Abstract
Non-cystic fibrosis bronchiectasis represents a heterogenous spectrum of disorders characterised by an abnormal and permanent dilatation of the bronchial tree associated with respiratory symptoms. To date, diagnosis relies on computed tomography (CT) evidence of dilated airways. Nevertheless, definite radiological criteria and standardised CT protocols are still to be defined. Although largely used, current radiological scoring systems have shown substantial drawbacks, mostly failing to correlate morphological abnormalities with clinical and prognostic data. In limited cases, bronchiectasis morphology and distribution, along with associated CT features, enable radiologists to confidently suggest an underlying cause. Quantitative imaging analyses have shown a potential to overcome the limitations of the current radiological criteria, but their application is still limited to a research setting.
In the present review, we discuss the role of imaging and its current limitations in non-cystic fibrosis bronchiectasis. The potential of automatic quantitative approaches and artificial intelligence in such a context will be also mentioned.
Introduction
The term bronchiectasis refers to a clinicoradiological entity characterised by an abnormal and permanent dilatation of the bronchial tree associated with respiratory symptoms, including productive cough and dyspnoea.1 A rather rough aetiological classification distinguishes two main groups of bronchiectasis: cystic fibrosis (CF) bronchiectasis and non-CF bronchiectasis. The latter recognises a broad spectrum of causes and associations, ranging from chronic obstructive pulmonary disease to allergic bronchopulmonary aspergillosis (ABPA) and tuberculous-associated lung destruction.2–4 Nevertheless, a non-negligible proportion of patients remains diagnosed with idiopathic bronchiectasis despite extensive testing, facing a potentially worse prognosis as compared to those affected by a known, treatable disease.4,5
Due to the increasingly larger use of computed tomography (CT)6,7 and greater awareness among physicians of its clinical and prognostic implications (e.g. hospitalisation for severe exacerbations, increased mortality rate, etc), the prevalence of non-CF bronchiectasis has been progressively rising over the last decades,8,9 reaching up to 566 per 100,000 inhabitants in some patient cohorts.10,11 The diagnosis of such an entity strongly relies on imaging, which is essential to identify abnormally widened airways.12,13 Additionally, imaging allows to: (i) quantify the airway dilatation; (ii) suggest the aetiology (in a few cases though), and (iii) evaluate the course of disease.14 Although chest X-ray (CXR) has been indicated as the first-line imaging modality for assessing bronchiectasis, it has substantially lower sensitivity and specificity compared to chest CT, which now represents the diagnostic reference standard.15,16 The diagnosis of bronchiectasis on CT, however, is based on the visual assessment of bronchial dilatation and is subjected to radiologist expertise as standardised diagnostic criteria are still to be defined.17,18 Several visual scoring systems have been used to assess the severity of bronchiectasis, showing substantial limitations.19–21 More recently, quantitative post-processing methods have been developed to objectively define bronchiectasis and quantify disease severity, but their application is mostly limited to a research setting.18
In the present review article, we discuss the role of imaging and its current limitations in non-CF bronchiectasis, hereafter named bronchiectasis. The potential of automatic quantitative approaches and artificial intelligence in such context will be also mentioned.
Radiological definition of bronchiectasis
Chest radiography
Although CXR is considered the first-line imaging modality in the diagnosis of bronchiectasis, it has limited sensitivity in detecting airway dilatation. Even when pronounced, the radiographic features of bronchiectasis are usually non-specific, and rarely provide direct evidence of bronchial dilatation.22 These features include increased pulmonary markings and, in case of cystic bronchiectasis, thin-walled cysts with or without air-fluid levels.22,23 Increased lung markings are related to thick-walled bronchiectasis that fails to end in a tapered fashion towards the lung periphery. When such bands run parallel to each other result in the so-called “tram-track” (i.e. resembling a railway). If the dilated bronchus and the accompanying pulmonary artery branch are seen in cross-section, the “signet ring” sign can be appreciated.24 However, even other disorders such as bronchitis without bronchial dilation or purely vascular disorders may manifest with similar radiographic features.22 Other recognised radiographic features of bronchiectasis include tubular and branching opacities caused by mucus plugging within the bronchial lumen, hyperinflation, and atelectasis. Pleural thickening and adhesions have been described in a minor proportion of patients as a result of chronic inflammation and recurrent exacerbation.22
Computed tomography
CT, and high-resolution CT (HRCT) particularly, has radically changed the way by which the lungs can be viewed in vivo and is currently considered the most accurate non-invasive imaging modality for diagnosing numerous lung diseases, including bronchiectasis.16 The diagnosis of bronchiectasis, however, remains quite challenging in clinical practice. The relative ease of assessing severe bronchial dilation, observed in cystic bronchiectasis, strongly contrasts with the difficulty of depicting subtle earlier structural changes of cylindric bronchiectasis.13,25
CT protocols
There are no standardised HRCT protocols for the evaluation of bronchiectasis.18 For evaluating the airways, volumetric CT acquisition should be preferred, since it allows a precise assessment of the continuity of bronchial structures, while multiplanar reconstructions help differentiate bronchiectasis from cystic lung disorders (e.g. pulmonary Langerhans cell histiocytosis; lymphangioleiomyomatosis).26 According to current recommendations, images should be reconstructed with a slice thickness ≤1 mm to avoid overestimation of bronchial wall thickness and preferably assessed at a window level of −450 Hounsfield Unit (HU) - higher levels were demonstrated to increase the airway–artery ratio. Moreover, reconstruction kernels ought to be standardised for a more accurate longitudinal evaluation of bronchiectasis.16,18,27,28 Technical details are reported in Table 1.
Table 1.
HRCT technical parameters for the assessment of airways (* kVp: Peak kilovoltage; **mAs: Milliampere seconds; # HU: Hounsfield unit)
| Parameter | Value |
|---|---|
| Volumetric acquisition | - |
| Tube potential | 120–80 kVp*; 100 or 80 kVp* to be preferred for small size patients |
| Tube current | ≤240 milliampere (mA);≤100 mAs** or effective mAs** |
| Slice thickness | ≤1.5 millimetres (mm) |
| Gantry rotation time | As short as possible (e.g. 200–500 ms), always ≤1 sec |
| Pitch | 1–1.5 |
| Reconstruction algorithm | High spatial frequency |
| Lung parenchyma windows mean/width | −400 to −700 HU#/>1000 HU # |
| Soft tissue windows mean/width | 50/350 HU |
The use of iodine contrast is triggered by haemoptysis, a well-known and potentially life-threatening complication associated with severe bronchiectasis.29 Haemoptysis is usually caused by the rupture of a bronchial or pulmonary artery into the bronchial lumen resulting from bronchial artery dilatation and neovascularity due to recurrent airway inflammation, whereas bleeding from a bronchial vein is rare.30–32 Angiographic CT is the established diagnostic imaging modality to identify sources of haemoptysis and for preprocedural planning,33 whereas the evaluation of pulmonary arterial enlargement, an indirect sign of pulmonary hypertension that has been found to be a significant prognostic marker in patients with bronchiectasis, does not require intravenous contrast material.34
It is worth emphasising that the calibre of both the airway and the accompanying pulmonary artery branch depends on lung volumes at the time of scan acquisition.35 Lung volume standardisation should be pursued to increase both objectiveness and reproducibility of radiological interpretation of bronchiectasis, whose definition is mostly based on the assessment of the airway–artery ratio.18 Spirometer-controlled HRCT acquisition appears to be the optimal strategy to ensure adequate lung volumes and has been successfully used in some centres, mostly in children.36,37 The application of such acquisition method, however, remains limited in clinical practice,17 requiring patients to be highly cooperative and several professional figures to be involved at the time of acquisition.
Of note, differences in lung volume between baseline and follow-up CT scans might cause bronchiectasis to “disappear”.18 The concept of “reversible bronchiectasis”, originally described in bronchography and then in few case reports38–40 and retrospective studies employing CT,41,42 refers to a reversible bronchial dilation resulting from infection, inflammation or obstruction.40,43 Underlying mechanisms such as increased intraluminal pressure due to retained secretions, negative pressure secondary to atelectasis, and ineffective cough44,45 can lead to temporary airway dilation persisting up to months after the acute setting.46 Most often described in patients with pneumonia,40,47 these phenomena demand cautious evaluation of bronchial dilatation, particularly in consolidated or atelectatic lung areas, to avoid misinterpreting potentially reversible airway dilatation with bronchiectasis. Imaging follow-up at an appropriate time interval is of value to avoid this pitfall, as it enables differentiating bronchial dilation that returns to normal over time from cases in which irreversible destructive changes in the musculoelastic tissues of the bronchial wall have occurred.45,48
CT features of bronchiectasis
The most used criterion to determine the presence of bronchiectasis is the increased ratio of the cross-sectional diameter of an airway and its adjacent artery (airway–artery, AA, ratio).13,16 Such diagnostic criterion is affected by a number of limitations. First, the cut-off values for the AA ratio vary among different studies, and no reference interval has yet been validated.49,50 Nevertheless, 1.0 to 1.5 represents the interval ratio most frequently used in the literature. Furthermore, cut-off values should be age- and sex-dependent, as the AA ratio tends to increase in older subjects and decrease in infants.51–53 An AA ratio >0.8 has recently been suggested to define bronchiectasis in children and adolescents, and a ratio >1–1.5 in adults.54
No consensus exists on whether the either inner or outer airway diameter ought to be used to compute this ratio. If the inner diameter is used, the rate of false-negative diagnosis of bronchiectasis may increase, for instance, in case of mucus attached to the airway wall folding of the mucosa or when the CT is acquired at lung volumes below total lung capacity. These conditions can reduce the internal bronchial diameter andthus, modify the AA ratio.55,56 Likewise, all conditions that affect arterial diameter limit the reliability of the AA ratio. The so-called hypoxic pulmonary vasoconstriction, an adaptive mechanism in which alveolar hypoxia causes local pulmonary vasoconstriction, can increase the AA ratio and mimic bronchiectasis. Such a condition may be secondary to smoking and high altitude.12,57 Conversely, pseudonormalisation of the AA ratio may occur in case of pulmonary arterial enlargement.24
The other two most used criteria to diagnose bronchiectasis are represented by lack of tapering, defined as unchanged airway diameter for 2 cm after branching, and visualisation of airways in the periphery of the lung, within 1 cm from the costal pleura or abutting mediastinal pleural.58,59 These two criteria seem more reliable than the AA ratio, though their visual assessment is still limited by some degree of subjectivity.17
Other CT findings commonly associated with bronchiectasis include bronchial wall thickening, airway plugging, mosaic attenuation, and volume loss. Bronchial wall thickening usually represents airway inflammation.13 However, the definition of the underlying cause of bronchial wall thickness is rather difficult; stasis, mucus, and longstanding structural changes caused by repeated cycles of injury and repair (i.e. the process of remodelling) can result in bronchial wall thickening and lead to different degrees of functional impairment.58,60,61 Notably, evaluation of bronchial wall thickness is largely subjective in everyday practice and thus, increases variability in differentiating normal from abnormal (e.g. in cases of slight, even diffuse, thickness increase). Even in patients with advanced bronchiectasis, visual subscoring systems of bronchial wall thickness have shown intra- and inter-reader agreement values ranging from moderate (intraclass correlation coefficient, ICC = 0.5–0.56) to good (ICC = 0.67–0.73), respectively.62–64
Airway plugging appearance varies according to the length and orientation of the abnormal airway relative to the scan plane. Filled dilated bronchi will manifest as tubular, Y-, or V-shaped opacities when seen along their long axis, while resulting in “nodules” or “dots” if perpendicular to the image plane. In this latter case, the tree-in-bud pattern is observed when mucus secretions fill distal airways65,66 (Figures 1 and 2).
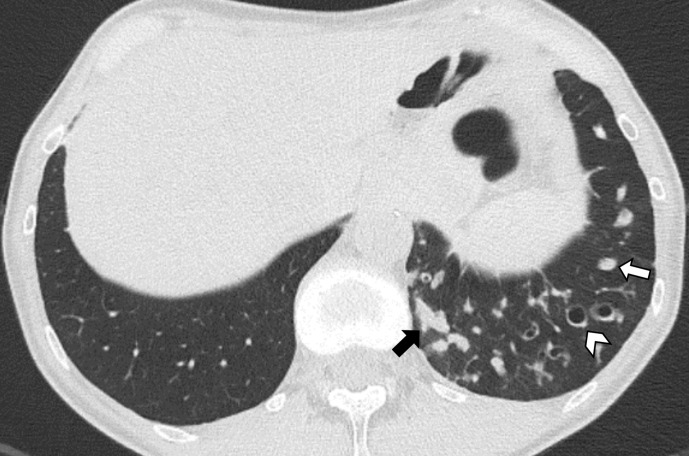
Figure 1.
Axial CT image shows cylindrical bronchiectasis in the left lower lobe. The disproportion between the bronchi and the corresponding pulmonary arteries running perpendicular to the axial plane recalls the “signet ring” appearance (arrowhead). Mucus plugging appears as tubular (black arrow) or nodular (white arrow) opacities depending on the bronchial orientation relative to the scan plane.
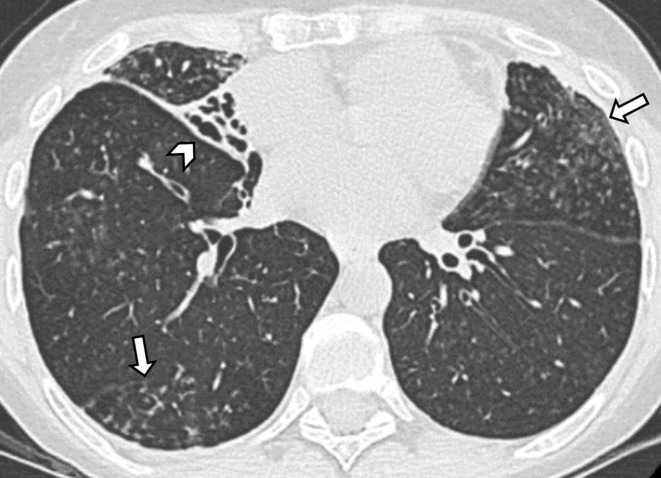
Figure 2.
Axial CT image in a patient diagnosed with non-tuberculous mycobacterial infection shows varicoid bronchiectasis and volume loss in the middle lobe (arrowhead). Bilateral centrilobular nodules and tree-in-bud opacities (arrows) due to distal airways involvement can be appreciated in the right lower lobe and in the left upper lobe.
Small airways involvement is regarded as an integral part of bronchiectasis.67 Air trapping is almost invariably associated with bronchiectasis, even in lobes without overt bronchiectasis, suggesting that obliterative bronchiolitis may be an early event in the pathogenesis of the disease. It was postulated that constrictive obliterative bronchiolitis might represent the first event that leads to proximal bronchial dilatation over time.68 Therefore, additional expiratory CT scanning is helpful in the presence of bronchiectasis to improve the interpretation of the mosaic attenuation pattern (i.e. to differentiate between air trapping and ground glass opacification)69 (Figure 3).
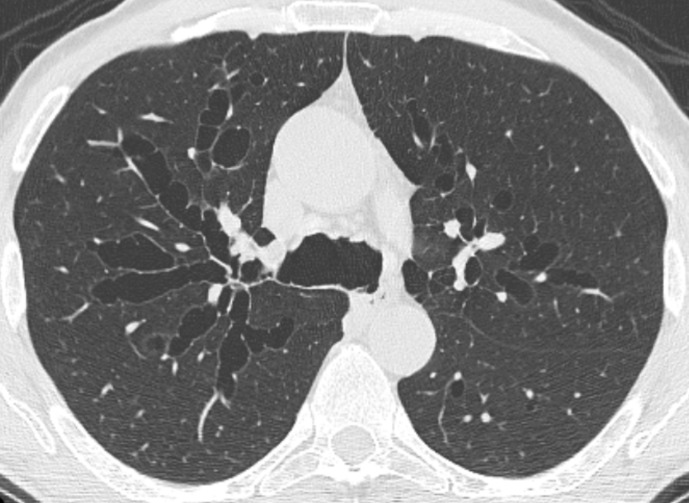
Figure 3.
(a) Inspiratory axial CT image shows thick-walled bronchiectasis within areas of slightly decreased attenuation (asterisks) in the upper lobes. (b) Expiratory axial CT image acquired at the same level showed no changes in density of the hypo-attenuated areas (asterisks), demonstrating the presence of air trapping.
Both signs of volume loss (e.g. bands of atelectasis, displacement of the fissures, etc.) and crowding of the airways are due to peribronchial inflammation and fibrosis. (Figure 2). Such distorted bronchiectasis should not be defined as traction bronchiectasis, a term that refers to airways irregularly dilated within CT features of lung fibrosis, such as peripheral reticular opacities and honeycombing.24
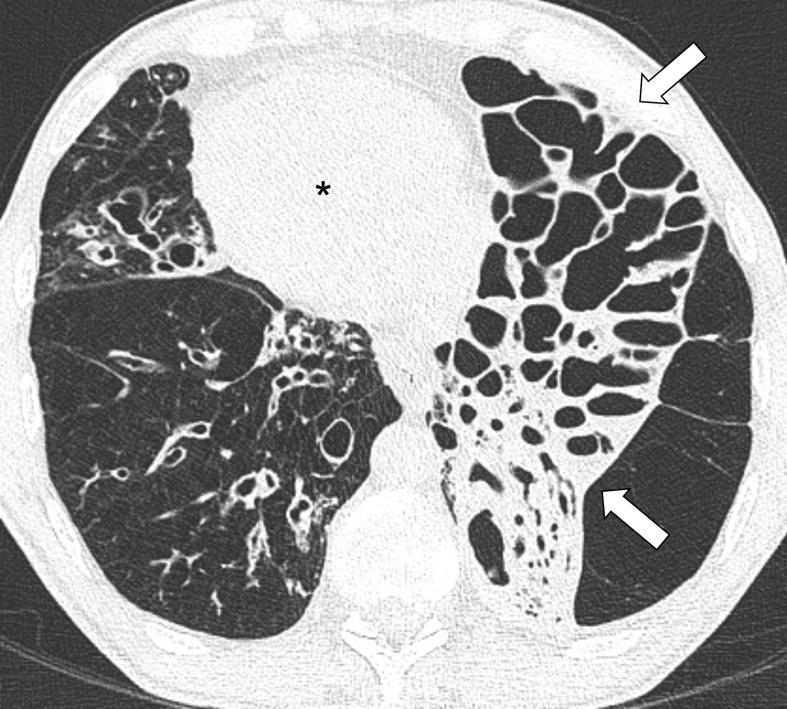
Thickening of interlobular septa has been described in a relevant proportion of patients diagnosed with idiopathic bronchiectasis (Figure 4). It was suggested that interlobular thickening might be secondary to septa infiltration by inflammatory cells or lymphatic congestion (e.g. due to increased or obstructed lymphatic flow). Notably, the extent of bronchiectasis correlated to the profusion of thickened interlobular septa.70
Figure 4.
Axial CT image shows extensive bilateral thick-walled bronchiectasis with mucus plugging (black arrows), consolidation (black arrowheads) and thickened interlobular septa (white arrowheads).
Severity of bronchiectasis: radiological scores
Over the decades, several radiological scoring systems have been proposed to quantify disease severity.
In 1991, Bhalla et al proposed the first CT-based scoring system, which allows a comprehensive characterisation of bronchiectasis, encompassing nine different features, also including the presence of emphysema.19 Although the Bhalla score was developed on only 14 CF patients, it was largely applicated to non-CF bronchiectasis in both clinical and research settings.
Subsequently, a simplified scoring system – the Reiff score – was derived from the Bhalla score. The Reiff score, whereby each lobe is assessed separately, was developed on a significantly larger number of patients (146), including both CF and non-CF ones.20
The more recent Bronchiectasis Radiologically Indexed CT Score (BRICS) was also derived from the Bhalla score but was developed in a specific cohort of non-CF patients: affected by either idiopathic or post-infective bronchiectasis with limited smoking history. Of note, this score is the only one that has been externally validated, showing consistent results in a validation cohort of more than 300 patients from 6 different European centres. The BRICS CT metrics of bronchiectasis were derived from multivariable models predictive of clinical disease severity markers. Interestingly, the multivariable models retained two CT features: bronchial dilation and number of bronchopulmonary segments affected by emphysema. These two combined CT features showed a significant correlation with clinicoprognostic markers, making the BRICS score an attractive easy-to-use method to assess non-CF bronchiectasis in routine practice.21
Nevertheless, these CT-based scoring systems have several limitations. Firstly, the Bhalla score from which the others were derived was developed for patients with CF, though applied to all types of bronchiectasis.71 Secondly, they all rely on a subjective judgement of the severity and extent of specific features of bronchiectasis (e.g. bronchial dilation, bronchial wall thickening and mucous plugging) and still do not capture the heterogeneity of the disease. For example, patients with structurally more abnormal but localised disease can be scored as high as those with widespread but less prominent structural abnormalities.
A substantial weakness of these scoring systems is represented by the lack of integration with clinical parameters. The BRICS takes into account clinicoprognostic aspects but fails to correlate specific CT features with the degree of disease activity, which influences treatment choice.1 Multidimensional scores, such as the Bronchiectasis Severity Index (BSI), and the FEV1, Age, Chronic colonisation, Extension and Dyspnoea (FACED) score, have attempted to integrate clinical and prognostic parameters with CT features of bronchiectasis,72,73 but include a rather limited number of clinical features, albeit relevant for the majority of the affected patients, potentially irrelevant for others. Moreover, these systems could not overcome the intrinsic limitations of the current CT criteria (i.e. the AA ratio measurement).
In this heterogenous clinical and radiological context, it is unlikely that a single scoring system would be suitable for all types of bronchiectasis, which does not represent a definite pathological entity, but rather a complex spectrum of disorders.
Confident radiological diagnosis of bronchiectasis
Although CT findings are usually of limited value to discriminate among different causes of bronchiectasis,20 it is worth reiterating that the type of bronchiectasis (i.e. cylindric, varicose or cystic), their distribution within the lung regions and concurrent abnormalities might help narrow the differential diagnoses.24,74 Among others, associated pathological features include abnormal tracheal dilatation, mucous plugging, tree-in-bud nodular pattern, consolidation, and atelectasis. Visual interpretation of CT images should entail the assessment of the location and spatial distribution of bronchiectasis (i.e. apical vs basal and central vs peripheral), their extent (i.e. focal vs diffuse), morphology (i.e. cylindric, varicose or cystic), and severity.75,76 Focal bronchiectasis, for instance, is frequently of acquired origin (e.g. extrinsic compression, endobronchial malignancies, foreign body aspiration) and is far less common than diffuse bronchiectasis.75 Bronchiectasis with upper and mid lobes predominance are typical of CF, sarcoidosis and non-tuberculous mycobacterial infections; a central predominance is more commonly encountered in ABPA, while a lower lobes predominance is characteristic of chronic aspiration or much rarer pathologies, such as primary ciliary dyskinesia (PCD) and congenital immunodeficiency.24,25,75
Non-CF bronchiectases recognise numerous causes, but there are only a handful of conditions whereby CT features are highly suggestive of a specific underlying cause. These conditions that share a low or extremely low prevalence are briefly discussed below. The main causes of non-CF bronchiectasis and associated CT features are reported in Table 2.
Table 2.
Main causes of non-CF bronchiectasis and associated CT features
| Causes | Type of bronchiectasis | Distribution | Associated and/or distinctive features |
|---|---|---|---|
| Congenital | |||
| Mounier-Kuhn Syndrome77 | NA (Trachea and main bronchi) |
Central lung regions | Bronchial diverticulosis, tracheal diverticula |
| Williams-Campbell Syndrome78 | Varicose, cystic | Sub segmental bronchi (fourth to sixth generations) |
Collapsed bronchi and distal air-trapping on expiratory CT |
| α 1-Antitrypsin deficiency79 | Cylindric, cystic | Mainly lower lobe | Panlobular emphysema |
| Immunologic | |||
| Allergic bronchopulmonary aspergillosis16 | Cylindric, varicose | Segmental and subsegmental bronchi of central-upper lungs regions | Mucous plugging (“finger-in-glove” sign) |
| Infectious or inflammatory | |||
| Bacterial, mycobacterial, viral | Various | Various | Depend on pathogens |
| Swyer-James Syndrome80 | Cylindric | Non-specific | Hyperlucent lobe or lung and air-trapping |
| Chronic aspiration80 | Cylindric | Basal lung regions | Bronchial wall thickening, tree-in-bud consolidations |
| Defective mucous transport | |||
| Primary Ciliary Dyskinesia81 | Varicose, cylindric | Middle and lower lobes | Situs inversus |
| Young’s Syndrome82 | Cystic | Little evidence | Little evidence |
| Primary immunodeficiency83 | Mainly cylindric | Upper and mid lung regions | Non-specific |
| Airways obstruction | |||
| Endobronchial malignancies75 | Various | Focal | Various |
| Broncholithiasis80 | Cylindric, varicose | Focal, more often middle lobe | Calcified lymph nodes |
| Extrinsic compression75 | Various | Focal | Various |
| Idiopathic | Various | Basal lung regions20 | Various |
Allergic bronchopulmonary aspergillosis (ABPA)
ABPA is a disorder characterised by chronic inflammation and airways damage due to persistent colonisation and sensitisation by Aspergillus species. It typically affects patients with asthma (up to 14% of corticosteroid-dependent asthmatic patients) and CF (6%).84 Radiological findings are quite specific and consist of bronchiectasis with a central-upper lungs predominance, bronchial wall thickening, mucoid impaction that frequently results in tubular branching opacities – the so-called “finger-in-glove” sign – and high attenuation mucus plugs (probably due to the contents of calcium). The recognition of these CT features allows a confident diagnosis in most cases in which ABPA is suspected.16,24
Tracheobronchomegaly (Mounier-Kuhn syndrome)
Tracheobronchomegaly, also known as “Mounier-Kuhn syndrome” is an extremely rare disorder of unknown prevalence characterised by an abnormal dilatation of the trachea and major bronchi. Most cases are congenital but acquired forms have been described in association with other disorders, such as pulmonary fibrosis.85 CT usually permits an accurate diagnosis of the disease, showing the marked increase of the calibre of the tracheobronchial tree, with a characteristic corrugated appearance of its walls77 (Figure 5).
Figure 5.
Axial CT image demonstrates enlarged main stem bronchi and thin-walled central bronchiectasis consistent with Mounier-Kuhn syndrome.
Williams-Campbell syndrome
Williams-Campbell syndrome is a rare disease characterised by a deficiency of cartilaginous tissue in subsegmental bronchi. CT findings consist of cystic bronchiectasis with thickened walls involving bronchi from the fourth to the sixth generations. Expiratory CT scan shows the collapse of cystic dilated bronchi, one of the most characteristic signs of such syndrome. These findings are quite peculiar, and their identification makes the diagnosis quite straightforward.78
Primary ciliary dyskinesia (PCD)
PCD accounts for up to 8% of adults non-CF bronchiectasis. In this inherited disorder, ultrastructural defects of the ciliary apparatus result in abnormal or absent beating of cilia.86 CT typically demonstrates the presence of varicose bronchiectasis, predominantly distributed in the middle and lower lung lobes and associated with atelectasis, tree-in-bud nodular consolidation, and mucous plugging.81 Moreover, half of the patients have Kartagener syndrome, which is defined by the triad of bronchiectasis, situs inversus totalis, and either nasal polyps or recurrent sinusitis86 (Figure 6). In such cases, the diagnosis of PCD-associated bronchiectasis is easily and confidently performed.
Figure 6.
Axial CT image of a patient diagnosed with Kartagener syndrome shows dextrocardia (asterisk) and bilateral bronchiectases involving the middle lobe and both lower lobes. Marked volume loss is evident in the left lower lobe (arrows).
Longitudinal evaluation of bronchiectasis
High-quality evidence in favour of repeated imaging in patients with bronchiectasis still lacks. Structural changes underlying fluctuations in pulmonary function such as the degree of bronchial wall thickening and mucous retention are not necessarily evident on CXR, making correlations of expected radiographic abnormalities with clinical and functional features of disease worsening of limited reliability.87,88 Repeated HRCT has otherwise shown promise for assessing physiologically relevant pulmonary changes, but it carries radiation risk and should be managed with caution.89 Current indications for repeated HRCT include chronic and acute clinical deterioration.54,87 According to the updated British Thoracic Society guidelines, in fact, a deteriorating patient should be assessed with chest CT and administration of iodine contrast ought to be considered when pulmonary embolism is suspected. Clinical deterioration is defined by significant and prolonged worsening of symptoms, rapid decline in lung function, increased frequency or severity of exacerbations, frequent hospital admissions and/or early relapse after treatment of an exacerbation episode.87 It is worth considering that patients who experience a slowly progressive clinical decline do not necessarily display the same CT features as those who present with acute clinical deterioration. For instance, morphological features of active disease, including increased bronchial wall thickening, mucous plugging with atelectasis, and parenchymal consolidation, are more likely to be depicted in acutely deteriorating patients, where the mere assessment of bronchial dilatation severity is of limited utility.
Regardless of the specific clinical setting, which has to be taken into account, establishing a radiological progression of bronchiectasis is quite difficult. Definite criteria still lack, and the limitations of current radiological scoring systems remain.90 To date, most studies that investigated the radiological evolution of bronchiectasis were performed on CF patients.89,91 Park et al, conversely, only enrolled non-CF patients, demonstrating that lower body mass index and isolation of Pseudomonas aeruginosa in respiratory specimen are associated with radiological progression of bronchiectasis.92 Such progression, however, was assessed using the Bhalla score, whose weaknesses have already been highlighted.
Quantitative imaging and future directions
Despite the large and consistent body of literature suggesting a prognostic role for HRCT in bronchiectasis, HRCT-based biomarkers are neither routinely used in clinical practice nor encompassed as a clinical end point in therapeutic trials. Quantitative evaluation of bronchiectasis suffers from a limited capability to reflect disease heterogeneity and activity, resulting in discrepancies between the radiological disease severity and prognostic information.93 The latter is particularly relevant since patients at a higher risk of recurrent exacerbations might benefit from more aggressive treatments, which are not free of risks.56 Moreover, visual assessment of HRCT findings and their extent has shown significant interobserver variation, even among expert radiologists, affecting both baseline and follow-up evaluation.94–96
Automated and semi-automated algorithms have the potential to overcome the limitations of the time-consuming manual annotations and visual scoring methods to quantify abnormal widening and thickening of airways. Significant differences in several parameters between subjects with bronchiectasis and controls have been described in the literature.56,97,98 These include either measurements that use artery size for normalisation or depend on the airway size only (i.e. wall thickness and lumen) or tapering. Their implementation in a clinical setting is still limited due to technical issues inherent to CT or related to algorithms’ performances: slight differences in attenuation values that affect the differentiation of wall thickening from mucous obstruction or peribronchial abnormalities; airways mislabelling; limited capability of extracting branches and pairing artery.99 Moreover, studies evaluating automatic and semi-automatic airways extraction in patients with bronchiectasis lack generalisability due to small and non-randomised study populations, lack of ground truth, and non-standardised protocols.62,97,98 Current evidence in supporting density-based CT scoring methods, a possible biomarker that does not rely on the complex workflow of airway extraction, mostly derives from the clinical setting of CF and suffers from similar limitations.100,101
Artificial intelligence has shown encouraging results in other fields of thoracic imaging,101 but if and how it will play a role in bronchiectasis remains to be determined. Learning techniques, such as convolutional neural networks, may have the potential to support radiologists in approaching bronchiectasis systematically, possibly minimising bias of subjective evaluation.102 Ideally, future studies should capture several aspects of the disease, including genomic, metabolomic, and clinical information, resulting in highly complex data sets to be mined with artificial intelligence to gain new knowledge regarding the diagnosis, classification, and treatment of bronchiectasis.
Footnotes
Roberta Eufrasia Ledda and Maurizio Balbi have contributed equally to this study and should be considered as co-first authors.
Contributor Information
Roberta Eufrasia Ledda, Email: robertaeufrasia.ledda@unipr.it.
Maurizio Balbi, Email: balbi.m@libero.it.
Francesca Milone, Email: francesca.milone1@gmail.com.
Andrea Ciuni, Email: aciuni@ao.pr.it.
Mario Silva, Email: mario.silva@unipr.it.
Nicola Sverzellati, Email: nicola.sverzellati@unipr.it.
Gianluca Milanese, Email: gianluca.milanese@unipr.it.
REFERENCES
- 1.Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629.09 09 2017. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 2.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 2006; 100: 2183–9. doi: 10.1016/j.rmed.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 3.Sethi GR, Batra V. Bronchiectasis: causes and management. Indian J Pediatr 2000; 67: 133–9. doi: 10.1007/BF02726189 [DOI] [PubMed] [Google Scholar]
- 4.Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000; 162(4 Pt 1): 1277–84. doi: 10.1164/ajrccm.162.4.9906120 [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekaran R, Mac Aogáin M, Chalmers JD, Elborn SJ, Chotirmall SH. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm Med 2018; 18: 83. doi: 10.1186/s12890-018-0638-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med 2018; 6: 715–26. doi: 10.1016/S2213-2600(18)30053-5 [DOI] [PubMed] [Google Scholar]
- 7.Hess EP, Haas LR, Shah ND, Stroebel RJ, Denham CR, Swensen SJ. Trends in computed tomography utilization rates: a longitudinal practice-based study. J Patient Saf 2014; 10: 52–8. doi: 10.1097/PTS.0b013e3182948b1a [DOI] [PubMed] [Google Scholar]
- 8.Chalmers JD. New insights into the epidemiology of bronchiectasis. Chest 2018; 154: 1272–3. doi: 10.1016/j.chest.2018.08.1051 [DOI] [PubMed] [Google Scholar]
- 9.Aliberti S, Sotgiu G, Lapi F, Gramegna A, Cricelli C, Blasi F. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med 2020; 20: 15. doi: 10.1186/s12890-020-1050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ringshausen FC, de Roux A, Pletz MW, Hämäläinen N, Welte T, Rademacher J. Bronchiectasis-associated hospitalizations in Germany, 2005-2011: a population-based study of disease burden and trends. PLoS One 2013; 8: e71109. doi: 10.1371/journal.pone.0071109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quint JK, Millett ERC, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–93. doi: 10.1183/13993003.01033-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz AA, Young TP, Maselli DJ, Martinez CH, Gill R, Nardelli P, et al. Quantitative CT measures of bronchiectasis in smokers. Chest 2017; 151: 1255–62. doi: 10.1016/j.chest.2016.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naidich DP, McCauley DI, Khouri NF, Stitik FP, Siegelman SS. Computed tomography of bronchiectasis. J Comput Assist Tomogr 1982; 6: 437–44. doi: 10.1097/00004728-198206000-00001 [DOI] [PubMed] [Google Scholar]
- 14.Saleh AD, Hurst JR. How to assess the severity of bronchiectasis. Eur Respir J 2014; 43: 1217–9. doi: 10.1183/09031936.00226913 [DOI] [PubMed] [Google Scholar]
- 15.Munro NC, Cooke JC, Currie DC, Strickland B, Cole PJ. Comparison of thin section computed tomography with bronchography for identifying bronchiectatic segments in patients with chronic sputum production. Thorax 1990; 45: 135–9. doi: 10.1136/thx.45.2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuinness G, Naidich DP. Ct of airways disease and bronchiectasis. Radiol Clin North Am 2002; 40: 1–19. doi: 10.1016/S0033-8389(03)00105-2 [DOI] [PubMed] [Google Scholar]
- 17.Meerburg JJ, Veerman GDM, Aliberti S, Tiddens HAWM. Diagnosis and quantification of bronchiectasis using computed tomography or magnetic resonance imaging: a systematic review. Respir Med 2020; 170: 105954. doi: 10.1016/j.rmed.2020.105954 [DOI] [PubMed] [Google Scholar]
- 18.Tiddens HAWM, Meerburg JJ, van der Eerden MM, Ciet P. The radiological diagnosis of bronchiectasis: what's in a name? Eur Respir Rev 2020; 29: 190120.30 Jun 2020. doi: 10.1183/16000617.0120-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, McCauley DI, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology 1991; 179: 783–8. doi: 10.1148/radiology.179.3.2027992 [DOI] [PubMed] [Google Scholar]
- 20.Reiff DB, Wells AU, Carr DH, Cole PJ, Hansell DM. Ct findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol 1995; 165: 261–7. doi: 10.2214/ajr.165.2.7618537 [DOI] [PubMed] [Google Scholar]
- 21.Bedi P, Chalmers JD, Goeminne PC, Mai C, Saravanamuthu P, Velu PP, et al. The BRICS (Bronchiectasis Radiologically Indexed CT Score): A Multicenter Study Score for Use in Idiopathic and Postinfective Bronchiectasis. Chest 2018; 153: 1177–86. doi: 10.1016/j.chest.2017.11.033 [DOI] [PubMed] [Google Scholar]
- 22.Gudbjerg CE. Roentgenologic diagnosis of bronchiectasis; an analysis of 112 cases. Acta radiol 1955; 43: 210–26. [PubMed] [Google Scholar]
- 23.Currie DC, Cooke JC, Morgan AD, Kerr IH, Delany D, Strickland B, et al. Interpretation of bronchograms and chest radiographs in patients with chronic sputum production. Thorax 1987; 42: 278–84. doi: 10.1136/thx.42.4.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milliron B, Henry TS, Veeraraghavan S, Little BP. Bronchiectasis: mechanisms and imaging clues of associated common and uncommon diseases. Radiographics 2015; 35: 1011–30. doi: 10.1148/rg.2015140214 [DOI] [PubMed] [Google Scholar]
- 25.Javidan-Nejad C, Bhalla S. Bronchiectasis. Thorac Surg Clin 2010; 20: 85–102. doi: 10.1016/j.thorsurg.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 26.Dodd JD, Souza CA, Müller NL. Conventional high-resolution CT versus helical high-resolution MDCT in the detection of bronchiectasis. AJR Am J Roentgenol 2006; 187: 414–20. doi: 10.2214/AJR.05.0723 [DOI] [PubMed] [Google Scholar]
- 27.Hansell DM. Thin-section CT of the lungs: the Hinterland of normal. Radiology 2010; 256: 695–711. doi: 10.1148/radiol.10092307 [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Cardona D, Nagle SK, Li K, Robinson TE, Chen G-H. Influence of radiation dose and reconstruction algorithm in MDCT assessment of airway wall thickness: a phantom study. Med Phys 2015; 42: 5919–27. doi: 10.1118/1.4930797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larici AR, Franchi P, Occhipinti M, Contegiacomo A, del Ciello A, Calandriello L, et al. Diagnosis and management of hemoptysis. Diagn Interv Radiol 2014; 20: 299–309. doi: 10.5152/dir.2014.13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruzzi JF, Rémy-Jardin M, Delhaye D, Teisseire A, Khalil C, Rémy J. Multi-detector row CT of hemoptysis. Radiographics 2006; 26: 3–22. doi: 10.1148/rg.261045726 [DOI] [PubMed] [Google Scholar]
- 31.Song JW, Im JG, Shim YS, Park JH, Yeon KM, Han MC. Hypertrophied bronchial artery at thin-section CT in patients with bronchiectasis: correlation with CT angiographic findings. Radiology 1998; 208: 187–91. doi: 10.1148/radiology.208.1.9646812 [DOI] [PubMed] [Google Scholar]
- 32.Walker CM, Rosado-de-Christenson ML, Martínez-Jiménez S, Kunin JR, Wible BC. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics 2015; 35: 32–49. doi: 10.1148/rg.351140089 [DOI] [PubMed] [Google Scholar]
- 33.Jeudy J, Khan AR, Mohammed T-L, Amorosa JK, Brown K, Dyer DS, et al. ACR appropriateness criteria hemoptysis. J Thorac Imaging 2010; 25: W67–9. doi: 10.1097/RTI.0b013e3181e35b0c [DOI] [PubMed] [Google Scholar]
- 34.Devaraj A, Wells AU, Meister MG, Loebinger MR, Wilson R, Hansell DM. Pulmonary hypertension in patients with bronchiectasis: prognostic significance of CT signs. AJR Am J Roentgenol 2011; 196: 1300–4. doi: 10.2214/AJR.10.5221 [DOI] [PubMed] [Google Scholar]
- 35.Bakker ME, Stolk J, Reiber JHC, Stoel BC. Influence of inspiration level on bronchial lumen measurements with computed tomography. Respir Med 2012; 106: 677–86. doi: 10.1016/j.rmed.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 36.Salamon E, Lever S, Kuo W, Ciet P, Tiddens HA. Spirometer guided chest imaging in children: it is worth the effort! Pediatr Pulmonol 2017; 52: 48–56. [DOI] [PubMed] [Google Scholar]
- 37.Kongstad T, Buchvald FF, Green K, Lindblad A, Robinson TE, Nielsen KG. Improved air trapping evaluation in chest computed tomography in children with cystic fibrosis using real-time spirometric monitoring and biofeedback. J Cyst Fibros 2013; 12: 559–66. doi: 10.1016/j.jcf.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 38.Yap VL, Metersky ML. Reversible bronchiectasis in an adult: a case report. J Bronchology Interv Pulmonol 2012; 19: 336–7. doi: 10.1097/LBR.0b013e31826ca79d [DOI] [PubMed] [Google Scholar]
- 39.Kucuk C, Turkkani MH, Arda K. A case report of reversible bronchiectasis in an adult: Pseudobronchiectasis. Respir Med Case Rep 2019; 26: 315–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachman AL, Hewitt WR, BEEKLEY HC, Bronchiectasis BHC. Bronchiectasis; a bronchographic study of sixty cases of pneumonia. AMA Arch Intern Med 1953; 91: 78–96. doi: 10.1001/archinte.1953.00240130086009 [DOI] [PubMed] [Google Scholar]
- 41.Cukier A, Stelmach R, Kavakama JI, Terra Filho M, Vargas F. Persistent asthma in adults: comparison of high resolution computed tomography of the lungs after one year of follow-up. Rev Hosp Clin Fac Med Sao Paulo 2001; 56: 63–8. doi: 10.1590/S0041-87812001000300001 [DOI] [PubMed] [Google Scholar]
- 42.Gaillard EA, Carty H, Heaf D, Smyth RL. Reversible bronchial dilatation in children: comparison of serial high-resolution computer tomography scans of the lungs. Eur J Radiol 2003; 47: 215–20. doi: 10.1016/S0720-048X(02)00122-5 [DOI] [PubMed] [Google Scholar]
- 43.Drapanas T, Siewers R, Feist JH. Reversible poststenotic bronchiectasis. N Engl J Med 1966; 275: 917–21. doi: 10.1056/NEJM196610272751702 [DOI] [PubMed] [Google Scholar]
- 44.Jennings GH. Re-expansion of atelectatic lower lobe. Br Med J 1937; 2: 963–78. doi: 10.1136/bmj.2.4010.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson SW, Christoforidis A. Reversible bronchiectasis. Radiology 1958; 71: 375–82. doi: 10.1148/71.3.375 [DOI] [PubMed] [Google Scholar]
- 46.Swensen SJ, Aughenbaugh GL, Douglas WW, Myers JL. High-Resolution CT of the lungs: findings in various pulmonary diseases. AJR Am J Roentgenol 1992; 158: 971–9. doi: 10.2214/ajr.158.5.1566699 [DOI] [PubMed] [Google Scholar]
- 47.Agarwal R. Bronchiectasis in acute pneumonia. Pseudobronchiectasis. Chest 2007; 132: 2054–5. [DOI] [PubMed] [Google Scholar]
- 48.Pontius JR, Jacobs LG. The reversal of advanced bronchiectasis. Radiology 1957; 68: 204–8. doi: 10.1148/68.2.204 [DOI] [PubMed] [Google Scholar]
- 49.Chalmers JD, Chang AB, Chotirmall SH, Dhar R, McShane PJ. Bronchiectasis. Nat Rev Dis Primers 2018; 4: 45. doi: 10.1038/s41572-018-0042-3 [DOI] [PubMed] [Google Scholar]
- 50.Juliusson G, Gudmundsson G. Diagnostic imaging in adult non-cystic fibrosis bronchiectasis. Breathe 2019; 15: 190–7. doi: 10.1183/20734735.0009-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuoka S, Uchiyama K, Shima H, Ueno N, Oish S, Nojiri Y. Bronchoarterial ratio and bronchial wall thickness on high-resolution CT in asymptomatic subjects: correlation with age and smoking. AJR Am J Roentgenol 2003; 180: 513–8. doi: 10.2214/ajr.180.2.1800513 [DOI] [PubMed] [Google Scholar]
- 52.Berend N, Woolcock AJ, Marlin GE. Relationship between bronchial and arterial diameters in normal human lungs. Thorax 1979; 34: 354–8. doi: 10.1136/thx.34.3.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapur N, Masel JP, Watson D, Masters IB, Chang AB. Bronchoarterial ratio on high-resolution CT scan of the chest in children without pulmonary pathology: need to redefine bronchial dilatation. Chest 2011; 139: 1445–50. doi: 10.1378/chest.10-1763 [DOI] [PubMed] [Google Scholar]
- 54.Chang AB, Fortescue R, Grimwood K, Alexopoulou E, Bell L, Boyd J, et al. Task force report: European respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur Respir J 2021;: 2002990.11 Feb 2021. doi: 10.1183/13993003.02990-2020 [DOI] [PubMed] [Google Scholar]
- 55.Lambert RK, Codd SL, Alley MR, Pack RJ. Physical determinants of bronchial mucosal folding. J Appl Physiol 1994; 77: 1206–16. doi: 10.1152/jappl.1994.77.3.1206 [DOI] [PubMed] [Google Scholar]
- 56.Kuo W, de Bruijne M, Petersen J, Nasserinejad K, Ozturk H, Chen Y, et al. Diagnosis of bronchiectasis and airway wall thickening in children with cystic fibrosis: objective airway-artery quantification. Eur Radiol 2017; 27: 4680–9. doi: 10.1007/s00330-017-4819-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim JS, Müller NL, Park CS, Lynch DA, Newman LS, Grenier P, et al. Bronchoarterial ratio on thin section CT: comparison between high altitude and sea level. J Comput Assist Tomogr 1997; 21: 306–11. doi: 10.1097/00004728-199703000-00028 [DOI] [PubMed] [Google Scholar]
- 58.Ooi GC, Khong PL, Chan-Yeung M, Ho JCM, Chan PKS, Lee JCK, et al. High-Resolution CT quantification of bronchiectasis: clinical and functional correlation. Radiology 2002; 225: 663–72. doi: 10.1148/radiol.2253011575 [DOI] [PubMed] [Google Scholar]
- 59.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 60.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest 2013; 144: 1026–32. doi: 10.1378/chest.12-3073 [DOI] [PubMed] [Google Scholar]
- 61.Lynch DA, Newell J, Hale V, Dyer D, Corkery K, Fox NL, et al. Correlation of CT findings with clinical evaluations in 261 patients with symptomatic bronchiectasis. AJR Am J Roentgenol 1999; 173: 53–8. doi: 10.2214/ajr.173.1.10397099 [DOI] [PubMed] [Google Scholar]
- 62.Kuo W, Andrinopoulou E-R, Perez-Rovira A, Ozturk H, de Bruijne M, Tiddens HAWM. Objective airway artery dimensions compared to CT scoring methods assessing structural cystic fibrosis lung disease. J Cyst Fibros 2017; 16: 116–23. doi: 10.1016/j.jcf.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 63.Loeve M, van Hal PTW, Robinson P, de Jong PA, Lequin MH, Hop WC, et al. The spectrum of structural abnormalities on CT scans from patients with CF with severe advanced lung disease. Thorax 2009; 64: 876–82. doi: 10.1136/thx.2008.110908 [DOI] [PubMed] [Google Scholar]
- 64.de Jong PA, Lindblad A, Rubin L, Hop WCJ, de Jongste JC, Brink M, et al. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax 2006; 61: 80–5. doi: 10.1136/thx.2005.045146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rossi SE, Franquet T, Volpacchio M, Giménez A, Aguilar G. Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 2005; 25: 789–801. doi: 10.1148/rg.253045115 [DOI] [PubMed] [Google Scholar]
- 66.Gruden JF, Webb WR. Identification and evaluation of centrilobular opacities on high-resolution CT. Semin Ultrasound CT MR 1995; 16: 435–49. doi: 10.1016/0887-2171(95)90030-6 [DOI] [PubMed] [Google Scholar]
- 67.Culiner MM. Obliterative BONCHITIS and bronchiolitis with bronchiectasis. Dis Chest 1963; 44: 351–61. doi: 10.1378/chest.44.4.351 [DOI] [PubMed] [Google Scholar]
- 68.Hansell DM, Wells AU, Rubens MB, Cole PJ. Bronchiectasis: functional significance of areas of decreased attenuation at expiratory CT. Radiology 1994; 193: 369–74. doi: 10.1148/radiology.193.2.7972745 [DOI] [PubMed] [Google Scholar]
- 69.Kligerman SJ, Henry T, Lin CT, Franks TJ, Galvin JR. Mosaic attenuation: etiology, methods of differentiation, and pitfalls. Radiographics 2015; 35: 1360–80. doi: 10.1148/rg.2015140308 [DOI] [PubMed] [Google Scholar]
- 70.Sibtain NA, Ujita M, Wilson R, Wells AU, Hansell DM. Interlobular septal thickening in idiopathic bronchiectasis: a thin-section CT study of 94 patients. Radiology 2005; 237: 1091–6. doi: 10.1148/radiol.2373041141 [DOI] [PubMed] [Google Scholar]
- 71.Brody AS, Kosorok MR, Li Z, Broderick LS, Foster JL, Laxova A, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging 2006; 21: 14–21. doi: 10.1097/01.rti.0000203937.82276.ce [DOI] [PubMed] [Google Scholar]
- 72.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–85. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martínez-García Miguel Á, de Gracia J, Vendrell Relat M, Girón R-M, Máiz Carro L, de la Rosa Carrillo D, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the faced score. Eur Respir J 2014; 43: 1357–67. doi: 10.1183/09031936.00026313 [DOI] [PubMed] [Google Scholar]
- 74.Cartier Y, Kavanagh PV, Johkoh T, Mason AC, Müller NL. Bronchiectasis: accuracy of high-resolution CT in the differentiation of specific diseases. AJR Am J Roentgenol 1999; 173: 47–52. doi: 10.2214/ajr.173.1.10397098 [DOI] [PubMed] [Google Scholar]
- 75.Cantin L, Bankier AA, Eisenberg RL. Bronchiectasis. AJR Am J Roentgenol 2009; 193: W158–71. doi: 10.2214/AJR.09.3053 [DOI] [PubMed] [Google Scholar]
- 76.Singh A, Bhalla AS, Jana M. Bronchiectasis revisited: imaging-based pattern approach to diagnosis. Curr Probl Diagn Radiol 2019; 48: 53–60. doi: 10.1067/j.cpradiol.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 77.Kwong JS, Müller NL, Miller RR. Diseases of the trachea and main-stem bronchi: correlation of CT with pathologic findings. Radiographics 1992; 12: 645–57. doi: 10.1148/radiographics.12.4.1636031 [DOI] [PubMed] [Google Scholar]
- 78.Di Scioscio V, Zompatori M, Mistura I, Montanari P, Santilli L, Luccaroni R, et al. The role of spiral multidetector dynamic CT in the study of Williams-Campbell syndrome. Acta Radiol 2006; 47: 798–800. doi: 10.1080/02841850600849084 [DOI] [PubMed] [Google Scholar]
- 79.Parr DG, Guest PG, Reynolds JH, Dowson LJ, Stockley RA. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2007; 176: 1215–21. doi: 10.1164/rccm.200703-489OC [DOI] [PubMed] [Google Scholar]
- 80.Hansell DAP, Lynch DA, et al. Imaging of diseases of the chest. Amsterdam, Elsevier 2005;: 143–81. [Google Scholar]
- 81.Dettmer S, Ringshausen F, Vogel-Claussen J, Fuge J, Faschkami A, Shin H-O, et al. Computed tomography in adult patients with primary ciliary dyskinesia: typical imaging findings. PLoS One 2018; 13: e0191457. doi: 10.1371/journal.pone.0191457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neville E, Brewis R, Yeates WK, Burridge A. Respiratory tract disease and obstructive azoospermia. Thorax 1983; 38: 929–33. doi: 10.1136/thx.38.12.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Obregon RG, Lynch DA, Kaske T, Newell JD, Kirkpatrick CH. Radiologic findings of adult primary immunodeficiency disorders. contribution of CT. Chest 1994; 106: 490–5. doi: 10.1378/chest.106.2.490 [DOI] [PubMed] [Google Scholar]
- 84.Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002; 121: 1988–99. doi: 10.1378/chest.121.6.1988 [DOI] [PubMed] [Google Scholar]
- 85.Woodring JH, Barrett PA, Rehm SR, Nurenberg P. Acquired tracheomegaly in adults as a complication of diffuse pulmonary fibrosis. AJR Am J Roentgenol 1989; 152: 743–7. doi: 10.2214/ajr.152.4.743 [DOI] [PubMed] [Google Scholar]
- 86.Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, et al. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med 2009; 11: 473–87. doi: 10.1097/GIM.0b013e3181a53562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.T Hill A, L Sullivan A, D Chalmers J, De Soyza A, Stuart Elborn J, Andres Floto R, Elborn SJ, Floto AR, et al. British thoracic Society guideline for bronchiectasis in adults. Thorax 2019; 74(Suppl 1): 1–69. doi: 10.1136/thoraxjnl-2018-212463 [DOI] [PubMed] [Google Scholar]
- 88.Pasteur MC, Bilton D, Hill AT, .British Thoracic Society Bronchiectasis non-CF Guideline Group . British thoracic Society guideline for non-CF bronchiectasis. Thorax 2010; 65 Suppl 1(Suppl 1): i1–58. doi: 10.1136/thx.2010.136119 [DOI] [PubMed] [Google Scholar]
- 89.Sheehan RE, Wells AU, Copley SJ, Desai SR, Howling SJ, Cole PJ, et al. A comparison of serial computed tomography and functional change in bronchiectasis. Eur Respir J 2002; 20: 581–7. doi: 10.1183/09031936.02.00284602 [DOI] [PubMed] [Google Scholar]
- 90.Crivelli P, Sverzellati N, Sotgiu G, Aliberti S. Is it feasible to radiologically monitor the evolution of non-CF bronchiectasis? Respirology 2016; 21: 1137. doi: 10.1111/resp.12837 [DOI] [PubMed] [Google Scholar]
- 91.Sanders DB, Li Z, Brody AS, Farrell PM. Chest computed tomography scores of severity are associated with future lung disease progression in children with cystic fibrosis. Am J Respir Crit Care Med 2011; 184: 816–21. doi: 10.1164/rccm.201105-0816OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park J, Kim S, Lee YJ, Park JS, Cho Y-J, Yoon HI, et al. Factors associated with radiologic progression of non-cystic fibrosis bronchiectasis during long-term follow-up. Respirology 2016; 21: 1049–54. doi: 10.1111/resp.12768 [DOI] [PubMed] [Google Scholar]
- 93.Wielpütz MO, Eichinger M, Weinheimer O, Ley S, Mall MA, Wiebel M, et al. Automatic airway analysis on multidetector computed tomography in cystic fibrosis: correlation with pulmonary function testing. J Thorac Imaging 2013; 28: 104–13. doi: 10.1097/RTI.0b013e3182765785 [DOI] [PubMed] [Google Scholar]
- 94.Sverzellati N, Devaraj A, Desai SR, Quigley M, Wells AU, Hansell DM. Method for minimizing observer variation for the quantitation of high-resolution computed tomographic signs of lung disease. J Comput Assist Tomogr 2011; 35: 596–601. doi: 10.1097/RCT.0b013e3182277d05 [DOI] [PubMed] [Google Scholar]
- 95.de Brito MCB, Ota MK, Leitão Filho FSS, Meirelles GdeSP. Radiologist agreement on the quantification of bronchiectasis by high-resolution computed tomography. Radiol Bras 2017; 50: 26–31. doi: 10.1590/0100-3984.2015.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh SLF, Calandriello L, Sverzellati N, Wells AU, Hansell DM, Consort U, .UIP Observer Consort . Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax 2016; 71: 45–51. doi: 10.1136/thoraxjnl-2015-207252 [DOI] [PubMed] [Google Scholar]
- 97.Kuo W, Perez-Rovira A, Tiddens H, de Bruijne M, CTsg NC, .Normal Chest CT study group . Airway tapering: an objective image biomarker for bronchiectasis. Eur Radiol 2020; 30: 2703–11. doi: 10.1007/s00330-019-06606-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez-Rovira A, Kuo W, Petersen J, Tiddens HAWM, de Bruijne M. Automatic airway-artery analysis on lung CT to quantify airway wall thickening and bronchiectasis. Med Phys 2016; 43: 5736–44. doi: 10.1118/1.4963214 [DOI] [PubMed] [Google Scholar]
- 99.Lo P, van Ginneken B, Reinhardt JM, Yavarna T, de Jong PA, Irving B, et al. Extraction of airways from CT (EXACT'09. IEEE Trans Med Imaging 2012; 31: 2093–107. doi: 10.1109/TMI.2012.2209674 [DOI] [PubMed] [Google Scholar]
- 100.de Lavernhe I, Le Blanche A, Dégrugilliers L, Carette M-F, Bayat S. Ct density distribution analysis in patients with cystic fibrosis: correlation with pulmonary function and radiologic scores. Acad Radiol 2015; 22: 179–85. doi: 10.1016/j.acra.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 101.Chassagnon G, Vakalopoulou M, Paragios N, Revel M-P. Artificial intelligence applications for thoracic imaging. Eur J Radiol 2020; 123: 108774. doi: 10.1016/j.ejrad.2019.108774 [DOI] [PubMed] [Google Scholar]
- 102.Aliboni L, Pennati F, Gelmini A, Colombo A, Ciuni A, Milanese G, et al. Detection and classification of bronchiectasis through Convolutional neural networks. J Thorac Imaging 2021;24 Mar 2021. doi: 10.1097/RTI.0000000000000588 [DOI] [PubMed] [Google Scholar]