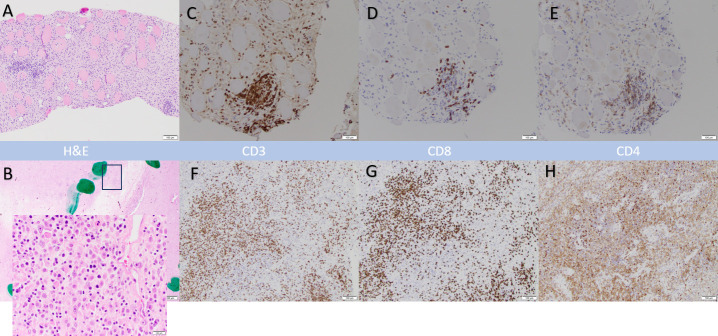
Our results revealed several novel observations. Increased lymphoplasmacytic infiltrate was reported in TVEC-treated specimens compared with that in specimens historically treated with EBRT alone. On further examination, certain tumor specimens showed increased CD8 T cell subset infiltration in comparison to pretreatment biopsies (figure 6). One patient with metastatic undifferentiated pleomorphic sarcoma of the trunk who received protocol therapy but later opted against primary tumor resection showed PD on the preoperative disease assessment scan. A follow-up scan 3 months later without interim treatment showed PR both in the primary tumor and metastatic disease sites in the lung, suggesting a delayed immune response. Given the curative intent, standard surgical timelines were followed in this study, which could have limited treatment efficacy especially in patients with metastatic disease. One patient with undifferentiated pleomorphic sarcoma of the extremities who was treated using protocol therapy but developed lung metastases later underwent metastasectomy. The resected specimen showed lymphoplasmacytic infiltration, suggesting an off-target immune response. This immune-driven phenomenon in the presence of radiation therapy is difficult to characterize and attribute to TVEC or EBRT alone.
Figure 6.
Example of myxofibrosarcoma histopathology sections after TVEC and preoperative EBRT. Hematoxylin and eosin staining showing the histology in the biopsy specimen (Panel A) and a residual tumor (11% necrosis) with admixed lymphocytic infiltrate in panel B (TVEC plus EBRT). The inset in Panel B shows a high power view of dense immune infiltrate. Panel C, D and E show CD3, CD8 and CD4 positive infiltrating T cells in pretreatment biopsy specimens. Panels F, G and H show corresponding changes in those T cell subtypes in post treatment tumor specimens. UPS, Undifferentiated pleomorphic sarcoma; TVEC, Talimogene laherparepvec; EBRT, external beam radiation therapy.
While trying to draw conclusions about the STS subtype-specific response is difficult given the small number of subjects in the study, some trends were noted. Five patients with low-grade myxoid liposarcoma remain free from local recurrence or distant metastasis, which may reflect the increased radiation sensitivity of this subtype or a good local immune control secondary to TVEC. Two patients with intermediate-grade myxofibrosarcoma (involving the trunk or extremities), one with locally recurrent disease at enrollment and both with microscopic positive margins postoperatively, have not had local or distant relapse >24 months since treatment, suggesting good local disease control similar to TVEC responses noted for in-transit metastasis in melanoma (stage IVM1a).13 A major concern with intratumoral injection was skin infiltration by tumor cells at the injection sites. During surgical resection, the ink marks used to direct injections were removed en bloc with the tumor specimen, and the sites were not infiltrated with tumor cells.
Our study adds to the growing literature on immune response in patients with an inherent resistance to immunotherapy, which needs to be further explored. There are several limitations of this study. A fixed TVEC dose was injected irrespective of tumor size. Whether this was an appropriate approach was not explored. This limitation could perhaps be overcome by evaluating phased dose escalations of TVEC plus preoperative EBRT based on sarcoma size (eg, 4 mL of TVEC in tumors 5–10 cm in size, 8 mL of TVEC in tumors >10 cm). An ongoing trial is evaluating 8 mL dose of intratumoral TVEC (NCT04599062) in combination with preoperative radiation is currently enrolling patients. Standard EBRT doses were used. Perhaps hypofractionated dosing of EBRT regimens that result in greater tumor cell killing and immune responses may elicit a better immune response.35 36 Despite careful ultrasound-guided injection to ensure good TVEC distribution to every tumor region, the drug effect could not be confirmed histologically in all parts of the tumor specimens. Whether good drug distribution within the tumor is important to elicit more potent antitumor immune responses remains to be determined. Visceral-site TVEC injections could be considered for metastatic disease. Given the possibility of delayed responses with immunotherapy, as seen in one of our subjects, allowing continued treatment despite first progression may be considered, provided there are limited symptoms. Addition of other immune stimulants, such as systemic anti-PD-137–39 or intratumoral TLR 9 receptor agonists, could be considered for more robust localized immune responses. A recent study of TVEC in combination with pembrolizumab in locally advanced or metastatic soft tissue sarcoma showed promising objective responses and the combination is being explored in select sarcoma subtypes.40 Exploring TVEC in combination with anti-PD1 together with radiation, which can further enhance immunotherapy effects, may be a very valuable approach to be studied in future clinical trials. Radiographic responses determined by comparing pretreatment and preoperative imaging (MRI or CT) did not correspond to pathological necrosis in all cases, which again highlights the lack of good radiographic disease assessment in the neoadjuvant therapeutic space.
Immunohistochemistry revealed significant differences in T cell infiltration into tumor specimens. Total T cells, as measured by CD3 staining, were increased in post-treatment tumor samples compared with pretreatment levels. Additionally, staining of CD4 and CD8 was significantly increased, indicating that there was increased infiltration of both CD4 and CD8 T cells into the tumor after TVEC treatment. Enhanced T cell infiltration to tumor sites, in particular higher numbers of CD8 T cells, correlates with better clinical outcomes in numerous cancer types.41 42 There is a significant increase in CD56 stained cells in TVEC plus EBRT treated resection specimens as compared with pretreatment biopsy specimens which suggests enhanced NK cell function. The change in CD56 is subtle, while the changes in CD3, CD4 and CD8 are much more substantial. This could also be due to the increased T cell infiltration since some T cell subsets are known to express CD56.
Immunostaining of tissue sections revealed significant differences in multiple immune markers. Expression of granzyme-B was significantly increased in post-treatment tissue specimens compared with pretreatment, although no difference was observed between patients that received TVEC compared with radiation alone controls. Granzyme-B is an effector serine-protease released by cytotoxic CD8 T cells and NK cells that function as a primary mechanism of tumor cell death.43 Caspase-3 is a protease involved in apoptosis and is downstream of many molecules including granzyme-B. Caspase-3 expression can correlate with increased tumor cell death in a number of cancer types.44–47 Staining for caspase-3 was increased in the post-treatment specimens. Additionally, caspase-3 staining was significantly increased in those patients that received TVEC treatment compared with those that received radiation alone. These combined data indicate that the TVEC treatment may enhance apoptotic tumor cell death.
No difference in TUNEL staining was demonstrated between TVEC treated patients and those treated with radiation alone. TUNEL staining detects double-stranded DNA breaks, a characteristic of both necrotic and apoptotic cell death; however, recent literature favors it to be a marker of apoptosis.48 Given the lack of significant difference as compared with radiation alone controls suggest that the response to therapy was maybe due to TVEC’s action as an immune stimulant and not as a radiosensitizer. While it is possible that apoptosis may be secondary to radiation therapy alone, the caspase data suggest that there is an added induction of apoptosis with the combination therapy with increased Granzyme being the major mediator of apoptosis. The reasons for the discrepancy between the TUNEL and caspase data are not readily apparent, but it is possible that caspase-3 measures apoptosis at a ‘reversible’ stage.49 Thus, caspase-3 staining may be a more reliable marker for apoptotic cancer cell death related to TVEC use.
Conclusion
Preoperative OV treatment with TVEC and concurrent EBRT for locally advanced STS is safe and well-tolerated. The combination however did not increase the proposed pathological necrosis rate. No DLTs were observed. While the combination of TVEC plus EBRT may enhance immune responses, as observed in some cases, long-term survival benefits will be awaited and reported in future. A trial evaluating greater dose level of TVEC corresponding to the tumor size and various combination strategies should be considered.
Acknowledgments
We thank the research coordinators, data managers, regulatory team and the cancer center pharmacy at Holden Comprehensive Cancer Cenrter for supporting this study. Special thanks to Dr Zuhair Ballas for his expert opinions on this subject. Above all thanks to all the patients and their families for their participation in this study. Research specimen were obtained through University of Iowa Holden Comprehensive Cancer Center's "Connective Tissue Proliferative Disorder Clinical Data and Tissue Sample Collection Project (STiR), an Institutional Review Board approved biospecimen repository and data registry.
Footnotes
Presented at: The safety data of patients treated in the Phase Ib part of the study was presented as an abstract at the Connective Tissue Oncology Society Meeting at Lisbon, Portugal in 2016.
Contributors: VM, MM, BJM, BA, CAn, SB, DK, MR, CM, and CAb were involved in actively treating the patients on the study. MT, SB, OJ, and JT reviewed and interpreted the histopathology data. LS, SH, SV, JH, LW, and WZ analyzed blood specimens and tissue specimens for immunological markers and interpreting the data. GZ and SM were the biostatisticians involved with this study. All authors reviewed and edited the final manuscript.
Funding: NIH grants R01 CA200673 (PI: W.Z.) and R01 CA203834 (PI: WZ). WZ was also supported by DOD/CDMRP grant BC180227 (PI: WZ) and an endowment from the Dr and Mrs James Robert Spencer Family Cancer Research Fund (PI: WZ).
Competing interests: MM has had an advisory role and received research funding from Amgen. VM receives research funding from Amgen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. All of the individual participant data collected during the trial, the study protocol, statistical analysis plan, informed consent, and clinical study report after deidentification have been made available indefinitely at https://clinicaltrials.gov/ct2/show/NCT02453191?term=NCT02453191&draw=2&rank=1.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol was approved by the University of Iowa Institutional Review Board.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.ESMO/European Sarcoma Network Working Group . Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii102–12. 10.1093/annonc/mdu254 [DOI] [PubMed] [Google Scholar]
- 3.von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2014. J Natl Compr Canc Netw 2014;12:473–83. 10.6004/jnccn.2014.0053 [DOI] [PubMed] [Google Scholar]
- 4.Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812–22. 10.1016/S1470-2045(17)30334-0 [DOI] [PubMed] [Google Scholar]
- 5.Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol 2016;17:671–80. 10.1016/S1470-2045(16)00010-3 [DOI] [PubMed] [Google Scholar]
- 6.Bharatan NS, Currier MA, Cripe TP. Differential susceptibility of pediatric sarcoma cells to oncolysis by conditionally replication-competent herpes simplex viruses. J Pediatr Hematol Oncol 2002;24:447–53. 10.1097/00043426-200208000-00008 [DOI] [PubMed] [Google Scholar]
- 7.Cripe TP, Dunphy EJ, Holub AD, et al. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res 2001;61:2953–60. [PubMed] [Google Scholar]
- 8.Leddon JL, Chen C-Y, Currier MA, et al. Oncolytic HSV virotherapy in murine sarcomas differentially triggers an antitumor T-cell response in the absence of virus permissivity. Mol Ther Oncolytics 2015;1:14010. 10.1038/mto.2014.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segal NH, Blachere NE, Shiu HYA, et al. Antigens recognized by autologous antibodies of patients with soft tissue sarcoma. Cancer Immun 2005;5:4. [PubMed] [Google Scholar]
- 10.Touchefeu Y, Vassaux G, Harrington KJ. Oncolytic viruses in radiation oncology. Radiother Oncol 2011;99:262–70. 10.1016/j.radonc.2011.05.078 [DOI] [PubMed] [Google Scholar]
- 11.Advani SJ, Mezhir JJ, Roizman B, et al. Revolt: radiation-enhanced viral oncolytic therapy. Int J Radiat Oncol Biol Phys 2006;66:637–46. 10.1016/j.ijrobp.2006.06.034 [DOI] [PubMed] [Google Scholar]
- 12.Havunen R, Santos JM, Sorsa S, et al. Abscopal effect in non-injected tumors achieved with cytokine-armed oncolytic adenovirus. Mol Ther Oncolytics 2018;11:109–21. 10.1016/j.omto.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780–8. 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 14.Puzanov I, Milhem MM, Minor D, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol 2016;34:2619–26. 10.1200/JCO.2016.67.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conry RM, Westbrook B, McKee S, et al. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum Vaccin Immunother 2018;14:839–46. 10.1080/21645515.2017.1412896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res 2010;16:4005–15. 10.1158/1078-0432.CCR-10-0196 [DOI] [PubMed] [Google Scholar]
- 17.US.department Of Health And Human Services . Common terminology criteria for adverse events (CTCAE) version 4.0, 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf [Google Scholar]
- 18.National Comprehensive Cancer Network (NCCN) . Soft tissue sarcoma (Version 6.2019), in sarcoma NCCNNCPGIOSt. Clinical Practice Guidelines. In: Oncology, 2020. https://www.nccn.org/patients/guidelines/content/PDF/sarcoma-patient.pdf [Google Scholar]
- 19.Wardelmann E, Haas RL, Bovée JVMG, et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European organization for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur J Cancer 2016;53:84–95. 10.1016/j.ejca.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 20.Canter RJ, Martinez SR, Tamurian RM, et al. Radiographic and histologic response to neoadjuvant radiotherapy in patients with soft tissue sarcoma. Ann Surg Oncol 2010;17:2578–84. 10.1245/s10434-010-1156-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res 2012;32:3911–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 23.Hu JCC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006;12:6737–47. 10.1158/1078-0432.CCR-06-0759 [DOI] [PubMed] [Google Scholar]
- 24.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 2009;27:5763–71. 10.1200/JCO.2009.24.3675 [DOI] [PubMed] [Google Scholar]
- 25.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005;75:48–53. 10.1016/j.radonc.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 26.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002;359:2235–41. 10.1016/S0140-6736(02)09292-9 [DOI] [PubMed] [Google Scholar]
- 27.Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81. 10.1002/cncr.23592 [DOI] [PubMed] [Google Scholar]
- 28.Verma S, Younus J, Stys-Norman D, et al. Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev 2008;34:339–47. 10.1016/j.ctrv.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 29.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006;26:154–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Zamarin D, Ricca JM, Sadekova S, et al. Pd-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy. J Clin Invest 2018;128:1413–28. 10.1172/JCI98047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeh HJ, Downs-Canner S, McCart JA, et al. First-In-Man study of Western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther 2015;23:202–14. 10.1038/mt.2014.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ott PA, Hodi FS. Talimogene Laherparepvec for the treatment of advanced melanoma. Clin Cancer Res 2016;22:3127–31. 10.1158/1078-0432.CCR-15-2709 [DOI] [PubMed] [Google Scholar]
- 33.Moore J, Isler M, Barry J, et al. Major wound complication risk factors following soft tissue sarcoma resection. Eur J Surg Oncol 2014;40:1671–6. 10.1016/j.ejso.2014.10.045 [DOI] [PubMed] [Google Scholar]
- 34.Griffin AM, Dickie CI, Catton CN, et al. The influence of time interval between preoperative radiation and surgical resection on the development of wound healing complications in extremity soft tissue sarcoma. Ann Surg Oncol 2015;22:2824–30. 10.1245/s10434-015-4631-z [DOI] [PubMed] [Google Scholar]
- 35.Kalbasi A, Kamrava M, Nelson SD, et al. 5-Day Hypofractionated preoperative radiation therapy in soft tissue sarcoma: preliminary toxicity and pathologic outcomes from a prospective phase 2 study. Int J Radiat Oncol Biol Phys 2017;99:E753–4. 10.1016/j.ijrobp.2017.06.2414 [DOI] [Google Scholar]
- 36.Koseła-Paterczyk H, Szacht M, Morysiński T, et al. Preoperative hypofractionated radiotherapy in the treatment of localized soft tissue sarcomas. Eur J Surg Oncol 2014;40:1641–7. 10.1016/j.ejso.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 37.Dummer R, Hoeller C, Gruter IP. Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors. 66. Cancer immunology, immunotherapy: CII, 2017: 683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell AM, Herbst RS, Gettinger SN, et al. Final results of a phase I prospective trial evaluating the combination of stereotactic body radiotherapy (SBRT) with concurrent pembrolizumab in patients with metastatic non-small cell lung cancer (NSCLC) or melanoma. JCO 2018;36:9099. 10.1200/JCO.2018.36.15_suppl.9099 [DOI] [Google Scholar]
- 39.Cushman TR, Gomez D, Kumar R, et al. Combining radiation plus immunotherapy to improve systemic immune response. J Thorac Dis 2018;10:S468–79. 10.21037/jtd.2018.01.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly CM, Antonescu CR, Bowler T, et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: a phase 2 clinical trial. JAMA Oncol 2020;6:402–8. 10.1001/jamaoncol.2019.6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Gruosso T, Zuo D, et al. Infiltration of CD8+ T cells into tumor cell clusters in triple-negative breast cancer. Proc Natl Acad Sci U S A 2019;116:3678–87. 10.1073/pnas.1817652116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fridman WH, Zitvogel L, Sautès-Fridman C, et al. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 2017;14:717–34. 10.1038/nrclinonc.2017.101 [DOI] [PubMed] [Google Scholar]
- 43.Rousalova I, Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation (review). Int J Oncol 2010;37:1361–78. 10.3892/ijo_00000788 [DOI] [PubMed] [Google Scholar]
- 44.Lee MS, Lim SH, Yu A-R, et al. Carfilzomib in combination with bortezomib enhances apoptotic cell death in B16-F1 melanoma cells. Biology 2021;10. 10.3390/biology10020153. [Epub ahead of print: 15 Feb 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Chi F, Qin K, et al. Chidamide induces apoptosis in DLBCL cells by suppressing the HDACs/STAT3/Bcl‑2 pathway. Mol Med Rep 2021;23. 10.3892/mmr.2021.11947. [Epub ahead of print: 02 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo Y-S, Kim T-H, Lim H, et al. Phenformin induces caspase-dependent apoptosis of FaDu head and neck squamous cell carcinoma cells. Anticancer Res 2019;39:3499–506. 10.21873/anticanres.13496 [DOI] [PubMed] [Google Scholar]
- 47.Pati ML, Hornick JR, Niso M, et al. Sigma-2 receptor agonist derivatives of 1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) induce cell death via mitochondrial superoxide production and caspase activation in pancreatic cancer. BMC Cancer 2017;17:51. 10.1186/s12885-016-3040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohan C, Long K, Mutneja M, et al. Detection of end-stage apoptosis by ApopTag® TUNEL technique. Methods Mol Biol 2015;1219:43–56. 10.1007/978-1-4939-1661-0_5 [DOI] [PubMed] [Google Scholar]
- 49.Geske FJ, Lieberman R, Strange R, et al. Early stages of p53-induced apoptosis are reversible. Cell Death Differ 2001;8:182–91. 10.1038/sj.cdd.4400786 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-003119supp001.pdf (183.8KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. All of the individual participant data collected during the trial, the study protocol, statistical analysis plan, informed consent, and clinical study report after deidentification have been made available indefinitely at https://clinicaltrials.gov/ct2/show/NCT02453191?term=NCT02453191&draw=2&rank=1.