Supplemental Digital Content is available in the text.
Key Words: sacrococcygeal region, endodermal sinus tumor, combined modality therapy, relapse-free survival, Pediatrics
Abstract
The aim of the study was to explore the clinicopathologic characteristics of sacrococcygeal yolk sac tumor (SYST) associated with relapse and the role of sensitivity to neoadjuvant chemotherapy in predicting outcome. The authors investigated prognostic factors of age, stage, initial tumor size, pathologic response to neoadjuvant chemotherapy, and alfa fetoprotein. A total of 26 patients with SYST were enrolled. Neoadjuvant chemotherapy was administered to 20 cases. Six patients underwent resection as initial therapy. Recurrence occurred in 12 patients. Nine patients with specimens exhibiting no malignant component after chemotherapy did not experience recurrence. By contrast, relapses occurred in 7 of 11 patients with viable residual tumor after neoadjuvant chemotherapy. All relapsed patients still achieved partial remission or complete remission after salvage therapy. Five-year relapse-free survival and overall survival rates were 55.2% and 100%, respectively (median follow-up, 59.5 mo; range, 16 to 155). Patients with complete necrosis after neoadjuvant chemotherapy had a better outcome compared with children with viable residual tumor. Relapse-free survival of pediatric SYSTs in this cohort were still low and warrants the multidisciplinary effort.
Germ cell tumors (GCTs) are formed by aberrant migration of primitive germ cells arising in midline sites including brain, head/neck, mediastinum, gonads, retroperitoneum, sacrococcygeal region, and vagina. GCTs are classified as gonadal and extragonadal GCTs on the basis of origin. Sacrococcygeal GCT represents ~40% of primary extragonadal and extracranial GCTs among children.1 Most pediatric tumors stemming from the region are benign teratomas followed by malignant yolk sac tumor (YST) (endodermal sinus tumor). Sacrococcygeal yolk sac tumors (SYSTs) are generally characterized by external mass growing around the sacrum and coccyx accompanied by elevation of alfa fetoprotein (AFP). A certain proportion of patients still developed local relapse although they received effective multidisciplinary treatment. Most of the clinical explorations of sacrococcygeal GCT were confined to a few case reports. The prognostic factors in relation to sacrococcygeal GCTs were poorly understood.
Here, we studied the relapse-free survival (RFS) rates, prognostic factors, and therapeutic effect of salvage treatment in a retrospective cohort of patients with SYST. Finally, we evaluated the combined prognostic factors of clinical significance on RFS.
METHODS
Eligibility
All patients 18 years of age or younger with newly diagnosed primary SYST at Sun Yat-sen University Cancer Center between January 2007 and December 2018 were eligible. The initial diagnosis was established on the basis of confirmed histology and AFP exceeding the age-related normal range. The predominant malignant element was pure YST. Patients with diagnosed pure teratoma were excluded from the analysis. Staging after initial resection was performed according to the classification system developed by Children’s Oncology Group (Supplement 1, Supplemental Digital Content 1, http://links.lww.com/JPHO/A426). Clinical variables were recorded regarding age, sex, AFP level, stage, metastasis site, primary tumor size, treatment, and pathologic response. The date of the last follow-up was April 18, 2020. In the cohort, the initial treatment decision was made according to tumor size, stage, and risks by the treating physician. The present study was approved by the Ethics Board of Sun Yat-sen University Cancer Center (2020-FXY-115) and conducted in accordance with the Helsinki Declaration. All the original data were deposited at http://www.researchdata.org.cn (RDD number RDDA2020001555).
Chemotherapy and Evaluation
Preoperative courses of chemotherapy were administered to patients with bulky disease or metastasis. Initial chemotherapy comprised cisplatin, etoposide, and bleomycin (PEB regimen): bleomycin 15 mg/m2 on day 1, etoposide 100 mg/m2 on days 1 through 5, and cisplatin 20 mg/m2 on days 1 through 5. Every cycle was repeated at the 3-week interval. Chemotherapy doses were adjusted for infants less than 12 months of age: cisplatin 0.7 mg/kg/dose, etoposide 3 mg/kg/dose, and bleomycin 0.5 mg/kg/dose.
Complete remission (CR) was considered as normal serum tumor markers (<25 ng/mL or below the age-related reference value) and absence of all imaging abnormalities in computed tomography or magnetic nuclear resonance imaging. Partial remission was defined as AFP decline and shrinkage of tumor size by at least 50%. Relapse was defined as the detection of new lesions in local or distant sites and tumor marker elevation in terms of CR.
AFP was monitored before every cycle of chemotherapy. Patients underwent a thorough evaluation of every 2 cycles of chemotherapy by imaging workup and tumor marker determinations. We proposed 2 to 4 preoperative chemotherapy courses before a radical complete resection without major morbidity was achieved. If the resected specimen showed no viable tumor cells and AFP declined within a reasonable range of half-life, then chemotherapy was considered effective. Additional 2 courses of identical chemotherapy were administered to good responders.2 Patients with malignant residual disease in the pathologic specimen continued to receive 4 cycles of platinum-based chemotherapy right away. Patients with progressive disease or no response to the initial chemotherapy were considered treatment failures and received second-line chemotherapy. Total cycles of chemotherapy varied between 6 and 8 courses.
Tumor Resection
Patients unable to receive complete resection or at high risk of rupture underwent biopsy before treatment. Tumor resection was considered when the primary tumor shrank approximately to 2 cm×3 cm to minimize the risk of rupture or spillage during resection. Management of residual masses with surgery should be performed in patients with tumor markers persisting at low levels after completion of first-line treatment. Tumor resection field included the tumor pseudocapsule and en-bloc coccyx bone as possible. Tumors were resected in toto without proof of rupture as possible as surgeons could.3 Pathologic response was assessed among 20 patients undergoing preoperative chemotherapy as follows: good pathologic response—complete necrosis observed in the resected specimen; poor pathologic response—viable residual tumor cells in resected tumors.
Salvage Therapy After Relapse
Therapeutic approach for patients with recurrent SYST was not homogenous. Therapeutic strategies were developed on the basis of the response to the previous treatment. The salvage strategy consisted of platinum-based chemotherapy basically followed by resection of residual masses when possible, radiotherapy, or maintenance treatment.
Statistical Analysis
Statistical analysis was conducted using the statistical software SPSS 25 (IBM Corp., Armonk, NY). A χ2 test was used to analyze the associations between pathologic response and patient characteristics. The RFS was generated according to the Kaplan-Meier method. The influences of suspected prognostic factors associated with RFS were analyzed with the Breslow test because of most relapse occurring in a short time. A log-rank test was performed to determine the survival difference in the long term. The prognostic value in affecting RFS was assessed using univariate analysis. RFS was calculated as the time from diagnosis to the first relapse or death (death related to disease or therapy-oriented complication) or from the first relapse to the next relapse. All statistical tests were 2-sided and the P-values <0.05 were considered statistically significant.
RESULTS
Patient Demographics
In total, 26 eligible patients were enrolled with SYST in the present study between March 2008 and November 2018, representing 20.6% of the total 126 GCT diagnoses in the period. Patient characteristics are listed in Table 1. Briefly, patients had a median age of 1.7 years (range, new born to 5 y). The median AFP levels were 50,480 ng/mL (range, 1200 to 80,300,000 ng/mL) at diagnosis. The stage distribution was 3 patients with stage II, 11 with stage III, and 12 with stage IV tumors. In patients with stage IV, metastatic sites were lung alone in 6 cases, bone and lung in 3 cases, liver and lung in 2 cases, and liver alone in 1 case (Table 1).
TABLE 1.
General Clinical Characteristics of Patients With Sacrococcygeal Yolk Sac Tumor
| Characteristics | No. Patients (%) |
|---|---|
| Sex | 26 |
| Male | 11 (42.3) |
| Female | 15 (57.8) |
| Age (y) | |
| Median | 1.7 |
| Range | 0-5 |
| Stage | |
| II | 3 (11.5) |
| III | 11 (42.3) |
| IV | 12 (46.2) |
| Initial tumor size | |
| ≤3 cm×4 cm | 10 (38.5) |
| >3 cm×4 cm | 16 (61.5) |
| Meatstasis | |
| Lung | 6 (50.0) |
| Liver | 1 (8.3) |
| Lung/liver | 2 (16.7) |
| Lung/bone | 3 (25.0) |
| AFP (ng/mL) | |
| ≤60,000 | 12 (46.2) |
| >60,000 | 14 (53.8) |
| Surgery | |
| Upfront surgery | 6 (23.1) |
| After chemotherapy | 20 (76.9) |
| Pathologic response to neoadjuvant chemotherapy | |
| Necrosis | 9 (45.0) |
| Microscopic residual disease | 11 (55.0) |
| No. relapse | |
| 0 | 14 (53.8) |
| 1 | 5 (19.2) |
| 2 | 3 (11.5) |
| 3 | 2 (7.7) |
| 4 | 1 (3.8) |
| 6 | 1 (3.8) |
AFP indicates alpha-fetoprotein; No, number.
Treatment and Response
Upfront surgical resections were undergone in 6 (23.1%) patients: stage II in 1 case, stage III in 4 cases, and stage IV in 1 case (unknown the pathologic diagnosis). Resected tumor size for all patients who underwent upfront surgery (defined as the greatest extent measured by magnetic resonance imaging scan) was measured to be ≤4 cm×3 cm. The median of chemotherapy cycles after resection was 4 (range, 4 to 6). One of these patients remained in continuous CR, and 5 patients relapsed. The local stage II was diagnosed in the patient with continuous CR and the AFP tumor marker was 35,031 ng/mL. Serum AFP ranged from 52,100 to 80,325 ng/mL among the other 5 relapsed patients. Twenty (76.9%) patients with initial tumor size >4 cm×3 cm received a median of 4 courses (range, 2 to 8) neoadjuvant chemotherapy as first-line treatment (Table 1). Primary tumor shrank approximately to 2 cm×3 cm and AFP declined to <100 ng/mL before surgery.
Histopathology after neoadjuvant chemotherapy was notable for nonviable cells in 9 patients. None of them developed relapse. Among the remaining 11 cases with confirmed malignant disease, 4 patients had no relapse and 7 patients experienced a relapse.
To evaluate assumed prognostic parameters in pathologic response to neoadjuvant chemotherapy, we compared sex, age, AFP level, stage at diagnosis, number of neoadjuvant chemotherapy cycles, and relapse between good and poor responders. In this analysis, these variables were comparable for both groups (Table 2).
TABLE 2.
Patient Characteristics at Diagnosis Between Good and Poor Pathologic Response to Neoadjuvant Chemotherapy
| Patient Characteristics | Good Response, n/N (%) | Poor Response, n/N (%) | χ2 | Significance (P) |
|---|---|---|---|---|
| Age (y) | ||||
| ≤2 y | 6/9 (66.7) | 5/11 (45.5) | 0.90 | 0.41 |
| >2 y | 3/9 (33.3) | 6/11 (54.5) | ||
| Sex | ||||
| Male | 3/9 (33.3) | 6/11 (54.5) | 0.90 | 0.41 |
| Female | 6/9 (66.7) | 5/11 (45.5) | ||
| Stage | ||||
| I-II | 1/9 (11.1) | 1/11 (9.1) | 0.02 | 1.00 |
| III-IV | 8/9 (88.9) | 10/11 (90.9) | ||
| AFP (ng/mL) | ||||
| ≤60,000 | 4/9 (44.4) | 4/11 (36.4) | 0.14 | 1.00 |
| 60,000 | 5/9 (55.6) | 7/11 (63.6) | ||
| Neoadjuvant chemotherapy cycles | ||||
| >4 | 3/9 (33.3) | 5/11 (45.5) | 0.30 | 0.67 |
| ≤4 | 6/9 (66.7) | 6/11 (54.5) | ||
| Relapses | 0/9 (0) | 7/11 (63.6) | 8.81 | 0.01* |
AFP indicates alpha-fetoprotein; N, number.
P < 0.05.
Survival
No patients died of relapse and therapy-associated complications at the last follow-up. Twelve patients developed relapses. Total frequencies of recurrence were once in 5 patients, 2 in 3 patients, 3 in 2 patients, 4 in 1 patient, and 6 in 1 patient, respectively (Table 1). Among these, 11 patients developed local relapse (42.3%) and 1 patient displayed a distant relapse at the right cerebral parietal lobe.
The 5-year overall survival (OS) and RFS rates of the whole cohort were 100.0% and 55.2% with a median follow-up of 59.5 months (range, 16 to 155 mo, Fig. 1). The estimated 5-year RFS rates for stage III and stage IV were 50.9% and 62.5%, respectively (P=0.40). There was a trend toward better 5-year RFS for girls (64.2%±17.6%) compared with boys (40.0%±15.5%, P=0.02). The RFS between the 2 age groups (≤2 vs. >2 y) was not significantly different (P=0.82, Table 3). Regarding AFP level, the 5-year RFS rate was 56.3% for ≤60,000 ng/mL, and this rate was higher than for >60,000 ng/mL (P=0.91, Table 3). Hence, stage, AFP level, age, and initial tumor size were not predictive of an adverse RFS prognosis. The 5-year RFS of patients with poor pathologic response was impaired as compared with good pathologic response (46.7%±16.6%; 11/20 patients vs. 100%; 9/20 patients) (P=0.03, Table 3). The median duration from surgery to relapse for patients with viable residual tumor was 6 months (range, 3 to 24 mo).
FIGURE 1.
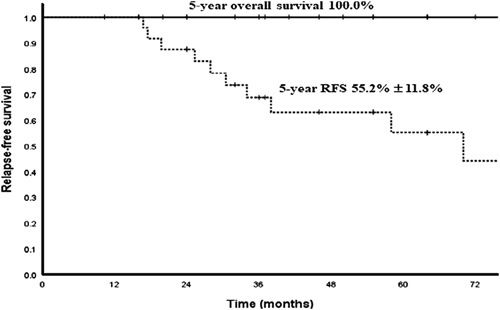
Kaplan-Meier estimates of 5-year survival of the sacrococcygeal yolk sac tumor: overall survival 100%; RFS 55.2%±11.8% (n=26). RFS indicates relapse-free survival.
TABLE 3.
Prognostic Factors Associated With 5-year Relapse-free Survival
| 5-year RFS (%) | |||||
|---|---|---|---|---|---|
| Patient Cohort | Patients, n (%) | % | SE | Breslow | P |
| Sex | |||||
| Male | 11 (42.3) | 40.0 | 15.5 | 5.14 | 0.02* |
| Female | 15 (57.7) | 64.2 | 17.6 | ||
| Age (y) | |||||
| 0-2 | 15 (57.7) | 60.1 | 14.2 | 0.05 | 0.82 |
| >2 | 11 (42.3) | 50.6 | 18.7 | ||
| Stage | |||||
| II | 3 (11.5) | ||||
| III | 11 (42.3) | 50.9 | 16.3 | 0.71 | 0.40 |
| IV | 12 (46.2) | 62.5 | 17.1 | ||
| AFP (ng/mL) | |||||
| ≤60,000 | 12 (46.2) | 56.3 | 18.8 | 0.01 | 0.91 |
| >60,000 | 14 (53.8) | 50.9 | 15.8 | ||
| Initial tumor size | |||||
| ≤3 cm×4 cm | 10 (38.5) | 62.2 | 17.8 | 1.28 | 0.26 |
| >3 cm×4 cm | 16 (61.5) | 51.6 | 14.7 | ||
| Pathologic response | |||||
| No malignant residues | 9 (45.0) | 100.0 | 0 | 4.63 | 0.03* |
| Malignant residues | 11 (55.0) | 46.7 | 16.6 | ||
AFP indicates alpha-fetoprotein; RFS, relapse-free survival.
P<0.05.
Regarding the prognosis according to pathologic response to neoadjuvant chemotherapy, the 5-year RFS rate was 100% for good responders, and this rate was significantly lower for the group with viable residual tumor (P<0.05, Fig. 2). There were no differences in age, sex, AFP level, and stage distribution between relapse group and relapse-free group among patients with viable residual tumor (Table 4). We did not identify prognostic factor for relapse among patients with viable residual tumor.
FIGURE 2.
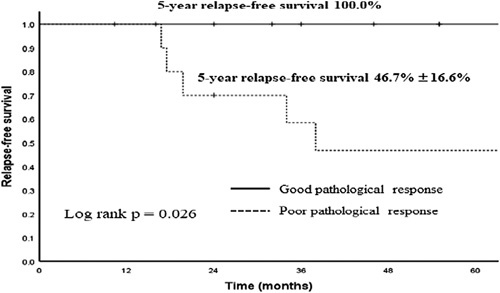
Kaplan-Meier estimates of 5-year relapse-free survival of the sacrococcygeal yolk sac tumor by pathologic response to neoadjuvant chemotherapy. The 5-year relapse-free survival rate of group good response (n=9) was significantly higher than that of group poor response (n=11) (P=0.026).
TABLE 4.
Characteristics of Patients With Viable Tumor Cells After Neoadjuvant Chemotherapy
| Variable | Relapse | No Relapse | Total | % |
|---|---|---|---|---|
| Age (y) | ||||
| 0-2 | 5 | 3 | 8 | 72.7 |
| >2 | 2 | 1 | 3 | 27.3 |
| Sex | ||||
| Male | 4 | 2 | 6 | 54.5 |
| Female | 3 | 2 | 5 | 45.5 |
| AFP (ng/mL) | ||||
| ≤60,000 | 3 | 1 | 4 | 36.4 |
| >60,000 | 4 | 3 | 7 | 63.6 |
| Stage | ||||
| II | 1 | 0 | 1 | 9.1 |
| III | 4 | 1 | 5 | 45.5 |
| IV | 2 | 3 | 5 | 45.5 |
AFP indicates alpha-fetoprotein.
Salvage Treatment After Relapse
Resection and chemotherapy represented the cornerstone of salvage treatment. All 12 relapsed patients underwent second-line carboplatinum-based chemotherapy. Other chemotherapy protocols contained docetaxel/vinorelbine, paclitaxel/nedaplatin, and high-dose paclitaxel/ifosfamide/cisplatin. Patients with local relapse underwent resection. Two patients with recurrent relapse received a total irradiation dose at 50 Gy.2 Eight patients achieved complete response and were alive. Four patients were alive with viable residual tumor in partial response after salvage therapy. Oral cyclophosphamide and vinorelbine-containing maintenance regimens or etoposide alone was administered to 4 repeated relapsed patients with inoperable residual disease after salvage chemotherapy or residual disease after resection. The schedule was as follows: cyclophosphamide 50mg/m2, d1 to 28; vinorelbine 25 mg/m2, on days 1, 8, 15 or oral etoposide 50 mg/m2, d1 to 14, 2 weeks break meaning 2 weeks on and 2 weeks off. Each cycle of treatment was repeated every 4 weeks.
DISCUSSION
The treatment approach used in our center followed the Children’s Oncology Group protocol. The 5-year OS and RFS rates of the whole cohort were 100.0% and 55.2%. After 2 to 4 preoperative chemotherapy courses, resection for stage IV or primary large mass was considered. In North America, the most current protocols recommended a treatment option for stage IV disease was surgery and chemotherapy with 4 cycles of standard PEB.4 However, more cycles of chemotherapy administered did not seem to benefit surgical resection for surgeons because of high chemotherapy sensitivity resulting in no definite tumor margin left. Chemotherapy after incomplete resection has the benefit for survival; however, complete resection of the coccyx is still the basic principle.3,5 Event-free survival (EFS) rate for patients with negative microscopic margins after resection was >90%. For patients with microscopic margins, EFS rates ranged from 75% to 85%. EFS rates declined to <40% for patients with macroscopic residual disease. Completeness of surgical resection is a key prognostic factor.3,5,6 The role of consolidation (the added cycles of chemotherapy) remained a controversy. A retrospective analysis was conducted to show that adjuvant chemotherapy could improve progression-free survival, but failed to demonstrate an improvement in OS. Therefore, the strategy of “wait-and-watch” may also be recommended. Regarding the factors indicating progression, International Germ Cell Cancer Collaborative Group classification defined complete resection of residual masses and <10% viable tumor cells in the resected specimens to be a favorable factor for prognosis. Adjuvant chemotherapy could be removed. If exceeding 10% of viable cancer remained with a completely resected tumor, or if completeness of the resection was not warranted, consolidation chemotherapy might be justified.7 In our clinical practice, complete resection of residual masses sometimes was not possible because of extensive local invasion at this site. Unlike osteosarcoma, we did not assess the ratio of necrosis in the resected specimens. The effectiveness of chemotherapy was defined as AFP decline, reduction of tumor size, or complete necrosis after neoadjuvant chemotherapy. Patients responding well to chemotherapy continued to receive the original chemotherapy. Two courses of adjuvant chemotherapy were administered in cases of good responders. None of them experienced relapse. According to the International Germ Cell Cancer Collaborative Group classification and our data, consolidation chemotherapy could be removed in good responders in the future. Although >2 courses of adjuvant chemotherapy were also administered to patients with viable residual tumor cells after neoadjuvant chemotherapy, relapse occurred in 7 of 11 patients. The role of adjuvant chemotherapy seemed to play a minor role in these patients. Further research into viable residual tumor cells after neoadjuvant chemotherapy is required to determine whether such patients warrant altered therapy or intensive therapy. We conducted a retrospective cohort study investigating the prognostic indicators related to relapse. Boys and poor pathologic response to neoadjuvant chemotherapy were at higher risk of early relapse in univariate analysis. Tumor marker AFP levels at diagnosis were not indicative of prognostic value. Initial tumor size did not seem to influence outcome although patients with tumor size >3 cm×4 cm had a marginal inferior RFS with no statistical significance (P=0.26, Table 3).
Although the OS of SYST tumor was optimal, the RFS remained in this cohort still low. Data from 2 large national pediatric clinical trial organizations have produced a conclusion that patients with extragonadal stage III to IV GCTs have a significantly poor prognosis with an estimated 4-year EFS of 61% for stage III extragonadal disease.8 Pure YST histology seemed to confer a better outcome with borderline significance (P=0.06).8 In our analysis, the 5-year RFS rate was 55.2%. Recurrences were evaluated on a combination of AFP elevations and the presence of new lesions in the sacrococcygeal region or other sites. The pediatric investigation on a small number of patients identified sacrococcygeal tumors as high risk.9 Furthermore, the inferior outcome has been attributed to delayed diagnosis and incomplete resection at the time of original surgery.10 However, a series of study from German (MAKEI) 83 of 86 and MAKEI 89 produced the conclusion that patients with sacrococcygeal primary tumors did not have a worse outcome compared with children with other primary sites, such as ovary or testis. The only difference described was the poorer outcome of children with stage IV disease and/or nonsacrococcygeal primary tumors. An International Collaborative study showed boys (11 y of age and older) with International Germ Cell Consensus Classification intermediate-risk or poor-risk features had inferior outcomes.11 Metastasis did not play a significant role in the outcome of children with advanced stage. The effectiveness of cisplatin-based chemotherapy outweighed adverse factors such as high AFP levels and metastasis. Literature reported risk factors associated with recurrence included gross or microscopic incomplete resection, unresected coccyx, tumor rupture, or spillage before or during surgery.12 However, some risk factors were still under controversy.13 Salvage therapy could still benefit patients when patients relapsed. Salvage chemotherapy was able to facilitate the completeness of relapse tumor resection. The OS of 100% until the last follow-up could be attributed to the effectiveness of second-line or third-line chemotherapy, secondary surgery, radiotherapy, or maintenance therapy. Within our cohort, the fact that 11 relapses were local and only 1 relapse was metastatic could be explained by challenges in local control because of extensive local invasion. Hence, the type of chemotherapy that is given after relapse may not be important compared with the type of surgery that can be achieved. A retrospective analysis of children with sacrococcygeal teratoma from 5 children’s hospitals in North America from 1999 to 2009 included 14 cases of stage I sacrococcygeal teratoma with malignant elements. Among the 14 patients managed with active surveillance-only, 12 survived with no relapse and the 2 relapsed patients were successfully salvaged with platinum-based chemotherapy. Hence, complete resection of GCTs at any site plays an important role in RFS.6 Incomplete resection of the tumor has an overt influence on prognosis.
Some limitations of our study lied in its retrospective character and small sample size. The small sample size did not allow multivariate analysis, and it is possible that the significance of gender in the univariate analysis may be confounded. All patients were not monitored for auditory. The experience of surgeons had an impact on decision making. Multicenter prospective studies are needed to determine prognostic factors in large sample groups.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jpho-online.com.
ACKNOWLEDGMENTS
The authors thank the staff in the Department of Pediatric Surgery, the First Affiliated Hospital of Sun Yat-Sen University for surgical support and Chunmei Chen for developing the study database.
Footnotes
Y.Z. and X.S. are co-corresponding author.
J.Z., H.C., and T.C. contributed equally to the article.
The authors declare no conflict of interest.
Contributor Information
Jia Zhu, Email: zhujia@sysucc.org.cn.
Zijun Zhen, Email: zhenzj@sysucc.org.cn.
Juan Wang, Email: wangjuan@sysucc.org.cn.
Suying Lu, Email: lusy@sysucc.org.cn.
Junting Huang, Email: huangjt@sysucc.org.cn.
Yizhuo Zhang, Email: zhangyzh@sysucc.org.cn.
REFERENCES
- 1. De Giorgi U, Rosti G, Slavin S, et al. Salvage high-dose chemotherapy for children with extragonadal germ-cell tumours. Br J Cancer. 2005;93:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider DT, Wessalowski R, Calaminus G, et al. Treatment of recurrent malignant sacrococcygeal germ cell tumors: analysis of 22 patients registered in the German protocols MAKEI 83/86, 89, and 96. J Clin Oncol. 2001;19:1951–1960. [DOI] [PubMed] [Google Scholar]
- 3. Gobel U, Schneider DT, Calaminus G, et al. Multimodal treatment of malignant sacrococcygeal germ cell tumors: a prospective analysis of 66 patients of the German cooperative protocols MAKEI 83/86 and 89. J Clin Oncol. 2001;19:1943–1950. [DOI] [PubMed] [Google Scholar]
- 4. Cushing B, Giller R, Cullen JW, et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study--Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol. 2004;22:2691–2700. [DOI] [PubMed] [Google Scholar]
- 5. Rescorla F, Billmire D, Stolar C, et al. The effect of cisplatin dose and surgical resection in children with malignant germ cell tumors at the sacrococcygeal region: a pediatric intergroup trial (POG 9049/CCG 8882). J Pediatr Surg. 2001;36:12–17. [DOI] [PubMed] [Google Scholar]
- 6. Egler RA, Gosiengfiao Y, Russell H, et al. Is surgical resection and observation sufficient for stage I and II sacrococcygeal germ cell tumors? A case series and review. Pediatr Blood Cancer. 2017. Doi: 10.1002/pbc.26311. [DOI] [PubMed] [Google Scholar]
- 7. Schmoll HJ, Souchon R, Krege S, et al. European consensus on diagnosis and treatment of germ cell cancer: a report of the European Germ Cell Cancer Consensus Group (EGCCCG). Ann Oncol. 2004;15:1377–1399. [DOI] [PubMed] [Google Scholar]
- 8. Frazier AL, Hale JP, Rodriguez-Galindo C, et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol. 2015;33:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marina N, London WB, Frazier AL, et al. Prognostic factors in children with extragonadal malignant germ cell tumors: a pediatric intergroup study. J Clin Oncol. 2006;24:2544–2548. [DOI] [PubMed] [Google Scholar]
- 10. Baranzelli MC, Kramar A, Bouffet E, et al. Prognostic factors in children with localized malignant nonseminomatous germ cell tumors. J Clin Oncol. 1999;17:1212. [DOI] [PubMed] [Google Scholar]
- 11. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594–603. [DOI] [PubMed] [Google Scholar]
- 12. Marina NM, Cushing B, Giller R, et al. Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: a Pediatric Oncology Group/Children’s Cancer Group Intergroup Study. J Clin Oncol. 1999;17:2137–2143. [DOI] [PubMed] [Google Scholar]
- 13. Yoshida M, Matsuoka K, Nakazawa A, et al. Sacrococcygeal yolk sac tumor developing after teratoma: a clinicopathological study of pediatric sacrococcygeal germ cell tumors and a proposal of the pathogenesis of sacrococcygeal yolk sac tumors. J Pediatr Surg. 2013;48:776–781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jpho-online.com.


