Abstract
Background
There is a critical need for objective and reliable biomarkers of outcome in meningiomas beyond WHO classification. Loss of H3K27me3 has been reported as a prognostically unfavorable alteration in meningiomas. We sought to independently evaluate the reproducibility and prognostic value of H3K27me3 loss by immunohistochemistry (IHC) in a multicenter study.
Methods
IHC staining for H3K27me3 and analyses of whole slides from 181 meningiomas across three centers was performed. Staining was analyzed by dichotomization into loss and retained immunoreactivity, and using a 3-tiered scoring system in 151 cases with clear staining. Associations of grouping with outcome were performed using Kaplan-Meier survival estimates.
Results
A total of 21 of 151 tumors (13.9%) demonstrated complete loss of H3K27me3 staining in tumor with retained endothelial staining. Overall, loss of H3K27me3 portended a worse outcome with shorter times to recurrence in our cohort, particularly for WHO grade 2 tumors which were enriched in our study. There were no differences in recurrence-free survival (RFS) for WHO grade 3 patients with retained vs loss of H3K27me3. Scoring by a 3-tiered system did not add further insights into the prognostic value of this H3K27me3 loss. Overall, loss of H3K27me3 was not independently associated with RFS after controlling for WHO grade, extent of resection, sex, age, and recurrence status of tumor on multivariable Cox regression analysis.
Conclusions
Loss of H3K27me3 identifies a subset of WHO grade 2 and possibly WHO grade 1 meningiomas with increased recurrence risk. Pooled analyses of a larger cohort of samples with standardized reporting of clinical definitions and staining patterns are warranted.
Keywords: H3K27, immunohistochemistry, meningioma, trimethylation
Key Point.
Loss of H3K27me3 identifies a subset of meningiomas at higher risk of recurrence.
Importance of the Study.
Previous studies have identified loss of H3K27me3 identified by immunohistochemistry as an independent prognostic factor in meningiomas across all WHO grades, however, its use has not been widely adopted due to lack of validation at other centers.1,2Our study represents the first independent validation of the use of H3K27me3 in meningiomas, encompassing samples from three international sites. We found that similar to the previous study from Katz et al., complete loss of H3K27me3 was a rare event in meningiomas, but when it was seen, it portended poorer recurrence-free survival in WHO grade 2 and 1 meningiomas, but not in WHO grade 3 tumors.2 Our experience and subsequent results with this IHC stain also demonstrate a need for standardization in the interpretation, analysis, and application of it for select groups of meningiomas, as well as the need for future mechanistic and molecular correlative studies.
Meningiomas arise from the arachnoid cap cells of the leptomeninges and are the most common primary brain tumor in adults.3–5 Currently, the mainstay of treatment is maximal surgical resection, with decisions of adjuvant radiotherapy guided by the histopathological World Health Organization (WHO) grade and extent of resection at surgery. The 2016 WHO classification relies entirely on histopathology and divides meningiomas into three grades (grades 1-3) with escalating rates of tumor recurrence after surgical resection as grade increases.6,7 The histological parameters assessed by WHO grading (number of mitosis and other features of cellular atypia) are based on subjective interpretation and thereby challenged by interrater variabilities. Therefore, for some patients, the histological grade does not accurately reflect the behavior of the tumor,8–10 with a subset of clinically aggressive meningiomas that recur rapidly independent of their histopathological grade and extent of resection at surgery.11–13 These imprecisions in reflecting tumor behavior highlight the critical need for reliable biomarkers to predict tumor behavior.
We and others have previously shown that epigenetic modifications in the form of DNA methylation alterations are reflective of tumor behavior for individual patients with meningiomas.8,12,14–16 Histone modification, primarily by acetylation and methylation, is an additional epigenetic mechanism of transcriptional regulation that has been implicated in carcinogenesis.17,18 In particular, trimethylation of lysine 27 (K27) of histone H3 has been found to play a significant role in tumorigenesis by affecting repair of DNA damage, particularly repair of double-stranded DNA breaks by homologous recombination repair.19–21 Trimethylation of H3K27 and subsequent silencing of genes in the region is mediated by the EZH2 subunit of the Polycomb repressive complex 2 (PRC2) which also contributes to chromatin compaction and is involved in several biological processes including cell differentiation, proliferation, and stem-cell plasticity.22 Dysregulation of H3K27 methylation has been implicated in different cancers including breast, prostate, colon, ovarian, malignant peripheral nerve sheath tumors, ependymomas, and gliomas.1,2,19,20,23–29
It has been previously demonstrated that complete loss of H3K27me3 by immunohistochemical (IHC) staining is associated with increased risk of radiological recurrence for WHO grade 1 and 2 meningiomas.1,2 The clinical utility of biomarkers is highly dependent on their robustness across multiple cohorts. Therefore, in this study, we sought to evaluate the reproducibility of the prognostic value of H3K27me3 loss in combination with histopathological features using a multicenter cohort of meningiomas enriched for WHO grade 2 and 3 tumors.
Materials and Methods
Patient Samples and Clinical Data
A total of 181 tissue samples from meningiomas were resected from the University of Toronto (Toronto, Canada), Foundation IRCCS Carlo Besta Neurological Institute (Milan, Italy), and Vancouver General Hospital (Vancouver, Canada) between December 2000 and November 2017 were initially included our analysis. Clinical data were collected for each sample using pre-established definitions that were established a priori by the International Consortium on Meningiomas. Hematoxylin and eosin (H&E) slides were reviewed from each case to confirm the diagnosis of meningioma, grade tumors according to the current 2016 WHO criteria by local experienced neuropathologists (K.A., B.P., S.Y.). Tumor recurrence and time to recurrence were the primary outcomes of interest in this study. Recurrence was defined as tumor growth following gross total resection or tumor progression following subtotal resection and the time to recurrence was determined by calculating the duration from the date of surgery to first postoperative imaging documenting tumor recurrence. The extent of resection was extracted from the surgeon’s operative report and checked with postoperative magnetic resonance imaging (MRI). For 13 patients, we were unable to confidently determine the extent of resection as immediate postoperative MRIs were unavailable to verify the surgeon’s operative report.
Immunohistochemistry
Briefly, 5-micron formalin-fixed, paraffin sections were rehydrated and heat-mediated antigen retrieval using sodium citrate buffer (pH 6) was performed. Slides were washed in 3% H2O2 in methanol and blocked in 5% bovine serum albumin in phosphate buffered saline for 1 h at room temperature followed by overnight incubation at 4°C with anti-H3K27me3 (Cell Signaling Technology, C36B11, rabbit monoclonal antibody, 1:200). The expression signal was developed using DAB Peroxidase Kit, and the slides were counterstained with H&E, dehydrated, and cover-slipped.
Immunohistochemical Analyses
Staining was assessed for nuclear expression on tumor cells by two independent clinical neuropathologists blinded to each other’s assessment (S.K., A.G.) using a 3-tiered scoring system with H3K27me3 staining considered lost (score of “0”) when there was complete lack of immunoreactivity in tumor cells with positive endothelial staining in intratumoral vessels as an internal positive control. When tumor cells demonstrated robust nuclear staining, these cases were assigned a score of “2” to denote trimethylation was retained. When tumor cells demonstrated an ambiguous pattern of staining, or when less than 50% of all the tumor cells on the slide had nuclear H3K27 staining, we considered trimethylation to be retained, but assigned this a score of “1.” Analysis was performed in a dichotomous manner dividing samples into either complete loss of trimethylation or retained trimethylation, as done previously by Katz et al.,23 as well as in a 3-tiered manner with scores of “0,” “1,” and “2.” Disagreements and discrepancies were reviewed by two independent clinicians (F.N., J.Z.W) blinded to the initial scoring, who then separately scored each slide in the same above manner. Any further disagreements were resolved with discussion and if unable to, a third independent neuropathologist reviewed the slides and made the final decision (H.N). Of the total 181 meningiomas, 30 cases (all from Milan) were excluded due to technically poor tissue staining in immunohistochemistry (IHC) in the form of no observable staining of normal brain (when present) or endothelial cells or very weak staining of tumor cells unable to be confidently differentiated from unstained cells. We repeated IHC staining using different slides of these same cases and observed similar results, leading us to suspect that poor staining was due to issues with tissue quality. The majority of these cases with unusable staining were slides that were over 10 years old. Therefore, a total of 151 meningiomas were used for analysis in this study.
Statistics
Categorical factors between H3K27me3 groups were compared using Fisher’s exact test. Student’s t-test was used to compare quantitative parameters between groups. Survival distributions of groups were compared using Kaplan-Meier estimates and log-rank tests. Cox proportional hazards regression was used to perform multivariable analyses of clinical parameters with outcome. A P value <.05 was considered to be statistically significant. All analyses were performed using R (v3.43).
Ethics
This study was approved by the Research Ethics Board of the University of Toronto (University Health Network, Research Ethics Board, 10th Floor, Room 1056, 700 University Ave. Toronto, Ontario M5G 1Z5 (416) 581-7849; REB #18-5820), the University of British Columbia (UBC Clinical Research Ethics Office, Room 210, Research Pavilion, 828 West 10th Avenue, Vancouver, BC, Canada, V5Z 1L8), and the Ethics Committees of the Fondazione IRCCS Istituto Neurologico Carlo Besta (Via Celoria 11, Milano, 20133, Italy; +39 (0)2 2394.2321; comitatoetico@instituto-besta.it). All patient data and specimens were deidentified prior to collection and analysis, therefore, individual patient consent was not required or obtained.
Results
Of the 151 meningioma cases, 21 (13.9%) demonstrated complete loss of H3K27me3 staining in tumor cell nuclei. A total of 11 (7.3%) demonstrated ambiguous staining whereby at least one pathologist scored a “1” denoting less than half of all tumor cells had nuclear H3K27 staining or the slide contained areas of tumor tissue with low-intensity nuclear staining with clear nuclear positivity in adjacent endothelial cells. These were considered to have retained H3K27me3. The remaining 119 meningiomas all demonstrated clearly retained trimethylation. Both independent pathologists (A.G., S.K.) agreed on all cases of complete H3K27me3 loss (Supplementary Table 1). Cohen’s κ, as a measure of interrater reliability, was 0.88, denoting strong agreement between the reviewers on their 3-tiered scoring.
Table 1 shows the baseline clinical information for all included patients. Of 151 meningioma patients with evaluable staining, 91 (60.3%) were female. The median age of all patients was 54 years (IQR 44-66). Our cohort was enriched for WHO grade 2 meningiomas (49.0%), as these tumors show the greatest variability with respect to local recurrence outcomes thereby making biomarker analyses perhaps the most clinically useful in this group. The majority of patients included in our cohort had gross total resection at surgery (67.5%). There was a significantly higher proportion of patients with WHO grade 3 tumors with complete loss of H3K27me3 (32.0%) compared to those with retained H3K27me3 (15.4%, P = .011). Recurrent tumors were more likely to show loss of trimethylation (19.4% vs 8.8%), although this did not reach statistical significance (P = .100). Rates of gross total resection and distribution of sex were similar in patients with loss vs retained H3K27me3 IHC (P = .990 and P = .330, respectively).
Table 1.
Baseline Clinical Characteristics of Included Patients
| Baseline Parameter | Total | Retained H3K27me3 | Loss of H3K27me3 | P a |
|---|---|---|---|---|
| N (%) | 151 | 130 (86.1%) | 21 (13.9%) | |
| Age (median) | 55 (IQR 44-66) | |||
| Sex | ||||
| Male | 60 (39.7%) | 49 (37.7%) | 11 (52.3%) | .300 |
| Female | 91 (60.3%) | 81 (62.3%) | 10 (47.7%) | |
| WHO grade | ||||
| 1 | 48 (31.7%) | 45 (34.6%) | 2 (24.0%) | .011a |
| 2 | 74 (49.0%) | 65 (50.0%) | 9 (44.0%) | |
| 3 | 29 (19.3%) | 20 (15.4%) | 8 (32.0%) | |
| Extent of resection | ||||
| GTR | 102 (67.5%) | 87 (72.5%) | 20 (80.0%) | .990 |
| STR | 36 (23.8%) | 30 (27.5%) | 5 (20.0%) | |
| Unknown | 13 (8.6%) | 13 (10%) | 0 (0%) | |
| Tumor | ||||
| Primary | 79 (52.3%) | 72 (55.3%) | 7 (33.3%) | .100 |
| Recurrent | 72 (47.6%) | 58 (44.7%) | 14 (66.7%) | |
| Median RFS (y) | 3.0 (95% CI 2.69-3.94) | 1.53 (95% CI 1.10-2.90) |
Abbreviations: CI, confidence interval; GTR, gross total resection; IQR, interquartile range; RFS, recurrence-free survival; STR, subtotal resection; WHO, World Health Organization.
aChi-square test of proportions with Yates correction retained vs loss of H3k27me3.
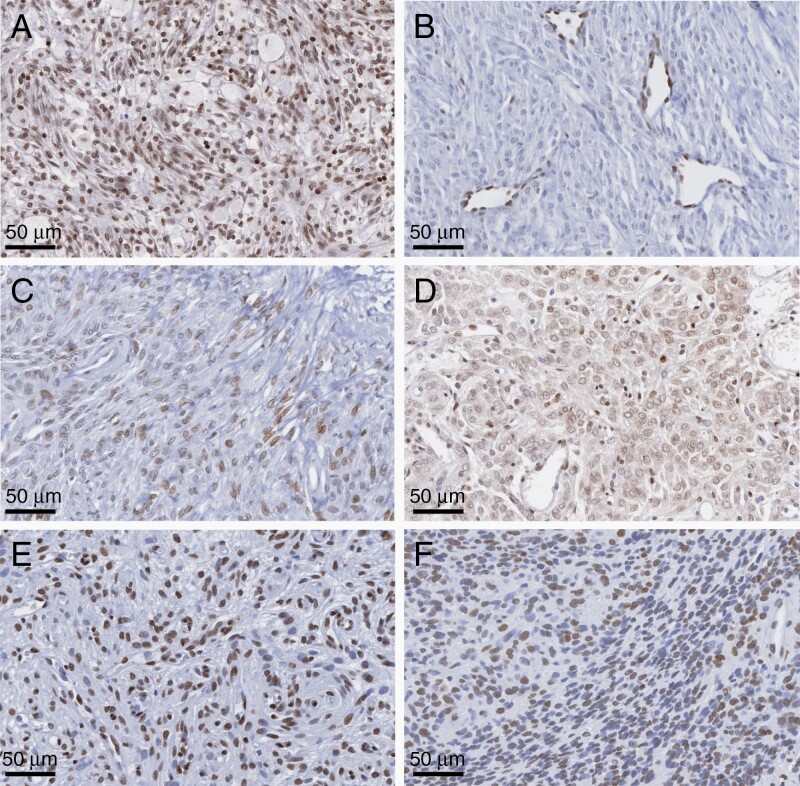
Overall, we noted two major patterns of H3K27me3 staining in meningiomas. One in which there was unequivocally positive staining, or retained trimethylation, denoted as clearly visible nuclear positivity in all or the majority of tumor cells and intratumoral vessels (Figure 1A), and the other in which all or nearly all tumor cells did not demonstrate a lack of nuclear immunopositivity while endothelial and other microenvironmental cells were clearly positive for H3K27me3 staining (Figure 1B). Tumors with the latter pattern of staining were designated to have clear loss of H3K27me3. There were also some tumors where mixed or ambiguous staining patterns were detected, defined as mainly low intensity of staining in tumor cells with clear nuclear positivity in endothelial cells (Figure 1C, D). In some cases, a subset of tumor cells showed nuclear positivity for H3K27me3 while other adjacent cells’ nuclei were immunonegative. In most of these instances, the majority of cells (≥50% of all tumor cells) showed retained positivity, which we scored as “2” in our predetermined scoring system (Supplementary Table 1, Figure 1E). In a small subset of cases, only a minority (<50% of cells) showed nuclear staining for H3K27me3, while the majority demonstrated apparent trimethylation loss, denoted as a “1” in our scoring system (Supplementary Figure 1F). All of these ambiguous patterns were classified as having retained trimethylation in our dichotomous analysis of H3K27me3 retained vs loss.
Fig. 1.
(A-F) Representative patterns of H3K27me3 staining in meningioma. (A) Distinct and strong brown nuclear and endothelial staining suggestive of retained trimethylation. (B) Clear loss of trimethylation with immunopositivity restricted only to endothelial cells as an internal positive control without any tumor cell staining. (C-F) Representative ambiguous staining whereby (C) some tumor cells stained weakly without consistent endothelial cell staining, (D) weak nuclear staining with some cytoplasmic background staining, (E) majority but not all tumor cells with clear immunopositivity, and (F) minority (less than half) of tumor cells demonstrating immunopositivity with other adjacent cells being immunonegative. Only (B) was considered to have unequivocal loss of trimethylation, with other representative images counted as having retained trimethylation.
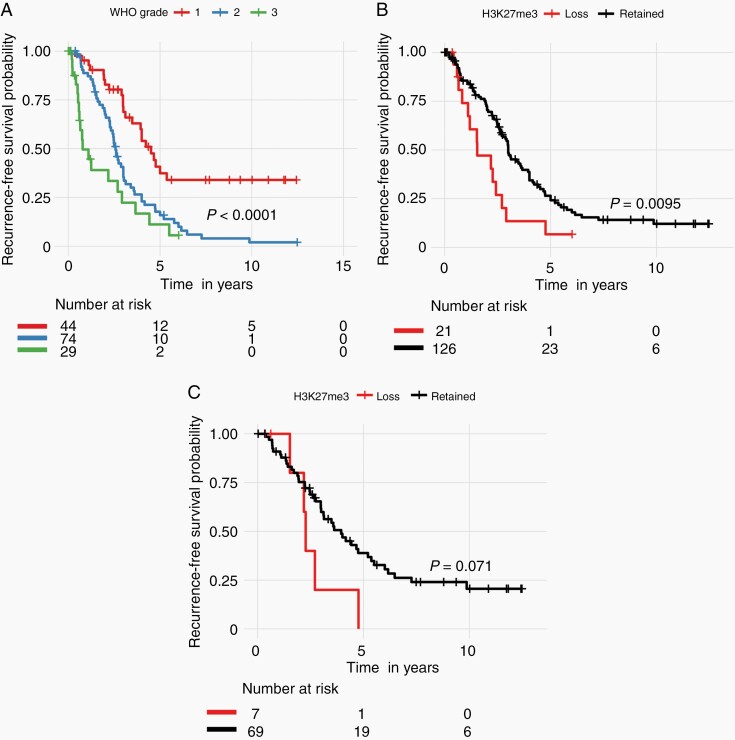
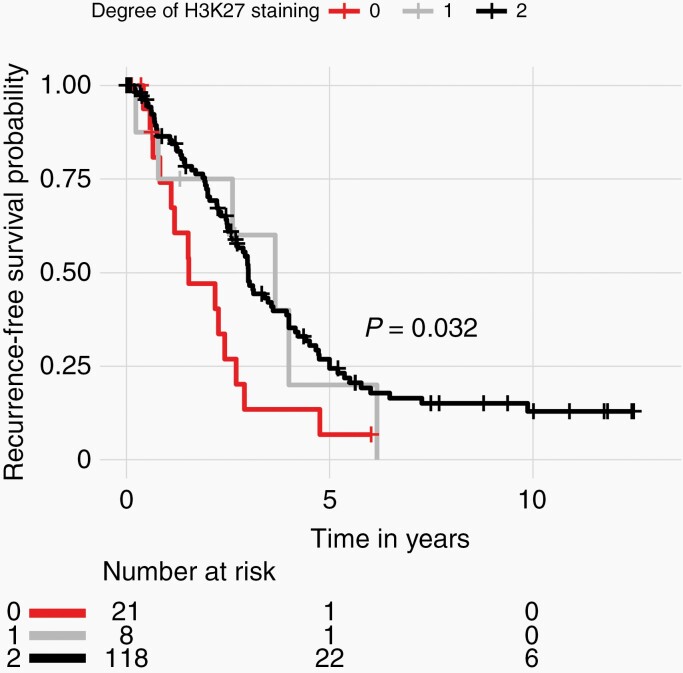
The samples included in this cohort showed the expected distribution patterns of recurrence-free survival (RFS) when stratified by WHO grade, with WHO grade 2 and 3 tumors showing significantly shorter times to recurrence when compared to WHO grade 1 tumors (Figure 2A, log-rank test P < .0001). Median RFS was 4.5 years (95% CI 3.47-5.24), 2.6 years (95% CI 2.271-3.00), and 0.78 years (95% CI 0.61-3.66) for WHO grade 1, 2, and 3 tumors, respectively. Tumors with complete loss of H3K27me3 also had significantly shorter RFS compared to tumors with retained trimethylation [H3K27me3 loss median RFS 1.53 years (95% CI 1.10-2.90) vs retained median RFS 3.0 years (95% CI 2.69-3.94), Figure 2B, log-rank test P = .0095]. As recurrent tumors may have exogenous alterations that are not reflective of the biology of the tumor, we evaluated the effect of trimethylation loss on only the primary tumors that had not received prior treatment within our cohort with sufficient survival data (N = 76). Only 7 primary meningiomas had complete loss of H3K27me3, but these tumors tended to have worse RFS in comparison to their counterparts with retained trimethylation (Figure 2C, P = .071). To determine whether cases where a minority of tumor cells showed H3K27me3 nuclear positivity had prognostic value, we partitioned our cohort into our predefined three classes: cases with H3K27me3 loss [“0,” median RFS 1.53 years (95% CI 1.10-2.90)], cases with minority stain retained [“1,” <50% of cells; median RFS 3.66 years (95% CI 2.61-3.94)], and cases with majority stain retained [“2,” ≥50% cells; median RFS 3.00 years (95% CI 2.61-3.94)]. Using this stratification, there was no difference in RFS between tumors with a minority (“1”) vs a majority (“2”) of tumor cells with retained H3K27me3 staining (Figure 3).
Fig. 2.
Kaplan-Meier survival curve with log-rank test showing recurrence-free survival stratified by (A) WHO grade and (B) H3K27me3 status in all patients, and (C) H3K27me3 status in primary tumors only.
Fig. 3.
Kaplan-Meier survival curves with log-rank test showing recurrence-free survival stratified by degree of H3K27me3 staining, with 0 denoting complete loss, 1 denoting minority staining (<50% of all tumor cells on the slide), and 2 denoting majority staining (>50% of all tumor cells on slide).
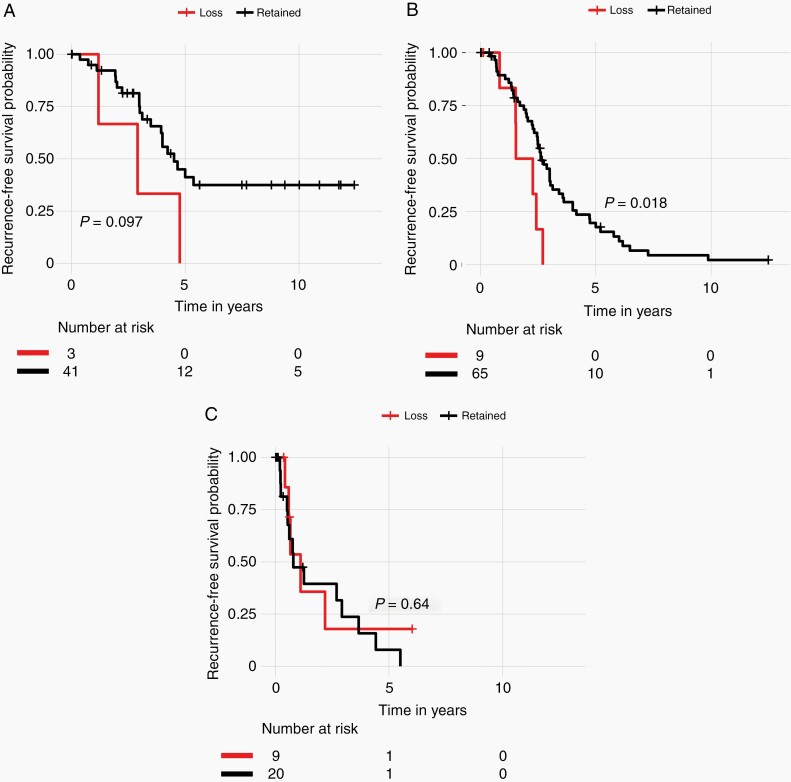
We next analyzed WHO grade 1, 2, and 3 tumors separately (Figure 4) and found that there was a statistically significant difference in the RFS in the WHO grade 2 tumors, which were enriched in our study, demonstrating that loss of trimethylation was associated with poorer RFS in this group (Figure 4B). There was also a notable trend toward poorer RFS in WHO grade 1 tumors with trimethylation loss, however, very few WHO grade 1 tumors (4%) showed loss of H3K27me3 overall. It should be noted that although these were WHO grade 1 meningiomas, 23/44 (52.7%) were noted to have recurrence during the course of follow-up, perhaps reflecting a more aggressive cohort of WHO grade 1 tumors. Interestingly, the outcomes of patients with WHO grade 3 meningiomas did not differ between tumors with retained vs loss of H3K27me3 (Figure 4C).
Fig. 4.
Kaplan-Meier survival curve with log-rank test showing recurrence-free survival stratified by H3K27me3 status in all WHO grade (A) 1, (B) 2, and (C) 3 patients.
We next sought to determine whether nuclear expression of H3K27me3 was an independent predictor of outcome in meningiomas. After controlling for WHO grade, sex, age, and primary/recurrence status of tumor, nuclear expression of H3K27me3 was not a significant independent prognostic factor in our cohort (0.59, 95%CI 0.31-1.09, P = .096, Table 2). Similarly, sensitivity analyses controlling for extent of resection in cases where this information was known (N = 138) did not change these results (Supplementary Table 2).
Table 2.
Multivariate Cox Regression Model on All Samples
| Parameter | HR | 95% CI | P Value |
|---|---|---|---|
| H3K27me3 | |||
| Retain vs loss | 0.58 | 0.31-1.09 | .093 |
| WHO grade | |||
| 2 vs 1 | 2.36 | 1.41-3.95 | <.001* |
| 3 vs 1 | 2.88 | 1.46-5.65 | <.001* |
| Recurrent sample | |||
| Yes vs No | 1.67 | 1.07-2.6 | .021 |
| Sex | |||
| Male vs Female | 1.61 | 1.07-2.43 | .022 |
| Age | |||
| 0.99 | 0.97-1.01 | .28 |
Abbreviations: CI, confidence interval; HR, hazard ratio; WHO, World Health Organization.
*Indicates a P value < .05.
Discussion
The variability in outcomes of patients with meningiomas using standard classifications is a major clinical challenge. Reliable, robust, and objective assessments that are easily interpretable across different institutions that can help refine prognostication are critically needed. Previous studies have tied clinical outcomes in meningiomas to alterations in the tumor epigenome, primarily by DNA methylation signatures.8,12,16 Consequently, there has been interest in investigating the role of other epigenetic changes, such as histone modifications in meningiomas, particularly clinically aggressive or higher-grade meningiomas.
Methylation of H3K27 is associated with gene repression and plays a notable role in mediating the expression of genes involved in lineage commitment and differentiation.22,30 It has therefore unsurprisingly been implicated in a number of oncogenesis processes and cancers. However, when present, it has not always been demonstrated to be of prognostic value. For example, although complete loss or global reduction of H3K27me3 in some cancers, such as breast cancer and pediatric posterior fossa ependymomas has been associated with worse clinical outcome, in other cases such as oral squamous cell carcinoma, a lower level of H3K27me3 has been associated with improved survival and decreased tumor invasiveness.25,31,32 These results highlight that our understanding of the clinical significance of H3K27me3 loss may be incomplete.
Three other studies have investigated the prognostic value of H3K27me3 loss in meningiomas. In the Heidelberg study of 232 meningioma cases enriched for more aggressive tumors, a total of 25 cases demonstrated complete loss of H3K27me3, of which the 1 was in a WHO grade 1 meningioma, 18 with WHO grade 2 meningiomas, and 6 with WHO grade 3 meningiomas. In this study, complete loss of H3K27me3 was associated with more rapid recurrence in WHO grade 1 and 2 tumors, but not in WHO grade 3 tumors.2 In a separate study of 66 anaplastic meningiomas, complete loss of H3K27me3 was associated with worse overall survival, although the impact of this on tumor recurrence was not investigated. Furthermore, “significant” loss of trimethylation in that study was defined as loss of staining in >50% of tumor cells, which was a significant departure from the much stricter classification of trimethylation loss in the Heidelberg study and our own.1 In comparison to prior studies as well as the present study, the recent report from Tübingen found loss of trimethylation in a smaller proportion (4.7%) of meningiomas, with increased rates of H3K27me3 loss in higher WHO grade tumors (3.1% for grade 1, 10.4% for grade 2, 17.7% for grade 3).33 The difference in proportion of H3K27me3 loss is likely explained by the differences in composition of the cohorts (49% WHO grade 2 and 19.3% WHO grade 3 in our study vs 20% WHO grade 2 and 1% WHO grade 3 in Tubingen cohort). The enrichment and robust representation for the more aggressive meningiomas in our study, as well as the WHO grade 2 tumors in the Tubingen study, allowed us to ascertain whether H3K27me3 would be truly prognostic across WHO grades, but also among the heterogenous groups of tumors within each WHO grade that behave with different degrees of aggressiveness.8,12 Both our study and the Tübingen study found trimethylation loss was notably increased in recurrent meningiomas (19.4% in our cohort, 11% in the Tübingen study) vs primary tumors (8.8%, 4%)33
Our study is the first independent study on a separate cohort of meningiomas enriched for WHO grade 2 tumors using IHC of whole slides as routinely performed in clinical practice as opposed to tissue microarrays which are typically utilized only in research settings. Our results validate those obtained by Katz et al.23 in that loss of H3K27me3 is predictive of poorer RFS in WHO grade 2, and likely grade 1 tumors, but not WHO grade 3 tumors. As well, our study is the first to show using statistical methods that the interpretation of H3K27me3 staining in meningioma is fairly reliable between independent reviewers.
Another similarity between our study and that of Katz et al. was the presence of ambiguous staining in our cohort with similar immunohistochemistry staining techniques (including the exact same antibody), analysis, and definitions. Katz et al. noted that in 13 cases, immunohistochemical staining was ambiguous in that there were regions of patchy staining where vessels were faintly stained or negative for H3K27me3 in areas with negative tumor cells.2 We found a similar number of these ambiguous staining patterns in our tumors (n = 11). These cases may represent artifact of staining, and similar to previous studies, we considered these ambiguous cases to have retained trimethylation. Gauchotte et al. encountered similarly heterogeneous immunolabelling patterns in their cohort of anaplastic meningiomas, and thereby set a threshold of 50% of tumor cells having lost trimethylation staining to be defined as significant loss of H3K27me3.1 On our tiered analysis, whether the majority or minority of tumor cells had H3K27 immunopositivity did not contribute to outcome, but rather the only meaningful prognosticator was whether tumors had complete loss of trimethylation or not. Interestingly there was one case in our cohort that demonstrated discrete areas of H3K27me3 loss and retention separated topographically whereas in all other cases, the heterogenous staining was scattered among tumor cells with no clear pattern. Given the relative rarity of this occurrence even in previous studies, it is unclear whether this reflects true intratumoral heterogeneity or how it correlates with focal anaplastic or atypical features that may affect outcome. However, without consistent reporting across centers and a universally accepted, predefined threshold for when tumors can be considered to have lost trimethylation, and the unclear significance of ambiguous H3K27me3 staining in some meningiomas (and pattern of heterogeneity), the interpretation and utility of this particular immunohistochemical marker on a prospective basis may be challenging.
Furthermore, our study, despite examining 151 total meningiomas enriched for WHO grade 2 and 3 tumors, still showed only a small subset of patients with loss of H3K27me3. When taken in the context that our cohort was enriched for patients with more aggressive tumors, including aggressive WHO grade 1 tumors that recur early, this suggests that loss of H3K27me3 remains a rare phenomenon in meningiomas. It is possible that the results of our multivariable analysis were a reflection of our smaller cohort size,33 however, the important finding that is consistent among studies is that for WHO grade 2 tumors, which confer the greatest degree of clinical uncertainty regarding recurrence risk and decision making for adjuvant treatment, loss ofH3K27me3 may in fact help stratify patients into those at higher and lower risk of recurrence. In fact, our study that looked at a slightly larger number of WHO grade 3 meningiomas (n = 29) compared to the Tübingen cohort (n = 17), found that RFS of WHO grade 3 meningiomas with and without loss of H3K27me3 loss are similar. A pooled analysis of data across multiple centers, with predetermined and standardized protocols and definitions for H3K27me3 positivity/negativity, including automated digital pathology approaches, may help better clarify the role of H3K27me3 in meningioma prognostication, particularly for grade 1 and grade 3 tumors.
Our study was designed to provide a qualitative assessment of H3K27me3 loss. Understanding the reasoning for poorer outcome with trimethylation loss in meningiomas in select WHO grades requires a more detailed mechanistic study. In pediatric posterior fossa ependymomas, low H3K27 methylation is associated with high levels of Enhancer of Zeste Homologs Inhibitory Protein which directly inhibits the EZH2 subunit of the PRC2, contributing to dysregulated gene silencing that drives tumorigenesis.34 H3K27M mutations and associated decrease in genome-wide H3K27 trimethylation are also a hallmark of pediatric high-grade gliomas, which result in elevated MYCN and increased expression of NOTCH pathway genes that promote oncogenesis.23,35–37 These pathways have not been similarly elucidated in meningiomas. However, given the higher proportion of trimethylation loss in higher grade meningiomas and that the outcomes of patients with a minority vs majority of immunopositive tumor cells are similar, the unfavorable outcomes of H3K27me3 loss may only be evident when there is complete loss of the EZH2 subunit of PRC2. It may be possible that a more precise quantitative threshold of positivity, using automated computation methods and machine learning algorithms is able to discriminate between more than two prognostic groups of meningiomas with greater accuracy. Additionally because the existing histopathological classification of meningiomas does not always reflect their clinical behavior, correlative studies of the prognostic value of H3K27me3 in the context of other known molecular prognosticators in meningiomas, such as TERT mutations, CDKN2A loss, and DNA methylation, are important next steps.8,12,38–42
As in previous studies, the number of meningiomas with unequivocal complete loss of H3K27me3 was low, particularly for WHO grade 1 tumor which also may limit our ability to make any definitive conclusions about the generalizability of this mark for low-grade meningiomas. Given the challenges of the interpretation of ambiguous cases, the low rates of loss of H3K27me3 in WHO grade 1 tumors, and the apparent lack of prognostic value in WHO grade 3 tumors, it is important for our community to form a consensus about the utility of this biomarker in meningiomas. Given the results of our study and others, H3K27me3 may be used as a useful adjunct to current standard of care clinical factors including WHO grade and extent of resection, to aid in predicting the risk of recurrence for WHO grade 1 and 2 meningiomas that demonstrate that most variability in outcome. Pooling of multiple samples across different centers and a round-robin assessment of stained tissue from multiple different institutions, comparison of whole slide analysis vs punch biopsies and tissue microarrays, as well as larger prospective studies stratifying patients based on H3K27me3 status, are warranted to consolidate the impact of this test in meningioma patients.
Supplementary Material
Contributor Information
The International Consortium on Meningiomas:
Kenneth Aldape, Karolyn Au, Jill Barnholtz-Sloan, Felix Behling, Wenya (Linda) Bi, Priscilla Brastianos, Nicholas Butowski, Chaya Brodie, Aaron Cohen-Gadol, Marta Couce, Francesco Dimeco, Kate Drummond, Ian Dunn, Aaron Cohen-Gadol, Eva Galanis, Norbert Galldiks, Caterina Giannini, Roland Goldbrunner, Oliver Hanemann, Christel Herold-Mende, Craig Horbinski, Raymond Huang, Mohsen Javadpour, Michael Jenkinson, Christine Jungk, Timothy Kaufmann, Boris Krischek, Sylvia Kurz, Daniel Lachance, Christian Lafougere, Katrin Lamszus, Ian Lee, Tathiana Malta, Serge Makarenko, Christian Mawrin, Michael McDermott, Christopher Millward, Jennifer Moliterno-Gunel, Andrew Morokoff, Farshad Nassiri, H K Ng, Houtan Noushmehr, Arie Perry, Laila Poisson, Bianco Pollo, Aditya Ragunathan, David Raleigh, Mirjam Renovanz, Franz Ricklefs, Felix Sahm, Andrea Saladino, Antonio Santacroce, Thomas Santarius, Christian Schichor, Nils Schimdt, Jens Schittenhelm, Warren Selman, Helen Shih, Jim Snyder, Matja Snuderl, Andrew Sloan, Suganth Suppiah, Erik Sulman, Ghazaleh Tabatabai, Marcos Tatagiba, Marcos Timmer, Joerg-Christian Tonn, Andreas Von Deimling, Michael Vogelbaum, Tobias Walbert, Justin Wang, Patrick Wen, Manfred Westphal, Stephen Yip, and Gelareh Zadeh
Funding
This study was funded by grants from the Canadian Institutes of Health Research (CIHR) and Brain Tumour Charity (BTC).
Conflict of interest statement. None to disclose.
Authorship statement. Collection of samples and assembly of clinical data was done by F.N. Data analysis, interpretation, and main manuscript preparation were done by F.N. and J.Z.W. IHC staining was performed by O.S., N.I., F.N., N.P., and J.Z.W. Pathology review and loss of H3K27me3 scoring done by S.K., A.G., and H.K.N. Tumor samples provided by A.S., B.P., F.D., S.Y., and G.Z. Manuscript editing and revisions by F.N., J.Z.W., K.A., and G.Z.
International Consortium on Meningioma Members. The generation of the manuscript has been supported by the membership of the consortium, which at the time of supplement generation includes: Kenneth Aldape, Karolyn Au, Jill Barnholtz-Sloan, Felix Behling, Wenya (Linda) Bi, Priscilla Brastianos, Nicholas Butowski, Chaya Brodie, Aaron Cohen-Gadol, Marta Couce, Francesco Dimeco, Kate Drummond, Ian Dunn, Aaron Cohen-Gadol, Eva Galanis, Norbert Galldiks, Caterina Giannini, Roland Goldbrunner, Oliver Hanemann, Christel Herold-Mende, Craig Horbinski, Raymond Huang, Mohsen Javadpour, Michael Jenkinson, Christine Jungk, Timothy Kaufmann, Boris Krischek, Sylvia Kurz, Daniel Lachance, Christian Lafougere, Katrin Lamszus, Ian Lee, Tathiana Malta, Serge Makarenko, Christian Mawrin, Michael McDermott, Christopher Millward, Jennifer Moliterno-Gunel, Andrew Morokoff, Farshad Nassiri, HK Ng, Houtan Noushmehr, Arie Perry, Laila Poisson, Bianco Pollo, Aditya Ragunathan, David Raleigh, Mirjam Renovanz, Franz Ricklefs, Felix Sahm, Andrea Saladino, Antonio Santacroce, Thomas Santarius, Christian Schichor, Nils Schimdt, Jens Schittenhelm, Warren Selman, Helen Shih, Jim Snyder, Matja Snuderl, Andrew Sloan, Suganth Suppiah, Erik Sulman, Ghazaleh Tabatabai, Marcos Tatagiba, Marcos Timmer, Joerg-Christian Tonn, Andreas Von Deimling, Michael Vogelbaum, Tobias Walbert, Justin Wang, Patrick Wen, Manfred Westphal, Stephen Yip, Gelareh Zadeh.
References
- 1. Gauchotte G, Peyre M, Pouget C, et al. Prognostic value of histopathological features and loss of H3K27me3 immunolabeling in anaplastic meningioma: a multicenter retrospective study. J Neuropathol Exp Neurol. 2020;79(7):754–762. [DOI] [PubMed] [Google Scholar]
- 2. Katz LM, Hielscher T, Liechty B, et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol. 2018;135(6):955–963. [DOI] [PubMed] [Google Scholar]
- 3. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perry A. Meningiomas. In: Practical Surgical Neuropathology: A Diagnostic Approach. Elsevier. 2018:259–298. [Google Scholar]
- 5. Marosi C, Hassler M, Roessler K, et al. Meningioma. Crit Rev Oncol Hematol. 2008;67(2):153–171. [DOI] [PubMed] [Google Scholar]
- 6. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 7. Harter PN, Braun Y, Plate KH. Classification of meningiomas - advances and controversies. Chin Clin Oncol. 2017;6(Suppl 1):S2. [DOI] [PubMed] [Google Scholar]
- 8. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 9. Nowosielski M, Galldiks N, Iglseder S, et al. Diagnostic challenges in meningioma. Neuro Oncol. 2017;19(12):1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–391. [DOI] [PubMed] [Google Scholar]
- 11. Lusis EA, Watson MA, Chicoine MR, et al. Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res. 2005;65(16):7121–7126. [DOI] [PubMed] [Google Scholar]
- 12. Nassiri F, Mamatjan Y, Suppiah S, et al. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019;21(7):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang RY, Bi WL, Griffith B, et al. Imaging and diagnostic advances for intracranial meningiomas. Neuro Oncol. 2019;21(Suppl 1):i44–i61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kishida Y, Natsume A, Kondo Y, et al. Epigenetic subclassification of meningiomas based on genome-wide DNA methylation analyses. Carcinogenesis. 2012;33(2):436–441. [DOI] [PubMed] [Google Scholar]
- 15. Lomas J, Bello MJ, Arjona D, et al. Genetic and epigenetic alteration of the NF2 gene in sporadic meningiomas. Genes Chromosomes Cancer. 2005;42(3):314–319. [DOI] [PubMed] [Google Scholar]
- 16. Suppiah S, Nassiri F, Bi WL, et al. Molecular and translational advances in meningiomas. Neuro Oncol. 2019;21(Suppl 1):i4–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bannister AJ, Schneider R, Kouzarides T. Histone methylation: dynamic or static? Cell. 2002;109(7):801–806. [DOI] [PubMed] [Google Scholar]
- 19. Ngollo M, Lebert A, Daures M, et al. Global analysis of H3K27me3 as an epigenetic marker in prostate cancer progression. BMC Cancer. 2017;17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoo KH, Hennighausen L. EZH2 methyltransferase and H3K27 methylation in breast cancer. Int J Biol Sci. 2012;8(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gehring M, Reik W, Henikoff S. DNA demethylation by DNA repair. Trends Genet. 2009;25(2):82–90. [DOI] [PubMed] [Google Scholar]
- 22. Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harutyunyan AS, Krug B, Chen H, et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat Commun. 2019;10(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J, Hart RP, Mallimo EM, Swerdel MR, Kusnecov AW, Herrup K. EZH2-mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia. Nat Neurosci. 2013;16(12):1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bayliss J, Mukherjee P, Lu C, et al. Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci Transl Med. 2016;8(366):366ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29(1):4–13. [DOI] [PubMed] [Google Scholar]
- 27. Hu S, Yu L, Li Z, et al. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo. Cancer Biol Ther. 2010;10(8):788–795. [DOI] [PubMed] [Google Scholar]
- 28. Tang G, Guo J, Zhu Y, et al. Metformin inhibits ovarian cancer via decreasing H3K27 trimethylation. Int J Oncol. 2018;52(6):1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zha L, Cao Q, Cui X, et al. Epigenetic regulation of E-cadherin expression by the histone demethylase UTX in colon cancer cells. Med Oncol. 2016;33(3):21. [DOI] [PubMed] [Google Scholar]
- 30. Mohn F, Weber M, Rebhan M, et al. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell. 2008;30(6):755–766. [DOI] [PubMed] [Google Scholar]
- 31. Hsieh IY, He J, Wang L, et al. H3K27me3 loss plays a vital role in CEMIP mediated carcinogenesis and progression of breast cancer with poor prognosis. Biomed Pharmacother. 2020;123:109728. [DOI] [PubMed] [Google Scholar]
- 32. Chen YW, Kao SY, Wang HJ, Yang MH. Histone modification patterns correlate with patient outcome in oral squamous cell carcinoma. Cancer. 2013;119(24):4259–4267. [DOI] [PubMed] [Google Scholar]
- 33. Behling F, Fodi C, Gepfner-Tuma I, et al. H3K27me3 loss indicates an increased risk of recurrence in the Tübingen meningioma cohort. Neuro Oncol. 2021;23(8):1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jain SU, Do TJ, Lund PJ, et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat Commun. 2019;10(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castel D, Philippe C, Kergrohen T, et al. Transcriptomic and epigenetic profiling of ‘diffuse midline gliomas, H3 K27M-mutant’ discriminate two subgroups based on the type of histone H3 mutated and not supratentorial or infratentorial location. Acta Neuropathol Commun. 2018;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Funato K, Major T, Lewis PW, Allis CD, Tabar V. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27M histone mutation. Science. 2014;346(6216):1529–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Solomon DA, Wood MD, Tihan T, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016;26(5):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sahm F, Schrimpf D, Olar A, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5):djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boström J, Meyer-Puttlitz B, Wolter M, et al. Alterations of the tumor suppressor genes CDKN2A (p16INK4a), p14ARF, CDKN2B (p15INK4b), and CDKN2C (p18INK4c) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159(2):661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guyot A, Duchesne M, Robert S, et al. Analysis of CDKN2A gene alterations in recurrent and non-recurrent meningioma. J Neurooncol. 2019;145(3):449–459. [DOI] [PubMed] [Google Scholar]
- 42. Sievers P, Hielscher T, Schrimpf D, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.