Abstract
Background
Our study estimated insurance payments and patient out-of-pocket (OOP) expenses associated with discarded weight-based intravenous antineoplastic drugs for privately insured US adult patients with cancer.
Methods
We identified patients who received weight-based antineoplastic drugs from a 2017 MarketScan health risk assessment (IBM Corp, Armonk, NY) linked to claims data. Using weight information in the health risk assessment, we derived the recommended dose and calculated the percentage of drugs discarded. We applied β-regression to determine factors associated with the discarded percentages. To compare patients with and without high-deductible plans, we employed a generalized linear model and a 2-part model to examine insurance payment and OOP expense, respectively. All statistical tests were 2-sided.
Results
Of 27 350 claims for 58 weight-based antineoplastic drugs from 1970 patients, the median discarded percentage was 9.8% (mean [SD] = 12.8% [10.5%]). Aside from drug and tumor type, statistically significantly higher discarded percentages were found for patients in the lowest weight group (5.5% [95% confidence interval = 4.7% to 6.4%]; P < .001; weight <150 lb [68.0 kg] vs ≥200 lb [90.7 kg]). Private payers spent $5090 per patient in 2017 on discarded weight-based antineoplastic drugs, and patients’ mean OOP expense on discarded drugs was $63. In total, 39.7% of patients had high-deductible plans. The adjusted mean OOP expense for discarded drugs was statistically significantly higher for those in high-deductible plans ($95 vs $47; P < .001).
Conclusions
Private insurers incurred substantial financial burden from discarded weight-based antineoplastic drugs. Although the OOP expenses of discarded drugs were modest for most privately insured patients with cancer, approximately 5% spent more than $400 on the discarded drugs. Policies designed to reduce drug waste from single-dose, weight-based antineoplastic drugs should evaluate their financial consequences for payers and patients.
Spending on cancer drugs in the United States has grown 64% from 2013 to 2018, reaching $57 billion in 2018, with the median annual list price of newly approved cancer drugs staying above $150 000 since 2014 (1). Many antineoplastic drugs are administered intravenously with the dose depending on a patient’s weight or body surface area (BSA). These weight-based antineoplastic drugs are often packaged as single-dose vials. Leftover drug is common with these single-dose vials because the recommended dose based on patients’ weight or BSA often does not exactly match the dose in the vial. Under the prevailing “buy and bill” payment model in the United States, insurance companies reimburse physicians for both the drug administered to a patient and the unused proportion.
Bach and colleagues estimated that the total revenues from discarded drugs for the top 20 antineoplastic drugs amounted to $1.8 billion in the United States in 2016 (2). A portion of the spending on discarded drugs is borne by patients because of the cost-sharing requirement in insurance plans. For patients with cancer who receive weight-based antineoplastic drugs, the out-of-pocket (OOP) payment of discarded drugs will depend on drug price, the amount discarded, and the insurance benefit design. The financial burden of discarded drugs is most perceptible for patients with cancer who are enrolled in high-deductible plans, which now account for more than 40% of adults with employer-based health insurance (3); high-deductible plans include consumer-driven health plans and high-deductible health plans (4). Importantly, previous research has not provided precise estimates of OOP expenses for discarded cancer drugs because such estimation would require information about each patient’s weight (and height, in the case of BSA).
This study linked biometric data from employee health surveys with claims of the employees to obtain refined estimates of the discarded percentage of weight-based antineoplastic drugs and estimate the associated insurance payments and OOP expenses. We distinguished patients with cancer enrolled in high-deductible plans because they are most vulnerable to high cost-sharing and OOP costs.
Methods
Data Sources
We linked employees who responded to the health risk assessment (HRA) data in the 2017 MarketScan Research Databases (IBM Corp, Armonk, NY) to their claims in the Commercial Claims and Encounters (CCAE) database through unique enrollee identifiers. The HRA contains biometrics, health risks, and behavior collected from employees’ risk assessment questionnaires administrated by US corporations and health plans contributing data to MarketScan. The CCAE covers private-sector health data from approximately 350 private payers and collects paid claims and enrollment information of active employees, early retirees, former employees with continued coverage through the Consolidated Omnibus Budget Reconciliation Act of 1985, and spouses and dependents covered by employer-sponsored plans (5). This study was exempt from the institutional review board at the authors’ institution.
Ascertainment of Study Cohort
We identified patients who had cancer through International Classification of Diseases, Tenth Revision, diagnosis code from the linked HRA-CCAE database. Next, we obtained the list of weight-based antineoplastic drugs and the associated Healthcare Common Procedure Coding System (HCPCS) codes from the 2017 Centers for Medicare & Medicaid Services (CMS) Part B Discarded Drug Units Report (6). We then identified patients who had 1 or more claims with a HCPCS code indicating weight-based antineoplastic drugs from the CCAE claims. We excluded claims with payment of 0 or less and with service dates occurring in the months without a valid record of insurance enrollment. The final study cohort consisted of 1970 privately insured patients with cancer who received weight-based intravenous antineoplastic drugs.
Calculation of Discarded Drugs and Associated Costs
For each drug, we obtained information about the available vial sizes and recommended doses from IBM Micromedex 2.0. We derived the recommended dose for each patient based on his or her cancer type, weight, or BSA (7). We then estimated the discarded dose as the difference between the full vial dose based on the vial size and the recommended dose per a patient’s weight or BSA and calculated the discarded percentage as discarded dose divided by the full vial dose. For drugs with multiple vial sizes, we took a conservative approach by using the smallest vial size. Information about the available vial sizes in the United States and the recommended dose for each drug included in our analysis is shown in Supplementary Table 1 (available online).
For each claim for a weight-based antineoplastic drug, we multiplied the discarded percentage by net payment and OOP expense (sum of deductible, copayment, and coinsurance) to quantify costs associated with the discarded drugs from the payers’ and patients’ perspective, respectively. We then aggregated these claim-level insurance payments and OOP expenses to the patient level to estimate the per-patient financial burden of discarded weight-based antineoplastic drugs for payers and patients over calendar year 2017.
Statistical Analysis
Using claims as the unit of analysis, we applied β-regression to determine factors associated with the discarded percentage (8). Beta-regression is well suited for regression models- with rates or proportions as the dependent variable, such as the percentage of drug discarded. Covariates included age category (<50 y, 50-59 y, ≥60 y), weight group (<150 lb [68.0 kg], 150-199 lb [68.0-90.3 kg], ≥200 lb [90.7 kg]), place of service (hospital vs office), geographic region, cancer type, antineoplastic drug, and whether a patient was enrolled in a high-deductible plan. We categorized cancers into 8 groups: breast, lung, gastrointestinal (GI), gynecologic, genitourinary, lymphoma, other blood cancer, and all others. Because certain types are gender specific, we did not include sex as a covariate. Because patients with cancer often have multiple claims for their treatment, our analysis also accounted for within-patient correlations.
For patient-level analysis, we compared the adjusted mean and median payments and the OOP expenses (for total and discarded drugs) between patients with and without high-deductible plans based on multivariable analysis that controlled for age category, weight group, place of service, geographic region, and cancer type. We obtained adjusted mean of insurance payment and OOP expense using the generalized linear model, with gamma family and log link (to account for skewed distribution of cost data) (9) and 2-part model (to account for the large number of patients with no OOP expenses) (10,11), respectively. The adjusted median was obtained from quantile regressions (12). We then categorized payment and OOP expense into 5 cost ranges using median, 75th, 90th, and 95th percentiles of the distribution from the study cohort (ie, full sample) as cut points and compared the distribution across these cost ranges between the subset of patients with high-deductible plans and those without.
We used SAS statistical software, version 9.4 (SAS Institute, Cary, NC) for data management and Stata statistical software, version 15.1 (StataCorp, College Station, TX) for statistical analysis. To calculate P values, we used χ2 tests, Wald tests, and t tests, as indicated. All statistical tests were 2 sided, and P ≤ .05 were considered statistically significant.
Results
Characteristics of the Study Cohort
Of the 1970 patients with cancer who received weight-based antineoplastic drugs, the mean (standard deviation [SD]) age was 51.8 (8.8) years, and 65.4% were women. Approximately 39.7% of patients were enrolled in high-deductible plans. Breast cancer, GI cancer, and lymphoma accounted for 33.8%, 17.6%, and 10.6% of the study cohort, respectively. More patients were in the higher weight categories (41.8% between 150 [68.0 kg] and 199 lb [90.3 kg] and 31.8% ≥200 lb [90.7 kg]) than the lowest weight category (26.4% <150 lb [68.0 kg]). The comparison between patients who enrolled in high-deductible plans and those who did not revealed similar patient characteristics, except that enrollees in high-deductible plans were more likely to receive infused therapy in the office and reside in the Northeastern and Southern US regions (Table 1).
Table 1.
Patient characteristics, total cohort and by enrollment in high-deductible plans
| Patient characteristics | Full sample | Enrolled in high-deductible plans | Not in high-deductible plans | P a |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| Total | 1970 (100.0) | 782 (100.0) | 1188 (100.0) | |
| Age, y | ||||
| <50 | 655 (33.2) | 269 (34.4) | 386 (32.5) | .51 |
| 50-59 | 904 (45.9) | 359 (45.9) | 545 (45.9) | |
| ≥60 | 411 (20.9) | 154 (19.7) | 257 (21.6) | |
| Sex | ||||
| Male | 681 (34.6) | 263 (33.6) | 418 (35.2) | .48 |
| Female | 1289 (65.4) | 519 (66.4) | 770 (64.8) | |
| Weight, lb (kg) | ||||
| <150 (68.0) | 520 (26.4) | 203 (26.0) | 317 (26.7) | .86 |
| 150-199 (68.0-90.3) | 824 (41.8) | 333 (42.6) | 491 (41.3) | |
| ≥200 (90.7) | 626 (31.8) | 246 (31.5) | 380 (32.0) | |
| Place of service | ||||
| Office | 934 (47.4) | 407 (52.1) | 527 (44.4) | .001 |
| Hospital outpatient | 1036 (52.6) | 375 (48.0) | 661 (55.6) | |
| Cancer type | ||||
| Breast | 666 (33.8) | 288 (36.8) | 378 (31.8) | .17 |
| GI | 346 (17.6) | 131 (16.8) | 215 (18.1) | |
| Genitourinary | 96 (4.9) | 40 (5.1) | 56 (4.7) | |
| Gynecologic | 167 (8.5) | 52 (6.7) | 115 (9.7) | |
| Lung | 101 (5.1) | 40 (5.1) | 61 (5.1) | |
| Lymphoma | 208 (10.6) | 78 (10.0) | 130 (10.9) | |
| Other blood cancers | 117 (5.9) | 50 (6.4) | 67 (5.6) | |
| Others | 269 (13.7) | 103 (13.2) | 166 (14.0) | |
| Region | ||||
| Northeast | 291 (14.8) | 140 (17.9) | 151 (12.7) | <.001 |
| North Central | 609 (30.9) | 193 (24.7) | 416 (35.0) | |
| South | 700 (35.5) | 309 (39.5) | 391 (32.9) | |
| West | 370 (18.8) | 140 (17.9) | 230 (19.4) | |
| Average mo in chemotherapy (SD) | 4.52 (2.98) | 4.60 (2.94) | 4.46 (3.02) | .28b |
P values from 2-sided χ2 tests. GI = gastrointestinal; SD = standard deviation.
P value from 2-sided t test.
Claim-Level Analysis of Discarded Percentage
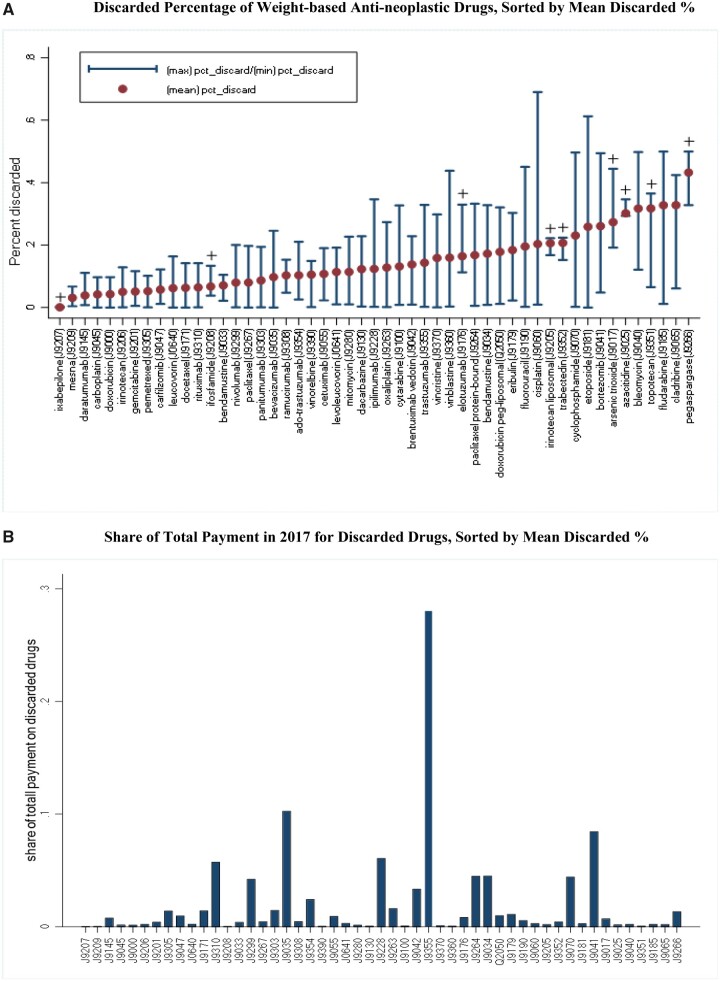
Of the 27 350 claims for 58 weight-based antineoplastic drugs, the mean discarded percentage was 12.8% (median [SD] = 9.8% [10.5%]). Figure 1, A plots the mean, minimum, and maximum discarded percentage, sorted by the mean values, for each drug that had 10 or more claims. The mean discarded percentages varied widely across drugs, ranging from less than 1.0% for ixabepilone (HCPCS code J9207) to 43.2% for pegaspargase (HCPCS code J9266). Figure 1, A also shows that for each drug, variations in patients’ weight or BSA resulted in a noticeable range between min and max percentages for some drugs. Figure 1, B depicts the share of each drug in the total payment for all discarded antineoplastic drugs in 2017 among our study cohort. As shown, drugs with higher mean discarded percentages do not necessary result in high cost share of discarded drugs. The top 6 drugs with the highest share were trastuzumab (28.0%), bevacizumab (10.2%), bortezomib (8.4%), ipilimumab (6.0%), rituximab (5.7%), and bendamustine (4.5%). These 6 drugs combined accounted for 62.8% of all payment for discarded drugs.
Figure 1.
Discarded percentage of each weight-based antineoplastic drug and its share in total payment for discarded drugs in 2017, sorted by mean discarded percentages. A) Mean, minimum, and maximum discarded percentage of each weight-based antineoplastic drug calculated from patients’ biometric information linked to drug claims and sorted by mean discarded percentages. A plus sign (+) indicates that fewer than 5 patients in our study cohort received the drug. B) The share of each weight-based antineoplastic drug among total payment for discarded drugs in 2017 varies widely across drugs.
Table 2 shows that after controlling for the list of antineoplastic drugs, 2 covariates statistically significantly associated with the discarded percentages were patients’ weight and cancer type. Specifically, the discarded percentage was 5.5% (95% confidence interval [CI] = 4.7% to 6.4%) and 1.1% (95% CI = 0.3% to 1.9%) higher for patients in the lowest (<150 lb [68.0 kg]) and middle (150-199 lb [68.3-90.3 kg]) weight categories compared with those in the highest weight category (>200 lb [90.7 kg]). In addition, compared with patients with GI cancer, the discarded percentage was 4.8% (95% CI = 2.0% to 7.5%) lower for those with lung cancer; breast cancer displayed a nearly statistically significant 1.7% increase in discarded percentage.
Table 2.
Claims-level analysis of factors associated with discarded percentage
| Covariatesa | Estimate (95% CI), % | P b |
|---|---|---|
| High-deductible plan | 0.01 (−0.7 to 0.7) | .98 |
| Age [Reference: age <50 y] | ||
| 50-59 y | −0.5 (−1.3 to 0.2) | .18 |
| ≥60 y | −0.1 (−1.0 to 0.8) | .84 |
| Weight [Reference: >200 lb (90.7 kg)] | ||
| <150 lb (68.0 kg) | 5.5 (4.7 to 6.4) | <.001 |
| 150-199 lb (68.0-90.3 kg) | 1.1 (0.3 to 1.9) | .008 |
| Place of service [Reference: office] | ||
| Hospital | 0.4 (−2.2 to 1.1) | .20 |
| Cancer type [Reference: GI] | ||
| Breast | 1.65 (−0.1 to 3.4) | .06 |
| Genitourinary | −0.03 (−3.1 to 3.0) | .98 |
| Gynecological | −1.4 (−3.2 to 0.3) | .11 |
| Lung | −4.8 (−7.5 to −2.0) | .001 |
| Lymphoma | −0.5 (−2.3 to 1.4) | .61 |
| Other blood cancers | −0.4 (−3.5 to 2.8) | .81 |
| Other cancer | 0.4 (−1.0 to 1.8) | .56 |
| Region [Reference: Northeast] | ||
| North Central | −0.7 (−1.7 to 0.4) | .22 |
| South | 0.4 (−0.6 to 1.4) | .43 |
| West | 0.6 (−0.4 to 1.7) | .25 |
Other covariates include the list of weight-based antineoplastic drugs. CI = confidence interval; GI = gastrointestinal.
P values from Wald tests; all P values were 2-sided.
Patient-Level Analysis of Insurance Payment and OOP Expense for Discarded Drugs
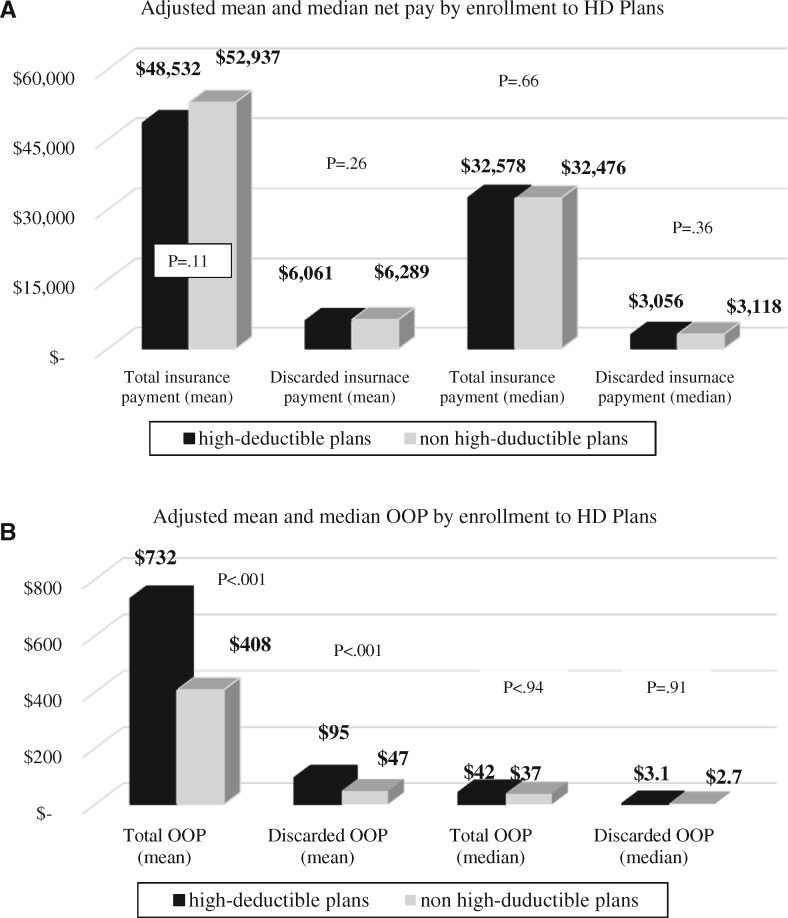
On average, private payers spent $43 902 per patient in 2017 on weight-based antineoplastic drugs. Of that, $5090 (11.6%) was spent on discarded drugs. Patients’ mean OOP expense was $522, with $63 spent on discarded drugs. After controlling for patient characteristics in the multivariable analyses, neither the adjusted mean nor the adjusted median insurance payment for total weight-based antineoplastic drugs as well as discarded drugs was statistically significantly different between patients enrolled in high-deductible plans and those not enrolled (Figure 2, A). The adjusted mean OOP expense, however, was statistically significantly higher for those in high-deductible plans ($732 vs $408, P < .001 [Wald test] for total amount; $95 vs $47, P < .001 [Wald test] for discarded amount). The adjusted median OOP was not statistically significantly different between patients who were in high-deductible plans and those who were not for total OOP ($42 vs $37, P = .94 [t test]) and OOP associated with discarded drugs ($3.13 vs $2.72, P = .91 [t test]) (Figure 2, B). Other covariates statistically significantly associated with the costs of discarded drugs were weight group and the total number of months in chemotherapy (Table 3).
Figure 2.
Comparison of the adjusted mean and median insurance payment and out-of-pocket (OOP) expenses for total and discarded amount of weight-based antineoplastic drugs between patients enrolled in high-deductible (HD) plans and those not in HD plans (N = 1970). A) No statistically significant difference by enrollment to HD plans was observed in the adjusted mean and median net payment for total and discarded amount of weight-based antineoplastic drugs. B) Mean OOP of total and discarded weight-based antineoplastic drugs was statistically significantly higher for patients enrolled in HD plans. No statistically significant difference in median OOP was found between patients in HD and non-HD plans.
Table 3.
Patient-level analysis of factors associated with insurance payment or out-of-pocket payment for discarded drugsa
| Covariate | GLM: insurance payment Estimate (95% CI) |
Quantile regression: insurance payment Estimate (95% CI) |
2-part model: OOP payment Estimate (95% CI) |
Quantile regression: OOP payment Estimate (95% CI) |
|---|---|---|---|---|
| High-deductible plan | −533 (−1463 to 397) | −234 (−725 to 257) | 40 (24 to 56) | 0.18 (−2.9 to 3.2) |
| Age [Reference: <50 y] | ||||
| 50–59 y | −855 (−2017 to 307) | −8.7 (−554 to 536) | −0.8 (−17 to 16) | −0.00001 (−3.4 to 3.4) |
| ≥60 y | −682 (−2132 to 768) | −56 (−735 to 622) | 13 (−9 to 34) | 0.08 (−4.1 to 4.3) |
| Weight [Reference: >200 lb (90.7 kg)] | ||||
| <150 lb (68.0 kg) | 1843 (661 to 3024) | 567 (−77 to 1211) | 34 (14 to 55) | 0.08 (−3.9 to 4.1) |
| 150-199 lb (68.0-90.3 kg) | 1420 (161 to 2680) | 193 (−370 to 755) | 21 (3 to 39) | −0.0001 (−3.5 to 3.5) |
| Place of service [Reference: office] | ||||
| Hospital | 3586 (2366 to 4806) | 775 (289 to 1262) | −17 (−32 to −2) | −0.9 (−3.9 to 2.1) |
| Cancer type [Reference: GI] | ||||
| Breast | 2158 (1020 to 3296) | 813 (110 to 1516) | 30 (8 to 51) | 0 (−4.4 to 4.4) |
| Genitourinary | −99 (−2609 to 2412) | 568 (−658 to 1793) | −14 (−50 to 21) | 2.1 (−5.5 to 9.7) |
| Gynecologic | −3321 (−5343 to −1298) | −468 (−1463 to 527) | −43 (−79 to −7) | −0.08 (−6.3 to 6.1) |
| Lung | 506 (−1379 to 2392) | 599 (−602 to 1801) | −17 (−59 to 25) | −0.69 (−8.1 to 6.8) |
| Lymphoma | 7679 (5837 to 9520) | 2071 (1143 to 2998) | 62 (37 to 87) | 1.0 (−4.8 to 6.8) |
| Other blood cancers | 9349 (683 to 11860) | 4520 (3389 to 5651) | 72 (41 to 103) | 0.92 (−6.1 to 7.9) |
| Other cancer | 4302 (1397 to 7206) | 309 (−549 to 1168) | −3 (−35 to 30) | 0 (−5.3 to 5.3) |
| Region [Reference: Northeast] | ||||
| North Central | 294 (−1185 to 1773) | −297 (−1060 to 466) | −3 (−29 to 23) | 0 (−4.7 to 4.7) |
| South | 311 (−930 to 1552) | 20 (−729 to 769) | 8 (−17 to 33) | 0.08 (−4.6 to 4.7) |
| West | 865 (−499 to 2228 | 210 (−628 to 1048) | −10 (−38 to 18) | −0.84 (−6.0 to 4.4) |
| Time on chemotherapy, mo | 1927 (1606 to 2247) | 902 (821 to 983) | 12 (10 to 15) | 0.92 (0.4 to 1.4) |
CI = confidence interval; GI = gastrointestinal; GLM = generalized linear model; OOP = out of pocket.
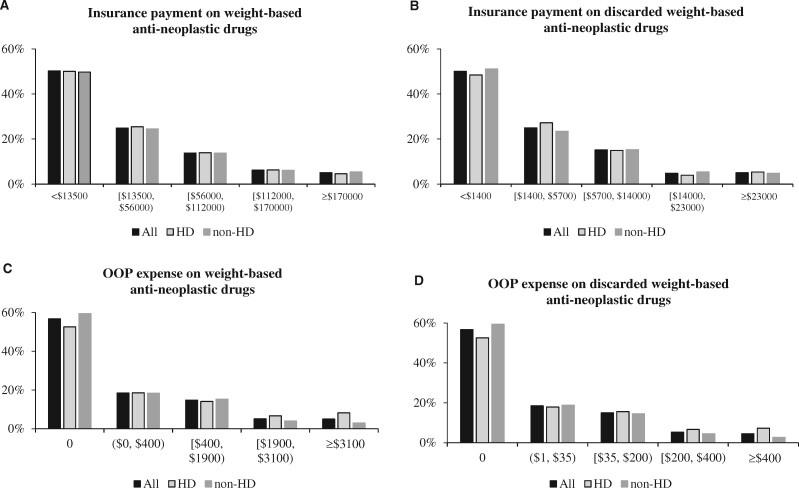
Figure 3 illustrates the distribution of insurance payment and OOP expense for weight-based antineoplastic drugs and for their discarded amount in 2017 for all patients in the full sample and stratified by whether patients enrolled in high-deductible plans. The distribution of insurance payment was similar between patients who enrolled in high-deductible plans and those who did not, with median payment (per patient) around $13 500 for antineoplastic drugs (Figure 3, A) and $1400 for discarded drugs (Figure 3, B). Distribution of OOP expense indicates that although close to 60.0% of patients had no expense, a higher proportion of patients in high-deductible plans incurred expenses higher than the 95th percentile. Figure 3, C shows 8.2% of patients in high-deductible plans spent $3100 or more OOP on weight-based antineoplastic drugs compared with 3.0% of patients who did not enroll in high-deductible plans. For OOP expense on discarded drugs, Figure 3, D shows that 7.3% of patients in high-deductible plans and 2.7% not in high-deductible plans spent $400 or more OOP on discarded drugs.
Figure 3.
Distribution of insurance payment and out-of-pocket (OOP) expense on total vs discarded weight-based antineoplastic drugs (N = 1970). A) Distribution of insurance payment on weight-based antineoplastic drugs was similar between patients in high-deductible (HD) and non-HD plans. B) Distribution of insurance payment on discarded weight-based antineoplastic drugs was similar between patients in HD and non-HD plans. C) Distribution of OOP expenses on weight-based antineoplastic drugs showed a higher proportion of patients with HD plans in higher-cost categories and a lower proportion of HD patients had no OOP expense. D) Distribution of OOP expenses on discarded weight-based antineoplastic drugs showed a higher proportion of patients with HD plans in higher-cost categories and a lower proportion of HD patients had no OOP expense. The cut point of each cost category corresponds to median, 75th, 90th, and 95th percentiles of the distribution of the full sample.
Discussion
Our study reports the financial burden of discarded weight-based antineoplastic drugs for payers and patients of commercial insurance. We found that although OOP expenses of discarded drugs were modest for the majority of privately insured patients, approximately 4.5% of patients paid more than $400 in 2017 for discarded drugs alone. The percentage was even higher (7.3%) for the subset of patients enrolled in high-deductible plans. These numbers are noteworthy because of both the trend of rising enrollment in high-deductible plans and the state of financial preparedness among American families. Indeed, a 2018 survey on the economic well-being of US households reported that 4 in 10 adults could not cover an unexpected expense of $400 (13). Another notable finding is the high costs of discarded antineoplastic drugs for third-party payers, with the estimated payment on the leftover amount averaging more than $5000 per patient in 2017. Although not directly paid by the patients, these costs are ultimately transmitted to the insured population as higher premiums. The National Academies of Sciences, Engineering, and Medicine (National Academies) recently released a consensus study report on discarded drugs (14). The report suggests that if regulatory action is taken to require rebates from manufacturers, the rebates should first be directed to cover patients’ OOP expense for the discarded drugs. Estimates from this study offer useful information for policy discussions regarding such rebates.
Undoubtedly, the high price tag of discarded weight-based antineoplastic drugs is driven by the high costs of cancer drugs in the United States. The definitive solution is to develop policies to lower the price of prescription drugs—a point emphasized in the above National Academies report (14). Still, incremental changes may help reduce drug waste caused by leftover from single-dose vials. Researchers have proposed to mitigate this problem through 3 channels: drug delivery and administration, manufacturing, and reimbursement (2,15). Proposed strategies under each channel can potentially reduce the costs of discarded drugs for some stakeholders in the US health-care system but also face unique regulatory and implementation challenges.
Vial sharing is a drug delivery practice that deploys strategies to safely administer the leftover portion of the drug in a single-use vial to a second patient instead of discarding it. The safety of vial sharing is enhanced by technologies such as closed-system drug-transfer devices that reduce the risk of microbial contamination (16). Per the infection-control guideline of the Centers for Disease Control and Prevention, vial sharing is prohibited because the guideline requires a new syringe and needle (as in the case of single-dose vials) to be used by 1 patient (17). The United States Pharmacopeia imposes less strict requirements than the Centers for Disease Control and Prevention. Under United States Pharmacopeia Chapter 797 standards, single-use drugs must be discarded within 6 hours of use if the drug was opened and kept in International Organization for Standardization 5 air conditions and within 1 hour if outside International Organization for Standardization 5 air conditions (18). These regulatory requirements contribute to the less frequent vial-sharing practices observed in the United States compared with other countries, despite evidence of the cost-effectiveness of the vial-sharing practice (19). From patients’ perspective, it is unclear whether vial sharing will ease the financial burden associated with discarded cancer drugs because providers may charge payers for the full vial under the buy-and-bill model (20).
Another proposed strategy urged regulators to require biopharmaceutical companies to manufacture drugs in multiple vial sizes. Bach and colleagues (2) estimated that this strategy could save the health-care system approximately $2 billion from wastage avoided for the top 20 infused cancer drugs. (2) Economists have cautioned that as long as drug manufacturers hold monopoly power in setting drug prices, an unintended consequence of regulating multiple vial sizes could be a compensatory increase in drug prices (21). In that case, patients could actually be worse off financially. In addition, it is possible that even with the option of multiple vial sizes, providers may choose to purchase only 1 size to reduce inventory costs and administrative burden.
On the reimbursement front, Bach et al. (2) suggested requiring drug manufacturers to refund the cost of leftover drug in exchange for allowing them to select their vial sizes (2); such a refund could be extended to patients. In fact, the JW modifier mandated by CMS may offer a viable mechanism to refund payers and patients for the discarded proportion of weight-based drugs. The JW modifier is a HCPCS Level II modifier created by CMS in July 2007 for providers to report drug amount discarded/not administered to any patient as a separate line in drug claims (22). Effective January 1, 2017, the CMS mandated providers to report the JW modifier for discarded drugs on Part B claims (23). Some private insurance plans, such as Blue Cross Blue Shield, have also adopted this CMS mandate (24). If providers adhere to the use of the JW modifier when submitting their claims, policy makers can use this information to mark claims to request refunds and waive the associated copayment for patients. Unfortunately, a recent study suggested wide variation across providers in the billing practice of using the JW modifier to report discarded drugs (25). Price transparency could potentially inform patients of the impact of discarded drugs on their OOP expense and incentivize policy actions. Despite efforts from state legislators and private sectors in promoting price transparency (26,27), however, price transparency in the context of OOP expense remains challenging (28).
Several study limitations warrant discussion. First, our study was based on a convenient sample of employees who responded to their employers’ health risk assessment questionnaire; thus, the study cohort may not be representative of the entirety of privately insured patients with cancer. Although the biometric information available in our data offers a rare opportunity to obtain patient-specific cost estimates, interpretations of the drug-to-drug variations should note that for drugs with a small number of users, the discarded percentages documented in our study would more likely reflect the biometric characteristics of patients than the available vial sizes. Second, weight and height information in the HRA were self-reported and static, and studies have found that self-reported weight tends to be underreported for both overweight and obese individuals (29,30). Third, our use of the smallest vial size to calculate discarded percentage could underestimate the discarded percentages and associated costs for weight-based drugs with multiple vial sizes. Lastly, our analysis did not consider dose rounding, because such practice cannot be reliably determined from claims data. This may overestimate costs associated with discarded drugs, with research showing that dose rounding can reduce drug wastage (31). Nevertheless, our estimates from the perspective of payers and patients in private insurance add important information to the literature as the study by Bach et al. (2) derived their estimates from Medicare payment (2), which tend to have lower markups than private payers. Another unique contribution of our study is our elucidation of the financial impact of discarded drugs for patients.
Private insurers incurred about $5000 per patient per year to reimburse oncology practices for the discarded proportion of weight-based antineoplastic drugs. Although OOP expenses of discarded drugs were modest ($63 on average), more than 7.0% of those enrolled in high-deductible plans spent more than $400 per year on the discarded drugs alone. Policies designed to reduce drug waste from single-dose, weight-based antineoplastic drugs should consider their financial impact at both the societal level (eg, waste is translated to higher insurance premiums for employees) and the individual level, where select patients may have high OOP expenses.
Funding
Dr Shih acknowledges funding from the National Cancer Institute (R01CA207216 and CCSG P30 CA016672) and Health Care Services Corporation/BCBSTX. Drs Shih and Yao acknowledge funding from National Cancer Institute R01CA225647.
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: Dr Shih received consulting fees, travel, and accommodations for serving on a grants review panel for Pfizer Inc and an advisory board for AstraZeneca in 2019. Dr Schrag acknowledges funding from the National Cancer Institutes (1UM1 CA233035-01). Dr Schrag received compensation for speaking at a Pfizer satellite symposium in 2019, receives services for editorial work for JAMA, and obtained research funding from the AACR for project GENIE. All other authors have no conflict of interest to disclose.
Acknowledgements: The authors thank Gary Deyter, technical writer from the Department of Health Services Research at The University of Texas MD Anderson Cancer Center, for proofreading the manuscript.
Prior presentations: Preliminary study findings were presented as a poster at the 2020 ASCO Annual Meeting.
Author contributions: Shih provided administrative support and acted as the overall guarantor. The authors made the following contributions: Conceptualization and study design: Shih, Xu; Data Acquisition: Shih; Data analysis: Shih, Xu; Data interpretation: Shih, Xu, Zhao, Schrag, Yao; Writing, Original Draft: Shih; Writing, Review & Editing: Shih, Xu, Zhao, Schrag, Yao.
Data Availability
Access to the data used in this study (ie, MarketScan) is strictly limited to members of the research team who signed the data use agreement at the corresponding author’s institution. Per the data use agreement, authors of this study cannot grant data access or distribute any subset of data to individual outside the research team.
Supplementary Material
References
- 1. Global oncology trends 2019. IQVIA Web site. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2019. Published May 30, 2019. Accessed May 19, 2021.
- 2. Bach PB, Conti RM, Muller RJ, Schnorr GC, Saltz LB.. Overspending driven by oversized single dose vials of cancer drugs. BMJ. 2016;352:i788. doi:10.1136/bmj.i788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kullgren JT, Cliff BQ, Krenz CD, et al. A survey of Americans with high-deductible health plans identifies opportunities to enhance consumer behaviors. Health Aff (Millwood). 2019;38(3):416–424. doi:0.1377/hlthaff.2018.05018. [DOI] [PubMed] [Google Scholar]
- 4. Cohen RA, Martinez ME, Zammitti EP. Health insurance coverage: early release of estimates from the National Health Interview Survey, January-March 2016. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/data/nhis/earlyrelease/insur201609.pdf. Published September 2016. Accessed May 19, 2021.
- 5. Truven Health MarketScan Research Databases. Ann Arbor, MI: Truven Health Analytics; 2016.
- 6. Medicare Part B Discarded Drug Units Report. CMS.gov Web site. https://www.cms.gov/research-statistics-data-systems/cms-drug-spending/medicare-part-b-discarded-drug-units-report. Updated December 22, 2020. Accessed May 19, 2021.
- 7. Du Bois D, Du Bois EF.. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5(5):303–311; discussion 312–313. [PubMed] [Google Scholar]
- 8. Ferrari SLP, Cribari-Neto F.. Beta regression for modelling rates and proportions. J Appl Stat. 2004;31(7):799–815. doi:0.1080/0266476042000214501. [Google Scholar]
- 9. Basu A, Manning WG, Mullahy J.. Comparing alternative models: log vs Cox proportional hazard? Health Econ. 2004;13(8):749–765. doi:10.1002/hec.852. [DOI] [PubMed] [Google Scholar]
- 10. Mullahy J. Much ado about two: reconsidering retransformation and the two-part model in health econometrics. J Health Econ. 1998;17(3):247–281. doi:10.1016/s0167-6296(98)00030-7. [DOI] [PubMed] [Google Scholar]
- 11. Deb P, Norton EC, Manning WG.. Health Econometrics Using Stata. College Station, TX: STATA Press; 2017. [Google Scholar]
- 12. Cameron AC, Trivedi PK.. Microeconometrics: Methods and Applications. 1st ed. New York, NY: Cambridge University Press; 2005. [Google Scholar]
- 13. Board of Governors of the Federal Reserve System. Report on the economic well-being of U.S. households in 2017. https://www.federalreserve.gov/publications/files/2017-report-economic-well-being-us-households-201805.pdf. Published May 2018. Accessed May 19, 2021.
- 14. Nass SJ, Lustig TA, Amankwah FK, Shortliffe EH, eds. Medications in Single-Dose Vials: Implications of Discarded Drugs. Washington, DC: National Academies Press; 2021. [PubMed] [Google Scholar]
- 15. Gilbar PJ, Chambers CR, Gilbar EC.. Opportunities to significantly reduce expenditure associated with cancer drugs. Future Oncol. 2017;13(15):1311–1322. doi:10.2217/fon-2017-0033. [DOI] [PubMed] [Google Scholar]
- 16. Gilbar PJ, Chambers CR, Vandenbrouche J, Sessink PJ, Tyler TG.. How can the use of closed system transfer devices to facilitate sharing of drug vials be optimised to achieve maximum cost savings? J Oncol Pharm Pract. 2019;25(1):205–209. doi:10.1177/1078155217753890. [DOI] [PubMed] [Google Scholar]
- 17. National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion. Guide to infection prevention for outpatient settings: minimum expectations for safe care. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/infectioncontrol/pdf/outpatient/guide.pdf. Published September 2016. Accessed May 19, 2021.
- 18. General chapter <797> pharmaceutical compounding—sterile preparations. United States Pharmacopeia Web site. https://www.usp.org/compounding/general-chapter-797. Accessed May 19, 2021.
- 19. Smith RSV. A 2-year retrospective review of vial sharing options for the compounding of cytotoxics. Eur J Hosp Pharm. 2015;22(3):161–164. doi:10.1136/ejhpharm-2014-000547. [Google Scholar]
- 20. Polite B, Conti RM, Ward JC.. Reform of the buy-and-bill system for outpatient chemotherapy care is inevitable: perspectives from an economist, a realpolitik, and an oncologist. Am Soc Clin Oncol Educ Book. 2015:e75–e80. doi:10.14694/EdBook_AM.2015.35.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glied S, Sampat B. Would a wider variety of vial sizes reduce the cost of chemotherapy? Not likely. Health Affairs Blog. Posted May 11, 2016. doi:10.1377/hblog20160511.054821.
- 22. CMS Manual System, Pub 100-04 Medicare claims processing. Transmittal 1248. CMS.gov Web site. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R1248CP.pdf. Published May 25, 2007. Accessed May 19, 2021.
- 23. Medicare program. JW modifier: drug/biological amount discarded/not administered to any patient. Frequently asked questions. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/JW-Modifier-FAQs.pdf. Published August 26, 2016. Accessed May 19, 2021.
- 24. Wasted/discarded drugs and biologicals guideline. Policy No. CPCP017, Version 3.0. Blue Cross Blue Shield of Illinois Web site. https://www.bcbsil.com/pdf/standards/wasted_discarded_drugs_biologicals.pdf. Published September 11, 2020. Accessed May 19, 2021.
- 25. Goldstein DA, Hirsch A.. A policy that encourages wastage of expensive medications—the JW modifier. JAMA Oncol. 2018;4(2):155–156. doi:10.1001/jamaoncol.2017.3997. [DOI] [PubMed] [Google Scholar]
- 26. de Brantes F, Delbanco S. Report card on state price transparency laws—July 2016. Newtown, CT: CT Health Care Incentives Improvement Institute; 2016.
- 27. Higgins A, Brainard N, Veselovskiy G.. Characterizing health plan price estimator tools: findings from a national survey. Am J Manag Care. 2016;22(2):126–131. [PubMed] [Google Scholar]
- 28. Shih YT, Nasso SF, Zafar SY.. Price transparency for whom? In search of out-of-pocket cost estimates to facilitate cost communication in cancer care. Pharmacoeconomics. 2018;36(3):259–261. doi:10.1007/s40273-018-0613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin CJ, DeRoo LA, Jacobs SR, Sandler DP.. Accuracy and reliability of self-reported weight and height in the Sister Study. Public Health Nutr. 2012;15(6):989–999. doi:10.1017/S1368980011003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowring AL, Peeters A, Freak-Poli R, Lim MS, Gouilllou M, Hellard M.. Measuring the accuracy of self-reported height and weight in a community-based sample of young people. BMC Med Res Methodol. 2012;12(1):175. doi:10.1186/1471-2288-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vandyke TH, Athmann PW, Ballmer CM, Kintzel PE.. Cost avoidance from dose rounding biologic and cytotoxic antineoplastics. J Oncol Pharm Pract. 2017;23(5):379–383. doi:10.1177/1078155216639756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the data used in this study (ie, MarketScan) is strictly limited to members of the research team who signed the data use agreement at the corresponding author’s institution. Per the data use agreement, authors of this study cannot grant data access or distribute any subset of data to individual outside the research team.