Abstract
Background
To determine if proton radiotherapy (PT), compared to intensity-modulated radiotherapy (IMRT), delayed time to cognitive failure in patients with newly diagnosed glioblastoma (GBM).
Methods
Eligible patients were randomized unblinded to PT vs IMRT. The primary endpoint was time to cognitive failure. Secondary endpoints included overall survival (OS), intracranial progression-free survival (PFS), toxicity, and patient-reported outcomes (PROs).
Results
A total of 90 patients were enrolled and 67 were evaluable with median follow-up of 48.7 months (range 7.1-66.7). There was no significant difference in time to cognitive failure between treatment arms (HR, 0.88; 95% CI, 0.45-1.75; P = .74). PT was associated with a lower rate of fatigue (24% vs 58%, P = .05), but otherwise, there were no significant differences in PROs at 6 months. There was no difference in PFS (HR, 0.74; 95% CI, 0.44-1.23; P = .24) or OS (HR, 0.86; 95% CI, 0.49-1.50; P = .60). However, PT significantly reduced the radiation dose for nearly all structures analyzed. The average number of grade 2 or higher toxicities was significantly higher in patients who received IMRT (mean 1.15, range 0-6) compared to PT (mean 0.35, range 0-3; P = .02).
Conclusions
In this signal-seeking phase II trial, PT was not associated with a delay in time to cognitive failure but did reduce toxicity and patient-reported fatigue. Larger randomized trials are needed to determine the potential of PT such as dose escalation for GBM and cognitive preservation in patients with lower-grade gliomas with a longer survival time.
Keywords: cognition, glioblastoma, proton therapy, radiation, randomized controlled trial
Key Points.
Proton therapy was not associated with a delay in time to cognitive failure.
Patients treated with proton therapy experienced fewer grade 2 or higher toxicities.
There was no difference in progression-free survival or overall survival between the study arms.
Importance of the Study.
Proton radiotherapy (PT) significantly decreases exposure of critical structures including uninvolved brain which may result in less cognitive deterioration after radiotherapy; yet, the role of PT remains controversial due to concerns regarding the increased costs and labor associated with PT. In this signal-seeking phase II trial, patients with newly diagnosed glioblastoma (GBM) were randomized to PT vs intensity-modulated radiotherapy (IMRT). There was no difference in progression-free survival or overall survival between study arms. PT significantly reduced the radiation dose for nearly all structures analyzed; despite this PT was not associated with a delay in time to cognitive failure possibly because the aggressive nature of GBM overshadows any potential improved cognitive outcomes with PT. PT was associated with reduced toxicity and patient-reported fatigue. Larger randomized trials are needed to determine the potential of PT for dose escalation for GBM and cognitive preservation in patients with better prognosis lower-grade gliomas.
Glioblastoma (GBM) accounts for approximately 25% of all primary central nervous system (CNS) tumors in adults and historically has been associated with median survival measured in months.1 However, the addition of temozolomide to radiotherapy has resulted in significant improvements in survival.2,3 With the improvement in survival time, there are growing concerns about the negative effects of treatment, especially the potential for cognitive deficits after cranial radiotherapy.
Decreasing the amount of brain exposed to radiation has a significant impact on cognitive function after radiation.4–7 Proton radiotherapy (PT) is a treatment modality that has been safely and effectively used in the treatment of GBM with low rates of toxicity.8 Previous studies have found that intensity-modulated proton therapy (IMPT) can allow for more conformal target coverage than photon intensity-modulated radiation therapy (IMRT) while minimizing doses to normal tissues such as the surrounding brain and contralateral hippocampus.9–11 Cognitive effect models predict improved cognitive function outcomes with PT,12 and a prospective single-arm trial of proton therapy for patients with low-grade glioma found stable cognitive function after proton therapy.13
However, to our knowledge, there are no prospective randomized controlled studies that assess the potential of protons to decrease cognitive toxicity in the treatment of GBM. To address these ongoing knowledge gaps, MD Anderson Cancer Center (MDACC) 2013-0097, a randomized phase II controlled trial, prospectively assessed the potential cognitive benefit of PT compared to IMRT in patients with newly diagnosed GBM.
Materials and Methods
Study Design and Random Assignment
The trial was approved by The University of Texas MDACC Institutional Review Board. All patients provided written informed consent before enrollment. On this phase II randomized trial (ClinicalTrials.gov identifier: NCT01854554), eligible patients were randomly assigned to one of the two groups, PT vs IMRT, in a 1:1 ratio and stratified by age (< and ≥65 years), Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) of gliomas14 class (III or IV vs V), and Mini-Mental Status Examination (MMSE) score (21-26 vs 27-30).15 Randomization was performed utilizing CORe clinical trials management system.
Patients
Adult patients (18 years of age or older) with newly diagnosed GBM or gliosarcoma (WHO grade IV), Karnofsky Performance Status (KPS) score 70 or greater, RPA class III, IV, or V were eligible for this trial. Eligibility criteria included MMSE score of 21 or greater, ability to complete an MRI with contrast, aspartate aminotransferase (AST) < 3 times normal limit, alanine aminotransferase (ALT) < 3 times normal limit, alkaline phosphatase < 3 times normal limit, creatinine < 1.7 mg/dl, blood urea nitrogen (BUN) < 35 mg/dl, absolute neutrophil count > 1800 cells/mm3, hemoglobin > 10 g/dl, and platelet count > 100 000, and able to adequately read, write, and speak to participate in the cognitive and patient-reported outcome (PRO) assessments, allowing for mild to moderate deficits in these functions due to tumor. Exclusion criteria included prior brain radiation, pregnancy, prior resection of other brain tumors, gliomatosis, or implantation of carmustine wafers.
Study Treatment
Radiation dose, target delineation, and organ-at-risk volume definitions were the same for both study arms. Planning was based on non-contrast CT images obtained at the time of simulation along with the fused postoperative MRI scan, which was obtained within 4 weeks of simulation. Gross tumor volume (GTV) was defined as tumor cavity and any residual T1 tumor enhancement. Clinical target volume (CTV) included GTV + 2-cm anatomically constrained margin (ie, excluded bone, fascia, and other anatomical barriers). Planning target volume PTV50 included the CTV + 3-5 mm and PTV60 (ie, the boost volume) included the GTV + 3-5 mm. Simultaneous integrated boost technique was used to treat both the PTV50 and PTV60 to 50 Gy and 60 Gy in 30 fractions, respectively. Fractionated radiation was delivered once daily Monday through Friday for all patients. Dose constraints for the hippocampi were not part of MDACC clinical practice at the time this protocol was conducted and tumor coverage was favored over sparing of these structures.
Chemotherapy was temozolomide, daily during radiotherapy followed by adjuvant temozolomide. Following randomization, insurance pre-authorization was obtained for the specified treatment modality. If a patient’s insurance did not cover payment for the assigned treatment arm, the patient was to be removed from the study and treated off protocol. For patients randomized to IMRT, plans used a configuration of 5-7 isocentric 6-MV photon beams using a linear accelerator to deliver the dose in a step-and-shoot technique. The relative biologic effectiveness (RBE) for proton irradiation was set at 1.1. For patients randomized to PT, the dose unit, Gy (RBE) is proton dose in Gy × RBE of 1.1. Treatment was delivered using the 250 MeV synchrotron (Hitachi Ltd., Power Systems, Ibaraki-ken, Japan) at the Proton Therapy Center at MDACC.
The majority of patients on the proton arm were treated with IMPT. IMPT was planned using either multi-field or single-field optimization. In single-field optimization, each field was optimized to deliver the prescribed dose to the target volume. Multi-field optimization used simultaneous spot optimization.16 If initiation of treatment wait times were too long, passive scatter was allowed for 7 of 26 patients treated with PT utilizing physical apertures or compensators to modify the intensity of the beam as the quality of radiation plans was similar to that of scanning beam plans.17
Assessments
At baseline, all patients underwent history and physical, including detailed neurological exam, MMSE, and determination of KPS. Patients also underwent a detailed neuropsychological evaluation, which included a battery of standardized tests and measures routinely used in clinical trials for patients with brain tumors by the neuropsychology team.18 This cognitive battery included Hopkins Verbal Learning Test-Revised (HVLT-R, learning and memory), Trail Making Test Part A (TMT-A, processing speed), Trail Making Test Part B (TMT-B, executive function), and Controlled Oral Word Association (COWA, verbal fluency).
Symptom evaluation and quality of life (QOL) measures were assessed with the MD Anderson Symptom Inventory Brain Tumor (MDASI-BT, symptom burden) and the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30/BN20 (EORTC QLQ C30/BN20, quality of life).18–20 All reasonable efforts were made to complete testing prior to the start of radiation therapy. However, in cases where this was not possible, evaluation was completed within the first 5 fractions of radiation treatment. Other baseline studies included complete blood count (CBC), AST, ALT, alkaline phosphatase, electrolytes, BUN, creatinine, and postoperative brain MRI.
All baseline assessments as well as assessment of adverse events (CTCAE v.4.0 scale) were repeated at 2-month intervals (±30 days) for a total of 24 months of follow-up after the completion of the assigned treatment. Progression-free survival (PFS) was assessed by contrast-enhanced brain MRI scan using Response Assessment in Neuro-Oncology (RANO) criteria.21
Endpoints
The primary endpoint was time to cognitive failure defined as a decline that meets or exceeds the reliable change index (RCI) for any of the six cognitive test variables (HVLT-R Total Recall, HVLT-R Delayed Recall, HVLT-R Delayed Recognition, TMT Part A or Part B, COWA).22 A cumulative incidence approach was used to estimate the risk of cognitive failure in order to account for the competing risks of disease progression and death. Patients experiencing disease progression or patients that died prior to experiencing cognitive failure were considered as having had a competing event.
Secondary endpoints included outcomes for each individual cognitive test, the Clinical Trial Battery Composite (determined by averaging standardized z scores from all cognitive tests),23 toxicity, overall survival (OS), PFS, PROs, and dosimetric parameters. For the MDASI-BT, a decline in symptom score of one point from baseline was classified as clinically significant and for EORTC QLQ-C30/BN20 a decline of 10 points from baseline on the standardized score (range, 0-100) was classified as clinically significant.24 Since the median PFS for GBM is approximately 7 months, the individual cognitive tests and QOL results are presented at the 2-, 4-, and 6-month time points as these earlier time points are more reflective of the effect of radiotherapy and less reflective of the effect of tumor progression.24
Statistical Considerations
With IMRT, the cognitive function failure rate at 4 months was estimated at 45%, based on comparative analyses of the cognitive failure rate of RTOG 0614 (NCT00566852) and compared to the cognitive impairment seen on RTOG 0525 (NCT00304031).23,24 A 33% reduction with PT would result in 30% cognitive function failure at 4 months. The time to cognitive failure in each treatment arm was assumed to follow an exponential distribution. A cognitive failure rate of 45% at 4 months implied a median time to cognitive failure of 4.6 months, while a cognitive failure rate of 30% at 4 months implied a median time to cognitive failure of 7.8 months. A 1-sided significance level of 0.20 was utilized as suggested by Rubinstein et al. for phase II screening trials.25
Expected enrollment was 2 patients per month to randomize a total of 60 evaluable patients (30 to each treatment arm). This would provide 80% power to detect this difference. Evaluable patients were defined as those who had received the assigned treatment and completed baseline and at least one follow-up cognitive test appointment. An over accrual of 33% was utilized to account for those patients not evaluable. Therefore, the target total enrollment sample size was 90 patients to reach a total of 60 evaluable patients with an estimation of 41 months to observe these events.
Study progress and analyses of safety data were presented to the MDACC Data Safety Monitoring Board (DSMB) on an annual basis, or as requested. As this was a phase II randomized trial, the primary objective (time to cognitive failure) was evaluated by treatment that was actually received. Data were summarized by descriptive statistics such as mean, standard deviation, median, and range for continuous variables and frequency and proportion for categorical variables. The difference in these variables between the treatment arms was evaluated by Wilcoxon rank-sum test and chi-square (or Fisher’s exact) test for continuous and categorical variables, respectively.26,27 The cumulative incidence rate of cognitive failure was estimated by considering death or disease progression as competing events and Gray’s test was applied to evaluate the difference between the two treatment arms.28
OS and PFS rates were estimated by Kaplan-Meier method and log-rank test was applied to compare OS and PFS between treatment arms.29,30 Univariate Cox regression model was employed to estimate the effect of treatment on OS and PFS times. Adverse events (assessed by the treating clinicians) experienced in each arm were summarized by attribution and grade. Adverse events that were grade 2 and higher were preferentially analyzed as the maximum grade for alopecia is grade 2 and alopecia is a common toxicity associated with cranial radiotherapy. There was no adjustment for multiple comparisons for the secondary endpoint analyses of cognitive function and PROs and so those results should be interpreted as exploratory.
Results
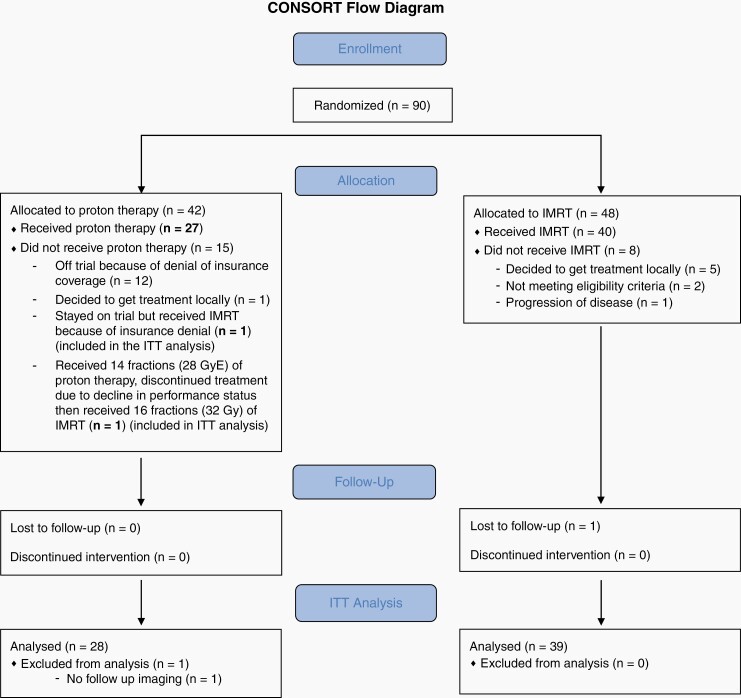
Between June 2013 and March 2016, 90 patients consented to participate (Figure 1) and 67 were treated (28 PT, 39 IMRT) with the majority of patients excluded before treatment began due to insurance denial of proton therapy (Figure 1). The median follow-up was 48.7 months (range [7.1, 66.7]). One patient stayed on trial but received IMRT because of insurance denial (n = 1) and another patient received 14 fractions (28 GyE) of proton therapy and then received 16 fractions (32 Gy) of IMRT. Both of these patients are included on the PT arm in the intention-to-treat (ITT) analysis, while they are included in the IMRT arm for the effective treatment analysis. There were no differences in baseline patient or tumor characteristics between study arms (Table 1; Supplementary Table 1). Baseline median cognitive scores ranged from average to mild impairment compared to population norms.
Fig. 1.
CONSORT diagram.
Table 1.
Patient Demographics and Clinical Characteristics (Effective Treatment)
| IMRT | PT | P Value | ||
|---|---|---|---|---|
| Overall | N | 41 (61.2%) | 26 (38.8%) | |
| Age at diagnosis | Mean ± Std | 52.1 ± 13.9 | 55.2 ± 11 | .46 |
| Median (Min, Max) | 53 (26, 82) | 54.5 (33, 72) | ||
| Ethnicity | White | 33 (80.5%) | 24 (92.3%) | .72 |
| Hispanic | 5 (12.2%) | 1 (3.8%) | ||
| African American | 2 (4.9%) | 1 (3.8%) | ||
| Asian | 1 (2.4%) | 0 (0%) | ||
| Gender | Male | 21 (51.2%) | 15 (57.7%) | .60 |
| Female | 20 (48.8%) | 11 (42.3%) | ||
| Mini-mental status score | Mean ± Std (N) | 27.7 ± 2 (41) | 28.2 ± 1.7 (26) | .30 |
| Median (Min, Max) | 28 (21, 30) | 28.5 (23, 30) | ||
| Extent of resection | Gross total | 20 (48.8%) | 19 (73.1%) | .14 |
| Subtotal | 17 (41.5%) | 5 (19.2%) | ||
| Biopsy | 4 (9.8%) | 2 (7.7%) | ||
| RPA | III | 12 (29.3%) | 5 (19.2%) | .25 |
| IV | 15 (36.6%) | 15 (57.7%) | ||
| V | 14 (34.1%) | 6 (23.1%) | ||
| Tumor hemisphere | Left | 14 (34.1%) | 13 (50%) | .36 |
| Right | 26 (63.4%) | 12 (46.2%) | ||
| Bilateral | 1 (2.4%) | 1 (3.8%) | ||
| Tumor lobe | Frontal | 14 (34.1%) | 9 (34.6%) | .71 |
| Temporal | 12 (29.3%) | 8 (30.8%) | ||
| Parietal | 9 (22%) | 8 (30.8%) | ||
| Occipital | 3 (7.3%) | 1 (3.8%) | ||
| Central/midbrain | 3 (7.3%) | 0 (0%) | ||
| MGMT | Unmethylated | 3 (50%) | 3 (75%) | .57 |
| Methylated | 3 (50%) | 1 (25%) | ||
| Not tested | 35 | 22 | ||
| IDH-1 | Normal | 19 (82.6%) | 18 (90%) | .67 |
| Mutated | 4 (17.4%) | 2 (10%) | ||
| Not tested | 18 | 6 | ||
| GTV volume (cc) | Mean ± Std | 48.5 ± 33.9 | 42.4 ± 30.6 | .63 |
| Median (Min, Max) | 40.3 (7.9, 143.9) | 40.1 (3.9, 133.8) | ||
| CTV volume (cc) | Mean ± Std | 235.6 ± 85.5 | 206.5 ± 84 | .24 |
| Median (Min, Max) | 232.9 (86, 471.2) | 215.6 (31.4, 404.4) | ||
| HVLT-R Total Recall | Median | −0.9 | −0.9 | .49 |
| HVLT-R Delayed Recall | Median | −1 | −1 | .96 |
| HVLT-R Recognition | Median | −0.2 | −0.6 | .98 |
| TMT Part A | Median | −0.4 | 0.4 | .12 |
| TMT Part B | Median | −1.9 | −0.7 | .12 |
| COWA | Median | −0.9 | −1 | .90 |
| CTB Composite | Median | −1.1 | −0.6 | .24 |
Abbreviations: COWA, Controlled Oral Word Association; CTB, Clinical Trial Battery; CTV, clinical target volume; GTV, gross tumor volume; HVLT-R, Hopkins Verbal Learning Test-Revised; IDH, isocitrate dehydrogenase; IMRT, intensity-modulated radiotherapy; Max, maximum; MGMT, O6-methylguanine-DNA methyltransferase; Min, minimum; N, number; PT, proton radiotherapy; Recognition, delayed recognition; RPA, recursive partitioning analysis; Std, standard deviation; TMT, Trail Making Test.
Cognitive tests were reported as standardized scores (z scores). CTB Composite score is the mean of standardized scores (HVLT-R-Total Recall, HVLT-R-Delayed Recall, HVLT-R-Delayed Recognition, TMT Parts A and B, and COWA).
Radiation Dosimetry by Treatment Modality
PT significantly reduced the minimum, average, and maximum radiation dose for all structures analyzed including the brain, right lens, left lens, right cochlea, left cochlea, pituitary, right hippocampus, and left hippocampus (Supplementary Table 2). Exceptions to this were a higher maximum dose to the brain with PT (6519 cGy vs 6406 cGy, P < .001), and no significant difference was found between study arms for maximum dose to left hippocampus.
Primary Endpoint Time to First Cognitive Failure
There was no significant difference in cumulative incidence rate of cognitive failure between treatment arms (HR, 0.88; 95% CI, 0.45-1.75; P = .74, IMRT vs PT, Figure 2).
Fig. 2.
Cumulative incidence rate of cognitive decline. Abbreviations: IMRT, intensity-modulated radiotherapy; PT, proton radiotherapy.
Cognitive Function and PROs
Testing of cognitive function was completed at baseline and at least one subsequent evaluation in 24/26 (92%) patients in the PT arm and 34/41 (83%) patients in the IMRT arm. There were no statistically significant differences in the rates of deterioration on the cognitive tests between the two treatment arms at 6 months (Table 2; additional findings Supplementary Table 3).24
Table 2.
Between-Arm Differences in Deterioration of Cognitive Function, QOL, and Symptoms at 6 Months
| Test/Measure and Component | IMRT | PT | P Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Cognitive function | |||||
| HVLT-R | |||||
| Total Recall | 5 | 21 | 2 | 12 | .68 |
| Delayed Recall | 0 | 0 | 0 | 0 | 1.00 |
| Recognition | 3 | 13 | 2 | 12 | 1.00 |
| TMT | |||||
| Part A | 2 | 8 | 1 | 6 | 1.00 |
| Part B | 4 | 17 | 4 | 24 | .70 |
| COWA | 2 | 8 | 2 | 12 | 1.00 |
| CTB Composite | 19 | 79 | 11 | 65 | .48 |
| EORTC QOL C30 | |||||
| Scale | |||||
| Global HS/QOL | 2 | 9 | 4 | 25 | .21 |
| Physical | 5 | 21 | 2 | 12 | .68 |
| Role | 3 | 13 | 2 | 12 | 1.00 |
| Emotional | 1 | 4 | 1 | 6 | 1.00 |
| Cognitive | 9 | 39 | 3 | 19 | .29 |
| Symptom items | |||||
| Social | 0 | 0 | 1 | 6 | .41 |
| Fatigue | 14 | 58 | 4 | 24 | .05 |
| Nausea/vomiting | 2 | 8 | 1 | 6 | 1.00 |
| Pain | 1 | 4 | 3 | 18 | .29 |
| Dyspnea | 0 | 0 | 2 | 12 | .17 |
| Insomnia | 5 | 21 | 2 | 12 | .68 |
| Appetite loss | 5 | 21 | 1 | 6 | .37 |
| Constipation | 3 | 13 | 4 | 24 | .42 |
| Diarrhea | 3 | 13 | 2 | 13 | 1.00 |
| Financial | 2 | 9 | 2 | 13 | 1.00 |
| BN20 | |||||
| Scale | |||||
| Future uncertainty | 4 | 17 | 2 | 12 | 1.00 |
| Visual disorder | 3 | 13 | 2 | 12 | 1.00 |
| Motor dysfunction | 7 | 29 | 1 | 6 | .11 |
| Comm deficit | 4 | 17 | 4 | 24 | .70 |
| Symptom items | |||||
| Headaches | 3 | 13 | 1 | 6 | .62 |
| Seizures | 1 | 4 | 1 | 6 | 1.00 |
| Drowsiness | 5 | 21 | 6 | 35 | .48 |
| Hair loss | 7 | 29 | 2 | 12 | .26 |
| Itchy skin | 4 | 17 | 3 | 18 | 1.00 |
| Weak legs | 3 | 13 | 1 | 6 | .63 |
| Bladder | 3 | 13 | 1 | 6 | .64 |
| MDASI-BT | |||||
| Subscales | |||||
| Core | 4 | 17 | 1 | 6 | .38 |
| Brain tumor | 3 | 13 | 1 | 6 | .63 |
| Interference | 4 | 17 | 2 | 12 | 1.00 |
| Severity | 4 | 17 | 1 | 6 | .38 |
| Factor groups | |||||
| Constitutional | 4 | 17 | 3 | 18 | 1.00 |
| Cognitive | 5 | 21 | 3 | 18 | 1.00 |
| Interference | 4 | 17 | 2 | 12 | 1.00 |
| Neurologic | 3 | 13 | 2 | 12 | 1.00 |
| Gastrointestinal | 2 | 8 | 4 | 24 | .21 |
| Affective | 6 | 25 | 2 | 12 | .43 |
| Work and walking | 4 | 17 | 2 | 12 | 1.00 |
| Mood-related | 4 | 17 | 4 | 24 | .70 |
Abbreviations: Comm, communication; COWA, Controlled Oral Word Association; CTB, Clinical Trial Battery; EORTC QOL C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30; HS, health status; HVLT-R, Hopkins Verbal Learning Test-Revised; IMRT, intensity-modulated radiotherapy; MDASI-BT, MD Anderson Symptom Inventory-Brain Tumor module; PT, proton radiotherapy; QOL, quality of life; Recognition, delayed recognition; Symptom interfere, symptom interference; TMT, Trail Making Test.
CTB Composite score is the mean of standardized scores (HVLT-R-Total Recall, HVLT-R-Delayed Recall, HVLT-R-Delayed Recognition, TMT Parts A and B, and COWA). Effective treatment and intent-to-treat analysis had identical results.
PROs were obtained at baseline and at least one subsequent evaluation in 24/26 (92%) patients in the PT arm and 34/41 (83%) patients in the IMRT arm. There were no statistically significant differences in the rates of deterioration between the two treatment arms at 6 months, except PT was associated with a lower rate of worsening fatigue (24% vs 58%, P = .05) on the EORTC QLQ-C30 (Table 2; additional findings Supplementary Table 3).
PFS and OS
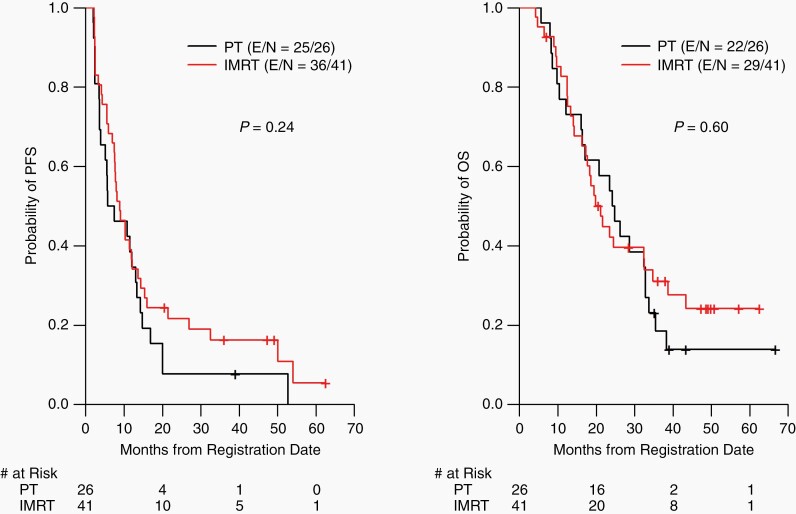
There was no significant difference in PFS (median 8.9 months in IMRT vs 6.6 months in PT; HR, 0.74; 95% CI, 0.44-1.23; P = .24) or OS (median 21.2 months in IMRT vs 24.5 months in PT; HR, 0.86; 95% CI, 0.49-1.50; P = .60) between the study arms (Figure 3; Supplementary Figure 1).
Fig. 3.
Kaplan-Meier estimates of progression-free survival (PFS) and overall survival (OS) according to treatment received. Abbreviations: IMRT, intensity-modulated radiotherapy; PT, proton radiotherapy.
Adverse Events
A total of 26 patients in the PT arm and 41 patients in the IMRT arm were evaluable for assessment of adverse events (Supplementary Tables 4 and 5). There were more patients with grade 2 or higher toxicities in the IMRT study arm, but this was not statistically significant (20 out of 41 patients vs 6 out of 26 patients; P = .06; Table 3). When evaluating the number of adverse event incidences for each patient, the average number of grade 2 or higher toxicities is significantly higher in patients who received IMRT compared to PT (mean 1.15, range [0, 6] in IMRT vs mean 0.35, range [0, 3]; P = .02). The most common grade 2 or higher adverse events were alopecia (7% IMRT vs 4%), fatigue (7% IMRT vs 0%), and headache (7% IMRT vs 4%). There were no incidents of radiation necrosis reported in either study arm.
Table 3.
Number of Patients Experiencing AEs by Maximum Grade (Grade 2 or Higher) by Effective Treatment Arm
| Grade | IMRT | PT |
|---|---|---|
| 4 | 2 | 1 |
| 3 | 4 | 1 |
| 2 | 14 | 4 |
| Total no. patients with AEs | 20 | 6 |
| Total treated | 41 | 26 |
Abbreviations: AE, adverse events; IMRT, intensity-modulated radiotherapy; PT, proton radiotherapy.
Discussion
In this signal finding trial, there was no indication of improved cognitive outcomes with PT as compared to photon radiotherapy in the treatment of patients with newly diagnosed GBM. Although the radiation exposure of normal tissues critical to cognitive function (eg, hippocampi) was significantly decreased with protons, this did not translate to improved cognitive outcomes. A possible explanation is that the aggressive nature of GBM so negatively impacts cognitive function that it overshadows any potential improved cognitive outcomes with the superior dosimetry of protons.15,31,32 Improved radiation dosimetry is associated with improved cognitive outcomes in patients with low-grade brain tumors.7,13,33 NRG BN005, an ongoing trial (NCT03180502), is assessing the potential cognitive benefits of PT in the treatment of better prognosis tumors, isocitrate dehydrogenase (IDH)-mutant grade II or III glioma. Studying a patient population with an extended PFS, such as those enrolled on NRG BN005, is especially important as large cohort studies of patients with low-grade glioma treated with conventional dose fractionation photons have found that cognitive decline, if it occurs, tends to be several years after radiotherapy.34,35
Possibly due to the dosimetric advantage of protons (ie, substantially less exit dose beyond the target compared to photons), patients in the proton treatment arm had less toxicity. Similar outcomes were seen in a trial that randomized patients with esophageal cancer to PT or IMRT and found less adverse events after PT compared to IMRT.36 It is possible that even greater benefit in toxicity rates, especially late toxicities, could be seen with PT in brain tumor patient populations with better prognosis such as IDH mutant tumors; hopefully, this will also be addressed by the ongoing trial NRG BN005 (NCT03180502).
In the current trial, the same dose of radiation was delivered to the same target volume parameters in both treatment arms, so it was not surprising that there was no difference in PFS or OS. However, the dosimetric advantages of PT in sparing dose to organs at risk (OARs) allows for dose escalation to potentially improve tumor control and survival outcomes. Based on promising single-arm prospective trials and other studies, NRG BN001, an ongoing randomized trial (NCT02179086), is assessing the potential survival benefit of dose-escalated PT compared to standard dose photons in the treatment of GBM.8,37
PT was associated with a lower rate of worsening fatigue on the EORTC QLQ-C30 at 6 months. While it is possible this is due to the dosimetric advantages of PT with significantly less dose to the surrounding brain and hippocampi, as there was no adjustment for multiple comparisons for the secondary endpoint analyses, this finding should be interpreted as exploratory.38
Limitations of the current trial include the relatively small sample size of this signal finding trial. A larger study could possibly find improved cognitive outcomes noting the significantly improved dosimetry with PT in patients with GBM. A larger trial would also increase the number of patients with prolonged survival, better enabling an estimation of the improvement in cognitive function in a patient subpopulation where early tumor progression does not compromise the analysis. A larger trial may have also seen benefit with PT for a subset of patients (eg, temporal lobe tumors) who would have otherwise received higher hippocampal doses with IMRT.33 O6-methylguanine-DNA methyltransferase (MGMT) status was not assessed for the majority of tumors as it was not part of clinical practice at MDACC at the time. Therefore, the analysis of survival outcomes could potentially have been compromised due to possible unrecognized imbalance in MGMT status between the study arms.2 Also, although there were no significant differences in baseline patient or tumor characteristics between study arms, unrecognized potential biases could have been introduced by the number of patients excluded by insurance denial of proton therapy. In addition, blinding was not possible since the PT and photons were delivered at different locations. The lack of blinding is especially pertinent to certain aspects of the trial such as the treating clinician’s assessment of toxicities and PROs. However, the lack of blinding to treatment arm in the current trial is consistent with the vast majority of clinical trials evaluating different forms of radiotherapy.
In summary, in this signal-seeking phase II trial, PT was not associated with a delay in time to cognitive failure but did significantly reduce dose to normal structures, toxicity, and patient-reported fatigue. Based on these findings, the off-trial use of protons to decrease the risk of cognitive decline after radiotherapy does not appear justified for patients with GBM. However, its use may be warranted for patients with other gliomas with better prognosis, and clinical trials addressing this specific indication are needed. Larger randomized trials are also needed to determine the potential of dose escalation with PT on GBM tumor control and survival.
Funding
Funding for this work was provided by the The University of Texas MD Anderson Cancer Center High Impact Clinical Research Support Program (HI-CRSP) and Dr. Marnie Rose Foundation, Houston, Texas, USA.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the efforts of Catherine Sullaway and Cristina Tortarolo in supporting the development of this work.
Conflict of interest statement. The authors have no conflicts of interest to declare.
Authorship statement. Conception and design: P.D.B., S.M., and J.S.W. Collection and assembly of data: P.D.B., C.C., D.G., D.D.L., and J.S.W. Neuroradiology review: N.G.T. Data analysis and interpretation: P.D.B., C.C., D.D.L., J.S.W. Manuscript writing and final approval: All authors contributed and approved.
Disclaimer statement. This study was conducted while Drs. Penas-Prado, Armstrong, and Gilbert were employed at The University of Texas MD Anderson Cancer Center. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the US government.
References
- 1. Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26(34):5603–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 3. Anderson MD, Liu D, Wu J, et al. Features of adult clinical trial participants with glioblastoma (GB) at The University of Texas MD Anderson Cancer Center (MDACC). J Clin Oncol. 2014;32:2038. [Google Scholar]
- 4. Brown PD, Buckner JC, Uhm JH, Shaw EG. The neurocognitive effects of radiation in adult low-grade glioma patients. Neuro Oncol. 2003;5(3):161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31(5):702–713. [DOI] [PubMed] [Google Scholar]
- 6. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295–1304. [DOI] [PubMed] [Google Scholar]
- 7. Jalali R, Gupta T, Goda JS, et al. Efficacy of stereotactic conformal radiotherapy vs conventional radiotherapy on benign and low-grade brain tumors: a randomized clinical trial. JAMA Oncol. 2017;3(10):1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizumoto M, Tsuboi K, Igaki H, et al. Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2010;77(1):98–105. [DOI] [PubMed] [Google Scholar]
- 9. Rosenschold PM, Engelholm S, Ohlhues L, et al. Photon and proton therapy planning comparison for malignant glioma based on CT, FDG-PET, DTI-MRI and fiber tracking. Acta Oncol. 2011;50:777–783. [DOI] [PubMed] [Google Scholar]
- 10. Adeberg S, Harrabi SB, Bougatf N, et al. Intensity-modulated proton therapy, volumetric-modulated arc therapy, and 3D conformal radiotherapy in anaplastic astrocytoma and glioblastoma: a dosimetric comparison. Strahlenther Onkol. 2016;192(11):770–779. [DOI] [PubMed] [Google Scholar]
- 11. Arvold ND, Niemierko A, Broussard GP, et al. Projected second tumor risk and dose to neurocognitive structures after proton versus photon radiotherapy for benign meningioma. Int J Radiat Oncol Biol Phys. 2012;83:e495–e500. [DOI] [PubMed] [Google Scholar]
- 12. Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51(1):110–117. [DOI] [PubMed] [Google Scholar]
- 13. Shih HA, Sherman JC, Nachtigall LB, et al. Proton therapy for low-grade gliomas: results from a prospective trial. Cancer. 2015;121(10):1712–1719. [DOI] [PubMed] [Google Scholar]
- 14. Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. [DOI] [PubMed] [Google Scholar]
- 15. Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. [DOI] [PubMed] [Google Scholar]
- 16. Zhu XR, Poenisch F, Li H, et al. A single-field integrated boost treatment planning technique for spot scanning proton therapy. Radiat Oncol. 2014;9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boehling NS, Grosshans DR, Bluett JB, et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2012;82(2):643–652. [DOI] [PubMed] [Google Scholar]
- 18. Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–1309. [DOI] [PubMed] [Google Scholar]
- 19. Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 20. Taphoorn MJ, Claassens L, Aaronson NK, et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 21. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. [DOI] [PubMed] [Google Scholar]
- 22. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. [DOI] [PubMed] [Google Scholar]
- 23. Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) . Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Armstrong TS, Wefel JS, Wang M, et al. Net clinical benefit analysis of Radiation Therapy Oncology Group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23(28):7199–7206. [DOI] [PubMed] [Google Scholar]
- 26. Wilcoxon F. Individual comparisons by ranking methods. Biometr Bull. 1945;1:8–83. [Google Scholar]
- 27. Fisher RA. On the interpretation of χ 2 from contingency tables, and the calculation of P. J Royal Stat Soc. 1922;85;87–94. [Google Scholar]
- 28. Gray RJ. A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]
- 29. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 30. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 31. Hall WA, Pugh SL, Wefel JS, et al. Influence of residual disease following surgical resection in newly diagnosed glioblastoma on clinical, neurocognitive, and patient reported outcomes. Neurosurgery. 2019;84(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flechl B, Sax C, Ackerl M, et al. The course of quality of life and neurocognition in newly diagnosed patients with glioblastoma. Radiother Oncol. 2017;125(2):228–233. [DOI] [PubMed] [Google Scholar]
- 33. Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85(2):348–354. [DOI] [PubMed] [Google Scholar]
- 34. Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 35. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 36. Lin SH, Hobbs BP, Verma V, et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J Clin Oncol. 2020;38(14):1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91(2):251–260. [DOI] [PubMed] [Google Scholar]
- 38. Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.