Abstract
Background
A loss of the trimethylation of lysine 27 of histone H3 (H3K27me3) in meningioma has been recently suggested as an adjunct to identify subsets of higher risk of recurrence. The aim of the present study was to assess the prognostic value of H3K27 histone trimethylation and its potential clinical utility in the “Tübingen meningioma cohort.”
Methods
Patients who underwent meningioma resection between October 2003 and December 2015 at the University Hospital Tübingen were included. Immunohistochemical stainings for H3K27me3 and the proliferation marker MIB1 were assessed and correlated with clinical parameters using univariate and multivariate Cox regressions as well as Pearson's chi-squared and log-rank test.
Results
Overall, 1268 meningiomas were analyzed with a female to male ratio of 2.6 and a mean age of 58.7 years (range 8.3–91.0). With 163 cases lost to follow up, 1103 cases were available for further analysis with a mean follow-up of 40.3 months (range 1.1–186.3). Male gender, younger age, intracranial tumor localization, progressive tumor, subtotal resection, higher WHO grade, increased MIB1 rate, and loss of H3K27me3 were significant negative prognostic factors in the univariate analysis. H3K27me3 status and all other prognostic factors, except age and tumor location, remained significant in the multivariate model. Furthermore, adjuvant radiotherapy was an independent positive prognostic factor.
Conclusions
Loss of H3K27me3 combined with MIB1 labeling index are independent prognostic factors in meningioma. These data from the Tübingen meningioma cohort support the clinical utility of H3K27me3 immunohistochemical staining in meningioma and its integration into the routine histopathological workup.
Keywords: H3K27me3, H3K27, histone methylation, meningioma, recurrence-free survival
Key Points.
1. Loss of H3K27me3 is rare in meningioma (<5%).
2. Loss of H3K27me3 in meningioma is an independent negative prognostic factor.
3. Adjuvant radiotherapy is an independent favorable prognostic factor in meningioma.
Importance of the Study.
Most meningiomas can be effectively treated by surgical resection and/or radiation therapy. An improvement of the prognostic evaluation is warranted for the clinical management of meningioma. In this regard, loss of the trimethylation of lysine 27 of histone H3 (H3K27me3) might be a potential novel prognostic candidate as indicated in a cohort of 232 meningiomas, enriched for higher grade tumors. We investigated the frequency and the prognostic role of H3K27me3 in the Tübingen meningioma cohort and performed a multivariate analysis including all established prognostic factors. We identified an independent negative prognostic impact of the loss of H3K27me3 and advocate for its inclusion in the routine neuropathological diagnostic workup for meningioma patients.
Meningiomas make up 36.8% of intracranial tumors.1 Many patients can be cured by surgical resection alone. Radiotherapy is an established treatment option for higher tumor grades, recurrent meningiomas or as a primary treatment in selected cases.2–4 Meningiomas can recur and infiltrate extensively into surrounding structures. The WHO grading system is very useful for risk stratification of meningiomas. Yet, the unmet clinical need of prognostic markers for recurrence-free survival has already been outlined in the 2016 update of the WHO classification of central nervous system tumors, which now includes histopathological evidence of brain invasion as an independent criterion for atypia and thus WHO grade II5. Molecular markers such as the telomerase reverse transcriptase (TERT) promotor and loss of the cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) are prognostically relevant but only present in a small subset of (mostly anaplastic) meningiomas.6,7 Therefore, further strengthening of prognostication is necessary to provide clinicians and their patients with information on the expected disease course.
The role of epigenetic modifications in cancer8 and more specifically in brain tumors has been in the focus of current research,9 especially in the light of the possibility to address such alterations therapeutically. Alterations of the methylation of lysine 27 of histone 3 (H3K27me) have been identified as pivotal modifications in different cancers10 and ways to target this therapeutically have been described.11 First studies in meningiomas have shed light on the role of epigenetics in this specific tumor entity.12 Recently, a loss of the histone trimethylation H3K27me3 has been suggested in meningiomas as a negative prognostic marker in a cohort of 232 meningiomas with a focus on differentiating the prognosis of WHO II meningiomas.13 Another study focused on the role of H3K27me3 loss in 47 anaplastic meningiomas and found an independent prognostic value as well.14 The H3K27m3e status has since been discussed as a promising prognostic marker with high clinical potential but its impact has not yet been investigated in a larger cohort. Furthermore, several additional factors might influence the prognostic value of H3K27me3 that were not included in the prior study,13 for example, tumor location, adjuvant radiotherapy, and tumor proliferation. In this study, we integrated these factors in the Tübingen meningioma cohort to investigate the clinical utility of the H3K27me3 status.
Material and Methods
Study Cohort
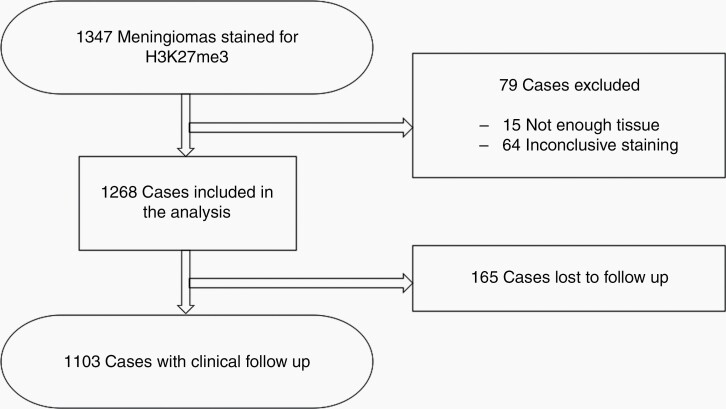
Tissue samples from 1347 meningiomas that were resected at the University Hospital Tübingen between October 2003 and December 2015 were included in this retrospective observational single center study. Fifteen cases were excluded due to lack of sufficient tissue material for further immunohistochemical evaluation. Sixty-four tissue samples were excluded due to technically unsatisfactory staining quality in immunohistochemistry. Overall, 1268 cases with sufficient tissue and staining quality and complete clinical data were included in the current analysis, with follow-up data for 1103 cases (Fig. 1). The clinical data set included age at diagnosis, gender, histopathological diagnosis (WHO classification of 2016), extent of resection (Simpson grade), tumor location, time to radiographic tumor recurrence/progression and radiotherapy treatment between surgery and tumor recurrence. Furthermore, a subgroup analysis was done restricted to patients with a follow-up of 5 years or longer.
Fig. 1.
Schematic overview of the study cohort.
Automated Assessment of MIB1
Data of MIB1 staining from the diagnostic evaluation after meningioma resection were assessed quantitatively on full slides. An automated evaluation of digitalized archived slides was done with the help of the Image J software (Version 1.51j8, NIH, Bethesda, MD, USA) and the plugins Bio-Formats (Release 5.4.1; Open Microscopy Environment, Madison, NJ, USA) and ImmunoRatio (Version 1.0c, Institute of Biomedical Technology, University of Tampere, Finland) (Supplementary Figure 1).
Tissue Microarray Construction and Immunohistochemistry
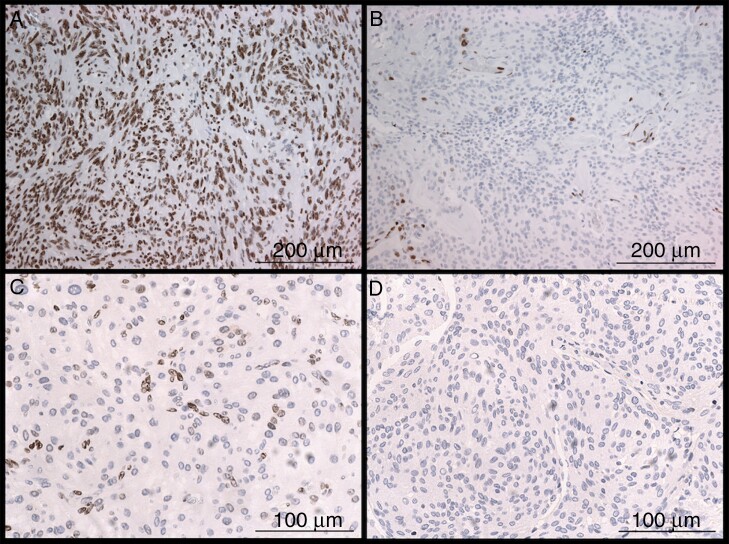
Archived formalin-fixed and paraffin-embedded tissue samples were used for the extraction of 2 biopsy punches of 1 mm diameter each to construct tissue microarrays with a conventional tissue microarrayer (Beecher Instruments, Sun Prairie, Wisconsin, USA). Tumor cylinder extraction was done after evaluation of hematoxylin and eosin slides for suitable areas. The new formed tissue blocks were cut to 4 μm slices with a microtome. After subsequent drying at 80° for 15 min, subsequent immunohistochemical staining for H3K27me3 (1:200, rabbit monoclonal antibody C36B11, Cell Signaling, Danvers, MA, USA) was done with a Ventana BenchMark immunostainer (Ventana Medical Systems, Tucson, Arizona, USA). Analysis of immunohistochemical stainings was done by two researchers. The interscorer reliability was measured and cases with non-matching results were reviewed and a consensus was found. Most cases showed a clear nuclear retention or loss. Nuclear staining of endothelia served as internal control in most cases. Cases with partial immunopositivity were scored as H3K27me3 retention while cases with complete immunonegativity (no positive internal control) were excluded (Fig. 2). A breast cancer metastasis and cerebral cortex as well as cerebellar cortex were used as controls on each TMA.
Fig. 2.
Immunohistochemistry of the histone trimethylation H3K27me3. Distinct differentiation of retained trimethylation with strong brown nuclear staining of all tumor cells (A) and loss of trimethylation with immunopositivity restricted to non-tumor cells like endothelium (B). Cases with partial staining of tumor cells were scored as retained (C) while samples with complete immunonegativity were excluded (D).
Statistical Analysis
Statistical analysis was done with JMP® (Cary, NC: SAS Institute Inc.; 1989) Statistical Discovery Software, version 15.1.0. Pearson's chi-squared and the log-rank test were used for univariate and the Wald test for multivariate analysis while a significance level of α < 0.05 was applied. A classification and regression tree (CART) analysis was done to determine the cut off for MIB1 expression and age regarding the biggest difference of tumor recurrence of the study cohort. The interscorer reliability was measured using Cohen's kappa coefficient. The study was approved by the Clinical Ethics Committee of the University of Tübingen (Project number: 618/2014BO2).
Results
Basic Clinical Characteristics of the Tübingen Meningioma Cohort
The female to male ratio was 2.62 with a mean age of 58.7 years ranging from 8.3 to 91.0. The cohort consisted mainly of patients with newly diagnosed meningiomas (89.3%) and the majority of tumors were located at the skull base (51.2%) followed by convexity/falx (38.5%) and the spine (10.3%). A complete resection (Simpson grade I, II, or III) was achieved in 70.1% of cases. A subtotal resection was done in 29.6% of meningiomas and 3 cases were biopsied (0.2%). Adjuvant radiotherapy was applied in 58 cases (5.3%) due to higher WHO grade and/or incomplete resection. WHO grading was performed according to the current WHO classification of 2016,5 and 78.9% of all tumors were classified as grade I (n = 1001), 19.7% were grade II (n = 250), and 1.3% grade III (n = 17). The meningothelial subtype was the most common in our cohort (51.2%, n = 649) followed by atypical (15.7%, n = 199), transitional (8.8%, n = 112), and fibroblastic meningiomas (7.6%, n = 96). A classification and regression tree (CART) analysis identified a prognostic cut off of digitally analyzed nuclear MIB1 expression at 6.9%, which was exceeded by 71 cases (5.6%). The subgroup with a follow-up of 5 years or longer consisted of 466 meningiomas. The characteristics of the subgroup are similar to the complete Tübingen cohort. All clinical characteristics are summarized in Supplementary Table 1.
Distribution of H3K27me3 Loss
We observed a loss of H3K27 staining in 60/1268 meningiomas (4.7%). The Cohen's kappa coefficient for interrater reliability was 0.734. The rate of trimethylation loss was significantly increased in recurrent meningiomas (11%) as opposed to newly diagnosed tumors (4%, P = 0.0003). A significantly higher rate of H3K27me3 loss was observed for male patients with 7.7% compared to 3.6% for female patients (P = 0.0020). In spinal meningiomas the trimethylation was lost in 0.8% compared to 3.7% in skull base and 7.2% in convexity/falx tumors (P = 0.0019). Tumor WHO grade also showed marked differences, with a trimethylation loss in 3.1% for grade I, 10.4% for grade II and 17.7% for grade III meningiomas (P < 0.0001). Variations among histological subtypes were observed as well (P = 0.0006). The highest rate of trimethylation loss was detected in anaplastic and rhabdoid meningiomas (16.7% and 20.0%, respectively), followed by atypical and chordoid meningiomas (9.9% and 14.3%, respectively). No cases with H3K27me3 loss were seen in angiomatous (n = 25), metaplastic (n = 16), microcystic (n = 25), and secretory meningiomas (n = 29) (Supplementary Table 1). Meningiomas with a higher MIB1 proliferation rate of more than 6.9% had a significantly higher rate of H3K27me3 loss (18.3% compared to 3.9%, P < 0.0001).
Univariate Analysis for the Risk of Tumor Recurrence
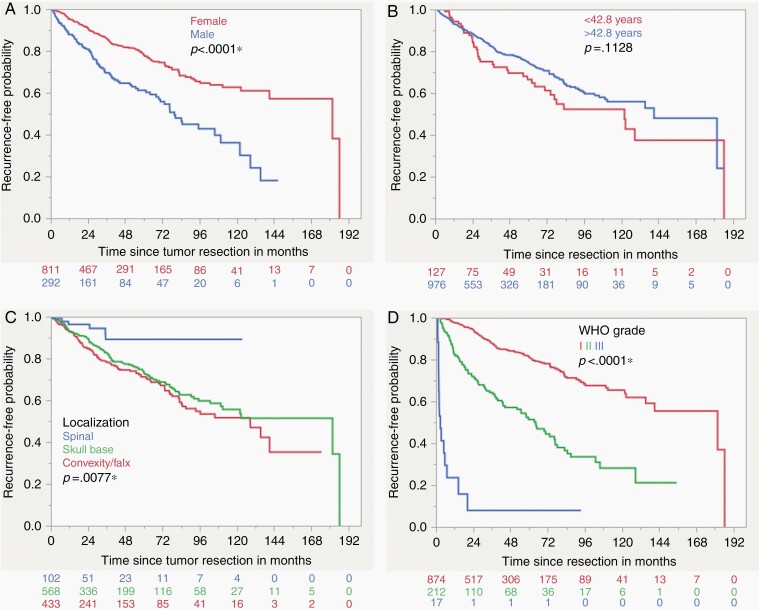
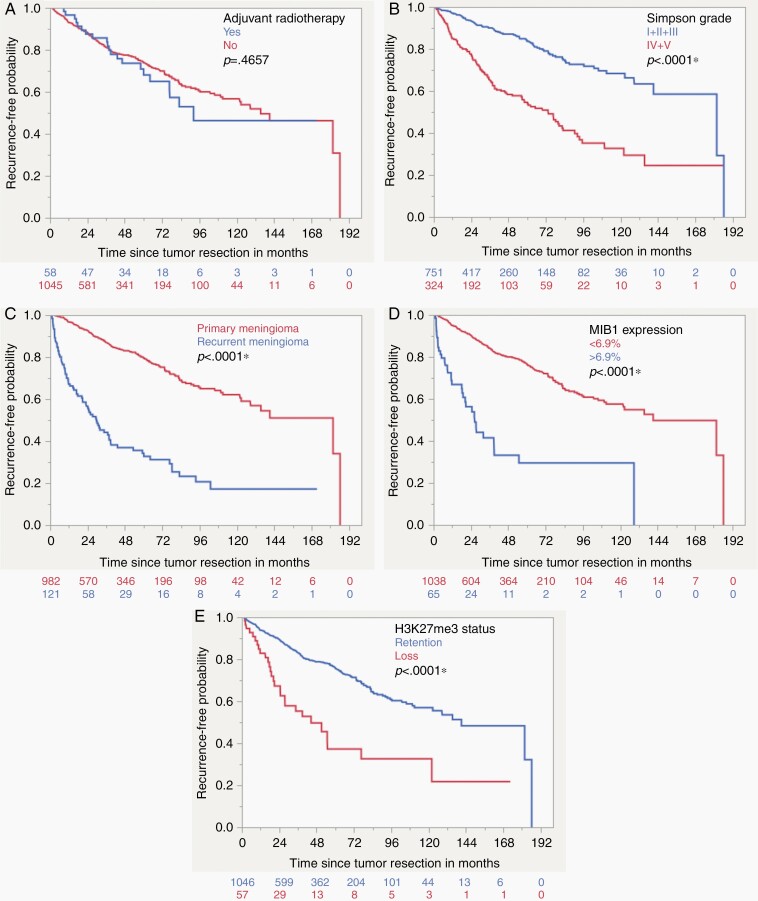
Overall, 165 cases were lost to follow up leaving 1103 meningiomas for prognostic analysis. As seen in other cohorts, the rate of tumor recurrence was significantly higher for male patients (32.9% compared to 17.3%, P < 0.0001). Tumors of patients that were younger than 42.8 years had a significantly higher rate of tumor recurrence with 31.5% compared to 20.1% for older patients (P = 0.0032, age cut off according to CART analysis). Recurrent meningiomas had an increased risk of another tumor recurrence with 62.8% compared to newly diagnosed meningiomas (16.3%, P < 0.0001). Regarding meningioma location, the lowest recurrence rate was seen for spinal meningiomas with 5.9% compared to skull base and convexity/falx tumors (21.8% and 24.5%, respectively). Incomplete tumor resection according to Simpson grade IV showed a higher rate of tumor recurrence (41.1% compared to 13.1% for Simpson grade I/II/III, P < 0.0001). Adjuvant radiotherapy was also a univariate risk factor for tumor recurrence (36.2%) compared to cases that did not receive radiation after surgery (20.6%, P = 0.0047). It should be noted that subtotal tumor resection or grading as WHO II or III led to the indication for adjuvant radiotherapy, making this a very distinct clinical subgroup with an increased risk of tumor recurrence. WHO grade III tumors recurred in 88.2% of cases, grade II in 43.9% and grade I meningiomas only in 14.7% (P < 0.0001). According to histopathology, the lowest rate of recurrence was observed for angiomatous and psammomatous meningiomas (5.0% and 5.4%, respectively) and the highest for anaplastic meningiomas (91.7%, P < 0.0001). More than one half of tumors with a MIB1 expression exceeding 6.9% recurred (55.4%, cut off according to CART analysis) compared to only 19.3% if the expressed proliferation rate was lower (P < 0.0001). The loss of H3K27me3 was also a significant univariate negative prognostic factor regarding tumor recurrence (49.1% compared to 19.9%, P < 0.0001). The significant results were observed in the Kaplan–Meier analysis as well, except for adjuvant radiotherapy and the age cut off at 42.8 years, that both did not show a significant univariate prognostic impact in the log-rank test (Supplementary Table 2. Figures 3 and 4). When regarding H3K27me3 loss in WHO grade I, II, and III meningiomas separately, the level of significance was only met for WHO grade II tumors (P = 0.0035) and missed by grade I and III lesions (P = 0.0543 and P = 0.8872, respectively. Supplementary Figure 2).
Fig. 3.
Kaplan–Meier curves demonstrating the results of the univariate survival analysis for gender (A), age (B), tumor location (C) and WHO grade (D) (Log-rank test).
Fig. 4.
Kaplan–Meier curves demonstrating the results of the univariate survival analysis for adjuvant radiotherapy (A), extent of resection (B), Primary/recurrent meningioma (C), MIB1 expression (D) and H3K27me3 status (E) (Log-rank test).
Multivariate Analysis for the Risk of Tumor Recurrence
All factors of the univariate analysis were integrated into the multivariate analysis. The loss of H3K27me3 nuclear expression remained an independent negative prognostic factor in meningiomas (P = 0.0084). The most pronounced risk ratios for tumor recurrence were observed for WHO grade and the extent of meningioma resection. For example, the risk ratio for WHO grade I meningiomas was 0.05 compared to grade III in the multivariate analysis (0.03–0.10, P < 0.0001). On the other hand, tumors that were resected subtotally had a risk ratio of 3.64 for tumor recurrence (2.73–4.85, P < 0.0001). In comparison to the univariate analysis, adjuvant radiotherapy was revealed as a protective factor regarding tumor regrowth in the multivariate analysis (RR 0.27 (0.17–0.44), P < 0.0001). Again, a higher risk of tumor recurrence was observed for recurrent meningiomas (P < 0.0001). Tumors exceeding a MIB1 labelling index of 6.9% were also at higher risk of recurrence (P = 0.0004). Male gender remained an independent negative prognostic factor (P = 0.0052), while age and tumor location did not reach statistical significance in the multivariate analysis. The level of significance of all prognostic factors was also reached in another multivariate analysis restricted to patients with a follow-up of 5 years or longer (Table 1).
Table 1.
Multivariate Analysis of Prognostic Factors of Tumor Recurrence of the Complete Cohort and the Subcohort of Patients with a Follow Up of 5 Years or Longer (Cox Proportional Hazard)
| Complete Cohort | 5-Y Follow-Up | |||
|---|---|---|---|---|
| Risk Ratio (95% CI) | P-value (Prob > Chisq) | Risk Ratio (95% CI) | P-value (Prob > Chisq) | |
| Male gender | 1.51 (1.13–2.02) | 0.0052* | 1.42 (1.06–1.90) | 0.0199* |
| Age < 42.83 | 1.26 (0.88–1.80) | 0.2079 | 1.19 (0.83–1.70) | 0.3472 |
| Location | ||||
| Skull base vs convexity/falx | 1.17 (0.88–1.55) | 0.2931 | 1.23 (0.92–1.63) | 0.1620 |
| Skull base vs spinal | 1.95 (0.85–4.48) | 0.1149 | 1.72 (0.75–3.96) | 0.2045 |
| Convexity/falx vs spinal | 1.67 (0.73–3.85) | 0.2260 | 1.40 (0.61–3.23) | 0.4304 |
| Recurrent meningioma | 3.37 (2.42–4.69) | <0.0001* | 2.96 (2.12–4.14) | <0.0001* |
| Simpson Grade 4/5 | 3.64 (2.73–4.85) | <0.0001* | 3.16 (2.37–4.21) | <0.0001* |
| WHO Classification 2016 | ||||
| I vs II | 0.32 (0.23–0.44) | <0.0001* | 0.35 (0.26–0.48) | <0.0001* |
| I vs III | 0.05 (0.03–0.10) | <0.0001* | 0.07 (0.03–0.13) | <0.0001* |
| II vs III | 0.16 (0.09–0.30) |
<0.0001* | 0.19 (0.10–0.34) |
<0.0001* |
| Adjuvant radiotherapy | 0.27 (0.17–0.44) | <0.0001* | 0.27 (0.17–0.44) | <0.0001* |
| MIB1 > 6.9% | 2.19 (1.42–3.40) | 0.0004* | 2.12 (1.37–3.27) | 0.0007* |
| H3K27m3 loss | 1.80 (1.16–2.80) | 0.0084* | 1.61 (1.04–2.50) | 0.0339* |
Discussion
The update of the WHO classification for meningiomas in 2016 identified an unmet clinical need, that is, the need for prognostic markers of recurrence-free survival.5 With the description of the negative prognostic impact of TERT promoter mutations and CDKN2A/B deletions, the integration of molecular markers into the risk stratification of meningiomas has been suggested.6,7,15 Furthermore, the use of DNA methylation profiling for improved prognostication in meningioma has been advocated.16,17 The role of histone modifications in cancer has gained increasing attention in the last decade18 and new insights were also reported in the field of neuro-oncology with the detection of alterations of histone demethylases in meningiomas.19
Recently, the immunohistochemical detection of H3K27me3 in meningioma was first described and its prognostic potential was suggested by Katz and colleagues. Their study focused on higher grade meningiomas, and the cohort was intentionally enriched for atypical meningiomas. An increased risk of tumor recurrence of cases with H3K27me3 loss was seen in a multivariate analysis of two cohorts, one with 232 tumors enriched for grade II meningiomas13 and another with 47 grade III tumors.14 The Tübingen meningioma cohort of 1103 meningiomas supports an independent prognostic role of the H3K27me3 status. We present a comprehensive cohort which includes recurrent meningiomas and information on tumor location. Furthermore, we integrated the prognostic effect of MIB1 labeling index and adjuvant radiotherapy to allow for a comprehensive multivariate prognostic analysis.
Radiotherapy is the only established therapeutic modality for meningiomas beyond surgery, particularly for selected invasive and recurrent meningiomas.2,3,20 The strongest independent predictors of tumor recurrence in the Tübingen cohort were WHO grade, the extent of resection and adjuvant radiotherapy. The negative prognostic effect of adjuvant radiotherapy can be explained by the constitution of this distinct subgroup. Most cases that received adjuvant radiation were of higher WHO grade, subtotally resected or recurrent meningiomas. After integration of all prognostic factors in the multivariate analysis, adjuvant radiotherapy was revealed as a strong independent positive prognostic factor (but not in the univariate analysis), indicating the necessity to integrate this factor in future prognostic evaluations of meningiomas.
We observed a significantly increased frequency of higher MIB1 expression (above the cut off of 6.9%) in cases with H3K27me3 loss (18.3% vs 3.9%, P < 0.0001). Consequently, we integrated the proliferation marker MIB1 in our multivariate analysis. After a comprehensive inclusion of all prognostic markers, H3K27me3 loss remained an independent marker for meningioma recurrence. Most importantly, the significance of all prognostic markers was the same for the subcohort of all cases with a follow-up of at least 5 years. Therefore, our results emphasize a prognostic relevance of H3K27me3 status in a large cohort of meningiomas with a long follow-up.
The incidence of H3K27me3 loss in the Tübingen cohort was 4.7% in comparison to the analysis by Katz et al., which showed loss of trimethylation in 10.8%. This difference can be explained by the fact that the prior study used a selected cohort mainly containing atypical meningiomas.13 The subgroup of atypical meningiomas based on histological criteria in our cohort showed a similar loss of H3K27me3 in 9.9%, only surpassed by chordoid, anaplastic and rhabdoid meningiomas (14.3%, 16.7%, and 20.0%, respectively). The analysis of 47 anaplastic meningiomas revealed loss in 21% which was seen in a similar rate in our cohort (16.7%).14 Besides a clear correlation with higher WHO grades, both factors remained independent prognostic factors in the multivariate analysis. An important result is the separate analysis of H3K27me3 loss in the different WHO grades which showed a significant result in the Kaplan–Meier curves only for WHO grade II tumors, while the level of significance was not reached for grade I meningiomas. It has to be noted, however, that loss of H3K27me3 was overall a rare event and a subgroup analysis according to WHO grade made the groups with methylation loss even smaller. This might certainly hamper the assessment of statistical significance in these subgroups.
Taken together, the Tübingen meningioma cohort indicates a prognostic potential of H3K27me3. The immunohistochemical staining is a standard procedure in neuropathological laboratories.21–23 Thus, the implementation of H3K27me3 seems therefore possible to optimize the prognostication for meningioma patients. Furthermore, distinct treatment options that target H3K27me3 and its regulators are being investigated in other cancer types24,25 and future developments may also be applicable in meningiomas.
Yet, our study has of course strengths and limitations. The Tübingen meningioma cohort includes >1000 clinically well-characterized meningiomas and 466 cases with a follow-up of 5 years or longer, which makes it a solid setting for assessing markers with a low incidence, like H3K27me3. In order to understand the prognostic impact of H3K27me3 in the context of other relevant clinical markers, we integrated all established prognostic factors in meningioma and furthermore MIB1 expression and adjuvant radiotherapy in a comprehensive multivariate analysis. Of course, the retrospective nature remains a limitation of the study, even though we managed to collect in most cases all clinical parameters that in our opinion were relevant for this study. Ongoing and future studies will be important to investigate the role of H3K27me3 loss in molecular subsets of meningioma or in different methylation classes. Another challenge was all gradings of H3K27me3 staining beyond a clear retention or a clear loss (Fig. 2C and D). Furthermore, we excluded cases that were completely immunonegative, that is, not displaying a positive endothelial signal as internal control (n = 64, Fig. 2D). These staining failures were more common in FFPE tissue older than 10 years. Overall, Cohen's kappa coefficient of 0.734 indicates a good interrater reliability. The TMA method enables a quick and structured analysis of immunohistochemical staining. Although, two sample cylinders measuring 1mm in diameter each were extracted from each tumor tissue block, it is possible that some aspects of the staining pattern in the tumor were missed. However, the sample cylinders were chosen after thorough analysis of HE slides in order to extract representative tumor tissue areas. For the automated assessment of MIB1 representative areas were chosen from full slides.
Taken together, we present here data from the Tübingen meningioma cohort on the role of H3K27me3 loss. With a loss in less than 5% of all meningiomas, alterations of this epigenetic marker are certainly a rare event, but given its prognostic value and potential clinical utility, especially in grade I WHO meningiomas, we advocate for further developments towards an integration into the routine diagnostic workup.
Supplementary Material
Acknowledgments
We thank Yeliz Donat, Sarah Hendel, Heike Pfrommer for excellent technical assistance.
Conflict of interest statement. F.B. received funding for industry sponsored workshops from Medac and Arbor Pharmaceuticals. I.G.T. received speaker fees and a travel grant from Novocure and funding from Medac for participation in industry sponsored workshops. G.T. has served on advisory boards of AbbVie, Bayer and BMS; received speaker fees from Medac and Novocure; received travel grants from Novocure, Medac and BMS; received research grants from Roche Diagnostics and Medac. The other authors do not have any COI to disclose.
Authorship statement. Study concept: F.B., J.S., G.T.; data acquisition: F.B., C.F., I.G.T., K.K., F.P., J.H., M.T., J.S., G.T.; tissue microarray construction: F.B., C.F., J.S. data analysis: F.B., C.F., F.P., M.S., J.H., M.T., J.S., G.T.; data interpretation: F.B., M.S., F.P., M.R., J.S., G.T.; writing of first draft: F.B., J.S., G.T. All authors reviewed and approved the final version of the manuscript.
Funding
Parts of the study were funded by the Medical Faculty Tübingen (Demonstratorprojekt Personalisierte Medizin) and by the Adolf Leuze Foundation.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brastianos PK, Galanis E, Butowski N, et al. ; International Consortium on Meningiomas . Advances in multidisciplinary therapy for meningiomas. Neuro Oncol. 2019;21(Suppl 1):i18–i31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 4. Rogers L, Zhang P, Vogelbaum MA, et al. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018;129(1):35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 6. Sahm F, Schrimpf D, Olar A, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5). doi: 10.1093/jnci/djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sievers P, Hielscher T, Schrimpf D, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. [DOI] [PubMed] [Google Scholar]
- 9. Maury E, Hashizume R. Epigenetic modification in chromatin machinery and its deregulation in pediatric brain tumors: Insight into epigenetic therapies. Epigenetics. 2017;12(5):353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nichol JN, Dupéré-Richer D, Ezponda T, Licht JD, Miller WH Jr. H3K27 methylation: a focal point of epigenetic deregulation in cancer. Adv Cancer Res. 2016;131:59–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan MR, Hsu MC, Chen LT, Hung WC. Orchestration of H3K27 methylation: mechanisms and therapeutic implication. Cell Mol Life Sci. 2018;75(2):209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Venza M, Visalli M, Beninati C, et al. Involvement of epimutations in meningioma. Brain Tumor Pathol. 2015;32(3):163–168. [DOI] [PubMed] [Google Scholar]
- 13. Katz LM, Hielscher T, Liechty B, et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol. 2018;135(6):955–963. [DOI] [PubMed] [Google Scholar]
- 14. Gauchotte G, Peyre M, Pouget C, et al. Prognostic value of histopathological features and loss of H3K27me3 immunolabeling in anaplastic meningioma: a multicenter retrospective study. J Neuropathol Exp Neurol. 2020;79(7):754–762. [DOI] [PubMed] [Google Scholar]
- 15. Mirian C, Duun-Henriksen AK, Juratli T, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91(4):378–387. [DOI] [PubMed] [Google Scholar]
- 16. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 17. Nassiri F, Mamatjan Y, Suppiah S, et al. ; International Consortium on Meningiomas . DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019;21(7):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodríguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17(3):330–339. [DOI] [PubMed] [Google Scholar]
- 19. Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li D, Jiang P, Xu S, et al. Survival impacts of extent of resection and adjuvant radiotherapy for the modern management of high-grade meningiomas. J Neurooncol. 2019;145(1):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karremann M, Gielen GH, Hoffmann M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018;20(1):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu VM, Marek T, Gilder HE, et al. H3K27 trimethylation loss in malignant peripheral nerve sheath tumor: a systematic review and meta-analysis with diagnostic implications. J Neurooncol. 2019;144(3):433–443. [DOI] [PubMed] [Google Scholar]
- 23. Panwalkar P, Clark J, Ramaswamy V, et al. Immunohistochemical analysis of H3K27me3 demonstrates global reduction in group-A childhood posterior fossa ependymoma and is a powerful predictor of outcome. Acta Neuropathol. 2017;134(5):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michealraj KA, Kumar SA, Kim LJY, et al. Metabolic regulation of the epigenome drives lethal infantile ependymoma. Cell. 2020;181(6):1329–1345 e1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.