Abstract
Background
Immune checkpoint inhibitors (ICI) have been a breakthrough for selected cancer patients, including those with brain metastases (BMs). Likewise, steroids have been an integral component of symptomatic management of BM patients. However, clinical evidence on the interaction between ICI and steroids in BM patients is conflicting and has not adequately been summarized thus far. Hence, the aim of this study was to perform a systematic literature review and meta-analysis on the association between steroid use and overall survival (OS) in BM patients receiving ICI.
Methods
A systematic literature search was performed. Pooled effect estimates were calculated using random-effects models across included studies.
Results
After screening 1145 abstracts, 15 observational studies were included. Fourteen studies reported sufficient data for meta-analysis, comprising 1102 BM patients of which 32.1% received steroids. In the steroid group, median OS ranged from 2.9 to 10.2 months. In the nonsteroid group, median OS ranged from 4.9 to 25.1 months. Pooled results demonstrated significantly worse OS (HR = 1.84, 95% CI 1.22-2.77) and systemic progression-free survival (PFS; HR = 2.00, 95% CI 1.37-2.91) in the steroid group. Stratified analysis showed a consistent effect across the melanoma subgroup; not in the lung cancer subgroup. No significant association was shown between steroid use and intracranial PFS (HR = 1.31, 95% CI 0.42-4.07).
Conclusions
Administration of steroids was associated with significantly worse OS and PFS in BM patients receiving ICI. Further research on dose, timing, and duration of steroids is needed to elucidate the cause of this association and optimize outcomes in BM patients receiving ICI.
Keywords: brain metastases, immune checkpoint inhibitors, meta-analysis, steroids, survival
Key Points.
-
1.
Steroid use is associated with shorter OS in brain metastases patients receiving ICI.
-
2.
Steroid use is associated with worse systemic PFS, but not with intracranial PFS.
Importance of the Study.
Immune checkpoint inhibitors (ICI) are increasingly being administered to cancer patients, including those with brain metastases (BMs). As steroids are prescribed routinely in many BM patients, there is an urgent need for a better understanding of the interaction with the effects of ICIs. Pharmacologically, ICIs and steroids exert opposite effects on the immune system. Preclinical evidence has indicated that a combination of steroids and ICI may diminish survival benefits, and 40% of ICI trials excluded patients on steroids. This systematic review and meta-analysis investigated the association between steroids and overall and progression-free survival (OS/PFS) in BM patients receiving ICI and demonstrated that steroids were associated with worse OS and systemic PFS. While a causal mechanism cannot be inferred from this study, our findings highlight the importance of further research into this question. Such investigations can help tailor steroid timing and dosing in ICI patients in the future.
The introduction of immune checkpoint inhibitors (ICI) in the treatment of different cancers including melanoma and non-small cell lung cancer (NSCLC) has been a breakthrough in oncology. These ICI target cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death (ligand) 1(PD-(L)1) molecules on T cells, resulting in prolonged activation of T-cell responses and subsequent stimulation of antitumor activity.1 In brain metastasis (BM) patients, intracranial response rates have been reported to range from 16% to 25% in melanoma BMs treated with ICI monotherapy,2–5 57% in melanoma BMs treated with combined anti-CTLA-4 and anti-PD-1 ICI, and 33% in NSCLC BMs treated with anti-PD-1.6 Moreover, the use of ICI in combination with stereotactic radiosurgery (SRS) in BM patients showed higher intracranial response rates and improved survival when compared to ICI alone.7–10
As a result of these ongoing advances in immunotherapy for BMs, the potential risks vs benefits of the concurrent use of ICI and steroids are becoming increasingly relevant. The immunosuppressive effects of steroids might counteract the working mechanism of ICIs; preclinical evidence suggested that a combination of these therapies might lead to diminished survival benefits.11 In part due to these concerns, 40% of ICI trials considered chronic steroid use as an exclusion criterion.12 This is problematic for BM patients because steroids such as dexamethasone have been an integral component of symptomatic treatment in this population since their introduction more than half a century ago.13,14 To date, the implications of these concerns for the treatment of BM patients are unclear, and studies reporting on an association between steroid use and survival in this population have produced conflicting results.
To shed light on this question, we performed a systematic review and meta-analysis of the current literature reporting on the association between the use of steroids and overall survival (OS) in BM patients treated with ICI.
Materials and Methods
Study Design and Search Strategy
A systematic literature search was performed in PubMed, Embase, Web of Science, Cochrane, Academic Search Premier, and PsycINFO on July 2, 2020. The complete search can be found in Supplementary Table S1. References of included studies were checked to identify additional relevant publications. Study screening and data extraction were conducted by two independent reviewers (C.A.C.J. and A.E.W.) according to the PRISMA checklist. In case of disagreement, a third reviewer (A.F.C.H.) was consulted.
In- and Exclusion Criteria
Studies were included if they (1) reported on at least five participants per group in any study design; (2) reported on BM patients as the entire study population or included a subgroup of BM patients with sufficient data for analysis; and (3) reported on the use of steroids in patients receiving ICI in relation to primary or secondary outcomes. The primary outcome of this study was median OS in months, whereas secondary outcomes were systemic progression-free survival (PFS) in months, intracranial PFS (IC-PFS) in months, and treatment response according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1,15 the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) or the immune-related response criteria (irRC),16 and occurrence of immunotherapy-related adverse events (IRAEs). Systemic PFS reflects time to death or progression at any intra- or extracranial location in the body, whereas IC-PFS is time to death or intracranial progression. Exclusion criteria were: (1) studies performed in animals, (2) studies including patients with primary brain tumors, (3) studies reporting exclusively on leptomeningeal disease in the absence of parenchymal BMs, (4) studies in which steroid use was supplied only for management of IRAEs, (5) studies with no full text available, and (6) non-English publications.
Data Extraction
The following information was extracted: study characteristics including study design and sample size; patient characteristics including sex, age, baseline Karnofsky Performance Status (KPS), and primary tumor site; treatment characteristics including previous craniotomy and/or radiation therapy and type of immunotherapy and steroids and clinical outcomes. Authors of the studies were contacted to obtain additional unpublished data if these were necessary for quantitative analysis.
Risk of Bias Assessment
A quality assessment of the cohort studies was performed based on the Newcastle-Ottawa Scale (NOS) grading studies with stars on selection, comparability, and outcome categories.17 The highest quality studies were awarded up to nine stars (the minimum is 0 stars). For case series, a modified NOS was used leaving out the comparability category and question 2 of the selection category (selection of the nonexposed cohort), resulting in a maximum of six stars that could be awarded.
Statistical Analysis
Data analysis was performed using R v 3.5.0 (R Core Team, Vienna, Austria). The random-effects model that used the DerSimonian-Laird method and Jackson method18 to account for variation between studies was used to obtain the overall hazard ratio (HR) and incidence rate ratio (IRR) estimates and 95% confidence intervals (CI).18 If the standard error was not reported in the studies, it was calculated using the number of deaths,19 the CI, or the P value.20 Heterogeneity among studies was assessed using Higgin’s and Thompson’s I2; >50% was considered to be high heterogeneity.21 The Cochrane Q test was used to assess the P value for heterogeneity (significant P value < .1). Pooled analysis was performed, both unstratified and stratified by primary tumor type and by receipt of concurrent SRS and ICI. Moreover, a leave-one-out analysis was performed to assess how each individual study affected the overall estimate of the rest of the studies. A sensitivity analysis was performed including only the studies that adjusted for confounders. A random-effects meta-regression analysis on different covariates that were reported by at least eight studies (age, % previous surgery, % previous upfront radiation therapy, type of immunotherapy, and mutational status) was used to explore sources of heterogeneity. To assess potential publication bias, funnel plots and Egger’s linear regression tests22 were used for outcomes that had at least 8 studies. Meta-analysis was conducted using the metagen function of the meta package in R.23 Unless specified otherwise, a P value of less than 5% was considered significant.
Results
Study Selection and Baseline Characteristics
Of 1145 publications identified by systematic search, 15 studies met the inclusion criteria (see Supplementary Figure S1 for flow chart). No additional studies were identified by reference check. The selected studies described a total of 1113 BM patients. One study reported insufficient outcome metrics for inclusion into quantitative analysis.24 Thus, 14 studies were ultimately included into the meta-analysis comprising a total of 1102 BM patients.2,4,25–32 For one study,25 additional data were obtained by directly contacting the authors.
Of the 15 included studies, 2 studies were case series,28,30 1 study was a prospective cohort,2 2 studies were both prospective and retrospective cohorts,26,27 and 12 were retrospective cohorts.4,24,25,28–36 Only one study25 reported on the use of corticosteroids and immunotherapy as the primary objective of the study, the rest of the studies reported this as a secondary outcome. Eight studies reported exclusively on BM patients,2,4,28,30–33,35 whereas seven included BM patients as a subgroup within a broader metastatic cancer cohort.24–27,29,34,36 Five out of 15 studies adjusted for potential confounders of which three adjusted for the presence of symptomatic BM (Table 1).26,29,32,33,36
Table 1.
Study Characteristics
| Study | Study Duration | Country | Study Design | Retrospective or Prospective or Both | Study Quality (NOS) | Single vs Multicenter | Total Sample Size (n) | BM Patients (n) | Adjustment for Potential Confounders | Primary Objective of Study |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies included in meta-analysis | ||||||||||
| Margolin et al. (2012) | 2009-2009 | USA | Cohort | Prospective | 6 | Multi | 72 | 72 | No | Investigate the safety and activity of ipilimumab in melanoma BM patients |
| Queirolo et al. (2014) | - | Italy | Cohorta | Retrospective | 6 | Multi | 146 | 146 | No | Evaluate the feasibility of ipilimumab in patients with stage III or IV melanoma and asymptomatic BM |
| Chasset et al. (2015) | 2010-2011 | France | Cohort | Retrospective | 8 | Single | 45 | 23 | Yes, including the presence of symptomatic BMs | Report survival outcomes and predictors of overall survival in patients treated with ipilimumab for stage III or IV melanoma |
| Jones et al. (2015) | 2008-2014 | USA | Case series | Retrospective | 6 | Single | 12 | 12 | No | Investigate the value of craniotomy for patients with melanoma BMs treated with ICI |
| Parakh et al. (2017) | 2012-2016 | Australia | Cohort | Retrospective | 6 | Multi | 66 | 66 | No | Report the clinical efficacy of anti-PD-1 antibodies in melanoma BM patients |
| Arbour et al. (2018) | 2011-2017 | USA. France | Cohort | Retrospective | 5 | Multi | 640 | 154 | No | Evaluate the potential impact of systemic corticosteroids at the start of ICI on the efficacy of PD-(L)1 blockade in advanced NSCLC patients |
| Banks et al. (2019) | 2012-2017 | Australia | Case series | Retrospective | 6 | Single | 12 | 12 | No | Report the use of bevacizumab as a steroid-sparing agent in melanoma BM patients treated with immunotherapy |
| Galli et al. (2019) | 2012-2016 | Italy | Cohorta | Retrospective | 5 | Single | 61 | 36 | No | Investigate the potential interaction between brain radiotherapy and immunotherapy in metastatic melanoma patients |
| Hendriks et al. (2019a) | 2012-2018 | the Netherlands. France | Cohorta | Both | 6 | Multi | 1288 | 14 | No | Evaluate survival of NSCLC patients with leptomeningeal metastases treated with ICI |
| Hendriks et al. (2019b) | 2012-2018 | France | Cohort | Both | 8 | Multi | 1025 | 255 | Yes, including symptomatic BMs at the start of ICI | Evaluate survival and prognostic factors in NSCLC BM patients treated with ICI |
| Kotecha et al. (2019) | 2010-2017 | USA | Cohort | Retrospective | 7 | Single | 150 | 150 | Yes, no adjustment for symptomatic BMs | Investigate the impact of timing of ICI in BM patients undergoing stereotactic radiosurgery |
| Minniti et al. (2019) | 2012-2017 | Italy | Cohorta | Retrospective | 5 | Multi | 80 | 80 | No | Investigate the efficacy and safety of concurrent stereotactic radiosurgery and ICI in patients with untreated melanoma BM |
| Carron et al. (2020) | 2013-2017 | France | Cohort | Retrospective | 8 | Single | 50 | 50 | Yes, including the presence of neurological symptoms | Assess the efficacy and radiotoxicity of the combination of SRS anti-PD1 therapy |
| Zhang et al. (2020) | 2016-2018 | China | Cohort | Retrospective | 7 | Multi | 73 | 32 | Yes, unclear which confounders | Determine whether there is a difference in the efficacy of nivolumab in patients with advanced NSCLC presenting with or without BMs |
| Studies not included in meta-analysis | ||||||||||
| Dumenil et al. (2018) | 2015-2016 | France | Cohort | Retrospective | 6 | Multi | 67 | 11 | No | Determine clinical factors associated with the efficacy and safety of nivolumab in advanced NSCLC patients |
Abbreviations: BM, brain metastasis; ICI, immune checkpoint inhibitor; NOS, Newcastle-Ottawa Scale; NSCLC, non-small cell lung cancer; PD-(L)1, programmed death (ligand) 1.
- Not reported.
aOriginal study is a cohort study designed for a different hypothesis than the one in the present meta-analysis; however, for the primary outcome of this meta-analysis, the study design was treated as a case series because data were extracted from 2 other groups than the original groups (that were not matched on any confounders).
Regarding patients’ and treatments’ characteristics, six studies reported on NSCLC BMs,24–27,33,36 eight on melanoma BMs,2,4,28–31,34,35 and one on BMs with various primaries including NSCLC and melanoma.32 In total, 354 patients received steroids, 328 patients received anti-CTLA-4 therapy, 769 patients received anti-PD1/PD-L1 therapy, and 13 patients received a combination of anti-CTLA-4 and anti-PD-(L)1 therapy. Some studies included patients that had also received local therapy including craniotomy and SRS.2,4,26–28,30,31,34,35 For the type of steroids, 10 studies did not specify the type of steroids2,24,30,33–35 or only described a specific dose of dexamethasone4 or prednisolone equivalent,25,26,36 three studies reported on dexamethasone,28,31,32 and two studies reported on (methyl)prednisolone or prednisone.27,29 Eight studies did not report on the indication for steroid use, 24–27,31–33,36 the rest mostly reported symptomatic BM as indication for steroid use.2,4,28–30,34,35 Regarding the timing of steroid use, seven studies reported on steroid use at the start of ICI treatment,2,4,24,26,27,29,35 two studies reported on steroid use within 30 days of start ICI treatment,25,36 two studies reported on steroid use overlapping ICI treatment,28,32 two studies reported on steroid use at a time around the start of ICI treatment,30,33 and two studies did not specify an exact time (Table 2).33,34
Table 2.
Patient and Treatment Characteristics
| Author | BM Patients (n) | Primary Tumor | Mean KPS | BRAF Mutation (%) | Male Patients (%) | Median Age | Craniotomy (%) | Upfront Radiation Therapy (%) | SRS as Type of Radiation (%) | Steroids (n) | Indication for Steroid Use | Timing of Steroids | Anti- CTLA-4 ICI (n) | Anti-PD-1/PD-L1 ICI (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies included in meta-analysis | ||||||||||||||
| Margolin et al. (2012) | 72 | Melanoma | 93 | - | 61.1 | - | 0 | 43.1 | 12.9 | 21 | Symptomatic BMs | At start ICI | 72 | 0 |
| Queirolo et al. (2014) | 146 | Melanoma | 100 | - | 52.1 | 54 | - | 4 | - | 26 | Symptomatic BMs | At start ICI | 146 | 0 |
| Chasset et al. (2015) | 23 | Melanoma | - | - | - | - | - | - | - | - | Symptomatic BMs, fever, bone metastases | At start ICI | 23 | 0 |
| Jones et al.(2015) | 12 | Melanoma | - | 41.67 | 66.7 | 59.2 | 100 | - | - | 9 | Perioperative use and symptomatic BMs | Time around start ICI | 12 | 0 |
| Parakh et al. (2017) | 66 | Melanoma | 80 | 45 | 68.2 | 62 | 31.8 | 32 | 42.9 | 15 | Symptomatic BMs | At start ICI | 0 | 66 |
| Arbour et al. (2018) | 154 | Lung | - | - | - | - | - | - | - | 41 | - | Within 30 days start ICI | 0 | 154 |
| Banks et al. (2019) | 12 | Melanoma | - | 66.67 | - | 58 | 58.3 | 100 | 33.3 | 8 | Symptomatic BMs | Overlap of steroids and ICI use | 7 | 3 |
| Galli et al. (2019) | 36 | Melanoma | - | 25 | 69.4 | 59 | 5.6 | 100 | - | 27 | Symptomatic BMs | - | 23 | 13 |
| Hendriks et al. (2019a) | 14 | Lung | 73 | 5.3 | 28.6 | 56.1 | - | 78.7 | - | 8 | - | At start ICI | 0 | 14 |
| Hendriks et al. (2019b) | 255 | Lung | - | 1.6 | 62.0 | 61.5 | 14.1 | 67.8 | 57.2 | 69 | - | At start ICI | 13b | 255b |
| Kotecha et al. (2019) | 150 | Mixeda | - | - | 49.3 | 61 | 19 | - | 69 | - | Overlap of steroids and ICI use | 0 | 150 | |
| Minniti et al. (2019) | 80 | Melanoma | - | 35 | 61.2 | - | 11.3 | 100 | 100 | 37 | - | - | 45 | 35 |
| Carron et al. (2020) | 50 | Lung | - | 58 | 60 | 66 | 0 | - | - | 8 | - | Time around start ICI | 0 | 50 |
| Zhang et al. (2020) | 32 | Lung | - | - | 78.1 | 57.7 | - | - | - | 11 | - | Within 30 days start ICI | 0 | 32 |
| Studies not included in meta-analysis | ||||||||||||||
| Dumenil et al. (2018) | 10 | Lung | - | - | - | - | - | 63.6 | 9.1 | 5 | - | At start ICI | 0 | 10 |
Abbreviations: BM, brain metastasis; CTLA4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; KPS, Karnofsky Performance Scale; PD-(L)1, programmed death (ligand) 1; SRS, stereotactic radiosurgery.
- Not reported. Some studies reported no baseline characteristics on the BM subgroup.
a66% lung, 16.7% melanoma, 1.3% breast, 0.7% gastrointestinal tract, 12% kidney, 3.4% other primary tumor site.
bThirteen patients received combination therapy with anti-PD1/PD-L1 and anti-CTLA-4.
Overall Survival
In the steroid group, median OS ranged from 2.9 months to 10.2 months across studies. In the nonsteroid group, median OS ranged from 4.9 to 25.1 months (Table 3).2,4,25–32 One study was not included in the meta-analysis, because no survival data were reported.
Table 3.
Outcome Characteristics
| Author | OS (in Months) | Systemic PFS (in Months) | Intracranial PFS | Criteria of Response | Complete/Partial Response (%) | Stable Disease (%) | Progressive Disease (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroids | No Steroids | Steroids | No Steroids | Steroids | No Steroids | Steroids | No Steroids | Steroids | No Steroids | Steroids | No Steroids | ||
| Studies included in meta-analysis | |||||||||||||
| Margolin et al. (2012) | 3.7 | 7 | 1.3 | 2.7 | 1.2 | 1.9 | irRC | 4.76 | 15.7 | 4.76 | 9.80 | 90.48 | 74.51 |
| Queirolo et al. (2014) | 2.9 | 4.9 | - | - | irRC | 8 | 12 | 8 | 16 | - | - | ||
| Chasset et al. (2015) | 4 | 7 | - | - | - | - | - | - | - | - | - | ||
| Jones et al.(2015) | 7 | 6 | - | - | - | - | - | - | - | - | - | ||
| Parakh et al. (2017) | 4.8 | 13.1 | - | - | 3.2 | 7.4 | - | - | - | - | - | - | - |
| Arbour et al. (2018) | 3.3 | 8.8 | - | - | - | - | - | - | - | - | - | ||
| Banks et al. (2019) | 5 | 5.9 | 1.8 | 1.9 | - | - | - | - | - | - | |||
| Galli et al. (2019) | 4 | 6 | 2.0 | 3.5 | - | - | - | - | - | - | - | ||
| Hendriks et al. (2019a) | 3.25 | 6.15 | 1.8 | 1.9 | - | - | - | - | - | - | - | ||
| Hendriks et al. (2019b) | - | - | - | - | Defined by investigator/local radiologist-assessed | - | - | 20 | 0 | 80 | 100 | ||
| Kotecha et al. (2019)a | 10.2 | 25.1 | - | - | RANO | 35 (≤60 mg) vs 23 (>60 mg) | 48 | 35.29 | - | 23 (≤60 mg) vs 18 (>60 mg) | 12 | ||
| Minniti et al. (2019) | 6 months: 91.2% 12 months: 57.3% |
6 months: 96% 12 months: 76.1% |
6 months: 42.3 12 months: 24.2 |
6 months: 73.6% 12 months: 35.9% |
- | - | - | - | - | - | - | ||
| Carron et al. (2020) | - | - | - | - | - | - | - | - | - | - | - | ||
| Zhang et al. (2020) | - | - | - | - | - | - | - | - | - | - | - | ||
| Studies not included in meta-analysis | |||||||||||||
| Dumenil et al. (2018) | - | - | - | - | RECIST 1.1 | 12.5 | 50 | 37.5 | 50 | 25 | 0 | ||
Abbreviations: irRC, immune-related response criteria; OS, overall survival; PFS, progression-free survival; RANO, Response Assessment in Neuro-Oncology Brain Metastases; RECIST 1.1, Response Evaluation Criteria in Solid Tumors.
- Not reported.
aTreatment response not used in the meta-analysis because of dichotomized outcomes.
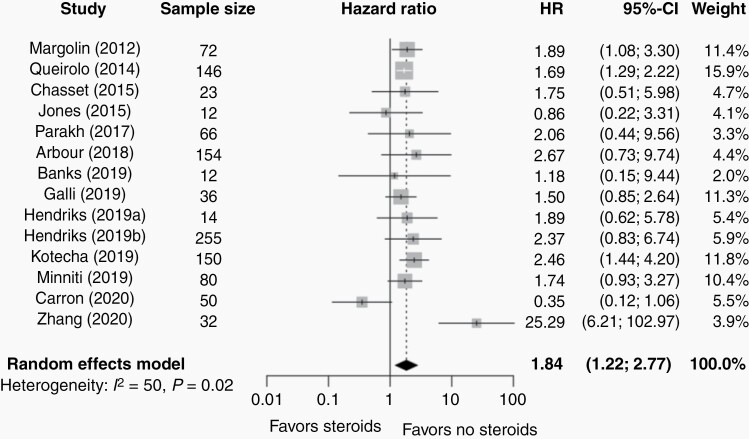
Four studies2,32,35,36 reported a significantly worse survival in the steroid group vs the no steroid group; whereas the remaining 104,25–31,33 reported no significant difference. Pooling the results, however, demonstrated a statistically significant mortality disadvantage of the use of steroids vs no steroids (HR = 1.84, 95% CI 1.22-2.77, P = .007; Figure 1). According to the Higgin’s and Thompson’s I2 value (I2 = 49.9%) and Cochrane Q test (P = .02), a significant heterogeneity was observed. Stratified analysis by primary tumor type indicated similar effect sizes for melanoma BM patients (HR = 1.67, 95% CI 1.49-1.87; Supplementary Figure S2) and BM patients with mixed primary tumors (HR = 2.46, 95% CI 1.44-4.20). For NSCLC BM patients, no significant association was seen between the use of steroids and OS (HR = 2.43, 95% CI 0.38-15.77; Supplementary Figure S2). Stratified analysis by receipt of concurrent ICI and SRS indicated similar effect sizes for BM patients receiving only ICI (HR = 1.97, 05% CI 1.28-3.05; Supplementary Figure S3); no significant association was seen between the use of steroids and OS in BM patients receiving concurrent ICI and SRS (HR = 1.30, 95% CI 0.11-14.77; Supplementary Figure S3). Additionally, leave-one-out analysis showed that the results were not driven by any single study (Supplementary Figure S4A). According to Egger’s test,22 no significant publication bias was identified for OS (P = .75), however, the funnel plot (Supplementary Figure S5) suggested the possibility of publication bias, knowing that the asymmetry could be due to reasons other than publication bias.
Fig. 1.
Forest plot of hazard ratios (HR) of overall survival (OS) comparing the steroid and nonsteroid group of patients with brain metastases.
The gray squares represent the point estimate of each study; the size of the squares is proportional to the weight of the study; horizontal lines show the 95% confidence intervals (CIs); the black diamond represents the pooled estimate for OS. The pooled HR for OS of all studies included in meta-analysis was 1.84 (95% CI: 1.22-2.77; I2 = 50%, P-heterogeneity > .01).
Sensitivity analysis only including the studies that adjusted for confounders26,29,32,33,36 showed no statistically significant difference for OS between the steroid and no steroid group. However, a trend was seen in disadvantage of the use of steroids (HR = 2.27, 95% CI 0.35-14.57; I2 = 89.5%; P-heterogeneity < .01).
Meta-regression did not show a significant effect modification by age, previous surgery, radiotherapy, or type of ICI therapy (all P values > .05; Supplementary Table S1). No meta-regression was performed for molecular alterations, ie, BRAF mutation, ALK rearrangement, or EGFR mutation, due to insufficient studies.
Systemic and Intracranial PFS
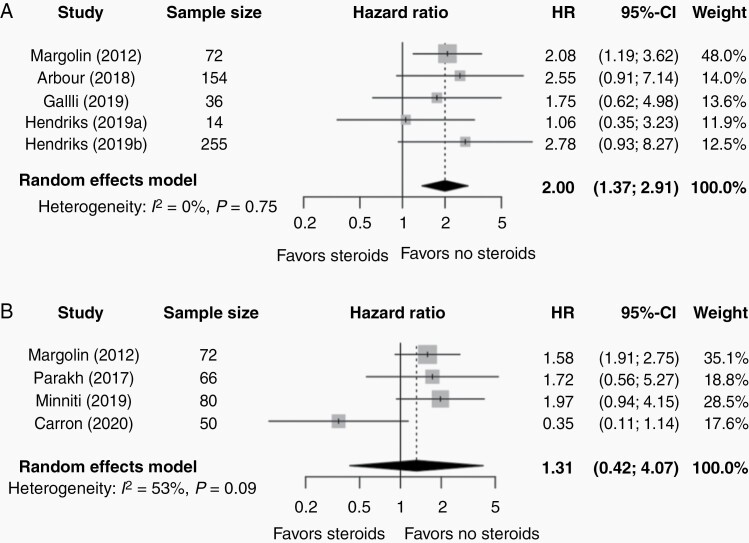
Eight studies reported on systemic and/or IC-PFS.2,4,25–27,31,33,34 In the steroid group, median systemic PFS ranged from 1.3 months to 2.0 months across studies and IC-PFS ranged from 1.2 to 3.2 months. In the nonsteroid group, median systemic PFS ranged from 1.9 months to 3.5 months and IC-PFS from 1.9 to 7.4 months. Pooling results of the five studies reporting on systemic PFS showed a statistically significant association between worse systemic PFS and the use of steroids in comparison with the nonsteroid group (HR = 2.00, 95% CI 1.37-2.91, P = .007; Figure 2A); no significant heterogeneity was observed according to the Higgin’s and Thompson I2 value (I2 = 0%) and Cochrane Q test (P = .75). There was no significant association between the use of steroids and IC-PFS (HR = 1.31, 95% CI 0.42-4.07, P = .50; Figure 2B). However, high heterogeneity was observed (I2 = 53%, P-heterogeneity = .09); leave-one-out revealed that results were driven by a single study (n = 50),33 exclusion of which would result in a significant association between steroid use and worse IC-PFS (Supplementary Figure S4C). Publication bias assessment was not feasible due to the paucity of the studies (<8) for each outcome.
Fig. 2.
Forest plot of hazard ratios (HR) of (A) systemic progression-free survival (PFS) and (B) intracranial progression-free survival (IC-PFS) comparing the steroid and nonsteroid group of patients with brain metastases. The gray squares represent the point estimate of each study; the size of the squares is proportional to the weight of the study; horizontal lines show the 95% confidence intervals (CIs); the black diamond represents the pooled estimate for each subgroup. (A) The pooled HR for PFS of all studies included in this meta-analysis is 2.00 (95% CI 1.37-2.91; I2 = 0%, P-heterogeneity = .75). (B) The pooled HR for IC-PFS of all studies included in this meta-analysis is 1.31 (95% CI 0.42-4.07; I2 = 53%, P-heterogeneity = .09). A P value for heterogeneity < 10% was considered significant.
Local Treatment Response
Five studies reported on treatment response of which two studies used the irRC, one study the RECIST 1.1, one study the RANO criteria, and in one study treatment response was defined by the investigator/local radiologist-assessed (Table 3).2,24,28,32,35
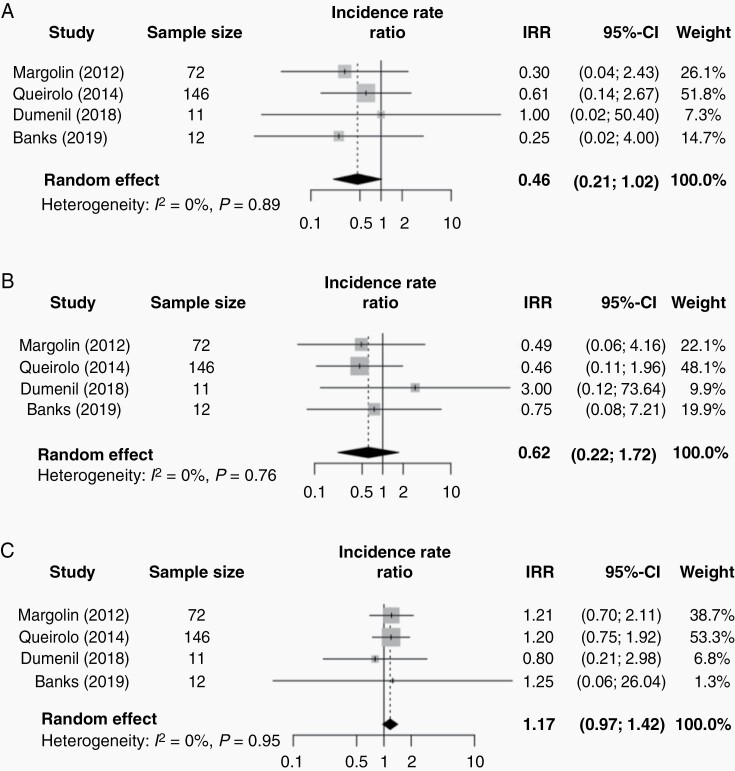
Complete and partial response was seen in 6.7% of patients receiving steroids vs 13.6% of patients not receiving steroids. A stable treatment response was seen in 11.7% of patients receiving steroids and 14.7% of patients not receiving steroids. Lastly, progressive disease was seen in 78.3% of the patients receiving steroids and 71.8% of the patients not receiving steroids. Pooling results of four studies showed no statistically significant association between the use of corticosteroids on complete response/partial response (IRR 0.46, 95% CI 0.21-1.02, P = .05, Figure 3A), stable disease (IRR 0.62, 95% CI 0.22-1.72, P = .23, Figure 3B) or progressive disease (IRR 1.17, 95% CI 0.97-1.42, P = .08, Figure 3C) (P-interaction comparing treatment response groups < .01).2,24,28,35 One study could not be included in meta-analysis due to insufficient data.32 No meta-regression or subgroup analysis by treatment response criteria was possible due to insufficient data.
Fig. 3.
Forestplot of the incidence rate ratio (IRR) of treatment response comparing the steroid and nonsteroid group of patients with brain metastases. The gray squares represent the point estimate of each study; the size of the squares is proportional to the weight of the study; horizontal lines show the 95% confidence intervals (CIs); the black diamond represents the pooled estimate for each subgroup. (A) The pooled IRR for complete/partial treatment response is 0.46 (95% CI 0.21-1.02; I2 = 0%, P-heterogeneity = .89). (B) The pooled IRR for stable disease is 0.62 (95% CI 0.22-1.72; I2 = 0%, P-heterogeneity = .76). (C) The pooled IRR for progressive disease is 1.17 (95% 0.97-1.42; I2 = 0%, P-heterogeneity = .95). A P value for heterogeneity <10% was considered significant.
Immunotherapy-Related Adverse Events
Only one study reported on the effect of corticosteroid use in patients receiving immunotherapy and the occurrence of IRAEs (Table 3).2 In this study, patients with vs without steroids experienced rash (28.6% vs 33.3%), pruritus (23.8% vs 31.4%), diarrhea (38.1% vs 43.1%), and elevated aspartate transaminase (19.0% vs 5.9%) (P value not reported).
Discussion
The present study aimed to investigate available literature on the association between the use of corticosteroids and OS in BM patients receiving ICI. While no study has previously addressed this issue as a primary research question, pooling of secondary and subset analyses allowed for quantitative meta-analysis, indicating that use of steroids was associated with a significantly worse OS and systemic PFS in BM patients receiving ICI. No significant association was seen between steroid use and IC-PFS.
A recent meta-analysis reported on the association between corticosteroid use and survival in metastatic cancer patients, predominantly melanoma and NSCLC, treated with ICI.37 The authors showed that patients using steroids had a significantly worse OS (HR = 1.54, 95% CI: 1.24-1.91) and PFS (HR = 1.34, 95% CI: 1.02-1.76). BMs as a reason for receiving corticosteroids were associated with worse OS (HR = 1.51, 95% CI 1.22-1.87). Because BMs, in general, are associated with worse survival,3,38 this begs the question whether this effect is due to the administration of steroids or merely due to the presence of BMs. A logical follow-up question, therefore, is whether steroids are still associated with worse survival within the BM population. The results of our meta-analysis answered this question in the affirmative.
Still, the question remains whether this was due to a causal effect of steroids. It could be argued that patients receiving steroids were more likely to have large, symptomatic BM and were therefore susceptible to a worse prognosis. A recent study by Ricciuti et al.39 showed that NSCLC patients receiving corticosteroids had worse outcomes than patients who received no or low-dose corticosteroids, but this difference seemed to be driven by a poor-prognosis subgroup of patients who received corticosteroids for palliative indications including symptomatic BM. In contrast, the study by Queirolo et al. included in this meta-analysis35 only reported on asymptomatic BM patients but did show a significant association between corticosteroid use and poorer OS. Moreover, three studies adjusted for the presence of symptomatic BM as a potential confounding variable. When considering other types of brain tumors, a glioblastoma study demonstrated that dexamethasone administration was an independent indicator of poor outcome in human patients. The investigators subsequently demonstrated that in mice treated with radiotherapy for glioblastoma, randomization to pretreatment with dexamethasone led to a decreased survival time, supporting a causal mechanism.40 However, ICIs were not administered in this experiment. Prospectively studying the causality of steroids in BM patients receiving ICI on survival would be difficult due to the essential role of steroids in symptom management and the paucity of alternatives, highlighting the need for studies such as the meta-analysis we performed and the studies included in this meta-analysis. A possible study design could involve bevacizumab, a vascular endothelial growth factor inhibitor which has shown benefit in patients with steroid-refractory edema/radionecrosis after irradiation of BMs and other brain tumors.41 Randomizing symptomatic BM patients on ICI between dexamethasone or bevacizumab could shed light on any causal detrimental effect of steroids on survival. However, this would first require more study on the safety of combining bevacizumab with ICI.
The results of the present study did not allow us to make statements about the cause for the association between steroids and survival. Steroid pharmacodynamics and preclinical studies may give some insight into this question. Steroids exert anti-inflammatory effects by influencing transcription of pro- and anti-inflammatory genes and inhibiting secretion of inflammatory cytokines.42,43 Exogenous corticosteroids have shown to be toxic to immature T cells and suppress interleukin-2-mediated T-cell proliferation, however, it is unclear if corticosteroids prevent T-cell differentiation or deplete already differentiated tumor-reactive lymphocytes. A study by Maxwell et al. demonstrated that mice bearing peripheral tumors had a diminished efficacy of anti-PD-1 therapy while receiving corticosteroids.11 However, the anti-PD-1-mediated antitumor immune responses remained intact after steroid administration in a murine glioma model. The authors suggested that anti-PD-1 responses might be influenced differentially depending on the location of the tumor within or outside the central nervous system. Potentially, the central nervous system plays a protective role against the immunosuppressive effect of corticosteroids. In patients with BMs, steroids might have different effects on intra- and extracranial cancer burden. According to another murine glioma study by Giles et al., naïve T cells were especially sensitive to dexamethasone-mediated suppression, as opposed to memory T cells.44 This suggests that negative corticosteroid effects may be diminished after developing a successful antitumor immune response. This could be an explanation for the observation that corticosteroids used in the treatment of IRAEs did not seem to influence the efficacy of ICI; for instance, ipilimumab was demonstrated to have persisting antitumor effects in melanoma patients receiving steroids for IRAEs.45–47 This meta-analysis did not allow us to make a statement about the timing of steroids and ICI therapy.
An interesting secondary finding of this study is that the use of steroids was not associated with IC-PFS, although the leave-one-out for IC-PFS revealed that these results were driven by only one study that adjusted for confounders and even found an opposite trend toward a benefit of steroid use for IC-PFS.33 A possible explanation of this outlier is that all patients received ICI in combination with SRS, which has been shown to have survival benefits and improved intracranial control compared with ICI alone.7–9 However, other studies in our meta-analysis that included patients receiving ICI in combination with SRS showed a trend toward a disadvantage of steroid use.2,4,26,31
Regarding the assessment of treatment response following immunotherapy, an important challenge merits discussion. Differentiating treatment response from tumor progression on anatomical contrast-enhanced magnetic resonance imaging (MRI) following ICI can be difficult in comparison with other treatment modalities like chemotherapy or targeted therapy.48 Unique radiological response patterns such as pseudoprogression are not adequately captured by traditional response criteria.49 Response criteria describing all patterns of antitumor activity associated with immunotherapy are lacking, however immune-related response criteria including the irRC, irRECIST, iRECIST, and immunotherapy RANO (iRANO) take into account pseudoprogression.48,50 Advanced MRI and positron emission tomography (PET) techniques can potentially assess the molecular, cellular, and structural components of the tumor and its microenvironment, resulting in valuable information for the differentiation of treatment response after immunotherapy and targeted therapy, either alone or in combination with radiotherapy.48 A curious secondary finding of this meta-analysis is that although not statistically significant (possibly due to lack of power), patients on steroids actually trended toward a higher complete/partial response rate than the patients that did not receive steroids. This counterintuitive effect might be explained by the observation that the anti-inflammatory effects of steroids might suppress the aforementioned response patterns. Unlike RECIST 1.1 and the irRC, RANO includes the use of steroids as a criterion, however, only one of the included studies reported on treatment response using the RANO criteria.32
The findings of this meta-analysis suggest that where possible, the administration of steroids in BM should be avoided. The immunosuppressive effects of corticosteroids are considered to be dose-dependent.44,51 Unfortunately, insufficient data were available to analyze a dose-response relationship between steroids and OS. There is relatively little evidence available regarding the optimal dosing scheme of corticosteroids in brain tumor patients.52 In BM patients, lower doses might be non-inferior to higher doses.53
This meta-analysis has some limitations. All included studies were observational studies, with only three studies adjusting for symptomatic BM as a possible confounder, which might have resulted in confounding by indication and residual confounding in the original studies included in this meta-analysis. Important covariates including the presence of symptomatic BM and KPS could not be included in meta-regression due to missing data. Because of this, independent and—ultimately—causal associations should not be concluded from our investigation and further research is needed in this area. Several studies reported BM as a subgroup of the entire study population; in some cases, reporting of BM-specific baseline characteristics was limited or absent, precluding meta-regression on these variables. Lastly, a significant statistical heterogeneity between studies was identified.
On the other hand, a major strength of our study is that, to our knowledge, it was the first in the literature that specifically assessed the question of ICI and steroids in BMs as a primary research question. Only 1 out of 13 included studies25 reported on the use of steroids and ICI as the primary objective of the study, including BM patients as a subgroup. The rest of the studies reported the impact of steroids as a secondary outcome. Moreover, a relatively large sample size strengthened our study.
There is a need for future investigation into the use of concurrent ICI therapy and corticosteroids in BM patients. Observational studies should aim to study these interactions taking into account confounding variables including the presence of symptomatic BM, and focus on the effect of dose and duration of steroid administration, as well as the optimal timing of ICI, steroids, and local treatments such as neurosurgery and radiosurgery in BMs. Finally, more preclinical research conducted specifically in BM models could help further elucidate the question of causality.
Conclusion
In this meta-analysis, the use of steroids was associated with significantly shorter OS and worse systemic PFS in BM patients receiving ICI. Further investigations on dose, timing, and duration of steroids are needed to elucidate this increasingly relevant question.
Supplementary Material
Acknowledgments
The authors thank J.W. Schoones for his contribution to the systematic search in the different databases and Dr K. Arbour, Dr G. Heller, and Dr M. Hellman for contributing additional data from their original studies.
Conflict of interest statement. The authors declare that they have no conflict of interest.
Authorship statement. All authors were involved in the design of the study, organization of the study, manuscript writing, and approval of final version of the manuscript. C.A.C.J. generated the search strategy and performed the search. C.A.C.J. and A.E.W. independently performed the article selection procedure, data extraction, and summarized the data. C.A.C.J. performed the data analyses, and R.A.M. checked the data analysis and validity. C.A.C.J. and A.F.C.H. wrote the manuscript.
Unpublished material. Not applicable.
Funding
This study received no external funding.
References
- 1. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Margolin K, Ernstoff MS, Hamid O, et al. . Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 3. Lorger M, Andreou T, Fife C, James F. Immune checkpoint blockade - how does it work in brain metastases? Front Mol Neurosci. 2019;12:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parakh S, Park JJ, Mendis S, et al. . Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br J Cancer. 2017;116(12):1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ajithkumar T, Parkinson C, Fife K, Corrie P, Jefferies S. Evolving treatment options for melanoma brain metastases. Lancet Oncol. 2015;16(13):e486–e497. [DOI] [PubMed] [Google Scholar]
- 6. Goldberg SB, Gettinger SN, Mahajan A, et al. . Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy B, Walker J, Bassale S, et al. . Concurrent radiosurgery and immune checkpoint inhibition: improving regional intracranial control for patients with metastatic melanoma. Am J Clin Oncol. 2019;42(3):253–257. [DOI] [PubMed] [Google Scholar]
- 8. Shepard MJ, Xu Z, Donahue J, et al. . Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: a matched cohort study. J Neurosurg. 2019;13(3):1–8. [DOI] [PubMed] [Google Scholar]
- 9. Chen L, Douglass J, Kleinberg L, et al. . Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell Carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(4):916–925. [DOI] [PubMed] [Google Scholar]
- 10. Diao K, Bian SX, Routman DM, et al. . Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neurooncol. 2018;139(2):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maxwell R, Luksik AS, Garzon-Muvdi T, et al. . Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. 2018;7(12):e1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connell CM, Raby S, Beh I, et al. . Cancer immunotherapy trial registrations increase exponentially but chronic immunosuppressive glucocorticoid therapy may compromise outcomes. Ann Oncol. 2017;28(7):1678–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet. 1961;81:46–53. [PubMed] [Google Scholar]
- 14. Palombi L, Marchetti P, Salvati M, Osti MF, Frati L, Frati A. Interventions to reduce neurological symptoms in patients with GBM receiving radiotherapy: from theory to clinical practice. Anticancer Res. 2018;38(4):2423–2427. [DOI] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 16. Wolchok JD, Hoos A, O’Day S, et al. . Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. [DOI] [PubMed] [Google Scholar]
- 17. Wells G, Shea B, O’Connell D, et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 19. Hackshaw A. A Concise Guide to Clinical Trials. 1st ed. Chicester: John Wiley and Sons Ltd.; 2009. [Google Scholar]
- 20. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. 2011;343:d2090. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 24. Dumenil C, Massiani MA, Dumoulin J, et al. . Clinical factors associated with early progression and grade 3-4 toxicity in patients with advanced non-small-cell lung cancers treated with nivolumab. PLoS One. 2018;13(4):e0195945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arbour KC, Mezquita L, Long N, et al. . Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. [DOI] [PubMed] [Google Scholar]
- 26. Hendriks LEL, Henon C, Auclin E, et al. . Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol. 2019;14(7):1244–1254. [DOI] [PubMed] [Google Scholar]
- 27. Hendriks LEL, Bootsma G, Mourlanette J, et al. . Survival of patients with non-small cell lung cancer having leptomeningeal metastases treated with immune checkpoint inhibitors. Eur J Cancer. 2019;116:182–189. [DOI] [PubMed] [Google Scholar]
- 28. Banks PD, Lasocki A, Lau PKH, Sandhu S, McArthur G, Shackleton M. Bevacizumab as a steroid-sparing agent during immunotherapy for melanoma brain metastases: a case series. Health Sci Rep. 2019;2(3):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chasset F, Pages C, Biard L, et al. . Single-center study under a French Temporary Authorization for Use (TAU) protocol for ipilimumab in metastatic melanoma: negative impact of baseline corticosteroids. Eur J Dermatol. 2015;25(1):36–44. [DOI] [PubMed] [Google Scholar]
- 30. Jones PS, Cahill DP, Brastianos PK, Flaherty KT, Curry WT. Ipilimumab and craniotomy in patients with melanoma and brain metastases: a case series. Neurosurg Focus. 2015;38(3):E5. [DOI] [PubMed] [Google Scholar]
- 31. Minniti G, Anzellini D, Reverberi C, et al. . Stereotactic radiosurgery combined with nivolumab or ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer. 2019;7(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kotecha R, Kim JM, Miller JA, et al. . The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol. 2019;21(8):1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carron R, Gaudy-Marqueste C, Amatore F, et al. . Stereotactic radiosurgery combined with anti-PD1 for the management of melanoma brain metastases: a retrospective study of safety and efficacy. Eur J Cancer. 2020;135:52–61. [DOI] [PubMed] [Google Scholar]
- 34. Galli G, Cavalieri S, Di Guardo L, et al. . Combination of immunotherapy and brain radiotherapy in metastatic melanoma: a retrospective analysis. Oncol Res Treat. 2019;42(4):186–194. [DOI] [PubMed] [Google Scholar]
- 35. Queirolo P, Spagnolo F, Ascierto PA, et al. . Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol. 2014;118(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang G, Cheng R, Wang H, et al. . Comparable outcomes of nivolumab in patients with advanced NSCLC presenting with or without brain metastases: a retrospective cohort study. Cancer Immunol Immunother. 2020;69(3):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petrelli F, Signorelli D, Ghidini M, et al. . Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers (Basel). 2020;12(3):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Di Giacomo AM, Valente M, Cerase A, et al. . Immunotherapy of brain metastases: breaking a “dogma”. J Exp Clin Cancer Res. 2019;38(1):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019;37(22):1927–1934. [DOI] [PubMed] [Google Scholar]
- 40. Pitter KL, Tamagno I, Alikhanyan K, et al. . Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levin VA, Bidaut L, Hou P, et al. . Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. [DOI] [PubMed] [Google Scholar]
- 43. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giles AJ, Hutchinson MND, Sonnemann HM, et al. . Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Downey SG, Klapper JA, Smith FO, et al. . Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harmankaya K, Erasim C, Koelblinger C, et al. . Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol. 2011;28(4):1140–1144. [DOI] [PubMed] [Google Scholar]
- 47. Horvat TZ, Adel NG, Dang TO, et al. . Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galldiks N, Kocher M, Ceccon G, et al. . Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro Oncol. 2020;22(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beer L, Hochmair M, Prosch H. Pitfalls in the radiological response assessment of immunotherapy. Memo. 2018;11(2):138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jia W, Gao Q, Han A, Zhu H, Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. 2019;16(4):655–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Min L, Hodi FS, Kaiser UB. Corticosteroids and immune checkpoint blockade. Aging (Albany NY). 2015;7(8):521–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jessurun CAC, Hulsbergen AFC, Cho LD, Aglio LS, Nandoe Tewarie RDS, Broekman MLD. Evidence-based dexamethasone dosing in malignant brain tumors: what do we really know? J Neurooncol. 2019;144(2):249–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vecht CJ, Hovestadt A, Verbiest HB, van Vliet JJ, van Putten WL. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994;44(4):675–680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.