Abstract
Background
To analyze the effect of treatment on neurocognitive functioning and the association of neurocognition with radiological abnormalities in primary central nervous system lymphoma (PCNSL).
Methods
One hundred and ninety-nine patients from a phase III trial (HOVON 105/ALLG NHL 24), randomized to standard chemotherapy with or without rituximab, followed in patients ≤60 years old by 30-Gy whole-brain radiotherapy (WBRT), were asked to participate in a neuropsychological evaluation before and during treatment, and up to 2 years posttreatment. Scores were transformed into a standardized z-score; clinically relevant changes were defined as a change in z-score of ≥1 SD. The effect of WBRT was analyzed in irradiated patients. All MRIs were centrally assessed for white matter abnormalities and cerebral atrophy, and their relation with neurocognitive scores over time in each domain was calculated.
Results
125/199 patients consented to neurocognitive evaluation. Statistically significant improvements in neurocognition were seen in all domains. A clinically relevant improvement was seen only in the motor speed domain, without differences between the arms. In the follow-up of irradiated patients (n = 43), no change was observed in any domain score, compared to after WBRT. Small but significant inverse correlations were found between neurocognitive scores over time and changes in white matter abnormalities (regression coefficients: −0.048 to −0.347) and cerebral atrophy (−0.212 to −1.774).
Conclusions
Addition of rituximab to standard treatment in PCNSL patients did not impact neurocognitive functioning up to 2 years posttreatment, nor did treatment with 30-Gy WBRT in patients ≤60 years old. Increased white matter abnormalities and brain atrophy showed weak associations with neurocognition.
Keywords: atrophy, cognition, radiation, rituximab, white matter lesions
Key Points.
1. Rituximab added to MTX-based chemotherapy does not impact neurocognitive functioning.
2. 30-Gy WBRT does not impact neurocognitive functioning in PCNSL patients ≤60 years.
3. Increased white matter abnormalities and atrophy were associated with neurocognition.
Importance of the Study.
The HOVON 105/ALLG NHL 24 study is one of the largest randomized controlled trials (RCTs) in adult primary central nervous system lymphoma (PCNSL) patients. This study did not show a significant difference in progression-free survival (PFS) between patients treated with and without rituximab. However, the evaluation of the effect of this treatment on neurocognitive functioning is required to determine the “net clinical benefit.” We found that neurocognition significantly improved after treatment in both arms, but improved only clinically relevant for the motor speed domain. There were no differences between the arms. Furthermore, in those who received whole-brain radiotherapy (WBRT) (30 Gy), neurocognitive functioning remained stable in the 2-year post-WBRT period, compared to directly after WBRT. The inverse relation between white matter abnormalities and brain atrophy on neurocognitive functioning was weak to moderate for all neurocognitive domains, up to 2 years posttreatment, suggesting that increased white matter abnormalities and atrophy in this setting imply worse neurocognitive functioning, but with a rather small effect size.
Primary central nervous system lymphoma (PCNSL) is a rare non-Hodgkin lymphoma confined to the brain, leptomeninges, spinal cord, and eyes. Over the last three decades the prognosis for patients with PCNSL improved significantly due to improvement of treatment, though mainly among patients below the age of 70 years.1,2 Preservation of neurocognitive functioning remains a major challenge in the treatment of PCNSL.3 Neurocognitive decline, along with other symptoms caused by the tumor and/or treatment, may subsequently compromise health-related quality of life (HRQoL).4,5
In systemic diffuse large B-cell lymphoma (DLBCL) patients, rituximab, a chimeric anti-CD20 monoclonal antibody that targets the CD20 cell surface protein, improves survival when added to standard chemotherapy.6,7 It has been hypothesized that rituximab added to standard high-dose methotrexate (HD-MTX)-based chemotherapy could also improve survival in PCNSL patients. The HOVON 105/ALLG NHL 24, a large international multicenter phase III randomized controlled trial (RCT), investigated the addition of rituximab to MBVP (methotrexate, tenoposide, BCNU, and prednisolone) chemotherapy, followed in patients ≤60 years old, by whole-brain radiotherapy (WBRT). This study showed that rituximab did not improve event-free, progression-free, and overall survival (OS), although OS data were still immature.8
For any new treatment regimen, information on the impact of this treatment on both the quantity and quality of life should be evaluated to determine the “net clinical benefit.” Neurocognitive impairment is an important factor that may negatively influence HRQoL in brain tumor patients and should therefore be considered in this evaluation. Although a direct effect of rituximab on neurocognition was not necessarily expected, improved efficacy of treatment resulting in fewer patients needing radiotherapy (boost) could influence the neurocognitive effect of the treatment. Combined survival and quality of survival will allow clinicians and patients to make informed decisions about the best treatment for each individual patient.
Radiological features, in particular brain volume and white matter lesions, have been found to correlate with worse neurocognitive functioning in patients treated for PCNSL in some, but not all, studies.9–13 Most of these studies were limited by a cross-sectional design and/or small cohorts (n = 16–28).11,12
Rituximab was found not to affect HRQoL in patients from the HOVON 105/ALLG NHL24 trial.14 The primary aim of this study was to determine the effect of rituximab, when added to standard treatment for PCNSL, on neurocognitive functioning, which was a predefined secondary endpoint of the HOVON 105/ALLG NHL 24 trial. In addition, we aimed to evaluate the effect of low-dose WBRT on neurocognitive functioning in irradiated patients. Lastly, we aimed to identify whether there is a relation between brain volume and/or white matter lesions and neurocognitive functioning over time in PCNSL patients.
Methods
Study Design and Patient Population
In the HOVON 105/ALLG NHL 24 trial 199 immunocompetent patients, aged 18–70 years, with newly diagnosed CD20-positive B-cell PCNSL were included from Dutch, Australian, and New Zealand hospitals between 2010 and 2016. Only patients who were fluent in English or Dutch and were treated in a center that was equipped for neuropsychological evaluation (NPE) were eligible for participation in this neurocognitive study. The trial design and treatment details were published elsewhere.8 In short, patients were randomized for two cycles of MBVP without or with rituximab (R-MBVP). Irrespective of treatment arm, this induction treatment was followed by consolidative HD-cytarabine chemotherapy. Patients ≤60 years old subsequently received 30-Gy (20 × 1.5 Gy) WBRT. An integrated boost of 10 Gy to the tumor-bed was given simultaneously with WBRT to patients who achieved only a partial response.15 All participants signed informed consent for the RCT and separately for undergoing neurocognitive assessments. The study and the neurocognitive testing part were approved by the ethics committees of all participating centers. The HOVON 105/ALLG NHL 24 trial was registered: EUdraCT number 2009-014722-42 and in the Netherlands Trial Register: Trial NL2321.
Neuropsychological Evaluation
All patients were tested by a trained research nurse or neuropsychologist using a standard test battery, as described in the assessment guidelines in PCNSL.3 For testing attention/executive functioning, the WAIS III digit span (DS) forward and backward16 and the Trail Making Test parts A and B17 were used. The written version of the Letter Digit Substitution Test (LDST)18 was used to determine information processing speed. Memory was tested with the Rey Auditory Verbal Learning Test (RAVLT),19 and motor speed with the Grooved Pegboard Test20 in the dominant and nondominant hand. To prevent practice effects, different versions were used at different visits for the RAVLT, LDST, and DS. At baseline, premorbid intelligence (IQ) was determined with the national adult reading test (NART) or Dutch adult reading test in English- and Dutch-speaking patients, respectively.21,22
According to protocol, patients underwent NPE before chemotherapy (baseline), after completion of chemotherapy, after radiotherapy (if given), and at 3, 6, 12, and 24 months posttreatment. NPEs were discontinued if a patient received <2 cycles of (R-)MBVP, when a relapse or progression occurred, or when a patient chose to withdraw from the either the RCT or NPE side-study.
Radiological Assessments
At baseline, after each treatment part (ie, (R-)MBVP, HD-cytarabine, WBRT [if applicable]), and thereafter every 3 months in the first 2 years of follow-up, all patients underwent cranial MRI. The degree of white matter abnormality (WMA) and brain atrophy were assessed centrally. For this evaluation the “end-of-treatment” MRI, irrespective of the final treatment modality, was considered the reference scan. MRI scans at 6, 12, and 24 months of follow-up were used to determine changes.
WMA were scored in five brain areas on both sides (frontal, temporal, parieto-occipital, basal ganglia, and infratentorial) according to the Fazekas score (range 0–3), with 0 denoting no lesions or symmetrical caps or bands, 1 indicating small focal lesions, and 2 and 3 indicating beginning or diffuse confluent lesions, respectively.23 The lobe or lobes in which the tumor lesion or lesions and its surrounding edema were located were not scored to prevent overestimation of WMA. The sum score (0–30) of WMA in all scored brain areas at each time point was used to calculate individual changes over time. The WMA ratio, defined as the sum score divided by the maximum possible sum score for each patient (excluding brain areas with tumor), was used to assess the correlation between WMA and neurocognitive functioning at each time point, for each domain. Grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) volume were calculated by automatic segmentation to determine brain volume, using a validated method.24 Total brain volume was defined as GM + WM volumes, while total intracranial volume was defined as GM + WM + CSF.
Statistical Analysis
Calculation of neurocognitive scores
For the adult reading test individual test scores were converted to a standardized score, corrected for age and sex, and transformed into an IQ score.25 For all other tests individual test scores were transformed into a z-score, corrected for age, sex, and/or level of education,26 using scores from the general population.27
Descriptive analysis
Clinical and sociodemographic characteristics were compared between treatment arms, and between patients who did and did not participate in the NPEs to address selection bias. Differences were tested using a chi-square test for categorical data, and a Mann–Whitney U or an independent t test for continuous data, depending on the distribution of the tested variable. Compliance with NPE at each time point was calculated, and defined as the number of completed NPEs (ie, all tests in at least one domain should have been completed) at a specific time point divided by the number of evaluations expected at that time point. A specified time window was defined for each evaluation point (Supplementary Methods). Only tests performed within the specified time windows were considered compliant with the assessment protocol and were analyzed. A domain score was calculated as the mean of the z-scores of the different tests within that domain. In line with previous research, a change in z-score over time or a difference between groups of ≥1 point (1 SD) was considered clinically relevant.
Neurocognitive scores over time
To estimate the impact of the treatment on neurocognitive functioning over time, linear mixed models were used, which allow the inclusion of all patients who underwent a NPE at least once, with fixed effects for treatment arm, time (as factor), and their interaction. Estimated marginal mean scores and their 95% confidence interval (CI) were calculated for each domain.
In patients who completed at least two NPEs, one of which was before treatment, we assessed the change from baseline in each domain for each individual patient. Next, patients were classified as improved, stable, or deteriorated, depending on a change in z-score of ≥1 SD in each time period.
Impact of WBRT
Using the same methods, we studied neurocognitive functioning after WBRT in irradiated patients. For these analyses the scores after WBRT measurement were considered as baseline.
Relation of neurocognition with brain volume and white matter abnormalities
Pearson correlation coefficients were calculated between the z-score of each neurocognitive domain and the WMA ratio of the Fazekas score, and the total brain volume at the 6, 12, and 24 months follow-up visits, cross-sectionally. Next, linear mixed model analyses were performed to assess the association between changes in WMA or atrophy up to 2 years posttreatment, and changes in neurocognitive functioning over time. These analyses were corrected for time (ie, visits), multiple lesions, sex, age, and education, and brain volume. The latter was also corrected for total intracranial volume. All analyses were conducted with Stata, version 15, and a two-tailed P-value < .05 was considered statistically significant.
Results
Patients
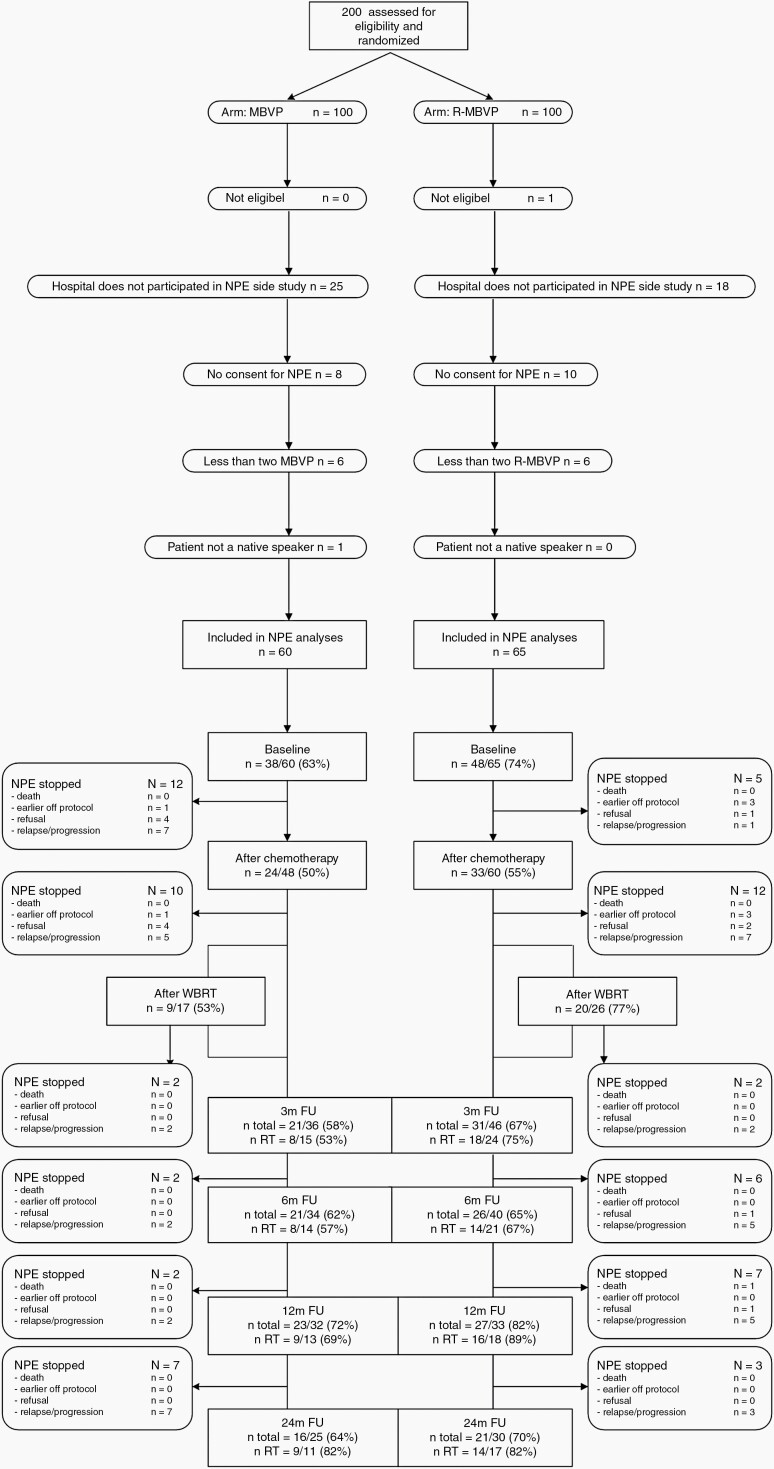
In the HOVON 105/ALLG NHL 24 trial 199 patients were included, of whom 125 (63%) signed informed consent for this side-study, participated, and were therefore included in the linear mixed models, addressing the primary objective of the study. Compliance with NPE was ≥50% at all evaluation points, Figure 1.
Fig. 1.
CONSORT diagram showing reasons for not-participating in the NPEs as well as the compliance rates at each time point, separately for the treatment arms. “After WBRT” was assessed only in those who had WBRT. Abbreviations: FU, follow-up; NPEs, neuropsychological evaluations; WBRT, whole-brain radiotherapy.
In the patients evaluated for neurocognitive functioning, there were no differences between treatment arms with respect to baseline clinical and sociodemographic characteristics and drug exposure, including baseline IQ. However, more patients in the R-MBVP-arm had cognitive impairments (<−1 SD) in at least one domain compared to the MBVP-arm (92% vs 87%; Table 1). No differences in sociodemographic or clinical characteristics were observed between patients who participated in NPEs (n = 125) and those who did not (n = 74). Patients included in this analysis had a median age of 61 years (interquartile range 55–66), 72% had a median WHO performance of <2, and 38% received WBRT, which is comparable to the total trial population8 (Supplementary Table 1). Mean baseline z-scores for each neurocognitive domain are shown in Table 1, and for each test in Supplementary Table 2.
Table 1.
Baseline Clinical and Sociodemographic Characteristics of the Patients Included in the Neuropsychological Evaluations, Per Treatment Arm and for the Total Study Population
| MBVP (n = 60) | R-MBVP (n = 65) | Total (n = 125) | |
|---|---|---|---|
| Sex (n, % male) | 35 (58%) | 29 (45%) | 64 (51%) |
| Age (median, IQR) | 60 (55–66) | 61 (55–67) | 61 (55–66) |
| WHO performance score (n, %) | |||
| WHO 0 | 16 (27%) | 16 (25%) | 32 (26%) |
| WHO 1 | 28 (47%) | 33 (51%) | 61 (49%) |
| WHO 2 | 11 (18%) | 9 (14%) | 20 (16%) |
| WHO 3 | 5 (8%) | 7 (11%) | 12 (10%) |
| Comorbidities active at baseline (n, % ≥2) | 29 (48%) | 33 (51%) | 62 (50%) |
| Baseline IQ (median, IQR) | 93 (76–106) | 92 (77–106) | 93 (77–106) |
| Level of education (years of education; n, %) | |||
| Low (≤6) | 10 (17%) | 11 (17%) | 21 (17%) |
| Average (7–9) | 30 (50%) | 36 (55%) | 66 (53%) |
| High (10–18+) | 12 (20%) | 11 (17%) | 23 (18%) |
| Missing | 8 (13%) | 7 (11%) | 15 (12%) |
| Solitary lesion (n, %) | 35 (58%) | 32 (49%) | 67 (54%) |
| Missing/NA | 3 (5%) | 3 (5%) | 6 (5%) |
| Bilateral involvement (n, %) | 22 (37%) | 27 (42%) | 49 (39%) |
| Missing/NA | 3 (5%) | 3 (5%) | 6 (5%) |
| Deep structures involved (n, %) | 39 (65%) | 43 (66%) | 82 (66%) |
| Study drug exposure | |||
| HD-cytarabine (n, %) | 55 (92%) | 59 (91%) | 114 (91%) |
| WBRT (n, %) | 22 (37%) | 26 (40%) | 48 (38%) |
| Radiation boost given (n, %) | 10 (17%) | 16 (25%) | 26 (21%) |
| Intrathecal treatment given (n, %) | 6 (10%) | 6 (9%) | 12 (10%) |
| Baseline score for each neurocognitive domain | |||
| Neurocognitive domain | MBVP | R-MBVP | Total |
| Attention/executive functioning (mean, SD) | −0.52 (0.95) | −0.85 (1.03) | −0.70 (1.00) |
| Information processing speed (mean, SD) | −0.99 (1.74) | −1.29 (1.72) | −1.16 (1.72) |
| Memory (mean, SD) | −1.52 (1.20) | −1.70 (1.19) | −1.62 (1.19) |
| Motor speed (mean, SD) | −2.78 (3.11) | −4.43 (5.57) | −3.62 (4.57) |
| Impaired cognitive functioning (<1 SD) in at least one domain | 33/38 (87%) | 44/48 (92%) | 77/86 (90%) |
Abbreviations: HD-cytarabine, high-dose cytarabine; IQ, intelligence quotient; IQR, interquartile range; NA, not applicable in case of no brain lesion(s); WBRT, whole-brain radiotherapy.
Neurocognitive Functioning Over Time
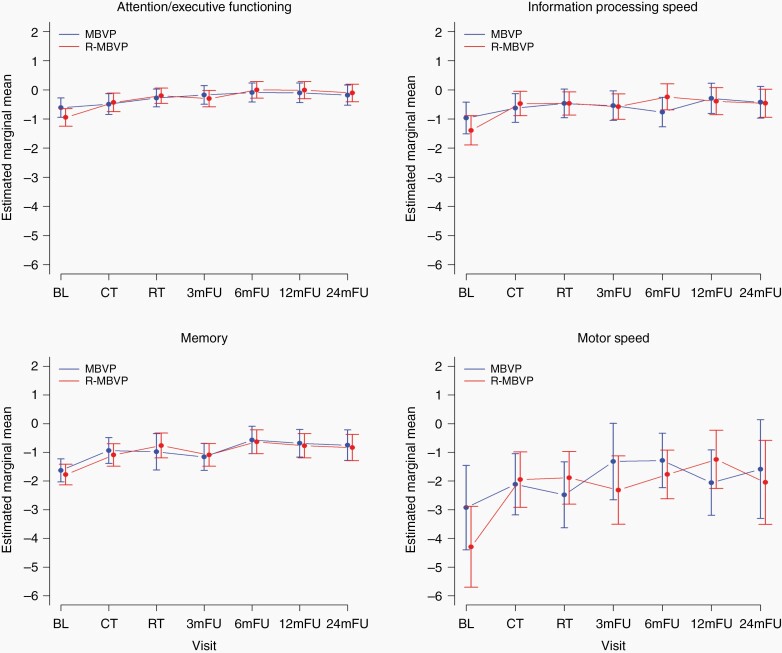
For 86 patients baseline neurocognitive data were available; by using linear mixed models all 125 patients with at least one NPE could be included in this evaluation. The results of the linear mixed models showed a statistically significant difference over time in all neurocognitive domains (all P < .01), without any significant or clinically relevant difference between treatment arms (Figure 2 and Supplemental Figure 2). The main change in most domains was improvement between baseline and after treatment, with a stabilization thereafter. Although these difference in all domains were statistically significant, only in the domain of motor speed this improvement was also clinically relevant. Nevertheless, the estimated marginal mean scores in the motor speed domain still remained below those of the norm population (ie, >−1 SD). For memory, scores did not quite improve to a clinically relevant extent, but over time the mean score improved from a clinically relevant impaired level to within the range of the norm population (ie, above −1 SD). Scores in attention/executive functioning and information processing speed remained within the range of the norm population up to 2 years posttreatment.
Fig. 2.
Mean z-scores for the different neurocognitive domains over time (A: attention/executive functioning; B: information processing speed; C: memory; D: motor speed), separately for the treatment arms. Estimated marginal means for each evaluation point separately for each treatment arm, with the vertical bars representing the 95% confidence interval of the group mean.
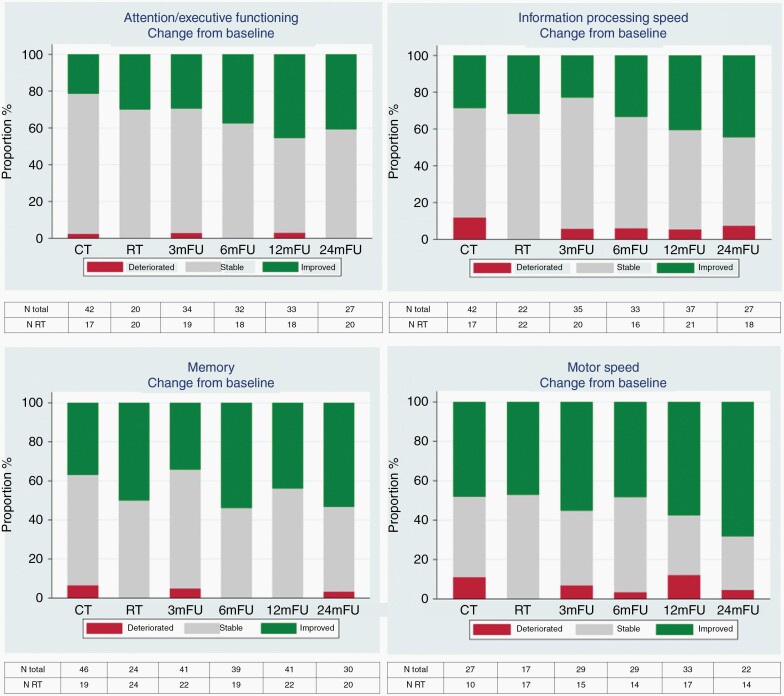
At the individual level, we combined both treatment arms since groups became too small and no differences were found between treatment arms. Compared to baseline, after 12 months the majority (52%–61%) remained stable in all neurocognitive domains, except for the domain of motor speed in which the majority improved (58%). After 24 months, 53% had improved scores in the memory and 68% in motor speed domain. For attention/executive functioning and information processing speed most patients remained stable: 59% and 48%, respectively. Only a minority of patients had worse neurocognitive functioning at 12 and 24 months (0%–7%), Figure 3, Supplementary Table 3 and Supplementary Figure 1.
Fig. 3.
Percentage of patients at each evaluation point with a clinically relevant change in neurocognitive domain scores compared to baseline combining both treatment arms.
Neurocognitive Functioning in Irradiated Patients
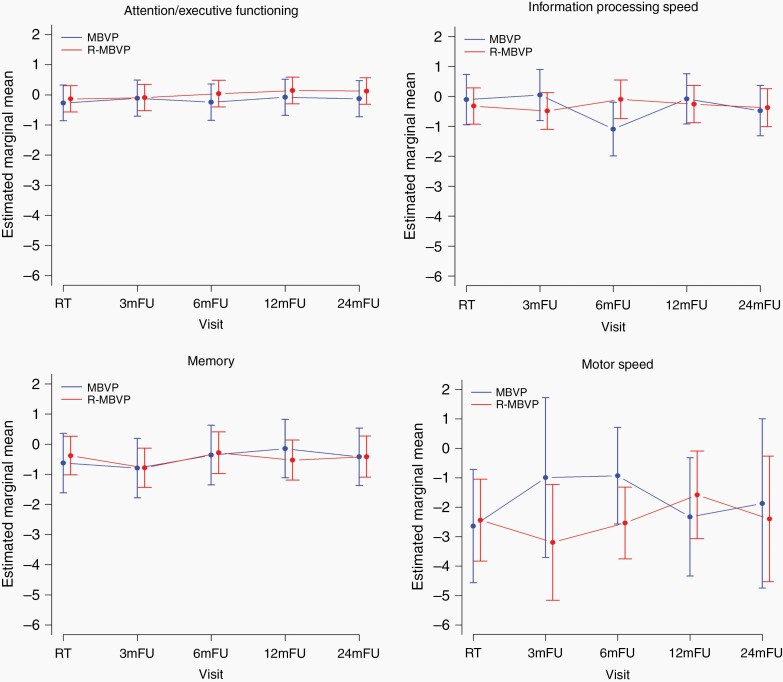
In the irradiated patients (n = 43) we assessed the effect of WBRT on neurocognitive functioning over time, up to 2 years post-radiotherapy. The results of the linear mixed model analyses showed no significant and clinically relevant changes over time, neither improvement or deterioration, except for a clinically relevant improvement in motor speed in the control-arm at 3 and 6 months posttreatment. There were no other differences between treatment arms (Figure 4 and Supplemental Figure 3).
Fig. 4.
Mean z-scores for each neurocognitive domain over time (A: attention/executive functioning; B: information processing speed; C: memory; D: motor speed), separately for the two treatment arms in the irradiated patients only. Estimated marginal means are shown for each evaluation point separately for each treatment arm, with the vertical bars representing the 95% confidence interval of the group mean.
At the individual level, again we combined both arms. Compared to baseline, the majority of patients improved or remained stable at 12 and 24 months of follow-up. Only a minority (0%–11%) decreased in neurocognitive functioning, and only in the domains of information processing speed and motor speed (Supplementary Table 4).
Neurocognitive Functioning in Relation to Radiological Features
For brain volumes and Fazekas score at group level at each time point, see Supplementary Table 5. In the cross-sectional analyses at 6, 12, and 24 months, without adjustment for the amount of WMA or brain volume at baseline on MRI, we observed weak correlations between neurocognitive scores and the degree of WMA (between +0.01 and −0.66) or brain atrophy (between +0.06 and −0.50; Supplementary Table 6).
In the longitudinal analysis we observed inverse associations between changes in the degree of WMA or brain atrophy, and changes in neurocognitive scores, with increasing WMA and atrophy correlating with a deterioration in neurocognitive functioning up to 2 years posttreatment (Table 2). Although significant in all domains except for memory, the changes in neurocognitive scores were rather small (regression coefficients ranged between −0.048 and −0.347), indicating that 1-point increase in the Fazekas sum score resulted in only a small, not clinically relevant, deterioration in neurocognitive functioning. For brain atrophy, a 10% decrease of brain volume was significantly associated with a deterioration in memory of −0.921 points (Table 2). Other associations were not significant or clinically relevant.
Table 2.
Regression Coefficients Showing the Adjusted Association Between a Change in the Fazekas Score and Brain Volume on the One Hand, and Changes in Neurocognitive Functioning Domain Scores on the Other Hand, up to 2 Years Posttreatment
| Fazekas Sum Scorea, β | P-value | Brain Atrophyb, β | P-value | |
|---|---|---|---|---|
| Attention/executive functioning | −0.048 | .029 | −0.212 | .505 |
| Information processing | −0.086 | .009 | −0.753 | .095 |
| Memory | −0.050 | .165 | −0.921 | .027 |
| Motor speed | −0.347 | .000 | −1.774 | .163 |
Correlation coefficients (β) reflect the association between a change in z-score and a change in the sum Fazekas score (max. 30) with 1 point, or a 10% change in brain volume.
aCorrected for sex, age, education, and multiple lesions at baseline.
bCorrected for sex, age, education, and multiple lesions and total skull volume at baseline.
Discussion
The addition of rituximab to standard MBVP chemotherapy did not improve the event-free survival and did not impact HRQoL.8,14 The current analysis shows that this treatment regimen resulted in a significant improvement in all neurocognitive domains, compared to baseline, although these differences were not clinically relevant except for motor speed. There were no significant or clinically relevant differences in neurocognitive functioning between those treated with and without rituximab.
Impairments in neurocognitive functioning or behavioral problems are reported to be presenting symptoms in 32%–48% of patients with PCNSL.28 In this study, 90% of the patients had impairments in at least one neurocognitive domain before treatment. The slightly better scores in all domains at the “end of treatment” compared to baseline, with a stabilization thereafter, is a pattern that has been described in multiple PCNSL cohorts.12,29–31 These findings suggest that neurocognitive functioning is mostly hampered by the tumor itself, and that treating the tumor results in improved neurocognitive functioning. Additionally, we found that the extent of improvement between baseline and “end of treatment” was not clinically relevant, except for motor speed.
The results at the individual patient level support the finding that neurocognitive scores improved over time, although most patients remained stable, and only a minority (0%–7%) deteriorated. Only in the motor speed domain the majority of the patients improved at 12 and 24 months, compared to baseline. The range of z-scores was wider for the Grooved Pegboard Test (GPT) in both treatment arms relative to the other cognitive tests. This is likely the result of fewer patients having been included in this part of the cognitive test, resulting in a wider confidence interval. Fewer patients were tested because the GPT was not performed in all centers because they were not equipped for this test. Nevertheless, this supports the results of the longitudinal analyses (linear mixed models) suggesting that motor speed improved over time to a clinically relevant extent. This finding is, however, in contrast with other studies showing less pronounced improvements or even deterioration in this domain, particularly when compared to other domains.32,33 An explanation for this may be that patients in our cohort had very low scores at baseline, allowing patients to improve. It should be noted though, that despite the improvement, the estimated marginal means continued to be lower than the norm population (ie, <−1 SD).
In irradiated patients, scores on all neurocognitive domains rather unexpectedly remained stable for up to 2 years after treatment with 30-Gy (20 × 1.5 Gy) WBRT in both arms, compared to the scores shortly after WBRT. We used “after WBRT” as baseline because we expected maximal reduction of tumor and tumor-related symptoms at that time point. Stable neurocognitive functioning was also found in a previously reported small cohort of PCNSL patients treated with HD-MTX-based chemotherapy combined with rituximab and followed by reduced dose (rd)WBRT (23.4 Gy).12 In that same cohort, a small but significant decline in the neurocognitive domains attention and memory was observed between 3 and 5 years posttreatment.34 This late, nonclinically relevant deterioration, however, was also observed in patients who received autologous stem cell transplantation (ASCT) instead of WBRT.34 Two randomized trials in adult PCNSL patients compared WBRT with ASCT as consolidation therapy.29,31 In the IELSG-32 study, 118 patients who achieved at least stable disease after induction chemotherapy were randomized for ASCT or 36-Gy WBRT as consolidation.29 After 2 years of follow-up, those who received WBRT had significantly worse scores in attention/executive functioning and memory domains.29 In the PRECIS study 104 patients aged 18–60 years were randomized between ASCT and 40-Gy WBRT as consolidation therapy.31 Similarly, significant deterioration was seen in attention/executive functioning in irradiated patients compared to “end of induction chemotherapy,” while those treated with ASCT remained stable for up to 3 years of follow-up.31 Several factors could explain the discrepancy between the above two contemporary studies and our study regarding neurocognitive functioning after WBRT. First, the lower total dose and fraction dose used in our study could reduce the negative impact of WBRT, as suggested by the findings of Morris et al.12 Second, it is possible that in our study longer follow-up will show deterioration of neurocognitive function. Such late deterioration has also been found to occur in patients with other brain tumors, such as low-grade glioma, in whom neurocognitive deterioration after (focal) radiotherapy was found after 12 years but not after 6 years.35,36 Lastly, the absence of published individual scores and/or z-scores of the neurocognitive tests in the IELSG-3229 and PRECIS31 studies do not allow estimation of the magnitude of changes in neurocognition, and consequently the clinical relevance of these changes, which may affect the interpretation of results.
Up to 2 years posttreatment, we observed that the increase in the degree of WMA was significantly associated with worsening in all neurocognitive domains except memory, while an increase of brain atrophy was associated with worsening in the memory domain only. The associations, however, were weak to moderate, indicating that in the first 2 years posttreatment the impact of WMA and brain atrophy on neurocognitive functioning seems modest, possibly partially because the extent of decrease in brain volume was limited. In a large (n = 80) long-term PCNSL survivors cohort (median follow-up of 5·5 years, range 2–26 years), the amount of WMA was significantly correlated with worse neurocognitive functioning on the long term. Moreover, those treated with WBRT (n = 15; 45–60 Gy) in this survivors cohort had twice as much WMA as those treated without WBRT (n = 65; P < .001), though this resulted in a clinically relevant difference in the motor speed domain only.10 In contrast, although more WMA were observed after rdWBRT (23.4 Gy) than after ASCT (70% vs 40%, P = .03), there was no difference in neurocognitive functioning between these groups, up to 5 years posttreatment.34 While assessed in small groups and with different durations of follow-up, these results suggest that radiation dose could be crucial for neurocognitive functioning in PCNSL patients and this is supported by our findings. Longer follow-up is nevertheless needed to determine the effect of 30-Gy WBRT and of white matter changes and cerebral atrophy on neurocognitive functioning in our cohort on the longer term. We were unable to investigate a direct effect of rituximab on WMA or cerebral atrophy because the number of patients in these subgroups became too small for a meaningful analysis. A recent, small (n = 47) retrospective study, however, found after a mean follow-up of 5 years that more patients treated with rituximab plus HD-MTX developed white matter lesions (68%), compared to rituximab naïve patients (46%).37 Although this finding does not necessarily support causation, further analysis might help to determine the etiology of these lesions.
The strengths of this study are the large, uniformly treated group of patients in which radiological assessments were done over time and neurocognitive functioning in multiple domains was assessed prospectively, allowing extensive analyses. Limitations of our study are the limited time of follow-up, that is, 2 years after end of treatment,38 and our relatively crude, visual measurement of the WMA with the Fazekas score as opposed to automatic exact measurements of white matter abnormalities, which may have masked an existing effect of WMA on neurocognition. Automatic segmentation of WMA was not possible due to different scan protocols in different including centers. Although we had some missing neurocognitive data over time, our longitudinal analyses were not hampered by this since we used linear mixed models, which deal with missing data in a sophisticated way. For all cognitive tests we used norm data from the Dutch population, since most patients were treated in Dutch centers. Although unlikely, it cannot be ruled out that other norms should have been applied to patients treated in Australia and New Zealand. Although this may have resulted in an over- or underestimation of the actual z-scores of patients recruited in Australia and New Zealand, interpretation of the results in terms of both a statistical and clinical significance will reduce this bias. Lastly, we could not compare irradiated with nonirradiated patients with respect to neurocognitive functioning and radiological changes, because these patients differed in age due to the study design (ie, irradiation in younger patients only). For this same reason we could not compare younger and older patients.
In conclusion, this analysis showed no effect of the addition of rituximab on neurocognitive functioning, neither positive nor negative at 2 years of follow-up. The lack of effect on event-free survival,8 the primary endpoint, as well as HRQoL14 and this neurocognitive study, as secondary endpoints, do not, however, support the use of rituximab in patients with newly diagnosed PCNSL, at least when treated with MBVP chemotherapy. Whether specific subgroups of patients benefit from this treatment regimen remains to be investigated. Moreover, in the first 2 years posttreatment, a lower dose of WBRT was not harmful for neurocognitive functioning, compared to just after WBRT. The association between white matter abnormalities and brain atrophy and neurocognitive functioning was modest and longer follow-up is needed to draw definitive conclusions.
Supplementary Material
Funding
The HOVON 105/ALLG NHL 24 trial was funded by Roche, the Dutch Cancer Society (grant number: 2009-4589), and Stichting STOPhersentumoren.nl.
Conflict of interest statement. Prof. Smits reports financial support from Parexel Ltd for independent trial review, paid to the institution, and from GE Healthcare for speaker fees, paid to the institution, outside the submitted work; Prof. van den Bent reports grants from Stichting STOPhersentumoren, during the conduct of the study; Dr. Bromberg reports grants from Roche, during the conduct of the study, all other authors report no disclosures.
Authorship statement. J.E.C.B., S.I., and J.K.D. initiated and designed the study, and were involved in data collection, data interpretation, and writing the manuscript. K.B. was involved in study design, data analysis and interpretation, and writing the manuscript. M.v.d.M., L.D., E.J.J.H., M.S., M.J.B.T., and M.J.v.d.B. were involved in designing the study, data collection, data interpretation, and writing the manuscript. H.C.A., T.S., G.C., H.S., J.M.Z., D.B., and R.H.E. were involved in data collection, data interpretation, and writing the manuscript. M.B. was involved in study design and writing the manuscript.
Data Availability Statement
Individual de-identified participant data, collected for this study, including the statistical analysis plan will be made available for other research to others upon request, after approval by the HOVON executive board. The data will be available until a maximum of 15 years after the study has ended.
Please find the trial protocol, a Data Request Form, and the criteria for data sharing on www.hovon.nl.
References
- 1. van der Meulen M, Dinmohamed AG, Visser O, Doorduijn JK, Bromberg JEC. Improved survival in primary central nervous system lymphoma up to age 70 only: a population-based study on incidence, primary treatment and survival in the Netherlands, 1989–2015. Leukemia. 2017;31(8):1822–1825. [DOI] [PubMed] [Google Scholar]
- 2. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Correa DD, Maron L, Harder H, et al. . Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol. 2007;18(7):1145–1151. [DOI] [PubMed] [Google Scholar]
- 4. Boele FW, Zant M, Heine EC, et al. . The association between cognitive functioning and health-related quality of life in low-grade glioma patients. Neurooncol Pract. 2014;1(2):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aaronson NK, Taphoorn MJ, Heimans JJ, et al. . Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. [DOI] [PubMed] [Google Scholar]
- 6. Coiffier B, Lepage E, Briere J, et al. . CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. [DOI] [PubMed] [Google Scholar]
- 7. Pfreundschuh M, Schubert J, Ziepert M, et al. . Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–116. [DOI] [PubMed] [Google Scholar]
- 8. Bromberg JEC, Issa S, Bakunina K, et al. . Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019;20(2):216–228. [DOI] [PubMed] [Google Scholar]
- 9. Harder H, Holtel H, Bromberg JE, et al. . Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology. 2004;62(4):544–547. [DOI] [PubMed] [Google Scholar]
- 10. Doolittle ND, Korfel A, Lubow MA, et al. . Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013;81(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neuwelt EA, Guastadisegni PE, Várallyay P, Doolittle ND. Imaging changes and cognitive outcome in primary CNS lymphoma after enhanced chemotherapy delivery. AJNR Am J Neuroradiol. 2005;26(2):258–265. [PMC free article] [PubMed] [Google Scholar]
- 12. Morris PG, Correa DD, Yahalom J, et al. . Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fliessbach K, Helmstaedter C, Urbach H, et al. . Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology. 2005;64(7):1184–1188. [DOI] [PubMed] [Google Scholar]
- 14. van der Meulen M, Bakunina K, Nijland M, et al. . Health-related quality of life after chemotherapy with or without rituximab in primary central nervous system lymphoma patients: results from a randomised phase III study. Ann Oncol. 2020;31(8):1046–1055. [DOI] [PubMed] [Google Scholar]
- 15. Abrey LE, Batchelor TT, Ferreri AJ, et al. . Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. [DOI] [PubMed] [Google Scholar]
- 16. Wechsler D. Wechsler Adult Intelligence Scale (WAIS-III). 3rd ed. San Antonio, TX: Harcourt Brace & Co; 1997. [Google Scholar]
- 17. Reitan RM. Trail Making Test. Manual for Administration and Scoring. Tuscon, AZ: Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- 18. Lezak MD. Neuropsychological Assessment. New York: York Oxford University Press; 1976. [Google Scholar]
- 19. Lezak MD, Howieson DB, Bigler ED, Tranel D.. Neuropsychological Assessment. 5th rev. ed. New York: Oxford Univerity Press; 2012. [Google Scholar]
- 20. Heaton RK, Grant I, Matthews CG.. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings and Clinical Applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 21. Nelson H. National Adult Reading Test (NART): Test Manual. Windsor, UK: Nfer-Nelson; 1982. [Google Scholar]
- 22. Schmand B, Bakker D, Saan R, Louman J. [The Dutch Reading Test for Adults: a measure of premorbid intelligence level] De Nederlandse Leestest voor Volwassenen: een maat voor het premorbide intelligentieniveau. Tijdschr Gerontol Geriatr. 1991;22(1):15–19. [PubMed] [Google Scholar]
- 23. Wahlund LO, Barkhof F, Fazekas F, et al. . A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–1322. [DOI] [PubMed] [Google Scholar]
- 24. de Boer R, Vrooman HA, van der Lijn F, et al. . White matter lesion extension to automatic brain tissue segmentation on MRI. Neuroimage. 2009;45(4):1151–1161. [DOI] [PubMed] [Google Scholar]
- 25. Mulder J, Bouma JM, Schmand B. Nederlandse leestest voor volwassenen. In: Bouma JM, Mulder J, Lindeboom J, Schmand B, eds. Handboek Neuropsychologische Diagnostiek. Amsterdam, The Netherlands: Pearson Assessment and Information B.V.; 2012:127–138. [Google Scholar]
- 26. Verhage F. Intelligentie En Leeftijd Onderzoek bij Nederlanders van Twaalf tot Zevenenzeventig Jaar [Intelligence and Age: Research Study in Dutch Individuals Aged Twelve to Seventy-Seven]. Assen, The Netherlands: Van Gorcum/Prakke & Prakke; 1964. [Google Scholar]
- 27. van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. The letter digit substitution test: normative data for 1,858 healthy participants aged 24–81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28(6):998–1009. [DOI] [PubMed] [Google Scholar]
- 28. Bataille B, Delwail V, Menet E, et al. . Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92(2):261–266. [DOI] [PubMed] [Google Scholar]
- 29. Ferreri AJM, Cwynarski K, Pulczynski E, et al. . Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4(11):e510–e523. [DOI] [PubMed] [Google Scholar]
- 30. van der Meulen M, Dirven L, Habets EJJ, van den Bent MJ, Taphoorn MJB, Bromberg JEC. Cognitive functioning and health-related quality of life in patients with newly diagnosed primary CNS lymphoma: a systematic review. Lancet Oncol. 2018;19(8):e407–e418. [DOI] [PubMed] [Google Scholar]
- 31. Houillier C, Taillandier L, Dureau S, et al. . Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol. 2019;37(10):823–833. [DOI] [PubMed] [Google Scholar]
- 32. Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62(4):548–555. [DOI] [PubMed] [Google Scholar]
- 33. Omuro A, Correa DD, DeAngelis LM, et al. . R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Correa DD, Braun E, Kryza-Lacombe M, et al. . Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol. 2019;144(3):553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klein M, Heimans JJ, Aaronson NK, et al. . Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 36. Douw L, Klein M, Fagel SS, et al. . Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 37. Estephan F, Ye X, Dzaye O, et al. . White matter changes in primary central nervous system lymphoma patients treated with high-dose methotrexate with or without rituximab. J Neurooncol. 2019;145(3):461–466. [DOI] [PubMed] [Google Scholar]
- 38. Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16(3):859–863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual de-identified participant data, collected for this study, including the statistical analysis plan will be made available for other research to others upon request, after approval by the HOVON executive board. The data will be available until a maximum of 15 years after the study has ended.
Please find the trial protocol, a Data Request Form, and the criteria for data sharing on www.hovon.nl.