Abstract
Objectives:
In the United States, trends in the initial treatment approach for ovarian cancer reflect a shift in paradigm towards increased use of neoadjuvant chemotherapy and interval cytoreductive surgery. The aim of this study was to evaluate the trends in surgical cytoreductive procedures in ovarian cancer patients who underwent either primary or interval cytoreductive surgery.
Methods:
This retrospective, population-based study examined patients with stage III/IV ovarian cancer diagnosed between January 2000 to December 2013 identified using SEER-Medicare. Small or large bowel resection, ostomy creation, and upper abdominal procedures were identified using relevant billing codes and compared over time. A 1:1 primary and interval cytoreductive propensity matched cohort was created using demographic and clinical variables. 30-day complications and use of acute care services were compared.
Results:
A total of 5,417 women were identified. 34% underwent bowel resections, 16% ostomy creation, and 8% upper abdominal procedures. There was an increase in bowel resections and upper abdominal procedures from 2000-2013 in patients who underwent primary cytoreductive surgery. Compared to patients who received primary cytoreduction, patients who underwent interval cytoreductive surgery were less likely to undergo bowel resection (OR=0.50; 95% CI [0.41, 0.61]) or ostomy creation (OR=0.48; 95% CI [0.42, 0.56]). Upper abdominal procedures did not differ between groups. For patients who underwent primary cytoreductive surgery, these procedures were associated with intensive care unit stay (4.6% vs <2%, p<0.01). In both primary and interval cytoreductive surgery patients, receipt of bowel and upper abdominal procedures was associated with multiple 30-day postoperative complications and higher rates of readmission and emergency room visits.
Conclusions:
Performance of upper abdominal procedures in ovarian cancer patients increased from 2000-2013. Interval cytoreductive surgery was associated with decreased likelihood of bowel surgery. In matched primary and interval cytoreductive surgery cohorts, receipt of these procedures were associated with increased likelihood of postoperative complications and use of acute care services.
Introduction
For advanced stage ovarian cancer, neoadjuvant chemotherapy followed by interval cytoreductive surgery is a studied and utilized treatment alternative to primary cytoreductive surgery followed by chemotherapy when there is a low likelihood of optimal cytoreduction or in the case of a poor surgical candidate1-4. A recent joint white paper by the Society of Gynecologic Oncology and American Society of Clinical Oncology recommended the initial approach to advanced ovarian cancer be tailored to the probability of achieving optimal cytoreduction of disease and the potential harm from surgery4. Observational studies in the United States have noted trends in initial approach to treatment for ovarian cancer that reflect a shift in paradigm towards increasing use of interval cytoreductive surgery. A recent multi-institutional study of advanced ovarian cancer patients treated at National Cancer Institute-designated cancer centers from 2003 to 2012 found increased use of neoadjuvant chemotherapy and interval debulking for stage IIIC and IV patients over the time period studied3. Similarly, Melamed et al. found a similar trend of increasing number of patients receiving neoadjuvant chemotherapy and interval surgery between 2004-2013 using the National Cancer Database5.
As neoadjuvant chemotherapy reduces disease burden, less extensive surgical procedures may be needed to achieve optimal cytoreduction with interval cytoreductive surgery6. This includes surgery in the upper abdomen, such as diaphragm stripping or resection, splenectomy, distal pancreatectomy, liver resection, and other procedures which increase the complexity of the cytoreductive surgery such as bowel resection or ostomy creation, which are associated with additional perioperative risk of morbidity and mortality7,8. There is currently a gap in knowledge in describing the trends of use of specific cytoreductive surgery in primary and interval debulking surgeries in the United States.
Given the published trend for increased use of interval cytoreductive surgery, this study sought to describe trends in the performance of extended surgical cytoreductive procedures in advanced stage ovarian cancer using a Medicare patient cohort. We also sought to describe any differences in morbidity that may be associated with extended surgical procedures in this cohort between patients who underwent initial management with neoadjuvant chemotherapy or primary cytoreductive surgery.
Methods
This was a retrospective, population-based study using the linked of Surveillance, Epidemiology, and End Results registry (SEER) database with Medicare health claims data from January 2000 to December 2013. The SEER program of the National Cancer Institute collects and publishes incidence and survival data from population-based cancer registries in the United States, covering a diversity of geographic areas across the U.S. and over a third of the U.S. population (https://seer.cancer.gov). Medicare is a large federal health insurance program that includes Americans 65 and older, and includes claims data for both inpatient and outpatient health services. This study was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board. Women who were 66 years or older with stage III or IV epithelial ovary cancer who received both an ovarian cancer-related surgery and a platinum and taxane-based chemotherapy were included. The primary cytoreductive surgery cohort included patients who received surgery prior to chemotherapy, and the interval cohort included patients who received chemotherapy prior to surgery. Patients who received chemotherapy alone or surgery alone were excluded. Patients who had received intraperitoneal chemotherapy were also excluded.
All socio-demographic variables and tumor characteristics were collected from SEER. This included year of diagnosis, age at diagnosis, race/ethnicity, socioeconomic status, marital status and geographic region. Socioeconomic status was estimated using census tract data and data from the 2000 Census including education, poverty level, and income level. Comorbidity index score was estimated using Klabunde-modified Charleson co-morbidity score9,10. Tumor characteristics included tumor size, histology, stage and grade.
Medical comorbidity data, all treatment and complications data was collected using Medicare claims data and ICD-9 diagnosis and procedure codes, Common Procedure Terminology (CPT) codes, Healthcare Common Procedure Coding System (HCPCS) codes, and other revenue center codes. Receipt of small or large bowel resection, ostomy creation, and/or upper abdominal procedures was identified using relevant billing codes. Outcomes included postoperative complications such as wound infection, cardiac and respiratory complications, infectious complications, fluid and electrolyte imbalances, ICU or emergency room admissions after surgery or readmissions within 30 days from receipt of surgery.
Two separate analyses were performed. First, all women in the sample were analyzed in order to describe the trends in receipt of surgical procedures over the time period studied in both primary and interval debulking surgery patients. We used frequency and percentages to describe categorical variables, and we used mean, and median for continuous variables. Differences in patient characteristics and treatments were evaluated using Chi-square test or F test. Second, propensity matching was used to create a matched cohort to compare treatment complications related to surgical procedures between those patients who underwent primary vs. those who underwent interval debulking surgery. Based on patients’ age, year of diagnosis, comorbidity index score, marital status, geographic region, socioeconomic status and tumor characteristics we used logistic regression modeling to generate a propensity score then used to match 1:1 patients who underwent primary surgery with patient who received interval surgery. The propensity matching was aimed to balance patients’ baseline characteristics by the treatment status. The matching process utilized a greedy algorithm as well as the nearest available pair matching method, where the cases were ordered sequentially according to propensity score. Once a control was matched to a case, the control was no longer considered to be available as a match for other cases. Complications and use of acute care by treatment group were then analyzed in propensity-score matched cohorts. All analyses were conducted with the SAS statistical software program (version 9.3, SAS Institute, Cary, NC). P-values of <0.05 were considered statistically significant.
Results
Our cohort included 5,417 patients with stage III/IV epithelial ovarian cancer diagnosed between 2000 and 2013 identified in the linked SEER-Medicare database. Of those patients, 22.5% (n=1221) received neoadjuvant chemotherapy followed by interval cytoreductive surgery and 77.5% (n=4196) underwent primary cytoreductive surgery followed by chemotherapy. In this unmatched cohort, several differences were seen between patients who received primary surgery compared with those who underwent interval surgery, including differences in geographic region, setting (urban/rural), comorbidities, and tumor characteristics (Table 1). Stage at presentation also differed between treatment groups. Patients with stage IV disease were more likely to undergo interval cytoreductive surgery; for patients with stage III disease, primary debulking was more common.
Table 1.
Patient characteristics
| Characteristics | Treatment Group |
P- value |
Total, n (%) | ||
|---|---|---|---|---|---|
| NACT, n (%) |
Primary Surgery, n (%) |
||||
| Patientsa | |||||
| Age at diagnosis (y) | 0.19b | ||||
| 66-70 | 387 (31.7) |
1326 (31.6) |
1713 | (31.6) | |
| 71-75 | 379 (31.0) |
1264 (30.1) |
1643 | (30.3) | |
| 76-80 | 270 (22.1) |
1038 (24.7) |
1308 | (24.1) | |
| 80+ | 185 (15.2) |
568 (13.5) |
753 | (13.9) | |
| mean (SD) | 74.1 ± 5.6 | 74.1 ± 5.5 | 0.90c | 74.1 ± 5.5 | |
| Year of diagnosis | <0.01b | ||||
| 2000 | 69 (5.7) |
363 (8.7) |
432 | (8.0) | |
| 2001 | 57 (4.7) |
372 (8.9) |
429 | (7.9) | |
| 2002 | 80 (6.6) |
371 (8.8) |
451 | (8.3) | |
| 2003 | 89 (7.3) |
365 (8.7) |
454 | (8.4) | |
| 2004 | 70 (5.7) |
342 (8.2) |
412 | (7.6) | |
| 2005 | 89 (7.3) |
335 (8.0) |
424 | (7.8) | |
| 2006 | 79 (6.5) |
333 (7.9) |
412 | (7.6) | |
| 2007 | 82 (6.7) |
257 (6.1) |
339 | (6.3) | |
| 2008 | 90 (7.4) |
311 (7.4) |
401 | (7.4) | |
| 2009 | 93 (7.6) |
274 (6.5) |
367 | (6.8) | |
| 2010 | 100 (8.2) |
234 (5.6) |
334 | (6.2) | |
| 2011 | 99 (8.1) |
223 (5.3) |
322 | (5.9) | |
| 2012 | 109 (8.9) |
206 (4.9) |
315 | (5.8) | |
| 2013 | 115 (9.4) |
210 (5.0) |
325 | (6.0) | |
| Comorbidity index | 0.05b | ||||
| 0 | 816 (66.8) |
2956 (70.4) |
3772 | (69.6) | |
| 1 | 278 (22.8) |
865 (20.6) |
1143 | (21.1) | |
| ≥2 | 127 (10.4) |
375 (8.9) |
502 | (9.3) | |
| Marital status at diagnosis | 0.05b | ||||
| Married | 585 (47.9) |
2096 (50.0) |
2681 | (49.5) | |
| Unknown | 25 (2.0) |
128 (3.1) |
153 | (2.8) | |
| Not Married | 611 (50.0) |
1972 (47.0) |
2583 | (47.7) | |
| Race | <0.01b | ||||
| African American, non-Hispanic | 67 (5.5) |
189 (4.5) |
256 | (4.7) | |
| Hispanic | 63 (5.2) |
184 (4.4) |
247 | (4.6) | |
| Other/Unknown | 57 (4.7) |
124 (3.0) |
181 | (3.3) | |
| White, non-Hispanic | 1034 (84.7) |
3699 (88.2) |
4733 | (87.4) | |
| Region | <0.01b | ||||
| Midwest | 108 (8.8) |
497 (11.8) |
605 | (11.2) | |
| Northeast | 271 (22.2) |
874 (20.8) |
1145 | (21.1) | |
| South | 264 (21.6) |
1048 (25.0) |
1312 | (24.2) | |
| West | 578 (47.3) |
1777 (42.3) |
2355 | (43.5) | |
| Area of residence | <0.01b | ||||
| Large metropolitan | 706 (57.8) |
2151 (51.3) |
2857 | (52.7) | |
| Metropolitan | 343 (28.1) |
1299 (31.0) |
1642 | (30.3) | |
| Urban | 73 (6.0) |
272 (6.5) |
345 | (6.4) | |
| Less urban | 74 (6.1) |
391 (9.3) |
465 | (8.6) | |
| Rural | 25 (2.0) |
83 (2.0) |
108 | (2.0) | |
| SEER registry | <0.01b | ||||
| Connecticut | 102 (8.4) |
235 (5.6) |
337 | (6.2) | |
| Detroit | 59 (4.8) |
224 (5.3) |
283 | (5.2) | |
| Hawaii | 11 (0.9) |
27 (0.6) |
38 | (0.7) | |
| Iowa | 49 (4.0) |
273 (6.5) |
322 | (5.9) | |
| New Mexico | 25 (2.0) |
98 (2.3) |
123 | (2.3) | |
| Seattle | 117 (9.6) |
247 (5.9) |
364 | (6.7) | |
| Utah | 26 (2.1) |
114 (2.7) |
140 | (2.6) | |
| Kentucky | 51 (4.2) |
279 (6.6) |
330 | (6.1) | |
| Louisiana | 58 (4.8) |
257 (6.1) |
315 | (5.8) | |
| New Jersey | 169 (13.8) |
639 (15.2) |
808 | (14.9) | |
| Georgia | 155 (12.7) |
512 (12.2) |
667 | (12.3) | |
| California | 399 (32.7) |
1291 (30.8) |
1690 | (31.2) | |
| Census tract percent below poverty (Census 2000), mean (SD) | 9.6 ± 8.4 | 10.1 ± 8.5 | 0.07c | 10.0 ± 8.5 | |
| Census tract median income (Census 2000), mean (SD) (US$) | 53469.0 ± 22695.1 | 52658.0 ± 24881.0 | 0.32c | 52835.8 ± 24418.6 | |
| Census tract percent non high school graduates (Census 2000), mean (SD) | 16.2 ± 11.6 | 16.9 ± 11.9 | 0.07c | 16.7 ± 11.9 | |
| Tumor | |||||
| Size (mm) | <0.01b | ||||
| 0 -25 | 99 (8.1) |
317 (7.6) |
416 | (7.7) | |
| 26 – 50 | 109 (8.9) |
446 (10.6) |
555 | (10.2) | |
| 51 -75 | 104 (8.5) |
476 (11.3) |
580 | (10.7) | |
| 76 + | 176 (14.4) |
1241 (29.6) |
1417 | (26.2) | |
| Unknown | 733 (60.0) |
1716 (40.9) |
2449 | (45.2) | |
| Mean (SD) | 69.6 ± 55.8 | 86.1 ± 66.9 | <0.01c | 83.4 ± 65.5 | |
| Histology groupings | <0.01b | ||||
| Clear cell | 17 (1.4) |
76 (1.8) |
93 | (1.7) | |
| Endometrioid | 18 (1.5) |
271 (6.5) |
289 | (5.3) | |
| Serous | 856 (70.1) |
3180 (75.8) |
4036 | (74.5) | |
| Mucinous/Other adenocarcinomas | 330 (27.0) |
669 (16.0) |
999 | (16.7) | |
| AJCC stage | <0.01b | ||||
| Stage III | 662 (54.2) |
2967 (70.7) |
3629 | (67.0) | |
| Stage IV | 559 (45.8) |
1229 (29.3) |
1788 | (33.0) | |
| Grade | <0.01b | ||||
| Grade I | 14 (1.1) |
93 (2.2) |
107 | (2.0) | |
| Grade II | 92 (7.5) |
565 (13.5) |
657 | (12.1) | |
| Grade III/IV | 667 (54.6) |
2875 (68.5) |
3542 | (65.4) | |
| Grade Unknown | 448 (36.7) |
663 (15.8) |
1111 | (20.5) | |
Values are censored to maintain patient confidentiality (n ≤ 11).
P values were derived using the chi-square test for comparing differences between two treatment groups.
P values were derived using the F test for comparing means between two treatment groups.
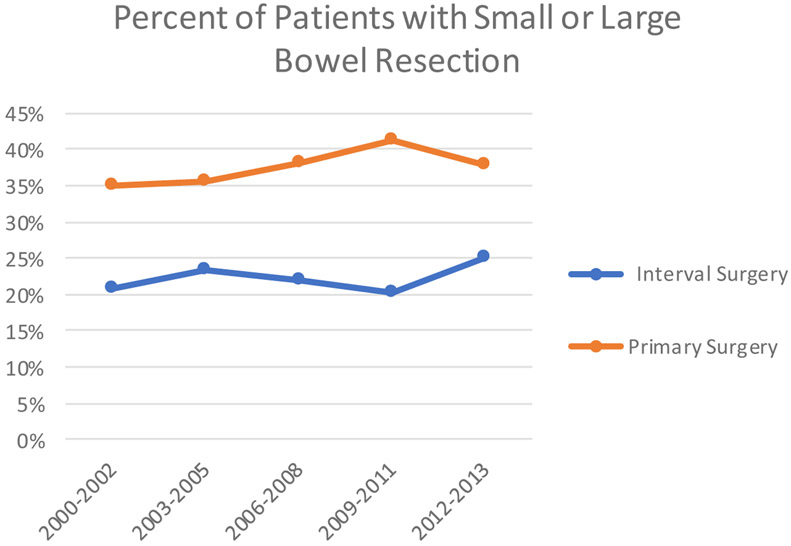
The overall rate of bowel or upper abdominal procedures was low with 34% of patients undergoing a large or small bowel resection, 16% undergoing ostomy creation, and 8% upper abdominal procedures (pancreatectomy, splenectomy, liver resection, or diaphragm resection/stripping). Interval cytoreductive surgery patients were less likely to have bowel resection (OR = 0.50, 95% CI 0.41, 0.61) or ostomy creation (OR = 0.48, 95% CI 0.42, 0.56) compared to primary cytoreductive surgery patients. The likelihood of undergoing upper abdominal procedures did not differ between treatment groups. When comparing trends in procedures over the time period studied, there was an increase in bowel resection (p=0.013) and upper abdominal procedures (p<.0001), but no change in rates of ostomy creation for patients who underwent primary surgery from 2000-2013 (Figure 1A-C). There were no statistically significant differences seen in ostomy creation, bowel resection, or upper abdominal procedures in patients undergoing neoadjuvant chemotherapy.
Figure 1A.
Trend in receipt of upper abdominal procedures in patients who underwent primary surgery and interval surgery in matched cohort, 2000-2013
Figure 1C.
Trend in receipt of bowel resection in patients who underwent primary surgery and interval surgery in matched cohort, 2000-2013
In the 1:1 matched cohort, bowel and upper abdominal procedures were associated with multiple 30-day complications and use of acute care services (Table 2). In both treatment groups, these surgical procedures were associated with wound infection, cardiac and respiratory complications, general infectious complications, other surgical complications, fluid and electrolyte imbalances. In the interval cytoreductive surgery group, bowel and upper abdominal surgery were associated with shock and acute renal failure. This association was not seen in the primary cytoreductive surgery cohort. In both treatment groups, Emergency Room visits and readmissions were higher in patients who underwent bowel surgeries or upper abdominal procedures. In the primary cytoreductive surgery cohort, patients who underwent these procedures as part of their surgery were also more likely to have a postoperative intensive care unit stay (4.6% vs <2%, p<0.01). This association was not seen in the interval cytoreductive surgery group.
Table 2.
30-day complications, by treatment, matched cohort
| Neoadjuvant chemotherapy (n=1,123) | Primary cytoreductive surgery (n= 1,123) | |||||
|---|---|---|---|---|---|---|
| No bowel or upper abdominal procedures (n=796) |
Bowel or upper abdominal procedures (n=327) |
p value | No bowel or upper abdominal procedures (n=580) |
Bowel or upper abdominal procedures (n=543) |
p value | |
| 30-day complications, n (%) | ||||||
| Wound infection | 80 (10.1) | 58 (17.7) | <0.01 | 84 (14.5) | 114 (21.0) | <0.01 |
| Pulmonary embolus/DVT | 92 (11.6) | 37 (11.3) | 0.91 | 53 (9.1) | 62 (11.4) | 0.21 |
| Hematoma/hemorrhage | 196 (24.6) | 93 (28.4) | 0.18 | 135 (23.3) | 147 (27.1) | 0.14 |
| Other surgical complications* | 222 (27.9) | 169 (51.7) | <0.01 | 217 (37.4) | 320 (58.9) | <0.01 |
| Cardiac | 106 (13.3) | 61 (18.7) | 0.02 | 92 (15.9) | 112 (20.6) | 0.04 |
| Respiratory | 136 (17.1) | 101 (30.9) | <0.01 | 188 (32.4) | 243 (44.8) | <0.01 |
| Acute renal failure | 31 (3.9) | 25 (7.6) | <0.01 | 47 (8.1) | 45 (8.3) | 0.91 |
| Shock | 40 (5.0) | 33 (10.1) | <0.01 | 51 (8.8) | 54 (9.9) | 0.51 |
| Fluid/electrolyte imbalances | 211 (26.5) | 114 (34.9) | <0.01 | 178 (30.7) | 202 (37.2) | 0.02 |
| Other infections** | 77 (9.7) | 68 (20.8) | <0.01 | 88 (15.2) | 129 (23.8) | <0.01 |
| Emergency Room Visit | 105 (13.2) | 67 (20.5) | <0.01 | 111 (19.1) | 163 (30.0) | <0.01 |
| ICU Stay | 14 (1.8) | 12 (3.7) | 0.05 | ---*** | 25 (4.6) | <0.01 |
| Hospital readmission | 121 (15.2) | 81 (24.8) | <0.01 | 120 (20.7) | 140 (25.8) | 0.04 |
Bold indicates statistical significance with a p<0.05
Peritonitis, reopening laparotomy, surgical complication NOS, injury to vessel of abdomen/pelvis, suture or laceration of ureter, foreign body, stoma complications
Pneumonia/respiratory infection, sepsis/bacteremia, urinary tract infection, Clostridium difficile
Censored due to small cell size to protect patients’ confidentiality
Discussion
In this study of Medicare patients undergoing either primary or interval cytoreductive surgery for treatment of stage III or IV ovarian cancer, the use of upper abdominal procedures and bowel surgery increased in those patients undergoing primary cytoreductive surgery from 2000-2013. Performance of these procedures did not change in the interval cytoreductive surgery group over the time period studied. Not unsurprisingly, increased complexity of surgery was associated with increased likelihood of multiple postoperative complications, use of acute care services, and readmissions, and ICU admission.
The findings from this study align with other findings in the literature also noting a trend of increased complex surgery in ovarian cancer patients. Jones et al., using the Nationwide Inpatient Sample database, found an increase in resection of the colon, small intestine, liver, diaphragm, spleen, stomach, and ostomy formation in women undergoing surgery for ovarian cancer from 2008-201311. However, the authors were unable to define which patients underwent primary or interval cytoreductive surgery, limiting their ability to describe trends within these treatment groups. Our overall rate of bowel and upper abdominal surgical procedures in our cohort of 30% is similar to that seen by other authors12. While the increased use of neoadjuvant chemotherapy and interval cytoreductive surgery in advanced ovarian cancer followed publication and provider awareness of two landmark randomized controlled trials, observed trends in aggressiveness of surgical cytoreduction either in upfront surgery or at time of interval cytoreduction may be related to recent presentations at conferences and publications demonstrating benefits of complete resection of all visible tumor. Several studies have highlighted that the incorporation of upper abdominal procedures into surgical management for ovarian cancer has been shown to increase the rate of optimally cytoreduced patients13,14.
In our cohort, undergoing bowel and upper abdominal procedures during cytoreductive surgery confers an increased likelihood of morbidity for patients with advanced ovarian cancer. Postoperative ICU admission following ovarian cancer cytoreductive surgery is historically as high as 30%, and has been found to be more likely in patients undergoing primary cytoreductive surgery compared with those who undergo interval cytoreductive surgery15. Age, medical comorbidities and more radical procedures have been cited as risk factors for postoperative ICU admission for ovarian cancer patients16. In our study, patients who underwent upper abdominal surgery were more likely to have an ICU admission if these procedures were performed as part of upfront surgery compared to at the time of interval cytoreductive surgery. While we were unable to capture extent of tumor at the time of surgery, this finding may be related to the decreased disease burden seen in patients who undergo interval cytoreductive surgery5. Others have reported increased likelihood of unplanned thirty-day readmission rates in primary cytoreductive surgery patients compared to those who underwent neoadjuvant chemotherapy and interval cytoreductive surgery17.
There are limitations to the current study inherent to its study design. We were limited by the inability to control for unobserved confounders in our cohort such as operative effort, further granularity regarding extent of disease during surgery or residual disease after surgery. Although the use of national SEER Medicare dataset offers a large number of patients from a diverse treatment settings and geographic locations, other mitigating factors were unable to be captured, such as a determination of surgeon skill, individual provider experience, or subspecialty training. As the databases utilized in this current study do not differentiate between training of surgeons performing ovarian cancer surgery, additional information could be gained from learning if these procedures were being performed by or in conjunction with general gynecologists, gynecologic oncologists, or surgical oncologists. While initial treatment approach may depend on several clinical and non-clinical factors18, we also were unable to determine the exact rationale for treatment choice of primary or interval surgery in our cohort. Given our inclusion criteria of receipt of both surgery and chemotherapy, we did not include those patients who underwent primary cytoreductive surgery without adjuvant therapy. If those surgery only patients represent a group who suffered significant morbidity or mortality precluding adjuvant chemotherapy, we may be underestimating the adverse outcomes in the surgery group. Furthermore, our cohort was limited to a Medicare-linked database, and thus all participants older than age 65. Use of the SEER Medicare care dataset was chosen due to the ability to obtain and analyze a wide range of population-based oncologic data and specific clinically relevant outcomes, despite the limitation that our findings might not be readily applicable to a younger cohort of patients.
Due to the low volume of individual procedures our analysis combined all upper abdominal procedures into a singular variable. Morbidity may differ according to each individual upper abdominal procedure. Others have demonstrated the wide range and rates complications from pancreatectomy, diaphragmatic resection, diaphragmatic peritoneal stripping and splenectomy19-21. Finally, while mortality was not captured in the present study, our group has previously published survival outcomes in advanced ovarian cancer patients undergoing primary and interval cytoreductive surgery using SEER-Medicare. While there was no difference in survival in stage IV patients, among women with stage III disease, primary cytoreductive surgery was associated with improved overall survival but with higher rates of perioperative morbidity22. In contrast, Horner et al. using the National Cancer Database found that an increase in the utilization of neoadjuvant chemotherapy over a similar time period was associated with decreased 30- and 90-day mortality and increase in five-year survival, with an overall increase in complexity of surgery in all ovarian cancer patients23.
As with any large series relying on national data, our findings may not be generalizable to all providers caring for ovarian cancer patients. Rates of complications and outcomes associated with incorporation of upper abdominal surgery into ovarian cancer cytoreductive surgery may be influenced by the experience of the surgeon performing these procedures and institutional capacity manage patients undergoing these procedures and their complications, as has been pointed out by Chi et al.6.
Our data demonstrates increased use of upper abdominal procedures in ovarian cancer patients from 2000-2013, likely reflecting increased adoption of the goal of removal of all visible tumor. Strengths of this study include the large, population-based cohort allowing for a study of trends over time, and use of propensity score matching to reduce bias when comparing postoperative outcomes in neoadjuvant and primary cytoreductive surgery cases. As others have demonstrated, use of upper abdominal and bowel related surgical procedures as part of cytoreductive surgery is not without risk, and when taken as a composite variable, performance of these procedures appears to be associated with greater morbidity, and in the upfront setting, intensive care unit admission. Moving forward, further studies of pre-operative assessment may help to triage which patients may derive the greatest oncologic benefit from cytoreductive surgery while minimizing potential intra- or post-operative morbidity.
Figure 1B.
Trend in receipt of ostomy in patients who underwent primary surgery and interval surgery in matched cohort, 2000-2013
Highlights.
There is limited data on U.S. trends in use of surgical procedures in cytoreductive ovarian surgery.
In the population studied, there was an increase in performance of upper abdominal procedures from 2000-2013.
Use of upper abdominal or bowel surgery as part of ovarian cancer surgery was associated with increased morbidity.
Research support:
This work was supported by the T32 training grant for training of academic gynecologic oncologists T32-CA101642 (Dottino, Suidan), NIH-NCI grant K07-CA201013 (Meyer), NIH-NCI grant K08-CA234333 (Rauh-Hain) and the MD Anderson Cancer Center Support Grant P30-CA016672
Footnotes
Conflict of interest statement: L. A M. received research funding from AstraZeneca for unrelated research, and has participated in an advisory board for Clovis Oncology in 2016. C.C.S. received research funding from AstraZeneca for unrelated research. All other authors report no conflict of interest.
Version of this abstract was presented at the Society for Gynecologic Oncology on March 24-27, 2018 in New Orleans, LA
References
- 1.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N Engl J Med. 2010;363(10):943–953. [DOI] [PubMed] [Google Scholar]
- 2.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257. [DOI] [PubMed] [Google Scholar]
- 3.Meyer LA, Cronin AM, Sun CC, et al. Use and effectiveness of neoadjuvant chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2016;34(32):3854–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol Oncol. 2016;143(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol. 2016;143(2):236–240. [DOI] [PubMed] [Google Scholar]
- 6.Hou JY, Kelly MG, Yu H, et al. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol Oncol. 2007;105(1):211–217. [DOI] [PubMed] [Google Scholar]
- 7.Chi DS, Zivanovic O, Levinson KL, et al. The incidence of major complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal, and peritoneal carcinomas. Gynecol Oncol. 2010;119(1):38–42. [DOI] [PubMed] [Google Scholar]
- 8.Wright JD, Lewin SN, Deutsch I, et al. Defining the limits of radical cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2011;123(3):467–473. [DOI] [PubMed] [Google Scholar]
- 9.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 10.Klabunde CN, Harlan LC, Warren JL. Data Sources for Measuring Comorbidity. Med Care. 2006;44(10):921–928. [DOI] [PubMed] [Google Scholar]
- 11.Jones NL, Chen L, Chatterjee S, et al. National trends in extended procedures for ovarian cancer debulking surgery. Int J Gynecol Cancer. 2018;28(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez N, Miller A, Richard SD, et al. Upper abdominal procedures in advanced stage ovarian or primary peritoneal carcinoma patients with minimal or no gross residual disease: An analysis of Gynecologic Oncology Group (GOG) 182. Gynecol Oncol. 2013;130(3):487–492. [DOI] [PubMed] [Google Scholar]
- 13.Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114(1):26–31. [DOI] [PubMed] [Google Scholar]
- 14.Aletti GD, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107(1):77–85. [DOI] [PubMed] [Google Scholar]
- 15.Pepin K, Bregar A, Davis M, et al. Intensive care admissions among ovarian cancer patients treated with primary debulking surgery and neoadjuvant chemotherapy-interval debulking surgery. Gynecol Oncol. 2017;147(3):612–616. [DOI] [PubMed] [Google Scholar]
- 16.Díaz-Montes TP, Zahurak ML, Bristow RE. Predictors of extended intensive care unit resource utilization following surgery for ovarian cancer. Gynecol Oncol. 2007;107(3):464–468. [DOI] [PubMed] [Google Scholar]
- 17.Clark RM, Rice LW, Del Carmen MG. Thirty-day unplanned hospital readmission in ovarian cancer patients undergoing primary or interval cytoreductive surgery: systematic literature review. Gynecol Oncol. 2018. August;150(2):370–377 [DOI] [PubMed] [Google Scholar]
- 18.Hinchcliff E, Melamed A, Bregar A, et al. Factors associated with delivery of neoadjuvant chemotherapy in women with advanced stage ovarian cancer. Gynecol Oncol. 2018;148(1):168–173. 8 [DOI] [PubMed] [Google Scholar]
- 19.Bacalbasa N, Balescu I, Dima S, Brasoveanu V, Popescu I. Splenectomy as part of cytoreductive surgery in recurrent epithelial ovarian cancer. Anticancer Res. 2015;35(9):5097–5102. [PubMed] [Google Scholar]
- 20.Kehoe SM, Eisenhauer EL, Abu-Rustum NR, et al. Incidence and management of pancreatic leaks after splenectomy with distal pancreatectomy performed during primary cytoreductive surgery for advanced ovarian, peritoneal and fallopian tube cancer. Gynecol Oncol. 2009;112(3):496–500. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti Panici P, Di Donato V, Fischetti M, et al. Predictors of postoperative morbidity after cytoreduction for advanced ovarian cancer: Analysis and management of complications in upper abdominal surgery. Gynecol Oncol. 2015;137(3):406–411. [DOI] [PubMed] [Google Scholar]
- 22.Meyer LA, He W, Sun CC et al. Neoadjuvant chemotherapy in elderly women with ovarian cancer: rates of use and effectiveness. Gynecol Oncol. 2018;150(3):451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horner W, Peng K, Pleasant V et al. Trends in surgical complexity and treatment modalities utilized in the management of ovarian cancer in an era of neoadjuvant chemotherapy. Gynecol Oncol. 2019; 154(2):283–289. [DOI] [PubMed] [Google Scholar]