Selective autophagy relies on adaptor proteins to bind and transport cargos (or substrates) to the lysosome or vacuole, yet the mechanisms for cargo recognition are not well understood. In this issue, Wang et al (2021) showed that in the fission yeast, Nbr1, a homolog of a mammalian selective autophagy adaptor, recognizes vacuolar hydrolases Ams1 and Ape4 through both versatile and cargo‐specific interactions with the Nbr1 ZZ1 domain.
Subject Categories: Autophagy & Cell Death, Membrane & Intracellular Transport, Structural Biology
A recent study identifies two novel substrates for Schizosaccharomyces pombe Nrb1 intracellular cargo adaptor and uncovers their recognition mechanism by Nbr1's ZZ1 domain.
Autophagy is a general term describing processes that deliver cytoplasmic components to the lysosome or vacuole for degradation. Of the three known forms of autophagy, macroautophagy assembles a double‐membraned structure—the autophagosome—which proceeds to capture part of the cytoplasm before fusing with the lysosome, whereas microautophagy creates membrane protrusions or invaginations on the endosomal or lysosomal membranes that directly engulf part of the cytosol (Mizushima & Komatsu, 2011). A specialized form of autophagy, named selective autophagy, requires adaptors (or receptors) that recognize specific cargos (or substrates) and mediates their uptake into the lysosome and vacuole (Johansen & Lamark, 2020). Polyubiquitin is a well‐known degradation signal recognized by selective autophagy adaptors such as NBR1 and p62/SQSTM1 (Johansen & Lamark, 2020) (Fig 1A, top).
Figure 1. The NVT and Cvt pathways, two selective autophagy‐like pathways, are involved in the biosynthesis of vacuolar hydrolases.
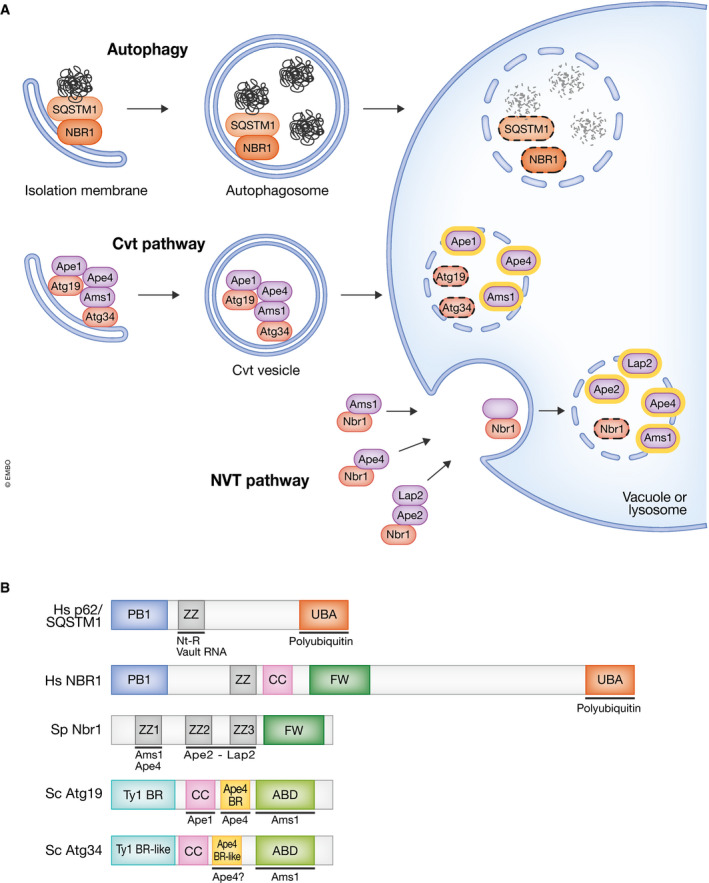
(A) Schematic diagram of the canonical autophagy, Cvt, and NVT pathways. The adaptor proteins p62/SQSTM1, Nbr1, and Atg19/Atg34 belong to the same family. The Cvt pathway transports Ams1 and Ape4 via macroautophagy‐like mechanisms, whereas the NVT pathway delivers Lap2–Ape2, Ams1, and Ape4 (the latter two are newly identified cargos in Wang et al (2021)) through microautophagy‐like mechanisms. (B) Domain structures of human p62/SQSTM1, human NBR1, Schizosaccharomyces pombe Nbr1, Saccharomyces cerevisiae Atg19 and Atg34, and cargo proteins recognized by each domain. PB1, Phox and Bem1; ZZ, ZZ‐type zinc finger; UBA, ubiquitin‐associated domain; Ty1 BR, Ty1 virus‐like particle‐binding region; CC, coiled‐coil; Ape4 BR, Ape4‐binding region; ABD, Ams1‐binding domain; and FW, four tryptophan (also known as NBR1) domain.
Pathways that are similar to selective autophagy include cytoplasm‐to‐vacuole targeting (Cvt) pathway in the budding yeast Saccharomyces cerevisiae (Yamasaki & Noda, 2017) and Nbr1‐mediated vacuolar targeting (NVT) pathway in the fission yeast Schizosaccharomyces pombe (Liu et al, 2015). In the Cvt pathway, Atg19 and Atg34 function as adaptors that transport Ape1, Ams1, and Ape4 to the vacuole via mechanisms shared with macroautophagy (Yamasaki & Noda, 2017). The NVT pathway, on the other hand, delivers Ape2 and Lap2 to the vacuole in a manner similar to microautophagy, which is dependent on the endosomal sorting complexes required for transport (ESCRT) machinery (Liu et al, 2015). This pathway utilizes Nbr1 as the sole adaptor. All the cargoes of the Cvt and NVT pathways are vacuolar hydrolases getting transported to their place of action, and therefore, these pathways are biosynthetic in nature (Fig 1A, middle and bottom).
In this issue, Wang et al (2021) performed a mass spectrometry‐based protein interactome analysis using Nbr1 as bait and identified Ams1 and Ape4, homologs of the Cvt pathway cargos in the budding yeast, as novel cargos for the NVT pathway in the fission yeast. The fission yeast Nbr1 has three ZZ domains (ZZ1–3). The same group previously showed that ZZ2 and ZZ3 recognize Ape2 and Lap2, but the function of ZZ1 remained unknown (Fig 1B) (Liu et al, 2015). Here, a domain‐deletion analysis revealed that ZZ1 is necessary and sufficient for binding Ams1 and Ape4. It was also found that Ams1 and Ape4 bind competitively to Nbr1 but independently of Lap2–Ape2.
In order to further characterize the mechanism of binding, Wang et al (2021) determined the cryo‐EM structure of Nbr1's ZZ1 domain in complex with Ams1 or Ape4. The resulting structures revealed two binding interfaces that work cooperatively, one which is common to ZZ domain‐containing proteins such as p62/SQSTM1, p300, ZZZ3, and HERC2 (Zhang et al, 2019), and the other one being cargo‐specific. The common interface is formed by the N‐terminal residues of Ams1 and Ape4 fitted into the acidic pocket of ZZ1, while the cargo‐specific interface requires P97 for Ams1 and L66 and A61 for Ape4 recognition, respectively. Even though the dissociation constants between ZZ1 and Ams1 or Ape4 are both on the order of nM, mutating residues at the suggested interfaces abolishes the interactions between these proteins and the vacuolar targeting of Ams1 and Ape4.
The selective autophagy adaptors Nbr1/NBR1, p62/SQSTM1, and Atg19 belong to the same family conserved in a wide range of eukaryotes (Kraft et al, 2010; Svenning et al, 2011). Mammalian NBR1 and p62 both contain ZZ and UBA domains (Fig 1B). The UBA domain, which is lacking in S. pombe Nbr1, interacts with polyubiquitin and therefore mediates the degradation of ubiquitinated proteins (Johansen & Lamark, 2020). On the other hand, Wang et al (2021) found that the ZZ domain is involved in the biosynthesis of hydrolases in the fission yeast (Liu et al, 2015; Wang et al, 2021). Thus, it might be hypothesized that the ZZ and UBA domains mediate biosynthetic and degradation pathways, respectively. However, it has been shown that the ZZ domain in mammalian p62 also has a degradative role, for example, by binding to the amino‐terminal arginine residue (Nt‐R), an N‐terminal degradation signal regulating protein lifespan (Zhang et al, 2019), or to vault RNA (Horos et al, 2019). Therefore, the role of the ZZ domain can be versatile, with potentially different binding modes.
While some Atg19 homologs have ZZ domains, they are lost in the budding yeast and its close relatives (Kraft et al, 2010; Svenning et al, 2011). Instead, the budding yeast uses other domains to bind Ams1 and Ape4 (Yamasaki & Noda, 2017) (Fig 1B). Therefore, the same vacuolar hydrolases are recognized by different regions of Atg19/Nbr1 and delivered to the vacuole via macroautophagy‐ and microautophagy‐like mechanisms in the budding yeast and the fission yeast, respectively. This is a curious case where the function is maintained while molecular mechanisms diverge.
Acknowledgements
This work was supported by Exploratory Research for Advanced Technology (ERATO) (No. JPMJER1702 to N.M.) from the Japan Science and Technology Agency (JST).
The EMBO Journal (2021) 40: e108777.
See also: YY Wang et al (August 2021)
References
- Horos R, Büscher M, Kleinendorst R, Alleaume A‐M, Tarafder AK, Schwarzl T, Dziuba D, Tischer C, Zielonka EM, Adak A et al (2019) The small non‐coding vault RNA1‐1 acts as a riboregulator of autophagy. Cell 176: 1054–1067 [DOI] [PubMed] [Google Scholar]
- Johansen T, Lamark T (2020) Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J Mol Biol 432: 80–103 [DOI] [PubMed] [Google Scholar]
- Kraft C, Peter M, Hofmann K (2010) Selective autophagy: ubiquitin‐mediated recognition and beyond. Nat Cell Biol 12: 836–841 [DOI] [PubMed] [Google Scholar]
- Liu XM, Sun LL, Hu W, Ding YH, Dong MQ, Du LL (2015) ESCRTs cooperate with a selective autophagy receptor to mediate vacuolar targeting of soluble cargos. Mol Cell 59: 1035–1042 [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147: 728–741 [DOI] [PubMed] [Google Scholar]
- Svenning S, Lamark T, Krause K, Johansen T (2011) Plant NBR1 is a selective autophagy substrate and a functional hybrid of the mammalian autophagic adapters NBR1 and p62/SQSTM1. Autophagy 7: 993–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Zhang J, Liu XM, Li Y, Sui J, Dong MQ, Ye K, Du LL (2021) Molecular and structural mechanisms of ZZ domain‐mediated cargo selection by autophagy receptor Nbr1. EMBO J 10.15252/embj.2020107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki A, Noda NN (2017) Structural biology of the Cvt pathway. J Mol Biol 429: 531–542 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mi W, Xue Y, Shi X, Kutateladze TG (2019) The ZZ domain as a new epigenetic reader and a degradation signal sensor. Crit Rev Biochem Mol Biol 54: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]


