Abstract
Background
We hypothesized that the addition of receptor tyrosine kinase inhibitors (RTKis, e.g., lapatinib, erlotinib, cetuximab, bevacizumab, panitumumab) to radiotherapy-based treatment for solid tumors does not increase overall survival but may increase toxicity.
Methods
Population, Intervention, Control, Outcome, Study Design; Preferred Reporting Items for Systematic Reviews and Meta-Analyses; and Meta-analysis of Observational Studies in Epidemiology methods were used to identify prospective randomized studies including patients with solid tumor cancers treated with radiotherapy with or without RTKis. Extracted variables included use of radiotherapy vs chemoradiotherapy, RTKi type (antibody vs small molecule), outcomes, and toxicities. The primary endpoint was overall survival; the secondary endpoint was grade 3+ toxicity. Random-effects meta-analyses were performed for each outcome measure. All statistical tests were 2-sided.
Results
A total of 405 studies met the initial search criteria, of which 13 prospective randomized trials of radiotherapy with or without RTKi met the inclusion criteria, encompassing 5678 patients. The trials included cancers of the head and neck (6 trials, 3295 patients), esophagus (3 trials, 762 patients), lung (2 trials, 550 patients), and brain (2 trials, 1542 patients). Three studies evaluated a small molecule and radiotherapy in 949 patients, and 10 studies evaluated antibodies and radiotherapy in 4729 patients. The addition of RTKis to radiotherapy-based treatment did not improve overall survival (hazard ratio = 1.02, 95% confidence interval = 0.90 to 1.15, P = .76) but increased grade 3+ toxicity (relative risk = 1.18, 95% confidence interval = 1.06 to 1.33, P = .009).
Conclusions
The addition of RTKis to radiotherapy does not improve survival and worsens toxicity.
Chemoradiotherapy has long been a cornerstone of cancer treatment because the combination of chemotherapy and external beam radiotherapy enhances cellular and tissue response to treatment (1). However, the survival benefit of chemoradiotherapy is often counterbalanced by increased toxicity because it is not selective for tumor cells (2-5). Thus, the combination of radiotherapy with tumor-specific receptor tyrosine kinase inhibitors (RTKis, e.g., lapatinib, erlotinib, cetuximab, bevacizumab, panitumumab) has emerged as a promising alternative to chemoradiotherapy, with the promise of a more “focused” antitumor effect and thus less toxicity (6,7).
The antitumor response may be enhanced from the combination of ionizing radiation with RTKis for a number of reasons (8). First, because ionizing radiation promotes RTK activity, RTKis can magnify radiation-induced antitumor effects when used in combination with radiation. Second, the DNA repair facilitated by RTKs would be inhibited by RTKis, thus inhibiting repair of damage caused by ionizing radiation. Finally, RTKs are involved in various steps of tumorigenesis such as increased cell proliferation, tumor progression, tumor angiogenesis, and increased cancer cell survival (1,9–11). Thus, the combination of RTK inhibitors with radiotherapy may serve to enhance the radiosensitivity of tumor cells. For the purposes of this work, we define RTKis as antibodies (eg, cetuximab, bevacizumab, panitumumab) or small molecules (eg, gefitinib, erlotinib, lapatinib), as illustrated in Supplementary Figure 1 (available online).
The purpose of this analysis is to evaluate the overall survival and toxicity of the addition of RTKis to standard-of-care radiotherapy-based treatment for solid tumors. We hypothesized that addition of RTKis to radiotherapy or chemoradiotherapy does not improve survival and worsens toxicity. The results of this work are applicable to patients receiving radiotherapy who may also be eligible for systemic therapy with RTKis. These results may also serve to affect the design of future studies exploring combination therapy.
Methods
Evidence Acquisition
The inclusion criteria for the literature search were defined using the Population, Intervention, Control, Outcome, Study Design (see Table 1) approach. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (see Figure 1) literature selection protocol was used for article selection, and the Meta-analysis of Observational Studies in Epidemiology reporting guidelines were followed (Supplementary Table 1, available online). The medical literature including clinical trials published in English from 2000 to 2018 was searched in PubMed, Ovid Medline, Cochrane, and CINAHL (search strategy; see Figure 1). The following terms were used in the search strategy: (“radiation”) AND (“bevacizumab” OR “cetuximab” OR “panitumumab” OR “trastuzumab” OR “pertuzumab” OR *mab OR “erlotinib” OR “gefitinib” OR “lapatanib” OR “imatinib” OR “nilotinib” OR “sorafenib” OR “sunitinib” OR “dasatinib” OR *nib) AND (“randomized”) AND (“survival”).
Table 1.
PICOS inclusion criteriaa
| Term | Definition |
|---|---|
| Population | Patients with a solid cancer of any disease site receiving definitive therapy |
| Intervention | Definitive radiotherapy or chemoradiotherapy plus receptor tyrosine kinase inhibitor (small molecule or antibody) |
| Control | Definitive radiotherapy or chemoradiotherapy |
| Outcome |
Overall survival, assessed using a hazard ratio Incidence of CTCAE acute and late grade 3, 4, and 5 toxicities, assessed based on crude counts, and relative risks calculated |
| Study design | Multi-arm prospective randomized controlled trial, with at least 10 individual patients |
CTCAE = Common Terminology Criteria for Adverse Events; PICOS = Population, Intervention, Control, Outcome, Study Design.
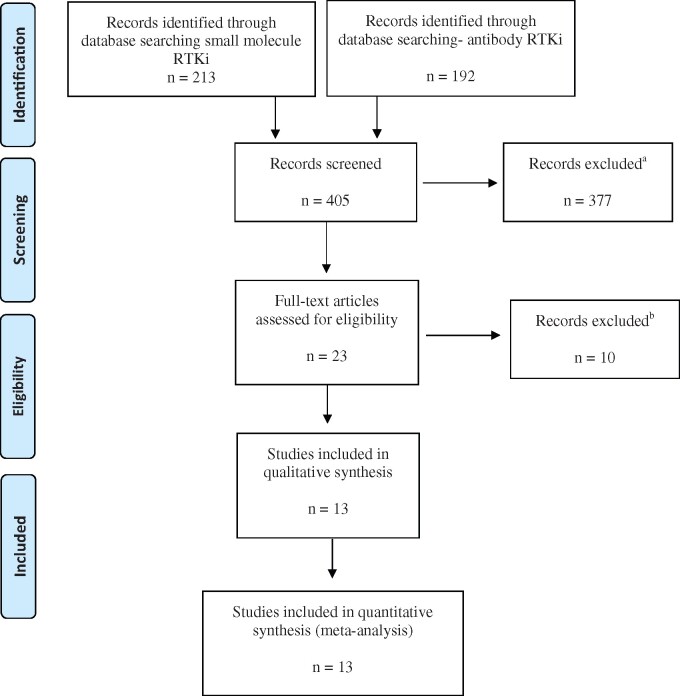
Figure 1.
Flow diagram describing the data collection process following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses convention. aExcluded studies included those that did not analyze mutually exclusive groups, did not provide survival data, were not in English, were not multi-arm randomized control trials, did not contain at least 10 individual patients, and evaluated combinations of drugs. bFollowing our initial screening process, studies were further excluded if the receptor tyrosine kinase inhibitor (RTKi) was not given concurrently with radiotherapy (n = 2), both arms of the study contained RTKi and radiotherapy (n = 3), survival data were not reported as a hazard ratio (n = 4), radiation was delivered using nonconventional dose or fractionation schema (n = 1).
Inclusion criteria were: 1) patients with solid cancer of any primary site receiving definitive therapy, 2) intervention arm included RTKi (either small molecule or antibody), 3) control arm included either radiotherapy or chemoradiotherapy, 4) the primary outcome measure (hazard ratio for overall survival) was reported, and 5) multi-arm randomized controlled trial. Both superiority and noninferiority trials were included in this analysis. Exclusion criteria included 1) retrospective or single-arm studies, 2) manuscripts involving nonhuman subjects, 3) works not published in English, 4) unfinished manuscripts, and 5) patients receiving palliative radiotherapy.
Twenty-three studies were found that met inclusion criteria. These were individually screened (N.Z., E.M., L.T.), and 10 trials were further excluded (Supplementary Table 2, available online), resulting in 13 trials eligible for further analysis (10,12–23) (Supplementary Figure 2, available online). With the exception of the trials by Bonner and Giralt (18,23), both the study and the experimental arms contained the same treatment regimen with or without the addition of RTKi. In these 2 trials, the experimental arms contained RTKi plus radiotherapy alone. However, these studies were included in our analysis because they met inclusion criteria requiring radiotherapy to be in both study arms. A sensitivity analysis was later performed excluding the trials.
Data Extraction
Extracted data include population size, cancer type, treatment type, overall survival, and Common Terminology Criteria for Adverse Events grade 3 or higher toxicity occurrence. For survival, hazard ratios and 95% confidence intervals (CI) were collected. Four authors contributed to data collection (N.Z., E.B., L.T., M.M.), and then data were reviewed among all authors. For toxicity, absolute numbers of grade 3+ toxicities were collected from the arms of the included studies, and relative risks (RRs) were calculated. All data from studies and discrepancies were reviewed and discussed by multiple authors to ensure reporting accuracy.
Intervention and Endpoints
The intervention was definitive conventionally fractionated radiotherapy, chemoradiotherapy, or the same treatment plus RTKi (either as an antibody or small molecule). The primary endpoint was overall survival, defined by the hazard ratio reported in each trial. The secondary endpoint was Common Terminology Criteria for Adverse Events grade 3+ toxicity. Four studies did not provide overall toxicity data (10,16,19). The corresponding author of 1 study was contacted to obtain the toxicity data (12).
Statistical Analysis
Stata (Stata Corp LLC; College Station, TX, USA) was used to conduct the meta-analyses and heterogeneity tests. Random effects meta-analyses were used to determine an overall summary estimate for each of the outcome measures. A random effects model was chosen over a fixed effects model because the studies included were performed over several years; among different populations; in a variety of countries; and using different systemic therapies, RTKis, and radiotherapy modalities and doses. Further, a random effects model is often preferred over fixed models when performing a meta-analysis to guide patient treatment decisions (24,25). Overall summary estimates for overall survival and toxicity were depicted on forest diagrams with their associated 95% confidence interval. We also performed subgroup analyses stratified by treatment type (chemoradiotherapy or radiotherapy) or RTKi type (small molecule or antibody). Heterogeneity was assessed using both the I2 statistic (26) and Cochran Q-Test (27). Statistically significant heterogeneity was considered to be present if I2 was greater than 50% and the P value of the Q-Test was less than .10. Funnel plots were generated to assess the potential publication bias. P values were calculated using t tests when indicated. The null hypothesis was rejected if the P value was less than .05. All tests were 2-sided.
Results
Study Characteristics
A total of 405 studies met our initial search criteria. Of these, 13 prospective randomized trials with a total of 5678 patients, published from 2006 to 2018, met our final inclusion criteria (Table 2). The trials included cancers of the head and neck (6 trials, 3295 patients), esophagus (3 trials, 762 patients), lung (2 trials, 550 patients), and brain (2 trials, 1542 patients). No study focused on very old (>80 years), very young (<18 years), or immunosuppressed patients.
Table 2.
Studies assessing RT with or without RTKisa
| Study | Site | Reference group | RTKi | Drug class | Control, No. of Patients | Experimental, No. of Patients | HR (95% CI) | No. of deaths |
No. of G3+ toxicity |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | ||||||||
| Harrington et al., 2015 (12) | HNSCC | CRT | Lapatinib | Small molecule | 342 | 346 | 0.96 (0.73 to 1.26) | 110 | 105 | 224 | 263 |
| Wu et al., 2018 (10) | Esophagus (conventional field arms) | CRT | Erlotinib | Small molecule | 88 | 88 | 0.76 (0.57 to 1.01) | 64 | 61 | NR | NR |
| Sperduto et al., 2013 (13) | NSCLC brain metastases | RT | Erlotinib | Small molecule | 44 | 41 | 1.46 (0.91 to 2.34) | 35 | 36 | 5 | 20 |
| Ang et al., 2014 (14) | HNSCC | CRT | Cetuximab | Antibody | 447 | 444 | 0.95 (0.74 to 1.22) | 183 | 190 | 90 | 99 |
| Bradley et al., 2015 (15) | NSCLC | RT | Cetuximab | Antibody | 228 | 237 | 1.07 (0.84 to 1.36) | 139 | 140 | 160 | 205 |
| Crosby et al., 2017 (16) | Esophagus | CRT | Cetuximab | Antibody | 129 | 129 | 1.25 (0.93 to 1.69) | 68 | 80 | 94 | 106 |
| Suntharalingam et al., 2017 (17) | Esophagus | CRT | Cetuximab | Antibody | 169 | 159 | 0.90 (0.70. to 1.16) | 122 | 105 | 113 | 112 |
| Gilbert et al., 2014 (20) | Glioblastoma | CRT | Bevacizumab | Antibody | 309 | 312 | 1.13 (0.96 to 1.34) | 195 | 215 | NR | NR |
| Bonner et al., 2006 (18) | HNSCC | RT | Cetuximab | Antibody | 213 | 211 | 0.74 (0.57 to 0.97) | 116 | 95 | NR | NR |
| Chinot et al., 2014 (19) | Glioblastoma | CRT | Bevacizumab | Antibody | 458 | 463 | 0.88 (0.76 to 1.02) | 126 | 156 | 238 | 306 |
| Mehanna et al., 2018 (21) | HNSCC | RT | Cetuximab | Antibody | 166 | 168 | 5.00 (1.70 to 14.70) | 4 | 18 | NR | NR |
| Geoffrois et al., 2018 (22) | HNSCC | CRT | Cetuximab | Antibody | 179 | 157 | 1.12 (0.86 to 1.46) | 100 | 81 | NR | NR |
| Giralt et al., 2015 (23) | HNSCC | CRT | Panitumumab | Antibody | 61 | 90 | 1.59 (0.91 to 2.78) | 18 | 38 | NR | NR |
CI = confidence interval; CRT = chemoradiotherapy; G3+ = grade 3+ toxicity; HNSCC = head and neck squamous cell cancer; HR = hazard ratio; NR = not reported; NSCLC = non-small cell lung cancer; RT = radiotherapy; RTKi = receptor tyrosine kinase inhibitor.
Three studies examined small-molecule RTKi and radiotherapy or chemoradiotherapy with 949 total patients (Table 3) (10,12,13). Ten studies examined RTKi antibodies and chemoradiotherapy or radiotherapy vs chemoradiotherapy or radiotherapy with 4729 patients total (Table 3) (14–23). Studies using small-molecule RTKis included 1 using lapatinib for head and neck cancers (12), 1 using erlotinib for esophageal cancer (10), and 1 using erlotinib for definitive treatment of 1-3 brain metastases from non-small cell lung cancer (13). Among the trials using antibody RTKis, 7 used cetuximab for cancers of the head and neck, lung, and esophagus (14–18,21,22). The authors of 2 of the antibody RTKi studies looked at bevacizumab for glioblastoma multiforme (19,20). Researchers of antibody RTKi trial looked at panitumumab for head and neck cancer (23). These results are shown in Table 2.
Table 3.
Outcomes and toxicities of RTKis added to RT-based therapy for the treatment of solid cancersa
| Subgroup stratification and randomization | Overall survival |
Toxicity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies, No. | Patients, No. | HR (95% CI) | I2, % | P | Studies, No. | Patients, No. | RR (95% CI) | I2, % | P | |
| RT or CRT | ||||||||||
| CRT ± any type of RTKi | 10 | 4835 | 1.00 (0.91 to 1.12) | 41.0 | .95 | 6 | 2970 | 1.18 (1.09 to 1.28) | 27.0 | .003 |
| RT ± any type of RTKi | 3 | 843 | 1.51 (0.66 to 3.45) | 87.0 | .33 | 1 | NA | NA | NA | NA |
| Drug type | ||||||||||
| RT or CRT ± small molecule RTKi | 3 | 949 | 0.97 (0.71 to 1.33) | 64 | .87 | 2 | NA | NA | NA | NA |
| RT or CRT ± antibody RTKi | 10 | 4729 | 1.04 (0.90 to 1.19) | 64.0 | .62 | 5 | 1942 | 1.18 (1.06 to 1.32) | 39.0 | .01 |
| Overall | 13 | 5678 | 1.02 (0.90 to 1.15) | 61.0 | .76 | 7 | 2715 | 1.18 (1.06 to 1.33) | 60.0 | .009 |
CI = confidence interval; CRT = chemoradiotherapy; HR = hazard ratio; NA = not applicable because too few studies; RR = relative risk; RT = radiation therapy; RTKi = receptor tyrosine kinase inhibitor.
Primary Endpoint: Overall Survival
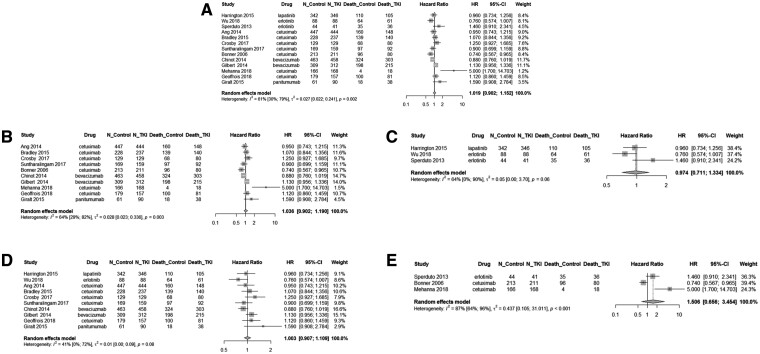
Figure 2 depicts the forest plots for hazard ratios for overall survival. On analysis of any RTKi plus chemoradiotherapy or radiotherapy, the overall hazard ratio was 1.02 (95% CI = 0.90 to 1.15, P = .76) (Figure 2, A). On subgroup analysis of patients receiving antibody RTKi, the hazard ratio for chemoradiotherapy or radiotherapy plus RTKi vs chemoradiotherapy or radiotherapy alone was 1.04 (95% CI = 0.90 to 1.19, P = .62) (Figure 2, B). On subgroup analysis of patients receiving a small-molecule RTKi, the hazard ratio for chemoradiotherapy or radiotherapy plus RTKis vs chemoradiotherapy or radiotherapy alone was 0.97 (95% CI = 0.71 to 1.33, P = .87) (Figure 2, C). Among patients receiving RTKi plus chemoradiotherapy vs chemoradiotherapy alone, the hazard ratio was 1.00 (95% CI = 0.91 to 1.12, P = .95) (Figure 2, D). Finally, among patients receiving RTKi plus radiotherapy vs radiotherapy alone, the hazard ratio was 1.51 (95% CI = 0.66 to 3.45, P = .33) (Figure 2, E). The overall I2 was 61.0% and Q-test P value was less than .01, indicating statistically significant heterogeneity between the studies in overall survival.
Figure 2.
Forest plot of overall survival in patients treated with chemoradiotherapy or radiotherapy with or without receptor tyrosine kinase inhibitor (RTKi). Forest diagrams are shown for patients overall (A) and organized by type of RTKi (small molecule vs antibody) (B-C), and by treatment type (chemoradiotherapy vs radiotherapy) (D-E). Hazard ratios (HRs), 95% confidence intervals (CIs), and weight, as related to survival, are shown. A) On analysis of any RTKi plus chemoradiotherapy or radiotherapy, the overall hazard ratio was 1.02 (95% CI = 0.90 to 1.15, P = .76). On analysis stratified by type of RTKi: (B) for studies using antibody RTKi, the hazard ratio was 1.04 (95% CI = 0.90 to 1.19, P = .62); (C) for studies using small molecules, the hazard ratio was 0.97 (95% CI = 0.71 to 1.33, P = .87). On analysis stratified by treatment type: (D) for studies using chemoradiotherapy, the hazard ratio was 1.00 (95% CI = 0.91 to 1.12, P = .95); (E) for studies using radiotherapy, the hazard ratio was 1.51 (95% CI = 0.66 to 3.45, P = .33). These data indicate that the addition of an RTKi (either small molecule or antibody) to standard treatment with either chemoradiotherapy or radiotherapy does not improve survival for cancer patients. All statistical tests were 2-sided. Squares represent individual studies with confidence intervals depicted as horizontal lines through each square. The lines are depicted as white if the confidence interval falls within the area of the square. The area of each square is proportional to the study’s weight in the meta-analysis. Diamonds represent the weighted random effects estimate for the combined studies in the meta-analysis. Vertical lines representing no effect are also depicted.
Secondary Endpoint: Toxicity
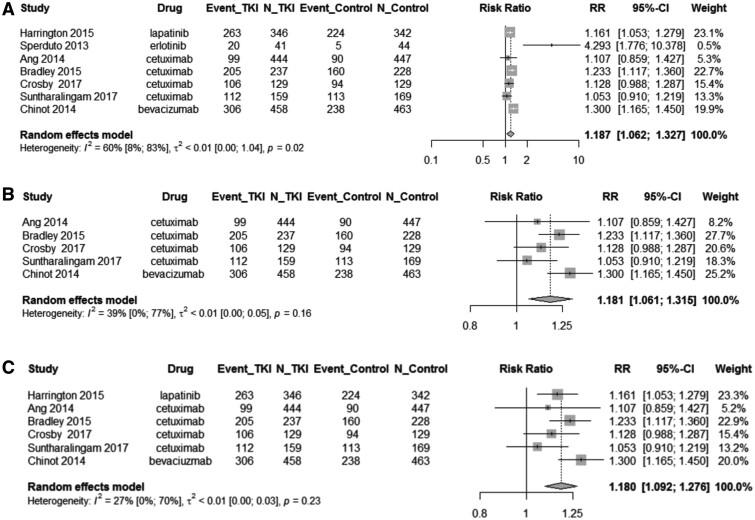
Rates of grade 3+ toxicity were reported in 7 of the studies (12–17,19). The RR of grade 3+ toxicities are shown in forest plots in Figure 3. Overall, among patients receiving any RTKi with chemoradiotherapy or radiotherapy, the relative rate was 1.18 (95% CI = 1.06 to 1.33, P = .009) (Figure 3, A). Among studies using antibody RTKi plus chemoradiotherapy or radiotherapy vs chemoradiotherapy or radiotherapy alone, the relative rate was 1.18 (95% CI = 1.06 to 1.32, P = .01) (Figure 3, B). On subgroup analysis of patients receiving any RTKi plus chemoradiotherapy vs chemoradiotherapy alone, the relative rate was 1.18 (95% CI = 1.09 to 1.28, P = .003) (Figure 3, C). We were unable to conduct subgroup analysis looking at the relative rate of grade 3+ toxicities in studies using a small-molecule RTKi plus chemoradiotherapy or radiotherapy vs chemoradiotherapy or radiotherapy alone or looking at any RTKi plus radiotherapy vs radiotherapy alone because there were too few studies with statistically significant heterogeneity. The overall I2 was 60.0% and Q-test P value was less than .01, indicating statistically significant heterogeneity between the studies in toxicity.
Figure 3.
Forest plot of grade 3+ toxicity in patients treated with chemoradiotherapy or radiotherapy with or without receptor tyrosine kinase inhibitor (RTKi). Forest diagrams shown for patients overall grade 3+ toxicity (A) and are organized by type of RTKi (small molecule or antibody) (B) and by treatment type (chemoradiotherapy vs radiotherapy) (C). The relative risk (RR), 95% confidence interval (CI), and weight as related to grade 3+ toxicity are provided. A) For patients overall, the RR of combination therapy vs chemoradiotherapy or radiotherapy alone was 1.18 (95% CI = 1.06 to 1.33, P =.009). B) On analysis stratified by type of RTKi (antibody or small molecule), studies using antibody RTKi had a relative risk of 1.18 (95% CI = 1.06 to 1.32, P = .01). Only 2 studies using small-molecule RTKi reported relative risk for toxicity, so a subgroup analysis could not be performed. C) On analysis of treatment type (chemoradiotherapy or radiotherapy), studies using chemoradiotherapy had a relative risk of 1.18 (95% CI = 1.09 to 1.28, P = .003). Only 1 study using radiotherapy reported relative risk for toxicity, so a subgroup analysis could not be performed. These data indicate that treatment with chemoradiotherapy or radiotherapy combined with an RTKi leads to increased risk of grade 3+ toxicity. Squares represent individual studies with confidence intervals depicted as horizontal lines through each square. The lines are depicted as white if the confidence interval falls within the area of the square. The area of each square is proportional to the study’s weight in the meta-analysis. Diamonds represent the weighted random effects estimate for the combined studies in the meta-analysis. Vertical lines representing no effect are also depicted. All statistical tests were 2-sided.
Discussion
Radiotherapy, with or without concurrent chemotherapy, is used in the definitive treatment of many solid-tumor cancers. The addition of systemic therapy to radiotherapy can enhance the efficacy of treatment, but at the expense of increased toxicity (3–5). It has been postulated that targeted agents, such as RTKis, can be used in addition to, or in place of, cytotoxic chemotherapy to provide a more targeted treatment approach, improving survival without a concomitant increase in toxicity (28). The results of this meta-analysis suggest that adding RTKis to radiotherapy-based treatment does not improve overall survival (hazard ratio = 1.02, 95% CI = 0.90 to 1.15, P = .76), and their addition increases toxicity (RR = 1.18, 95% CI = 1.06 to 1.33, P = .009).
Radiotherapy is the primary treatment modality in the management of a number of solid malignancies, including cancers of the lung and head and neck (29,30). Numerous randomized controlled clinical trials have demonstrated a survival benefit when chemotherapy is added to radiotherapy in the management of these cancers (31,32). The benefits of combining chemotherapy with radiotherapy, however, are limited by the toxicity associated with treatment. Limitations in the further advancement of chemoradiotherapy have resulted in widespread enthusiasm for the use of molecularly targeted agents in combination with either radiotherapy alone or chemoradiotherapy. RTKis play an important role in cell growth, differentiation, and survival, making them an attractive target for antitumor therapy. Thus, developing targeted agents to inhibit RTKis, in particular those targeting growth factor receptors, has been an active area of research over the last 2 decades (Supplementary Figure 1, available online) (33–35).
The combination of RTKis with radiotherapy has also been an active area of research because in theory their combination should improve tumor kill through a number of mechanisms. First, increased expression of EGFR has been correlated with radioresistance; thus, inhibition of EGFR would be expected to increase susceptibility of cancer cells to the damaging effects of radiation (36,37). Second, EGFR inhibition commonly produces cytostatic effects, which could potentiate the efficacy of fractionated radiotherapy by preventing tumor repopulation (38,39). Third, ionizing radiation has been shown to increase EGFR expression, thus promoting cancer cell growth and proliferation (40). Thus, inhibition of EGFR during radiotherapy could prevent EGFR activation and therefore enhance the efficacy of cancer treatment (38). Finally, growth factors play a role in DNA damage repair. Therefore, combining growth factor receptor inhibition with radiotherapy could further potentiate the effects of treatment by preventing the repair of DNA damage induced by radiation (38).
This study shows that the combination of RTKis and radiotherapy, with or without chemotherapy, does not improve survival of cancer patients undergoing definitive treatment for their disease. Further, we show that when RTKis are combined with chemoradiotherapy or radiotherapy, toxicity is increased. The rate of grade 3+ toxicity expected from treatment with RTKi alone is approximately 10%-40% (41–44); thus, it is not surprising that toxicity is increased when these drugs are added to standard chemoradiotherapy or radiotherapy treatment. The added toxicity might be warranted if survival outcomes are improved, but our study shows that they are not. Of the 13 studies we examined, 11 did not show improvement in survival when RTKi was added to chemoradiotherapy or radiotherapy for multiple disease sites. One of the 2 studies to show a survival benefit, the Bonner study for head and neck cancers, compared RTKi plus radiotherapy with radiotherapy alone, which is no longer a standard treatment for head and neck cancers where concurrent chemotherapy is now known to play an important role (18). This trial was included in the present analysis because it was an accepted treatment at the time the study was being conducted. A number of ongoing trials investigating the combination of RTKis with chemoradiotherapy or radiotherapy are actively recruiting patients (eg, NCT 02738983, NCT 00973310). We caution against further investigations into this combined modality therapy because of concerns over increased toxicity without concomitant improvements in survival outcomes for patients.
Not only is the combination of RTKis plus radiotherapy more toxic than radiotherapy alone, it is also more expensive. Treatment with RTKis increases the cost to the health-care system as well as out-of-pocket cost to the patient. A drug that exceeds a designated amount (45), approximately $600, can be placed in a “specialty tier” by Medicare Part D plans, which dictates how much patients pay per month to obtain the drug. RTKis fall within the “specialty tier” as designated by Medicare Part because of their increased cost of administration. Patients are required to pay a 25% to 33% copay during each year’s initial coverage phase to obtain these specialty drugs. Thus, the addition of RTKis to radiotherapy may cause undue financial burden on patients receiving care without concomitantly resulting in improved patient outcomes. High out-of-pocket costs are especially concerning for patients with multiple chronic health conditions needing several specialty tier prescriptions or prescriptions required for long periods of time.
Although this analysis failed to show a benefit to RTKi therapy concurrent with radiotherapy, there may still be a role for this combination of therapies that has not yet been elucidated. To benefit from the synergistic effects of radiotherapy in combination with RTKis, the sequencing of treatments may be relevant (38). Our study excluded trials that did not give RTKi concurrently with radiotherapy. It is possible that a benefit to RTKi plus radiotherapy exists if the therapies, including chemotherapy, are delivered in a particular sequence. Indeed, there are ongoing trials assessing the role of sequential without concurrent therapy (NCT 04111913, NCT 1553942), which may yield more promising results. The mode of delivery of radiotherapy may also affect the effect of combined therapy. Our analysis included only trials using conventionally fractionated radiotherapy; however, efforts are ongoing to evaluate the combination of stereotactic body radiotherapy and RTKi therapy (NCT 03727867, NCT 02314364, NCT 02599779). It should also be noted that our trial only investigated patients being treated with definitive intent. There may be a role for RTKi in combination with palliative radiotherapy for patients with metastatic disease. Further, it should be noted that the studies in this meta-analysis investigated only first-generation RTKis. Subsequent generations of drugs may improve outcomes relative to their first-generation counterparts. Finally, the trials we included did not perform biomarker analyses. Using biomarker analysis to select patients who are most likely to benefit from RTKis may enhance their effectiveness.
Bias is present in the studies evaluated in this meta-analysis (Supplementary Figures 2 and 3, available online). The Wu et al. (10) study, which analyzed the addition of erlotinib to chemoradiotherapy in the treatment of esophageal cancer, had a wide range of tumor stages and node positivity in each treatment group. Additionally, differences were present in treatment delivery for patients in each group, including the presence of interruptions of radiotherapy, varying doses of radiotherapy, and differences in receipt of chemotherapy. Wu et al. (10) showed a statistically significant improvement in overall survival with the addition of an RTKi to radiotherapy. Notably, the authors used a 2 × 2 factorial design in their study, and there may be interaction between the 2 randomization groups, conventional field irradiation, and elective nodal irradiation. The authors did not provide organized data regarding toxicities experienced by patients in either the treatment or control groups. The bias present in the studies analyzed contributes to the limitations of our study.
There are limitations to our study. First, we do not have access to individual patient–level data, and unmeasured factors could confound these results. Because individual patient–level data were not available, and tumor sites vary, a subgroup analysis among stage, histology, drug, and radiotherapy dose could not be performed. Second, not all studies evaluated provided complete information regarding individual toxicities, and some authors could not be reached for clarification. Studies reported overall survival and toxicity data at differing time points in treatment course. Additionally, the evaluated studies considered cancers only at particular sites with particular RTKi targets (head and neck, primary lung, esophageal, and primary brain). In addition, the type and dose of chemotherapy and the dose and fractionation of radiotherapy varied between the studies used in this meta-analysis. Finally, some cancer sites may be more or less responsive to RTKis, other chemotherapy drugs, and radiation, therefore skewing results; we did not perform an analysis by disease subsite.
Furthermore, the studies provided did not consider the molecular characteristics present (eg, presence of KRAS mutations when using cetuximab), making it difficult to understand how an imbalance in tumor molecular characteristics might affect survival and response to targeted therapies (46). RTKis may have different molecular targets, and therefore some tumors may respond differently than others. Additionally, some molecules inhibit multiple tyrosine kinase targets, and signaling output may vary; therefore, our analysis demonstrates a simplified classification of drugs. Statistically significant heterogeneity is in the data presented across the included studies. This heterogeneity calls to question the validity of extrapolating data from each study to a general population of cancer patients. Nonetheless, unlike the data for use of radiotherapy plus immunecheckpoint inhibitors, which show survival improvement and little toxicity with combination therapy (47–49), this study adds to the growing body of evidence of clinical harm for patients receving RT and RTKis (50,51).
The results of this study show that the addition of RTKis to conventionally fractionated radiotherapy, with or without chemotherapy, does not improve patient survival and worsens toxicity. The findings of this meta-analysis provide caution against initiation of further large-scale trials evaluating the safety and efficacy of RTKi-targeted therapies in combination with conventionally fractionated radiotherapy. Further study into the appropriate sequencing of these treatments and the optimal radiotherapy technique is needed.
Funding
This work did not receive any funding.
Notes
Role of the funder: Not applicable.
Disclosures: We have no financial conflicts of interests. NGZ received startup funding from Penn State Cancer Institute, is supported by the National Institutes of Health LRP 1 L30 CA231572-01. NGZ is supported by the American Cancer Society – Tri State CEOs Against Cancer Clinician Scientist Development Grant, CSDG-20-013-01-CCE. NGZ also received personal fees from Springer Nature, Inc and Weatherby Healthcare, unrelated to the current work. DMT received personal fees from Springer Nature, Inc and Novocure, unrelated to the current work. MM has received funding from Eli Lilly, not directly related to the current work. No author received payment by a pharmaceutical company or other agency. All authors had full access to the full data in the study and accept responsibility to submit for publication.
Author contributions: Conceptualization: NZ. Data Curation: LT, EB, MM, NZ. Formal Analysis: MW. Investigation: EB, LT, NZ. Methodology: MW, NZ. Project Administration: LT, MW, NZ. Supervision: NZ. Validation: All authors. Visualization: MW, LT, NZ. Writing—original draft: LT. Writing—review and editing: All authors.
Acknowledgements: We are not using any copyrighted information, patient photographs, identifiers, or other protected health information in this article. No text, text boxes, figures, or tables in this article have been previously published or owned by another party. This work was delivered as an oral presentation at the American Society for Radiation Oncology 2019 meeting by NGZ.
Data Availability
The data for this manuscript were extracted from the original manuscripts which have been cited in this work.
Supplementary Material
References
- 1.Mierzwa ML, Nyati MK, Morgan MA, Lawrence TS. Recent advances in combined modality therapy. Oncologist. 2010;15(4):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakubowicz J, Blecharz P, Skotnicki P, Reinfuss M, Walasek T, Luczynska E. Toxicity of concurrent chemoradiotherapy for locally advanced cervical cancer. Eur J Gynaecol Oncol. 2014;35(4):393–399. [PubMed] [Google Scholar]
- 3.Verma V, Simone CB, Werner-Wasik M. Acute and late toxicities of concurrent chemoradiotherapy for locally-advanced non-small cell lung cancer. Cancers (Basel). 2017;9(12):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sher DJ, Balboni TA, Haddad RI, et al. Efficacy and toxicity of chemoradiotherapy using intensity-modulated radiotherapy for unknown primary of head and neck. Int J Radiat Oncol Biol Phys. 2011;80(5):1405–1411. [DOI] [PubMed] [Google Scholar]
- 5.Gondi V, Bentzen SM, Sklenar KL, et al. Severe late toxicities following concomitant chemoradiotherapy compared to radiotherapy alone in cervical cancer: an inter-era analysis. Int J Radiat Oncol Biol Phys. 2012;84(4):973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1(2):117–123. [DOI] [PubMed] [Google Scholar]
- 7.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315(3):971–979. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya P, Shetake NG, Pandey BN, Kumar A. Receptor tyrosine kinase signaling in cancer radiotherapy and its targeting for tumor radiosensitization. Int J Radiat Biol. 2018;94(7):628–644. [DOI] [PubMed] [Google Scholar]
- 9.Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol. 2004;22(9):1743–1752. [DOI] [PubMed] [Google Scholar]
- 10.Wu S-X, Wang L-H, Luo H-L, et al. Randomised phase III trial of concurrent chemoradiotherapy with extended nodal irradiation and erlotinib in patients with inoperable oesophageal squamous cell cancer. Eur J Cancer. 2018;93:99–107. [DOI] [PubMed] [Google Scholar]
- 11.Prabhu VV, Devaraj N. Epidermal growth factor receptor tyrosine kinase: a potential target in treatment of non-small-cell lung carcinoma. J Environ Pathol Toxicol Oncol. 2017;36(2):151–158. [DOI] [PubMed] [Google Scholar]
- 12.Harrington K, Temam S, Mehanna H, et al. Postoperative adjuvant lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: a phase III, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2015;33(35):4202–4209. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85(5):1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crosby T, Hurt CN, Falk S, et al. Long-term results and recurrence patterns from SCOPE-1: a phase II/III randomised trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer. Br J Cancer. 2017;116(6):709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suntharalingam M, Winter K, Ilson D, et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the NRG oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol. 2017;3(11):1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. [DOI] [PubMed] [Google Scholar]
- 19.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geoffrois L, Martin L, De Raucourt D, et al. Induction chemotherapy followed by cetuximab radiotherapy is not superior to concurrent chemoradiotherapy for head and neck carcinomas: results of the GORTEC 2007-02 phase III randomized trial. J Clin Oncol. 2018;36(31):3077–3083. [DOI] [PubMed] [Google Scholar]
- 23.Giralt J, Trigo J, Nuyts S, et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16(2):221–232. [DOI] [PubMed] [Google Scholar]
- 24.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. [DOI] [PubMed] [Google Scholar]
- 25.Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44(2):127–139. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 27.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:110–129. [Google Scholar]
- 28.Cuneo KC, Nyati MK, Ray D, Lawrence TS. EGFR targeted therapies and radiation: optimizing efficacy by appropriate drug scheduling and patient selection. Pharmacol Ther. 154:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curran WJ, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–2190. [DOI] [PubMed] [Google Scholar]
- 32.Pignon JP, Bourhis J, Domenge C, Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer. Lancet. 2000;355(9208):949–955. [PubMed] [Google Scholar]
- 33.Ward WH, Cook PN, Slater AM, Davies DH, Holdgate GA, Green LR. Epidermal growth factor receptor tyrosine kinase. Investigation of catalytic mechanism, structure-based searching and discovery of a potent inhibitor. Biochem Pharmacol. 1994;48(4):659–666. [DOI] [PubMed] [Google Scholar]
- 34.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267(5205):1782–1788. [DOI] [PubMed] [Google Scholar]
- 35.Ciardiello F, Caputo R, Bianco R, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6(5):2053–2063. [PubMed] [Google Scholar]
- 36.Sheridan MT, O'Dwyer T, Seymour CB, Mothersill CE. Potential indicators of radiosensitivity in squamous cell carcinoma of the head and neck. Radiat Oncol Investig. 1997;5(4):180–186. [DOI] [PubMed] [Google Scholar]
- 37.Balaban N, Moni J, Shannon M, Dang L, Murphy E, Goldkorn T. The effect of ionizing radiation on signal transduction: antibodies to EGF receptor sensitize A431 cells to radiation. Biochim Biophys Acta. 1996;1314(1-2):147–156. [DOI] [PubMed] [Google Scholar]
- 38.Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6(11):876–885. [DOI] [PubMed] [Google Scholar]
- 39.Di Gennaro E, Barbarino M, Bruzzese F, et al. Critical role of both p27KIP1 and p21CIP1/WAF1 in the antiproliferative effect of ZD1839 ('Iressa'), an epidermal growth factor receptor tyrosine kinase inhibitor, in head and neck squamous carcinoma cells. J Cell Physiol. 2003;195(1):139–150. [DOI] [PubMed] [Google Scholar]
- 40.Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280(35):31182–31189. [DOI] [PubMed] [Google Scholar]
- 41.Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. [DOI] [PubMed] [Google Scholar]
- 42.Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. [DOI] [PubMed] [Google Scholar]
- 43.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 44.Fury MG, Sherman E, Lisa D, et al. A randomized phase II study of cetuximab every 2 weeks at either 500 or 750 mg/m2 for patients with recurrent or metastatic head and neck squamous cell cancer. J Natl Compr Canc Netw. 2012;10(11):1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doshi JA, Li P, Huo H, et al. High cost sharing and specialty drug initiation under Medicare Part D: a case study in patients with newly diagnosed chronic myeloid leukemia. Am J Manag Care. 2016;22(suppl 4):s78–s86. [PubMed] [Google Scholar]
- 46.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26(15):2450–2456. [DOI] [PubMed] [Google Scholar]
- 47.Lehrer EJ, Peterson J, Brown PD, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother Oncol. 2019;130:104–112. [DOI] [PubMed] [Google Scholar]
- 48.Sha CM, Lehrer EJ, Hwang C, et al. Toxicity in combination immune checkpoint inhibitor and radiation therapy: A systematic review and meta-analysis. Radiother Oncol. 2020;151:141–148. [DOI] [PubMed] [Google Scholar]
- 49.Lehrer E, McGee H, Peterson J, et al. Stereotactic Radiosurgery and Immune Checkpoint Inhibitors in the Management of Brain Metastases. Int J Mol Sci. 2018;19(10):3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh R, Lehrer EJ, Ko S, et al. Brain metastases from non-small cell lung cancer with EGFR or ALK mutations: A systematic review and meta-analysis of multidisciplinary approaches. Radiother Oncol. 2020;144:165–179. [DOI] [PubMed] [Google Scholar]
- 51.Patel RR, Ludmir EB, Augustyn A, et al. De-intensification of therapy in human papillomavirus associated oropharyngeal cancer: A systematic review of prospective trials. Oral Oncol. 2020;103:104608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this manuscript were extracted from the original manuscripts which have been cited in this work.