Abstract
Endosalpingiosis, a microscopic lesion composed of ectopic Fallopian tube epithelium, frequently involves the peritoneum and lymph nodes in patients with ovarian serous borderline tumour or low-grade serous carcinoma, but its pathogenic significance remains unclear. Using laser-capture microdissection and droplet digital PCR, we investigated whether endosalpingiosis harbours the driver mutations in BRAF and KRAS that characterise ovarian low-grade serous neoplasms. Somatic mutations were detected in 14 (33%) of 43 endosalpingiotic lesions analysed. Of 21 women with endosalpingiosis associated with a synchronous or metachronous ovarian low-grade serous tumour, mutations were identified in endosalpingiotic lesions from 11 (52%) women, with most cases (10/11, 91%) demonstrating identical mutations in both tumour and endosalpingiosis. In contrast, of 13 cases of endosalpingiosis not associated with an ovarian tumour, only one harboured a KRAS mutation. The proliferative activity as assessed by Ki-67 immunohistochemistry was lower in endosalpingiosis than in low-grade serous tumours, and endosalpingiosis with either a BRAF or KRAS mutation had a significantly lower Ki-67 index than those without. Ectopic expression of KRASG12V in Fallopian tube epithelial cells led to ERK phosphorylation, p21 induction, growth arrest and cellular senescence. In conclusion, we demonstrate that endosalpingiosis represents an interesting example of cancer driver mutations in deceptively normal-appearing cells, which may be prone to neoplastic transformation upon bypass of endogenous oncosuppressive mechanisms.
Keywords: endosalpingiosis, mutation, precursor lesion, serous borderline tumour, low-grade serous carcinoma
Introduction
Endosalpingiosis refers to the ectopic presence of morphologically benign glands lined by Fallopian tube-type epithelium, typically involving peritoneum and associated underlying soft tissues, and occasionally lymph nodes. While sharing some similarities with a related entity, endometriosis (i.e. ectopic endometrial glands), endosalpingiosis is typically asymptomatic, and is often detected incidentally during histological examination of specimens surgically removed for a variety of gynaecological conditions, especially in women with serous borderline tumour (SBT) [1].
Ovarian low-grade serous neoplasms are comprised of SBT and low-grade serous carcinoma (LGSC) and exhibit morphological and immunophenotypical features consistent with Fallopian tube epithelial differentiation [2,3]. They are characterised by a stable genomic landscape, with activating mutations in KRAS or BRAF in approximately 60% of tumours. Previous work from our group and others suggest that LGSC arises in a stepwise manner, starting as benign ovarian serous cysts (i.e. cystadenoma) and progressing to SBT. A small but noticeable proportion of SBTs transform into invasive LGSC [2,4]. A particularly unique feature of SBT is that despite being considered a benign entity, it is commonly associated with deposits of tumour cells on the peritoneum and other organs, known as ‘implants’ [5]. The majority of implants harbour identical KRAS or BRAF mutations as the associated SBT, supporting a clonal relationship between implants and the primary ovarian tumour [6]. Endosalpingiosis is frequently observed within the vicinity of implants and adjacent to tumour deposits in lymph nodes, suggesting that implants may in fact arise from endosalpingiosis rather than direct dissemination from the primary ovarian tumour [7,8].
Endosalpingiosis is also detected incidentally in women without ovarian tumours. In a retrospective series, endosalpingiosis was found in around 13% of surgically resected omenta from female patients [9]. In the ovaries, cortical inclusion cysts, which are lined by tubal epithelium, are thought to be a form of endosalpingiosis that may develop into serous cystadenomas/cystadenofibromas over time [10,11]. Mutations of KRAS or BRAF have been detected in the epithelial lining of ovarian cystadenomas that are directly adjacent to SBTs [4]. However, the significance of endosalpingiosis in lymph nodes and peritoneal sites and their potential for neoplastic transformation remain unknown.
In this study, we performed a molecular genetic analysis on endosalpingiosis with or without associated ovarian serous tumours. Proliferation indices were compared between endosalpingiosis, eutopic Fallopian tube and ovarian SBT/LGSC. Finally, the functional impact of KRAS mutation was studied in Fallopian tube epithelial cells in vitro. This represents the first comprehensive study into the pathobiology of endosalpingiosis, which establishes this entity as a clonal proliferation with neoplastic potential at least in some cases.
Materials and methods
Case selection
Diagnostic slides and formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved from the archives of the Johns Hopkins Hospital Department of Pathology. All work with human samples was approved by the institutional review board.
The study cohort was comprised of 21 cases of ovarian SBT or LGSC (8 BRAF mutated, 9 KRAS mutated, 4 wildtype for both genes) with synchronous (n=15) or metachronous (n=6) endosalpingiosis, and 13 cases of incidental endosalpingiosis in women without a borderline or malignant ovarian serous tumour. Most endosalpingiotic lesions were identified in the peritoneum or lymph nodes. Four cases with prominent ovarian cortical inclusion cysts were also included in this study.
Laser-capture microdissection and DNA extraction
Laser-capture microdissection was performed on FFPE tissue sections (10 μm thickness) using a Leica laser-capture microdissection microscope, as described previously [12], to enrich for epithelial cells from endosalpingiosis lesions. Tumour tissue (i.e. SBT or LGSC) was manually microdissected from 10-μm thick unstained sections in areas with >70% tumour cellularity identified on corresponding H&E slides. Microdissected tissues were subjected to genomic DNA extraction using the QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions.
Droplet digital PCR
Droplet digital PCR (ddPCR) was performed using the Bio-Rad QX200. The following validated ddPCR mutation assays were obtained from Bio-Rad (Hercules, CA, USA): BRAF p. V600E c. 1799T>A (dHsaMDV2010027); KRAS G12/13 Mutation Screening Kit (cat#1863506); KRAS p. G12C c. 34G>T (dHsaMDV2510584); KRAS p. G12V c. 35G>T (dHsaMDV2510592); KRAS p. G12D c. 35G>A (dHsaMDV2510596); and KRAS p. G12A c. 35G>C (dHsaMDV2510586). All samples were subjected to mutation analysis using the BRAF-V600E assay and the KRAS G12/G13 Mutation Screening Kit, a multiplex assay that screens for seven common mutations in codons 12 and 13. Samples were subjected to KRAS G12C, G12V, G12D and G12A mutation-specific ddPCR assays for definitive genotyping if found to carry a KRAS mutation by multiplex ddPCR.
The ddPCR reaction was comprised of 2× ddPCR Supermix (no dUTP), 0.5 μl uracil-DNA glycosylase (New England BioLabs, Ipswich, MA, USA), 1 μl primer/probe assay reagent and sample, made up to a total volume of 20μl. Droplets were generated using the Droplet Generator, with an eight-channel DG8 cartridge and cartridge holder. Droplets containing 70μl Droplet Generation oil per well and 20μl fluorescent PCR reaction mixture were transferred to a 96-well PCR plate, which was subsequently heat-sealed with foil. PCR amplification was performed with the following cycling conditions: initial incubation at 37 °C for 30min, then 10 min at 95 °C, followed by denaturation for 30s at 94 °C, annealing for 60s at 55 °C for 40 cycles; and final incubation for 10 min at 98 °C, ending at 4 °C. After amplification, the 96-well plate was placed into the Droplet Reader (Bio-Rad). Data were analysed using QuantaSoft analysis software (Bio-Rad). To avoid false-positive results, the threshold for a positive mutation call was set at a stringent allelic frequency of ≥1.0%, to account for low quantities of amplifiable DNA and potential artefacts intrinsic to formalin-fixed tissue samples isolated by laser-capture microdissection.
Immunohistochemistry
Immunohistochemical staining was performed, as described previously [6], using the following primary antibodies: Ki-67 rabbit monoclonal (Cell Signaling Technology, Danvers, MA, USA) and VE1 (Spring Biosciences/Abcam, Cambridge, MA, USA). Digitally scanned images of stained slides were manually annotated and analysed using Aperio ImageScope (LeicaBiosystems, Buffalo Grove, IL, USA). The Nuclear v9 algorithm was used for quantification of the Ki-67 labelling index.
Cell culture and lentiviral transduction
Following institutional review board approval, a primary Fallopian tube epithelial cell culture was established from a fresh salpingectomy specimen from a de-identified patient who underwent surgery for a benign gynaecological condition (Pt. X). After opening a segment of Fallopian tube longitudinally, the mucosal layer was scraped off using a scalpel blade and dissociated with TrypLE™ and collagenase. Cells were collected by centrifugation and plated onto a Petri dish in Advanced RPMI supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. After epithelial cells had adhered to the plate (24–48h), the medium containing cellular debris was removed and cells were washed with PBS, detached with trypsin, and re-plated for down-stream assays. Fallopian tube secretory cell differentiation was confirmed by Pax-8 staining. Cell cultures (primary cells from Pt. X and the immortalised Fallopian tube epithelial cell line, FT2821) were maintained in Advanced RPMI supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin.
The vector pCLXEBR-pTF-kRasV12 (Addgene plasmid #114318) was a gift from Dr P. Salmon and encodes the human KRAS gene, with the codon 12 glycine-to-valine modification, under a tetracycline-inducible promoter (henceforth abbreviated as tet-KRASG12V). Lentiviral packaging was performed using the cell line HEK293T. Trypsinised epithelial cell cultures were plated with medium containing viral supernatant and polybrene (8 μg/ml) and underwent selection with blasticidin (7 μg/ml) for 7 days.
Cell proliferation, colony formation and β-galactosidase staining
Cells were plated onto a 96-well plate at a density of 2000 cells per well. After cells had adhered to the plate, the culture medium was replaced with that containing 0, 0.1 or 0.5 μg/ml doxycycline. Viability was determined at baseline and every 24 h using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison WI, USA), following the manufacturer’s protocol. To assess clonogenic ability, cells were seeded onto 12-well plates at 500 cells per well. After 24 h, the culture media was replaced with medium containing 0, 0.1 or 0.5 μg/ml doxycycline. At 7 days post-treatment, colonies were stained with crystal violet and counted. Senescence-associated β-galactosidase staining was performed with a commercial assay kit (Cell Signaling Technology), following the manufacturer’s protocol.
Western blotting
Cells were washed with PBS and scraped directly into sample buffer and denatured at 95 °C for 5 min. Protein quantitation was performed using the Pierce 660nm Protein Assay (ThermoFisher, Waltham, MA, USA), following the manufacturer’s instructions. SDS-PAGE and immunoblotting were carried out using standard protocols and the following primary antibodies (all rabbit monoclonal antibodies from Cell Signaling Technology) at 1:1000 dilution: p-ERK1/2 (Thr202/Tyr204, clone D13.14.4E), ERK1/2 (clone 137F5), p21Waf1/Cip1 (clone 12D1) and vinculin (clone E1E9V).
Results
Morphological features of endosalpingiosis
Endosalpingiosis is characterised by gland-like structures lined by tubal-type epithelium, comprised of both secretory and ciliated cells (see supplementary material, Figure S1A–F). The morphological spectrum ranges from an isolated simple gland to a complex proliferation of glands, which may exhibit irregular contours, epithelial tufting and occasional intraluminal stromal papillae. In the most florid manifestation, there is morphological overlap with epithelial implants that are typically associated with SBT. In the present study, only lesions exhibiting unequivocal histological features of typical endosalpingiosis lacking significant nuclear or architectural atypia were included. The estimated proportion of ciliated cells was highly variable (median 30%, range: <1–90% of cells within a lesion).
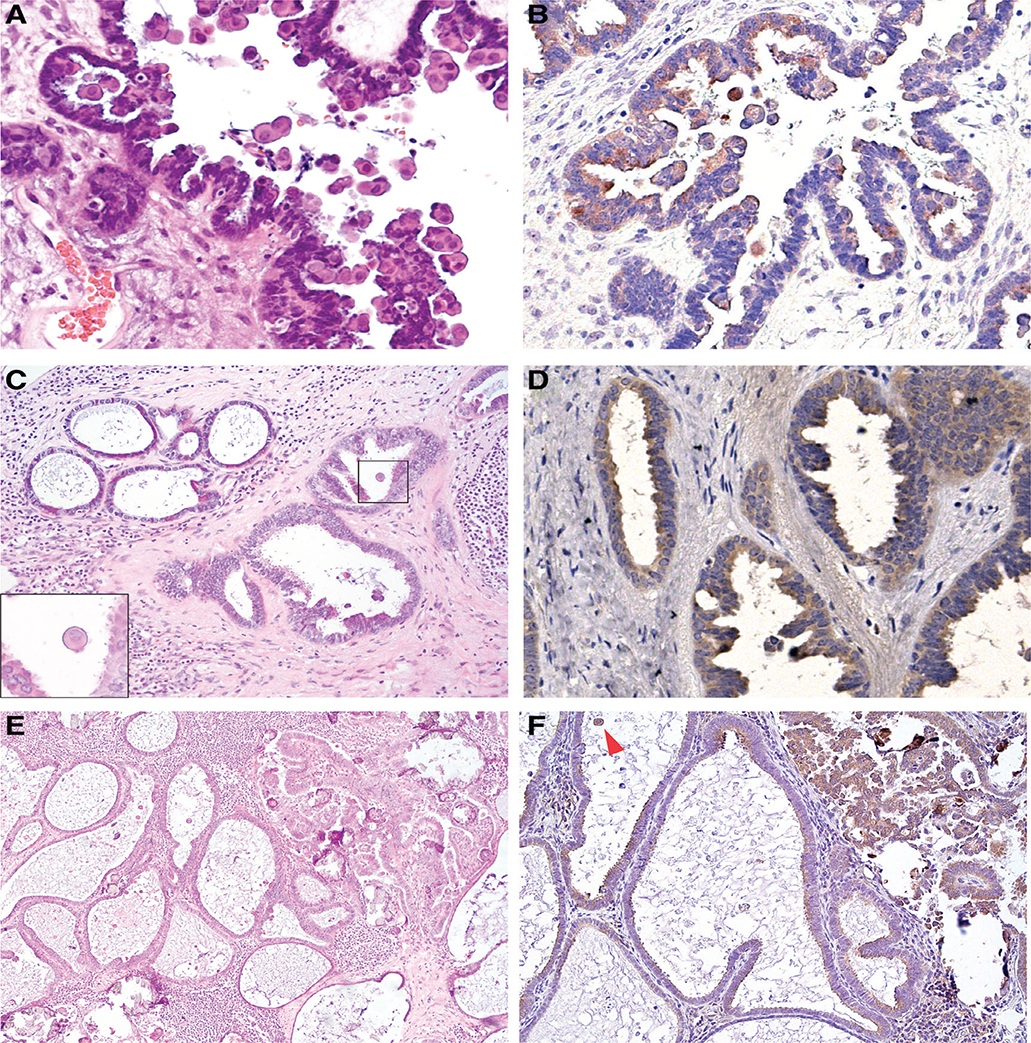
Previous work from our group has identified dense eosinophilic cytoplasm in epithelial cells to be a characteristic morphologic feature of BRAF-mutated SBTs [13], a finding confirmed by an independent study (Figure 1A,B) [14]. In some cases, scattered eosinophilic cells were also observed in foci of endosalpingiosis located away from the ovarian tumour (Figure 1C–F). Immunohistochemical staining with the BRAFV600E mutation-specific antibody, VE1, revealed positivity in associated endosalpingiosis from four of eight cases of SBT or LGSC with known BRAF mutation. Molecular analysis subsequently confirmed the presence of the BRAFV600E mutation in all VE1-positive lesions, but not in lesions with negative staining.
Figure 1.
Endosalpingiosis associated with BRAFV600E-mutated ovarian SBT (case #1). (A, B) Ovarian SBT, with cells exhibiting prominent eosinophilic cytoplasm, a histological feature associated with BRAFV600E mutation: (A) H&E (40×); (B) VE1 immunostaining (40×). (C, D) Associated nodal endosalpingiosis, composed of simple glands and adjacent glands with mild tufting and epithelial stratification: (C) H&E (4×), note scattered cells with dense eosinophilic cytoplasm (inset, 60×); (D) VE1 immunostaining (40×). (E, F) Lymph node involvement by SBT with adjacent endosalpingiosis: (E) H&E (10×); (F) VE1 immunostaining (20×). Arrowhead indicates positive staining in an exfoliated tumour cell with abundant cytoplasm.
BRAF/KRAS mutations are common in endosalpingiosis associated with ovarian low-grade serous neoplasms, but rare in those without
In total, 43 endosalpingiotic foci from 34 patients (21 with concurrent or prior ovarian low-grade serous tumour and 13 without) were isolated by laser-capture microdissection and assayed for BRAF and KRAS mutations by ddPCR (Tables 1 and 2). Somatic mutations were detected in 14 (33%) of 43 endosalpingiotic lesions from 12 (35%) of 34 patients. The median mutant allelic frequency (MAF) in mutation-positive lesions was 37% (range: 12–67%).
Table 1.
Mutation status of endosalpingiotic lesions associated with synchronous or metachronous ovarian low-grade serous tumour
| Case ID | Ovarian tumour |
Endosalpingiosis |
|||
|---|---|---|---|---|---|
| Type (stage) | Genotype | Site | Genotype | MAF | |
| 1 | SBT (II) | BRAFV600E | Lymph node (Es #1 - simple) | BRAFV600E | 54% |
| Lymph node (Es #2 - florid) | BRAFV600E | 58% | |||
| 2 | SBT (II) | BRAFV600E | Ovary (inclusion cysts)* | BRAFV600E | 38% |
| 3 | LGSC (III) | BRAFV600E | Peritoneum | BRAFV600E | 58% |
| 4 | Micropapillary SBT (III) | BRAFV600E | Peritoneum | BRAFV600E | 67% |
| 5 | Micropapillary SBT (II) | BRAFV600E | Lymph node | WT | – |
| 6 | SBT | BRAFV600E | Peritoneum | WT | – |
| 7 | SBT (I) | BRAFV600E | Peritoneum | WT | – |
| 8 | SBT (I) | BRAFV600E | Lymph node (Es #1) | WT | – |
| Lymph node (Es #2) | WT | – | |||
| Omentum | WT | – | |||
| 9 | SBT (II) | KRASG12V | Lymph node (Es #1 - simple) | KRASG12V | 36% |
| Lymph node (Es #2 - florid) | KRASG12V | 25% | |||
| 10 | LGSC (IV) | KRASG12D | Lymph node | KRASG12D | 43% |
| 11 | SBT (I) | KRASG12D | Ovary (inclusion cysts) | KRASG12D | 14% |
| 12 | SBT (I) | KRASG12D | Ovary (inclusion cysts) | KRASG12D | 19% |
| 13‡ | SBT (II) | KRASG12D | Peritoneum | KRASG12D | 32% |
| 14 | SBT (II) | KRASG12D | Peritoneum | KRASG12D | 43% |
| 15 | SBT (I) | KRASG12V | Lymph node | WT | – |
| 16 | SBT (III) | KRASG12D | Peritoneum (within noninvasive implant)† | WT | – |
| 17 | SBT (III) | KRASG12D | Peritoneum | WT | – |
| 18 | Micropapillary SBT (I) | WT | Omentum (Es #1) | WT | – |
| Omentum (Es #2) | KRASG12D+ KRASG12V | G12D: 12%; G12V: 13% |
|||
| 19 | SBT | WT | Lymph node | WT | – |
| 20 | Micropapillary SBT (III) | WT | Lymph node | WT | – |
| 21 | SBT (I) (focally arising in cystadenofibroma) | WT | Lymph node #1 | WT | – |
| Lymph node #2 | WT | – | |||
| Lymph node #3 | WT | – | |||
WT, wildtype.
Adjacent focus of tumour auto-implant also positive for BRAFV600E (MAF 57%).
Subclonal KRASG12D (MAF 10%) detected in tumour cells within the implant.
KRASG12V (MAF 1.9%) and KRASG12D (MAF 0.8%) detected in eutopic Fallopian tube.
Table 2.
Mutation status of endosalpingiotic lesions in women without ovarian low-grade serous tumour
| Endosalpingiosis |
|||
|---|---|---|---|
| Case ID | Site | Genotype | Other serous epithelial lesions (site) |
| NT-1 | Peritoneum | KRASG12D (MAF 37%) | Serous cystadenofibroma (ovary)* |
| NT-2 | Uterine serosa | WT | – |
| NT-3 | Lymph node | WT | – |
| NT-4 | Omentum | WT | Serous cystadenofibromas (ovary), LGSP (bladder) |
| NT-5 | Omentum | WT | Serous adenofibromas (ovary) |
| NT-6 | Lymph node | WT | – |
| NT-7 | Peritoneum | WT | – |
| NT-8 | Peritoneum | WT | – |
| NT-9 | Peritoneum | WT | – |
| NT-10 | Peritoneum | WT | – |
| NT-11 | Peritoneum | WT | – |
| NT-12 | Peritoneum (hernia sac) | WT | – |
| NT-13 | Ovary (inclusion cysts) | WT | – |
| Peritoneum | WT | – | |
| Omentum | WT | – | |
LGSP, low-grade serous proliferation, WT, wildtype.
KRASG12D is also present in the serous cystadenofibroma (MAF 21%).
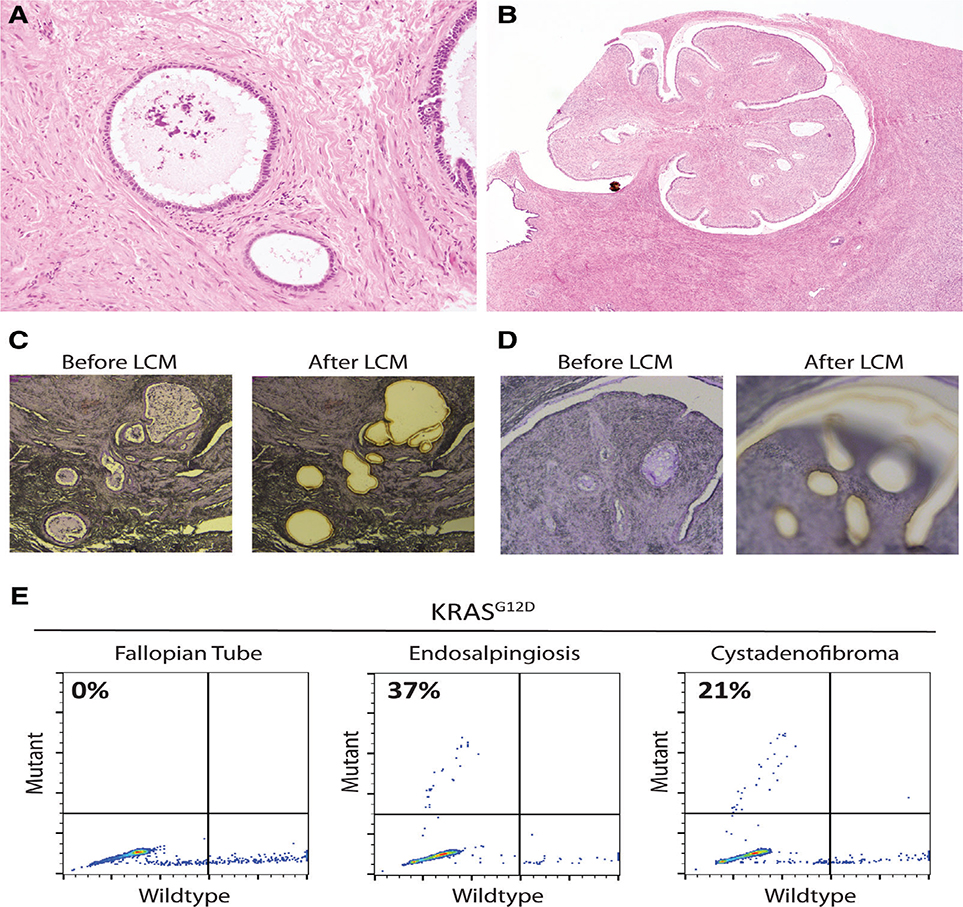
Only1(8%)of13casesofincidentalendosalpingiosis (without associated SBT or LGSC) harboured a somatic mutation (KRASG12D, MAF 37%; Figure 2A–E). Despite the bland cytological features, there was a florid proliferation of endosalpingiotic glands involving the right pelvic sidewall peritoneum in this unusual case. A microscopic serous cystadenofibroma (measuring 2.5mm) was also present in the ovary (which was submitted in its entirety for histological assessment) and carried a concordant KRASG12D mutation. After 3 years of clinical follow-up without any reported symptoms or imaging data, this patient died suddenly from an unknown cause.
Figure 2.
KRAS mutation in a case of incidental endosalpingiosis (case NT-1). (A) Endosalpingiosis involving right pelvic sidewall peritoneum (10×). (B) Cystadenofibroma in ipsilateral ovary (2×). (C, D) Haematoxylin-stained sections before and after laser-capture microdissection for lesions in (A) and (B), respectively. (E) KRASG12D ddPCR reveals mutant alleles in peritoneal endosalpingiosis and ovarian serous cystadenofibroma.
Mutations in either KRAS or BRAF were detected at a significantly higher frequency in endosalpingiosis associated with ovarian SBT or LGSC than in those without (11/21 [52%] versus 1/13 [8%] patients, p=0.011). In most cases (10/11, 91%), the identical mutation was detected in both tumour and associated endosalpingiosis. Regardless of the morphology of the endosalpingiosis (i.e. isolated simple glands or ‘hyperplastic’ foci with more complex glandular structures and epithelial tufting), the mutation status was usually concordant between spatially distinct lesions.
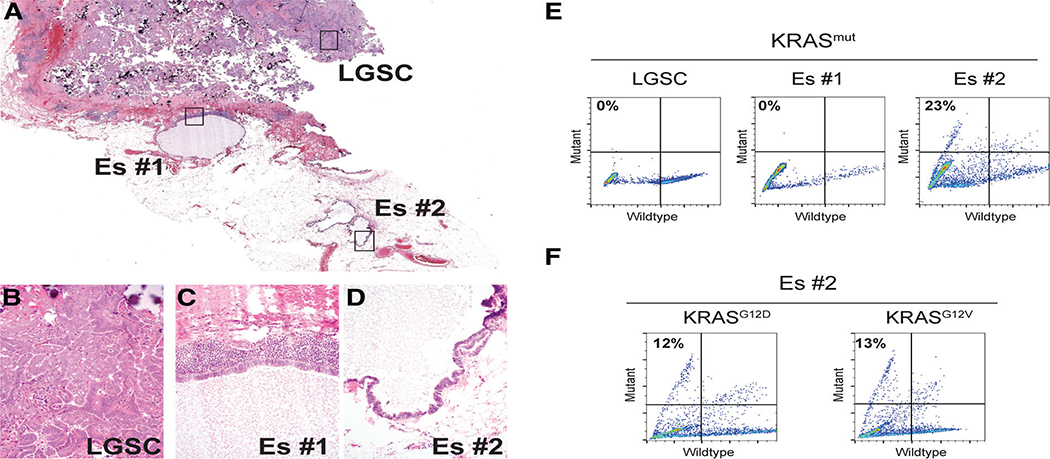
Case #18 was the only exception to this generalisation. Although associated with a metastatic LGSC wildtype for KRAS and BRAF, spatially distinct foci of endosalpingiosis were genetically heterogeneous: one gland was wildtype and the other harboured both KRASG12D and KRASG12V mutations, which were confirmed on repeat testing (Figure 3A–F). The finding of two different KRAS mutations within the same endosalpingiotic lesion recalls a similar observation recently reported in a case of endometriosis [15].
Figure 3.
Multifocal heterogeneity of KRAS mutation status (case #11). (A) Digital scan (1×) showing metastatic LGSC involving omentum and separate foci of endosalpingiosis. Note that for Es #2, the two adjacent cystic structures merge into a single cyst on deeper sections. (B–D) Lesions from (A) at 40×. (E) Multiplex KRAS mutation ddPCR screening assay for lesions isolated by laser-capture microdissection. (F) KRASG12D and KRASG12V ddPCR assays reveal the presence of both mutant alleles in Es #2.
Endosalpingiosis has been suggested to originate from the Fallopian tube [16]. In the present cohort, features of papillary tubal hyperplasia were identified in nine (43%) of 21 cases with Fallopian tube slides available for review. In case #13, showing papillary tubal hyperplasia, relatively low mutant allele frequencies of KRASG12V (1.9%) and KRASG12D (0.8%) were detected in microdissected eutopic Fallopian tube epithelium (see supplementary material, Figure S3B,C). Mutations were not detected in Fallopian tubes from five other cases (#1, 2, 17, NT-1, NT-5) with tissue available for mutation analysis.
Low proliferation in endosalpingiotic lesions, particularly those with BRAF or KRAS mutations
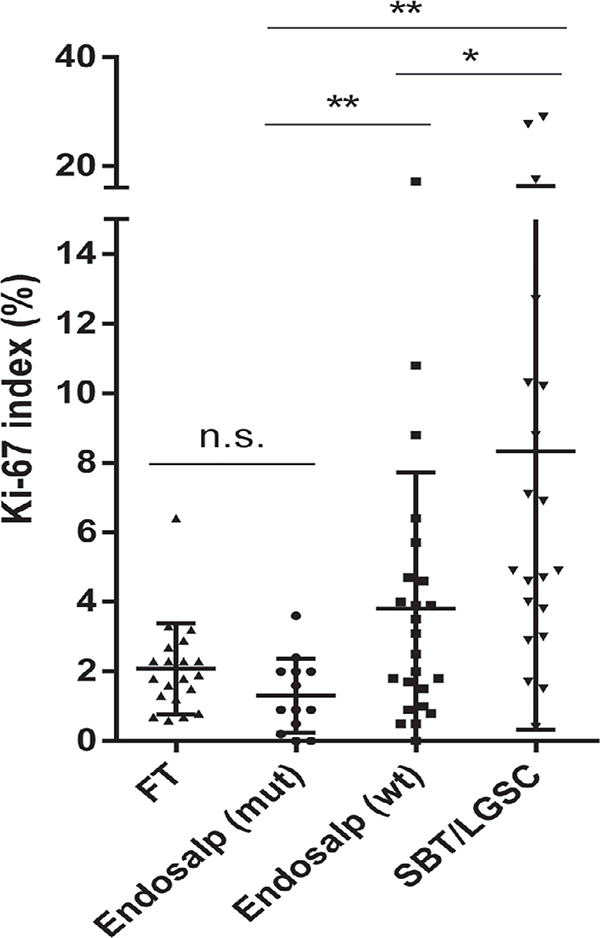
Immunohistochemical staining for Ki-67 was carried out in cases with remaining tissue available after laser-capture microdissection. The Ki-67 index was quantified by digital image analysis. As expected for a non-neoplastic lesion, we observed low proliferation in endosalpingiosis, relative to SBT/LGSC (Figure 4, see supplementary material, Figure S3A). There was even a trend towards a decreased Ki-67 index in endosalpingiosis compared with eutopic Fallopian tube epithelium (p=0.051). Of note, endosalpingiotic lesions with BRAF/KRAS mutations exhibited a significantly lower Ki-67 index than those that were wildtype for both genes.
Figure 4.
Summary of Ki-67 proliferation index, computed by digital image analysis, for Fallopian tube (FT), endosalpingiosis (with or without mutations in BRAF/KRAS) and low-grade serous tumours. Plot shows median ± SD. *p< 0.05, **p< 0.01, n.s. not significant.
Induction of KRASG12V mutation in Fallopian tube epithelial cells inhibits cellular growth
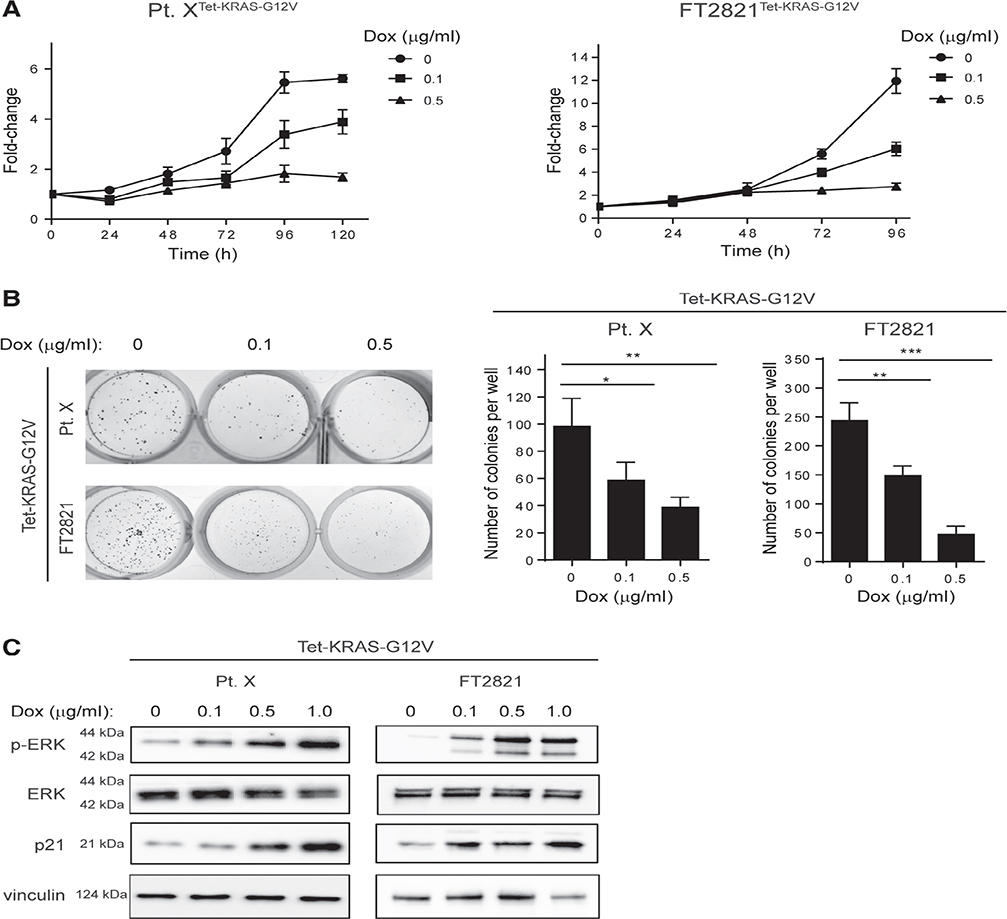
The low proliferation was somewhat paradoxical, given the commonly accepted view that oncogenes enhance proliferation. To investigate this further, a doxycycline-inducible KRASG12V mutation was engineered into Fallopian tube epithelial cells by lentiviral transduction. Primary patient-derived tubal epithelial cells were isolated from a benign salpingectomy specimen, and Müllerian epithelial differentiation was confirmed with Pax-8 and EpCAM staining (see supplementary material, Figure S4A). Following genetic modification by the pCLXBR-pTF-kRAS-V12 vector, in both primary short-term culture (designated Pt. Xtet-KRAS-G12V) and the FT2821 cell line (designated FT2821tetKRAS-G12V), treatment with doxycyline caused a dose-dependent decrease in proliferation rate and colony formation ability (Figure 5A,B). Increased senescence-associated β-galactosidase staining indicating cellular senescence was observed in doxycycline-treated compared with untreated cells (see supplementary material, Figure S4B). Immunoblotting showed that doxycycline-treated cells exhibited phosphorylation of ERK, an indicator of MAPK signalling activation, and increased p21 expression, a marker of cellular senescence (Figure 5C).
Figure 5.
KRASG12V induces growth arrest in Fallopian tube epithelial cells. Short-term primary culture of Fallopian tube epithelial cells (Pt. X) and immortalised Fallopian tube cell line (FT2821) were transduced with KRASG12V under the control of a tetracycline-inducible promoter. (A) Cell growth was monitored by an ATP luminescence assay every 24 h, following treatment with indicated concentrations of doxycycline (n= 3). (B) Colony formation over 7 days of exposure to indicated concentrations of doxycycline (n= 3). (C) Immunoblot indicates increasing levels of ERK phosphorylation and p21 expression with increasing concentrations of doxycycline after 48 h of treatment. Graphs show mean ± SD, *p< 0.05, **p< 0.01, ***p< 0.001.
Discussion
It has been established that Fallopian tube epithelium is the most probable origin of high-grade serous ovarian carcinoma [3,10,17], but the source of cells that give rise to low-grade serous neoplasms remains unclear. The term ‘papillary tubal hyperplasia’ was initially proposed to describe the distinctive morphological features of Fallopian tubes associated with ovarian low-grade serous tumours [16]. In this condition, the proliferative epithelial cells may be prone to detachment from the tubal mucosa and migration to the ovary and extra-ovarian sites. Colonisation of these ectopic sites results in ovarian inclusion cysts/cystadenomas and endosalpingiosis, respectively; these lesions may, in turn, initiate the development of ovarian SBTs and presumably give rise to peritoneal ‘implants’. This process is analogous to the pathogenesis of endometriosis, characterised by ectopic endometrial glands, probably originating from eutopic endometrium. Alternatively, endosalpingiosis may represent displaced embryonic Müllerian duct remnants, similar to endometriosis and endocervicosis [18].
Due to the minute size of endosalpingiotic lesions and their unremarkable morphology, the published literature on molecular alterations in endosalpingiosis is limited to KRAS mutational analysis on a handful of cases [19,20]. These initial observations demonstrated KRAS mutations in a subset of extra-ovarian ‘Müllerian inclusion cysts’ associated with ovarian SBT, corresponding to an identical mutation in the ovarian neoplasm. We expand upon these findings in a more substantial cohort, which included endosalpingiosis without associated ovarian serous neoplasm. Using ddPCR, which enables the quantification of MAF, we assayed for both BRAF and KRAS mutations in all cases.
Importantly, our results show that in most of the mutation-positive cases, a significant proportion of cells carry the mutation, indicating that the lesion represents a clonal expansion. Given that endosalpingiosis is typically comprised of secretory and ciliated cells, our data suggest a bipotential differentiation of a mutated precursor cell, a feature that is retained even in ovarian SBT. In contrast, high-grade serous ovarian carcinoma appears to exhibit a secretory cell phenotype, and even the earliest tubal precursor lesion, the so-called ‘p53 signature’, which refers to a stretch of morphologically normal Fallopian tube secretory-type epithelium with aberrant p53 staining (often corresponding to TP53 mutation), maintains this feature [21]. Hence, the intrinsic differences between high-grade and low-grade serous neoplasia appear to be manifested early on in pathogenesis.
Like the tubal p53 signature, KRAS/BRAF mutation in endosalpingiosis should not warrant any additional clinical management. The low Ki-67 proliferative indices observed in endosalpingiosis are similar to the levels observed in eutopic Fallopian tube epithelium and markedly less than in ovarian serous tumours. Proliferation is particularly low in endosalpingiotic lesions with KRAS or BRAF mutations. Previous work also did not show increased proliferation in KRAS-mutated endometriotic lesions [15]. Our functional studies have demonstrated that rather than promoting cell proliferation, the introduction of mutant KRAS into normal Fallopian tube epithelial cells causes growth arrest. Likewise, transfection of BRAFV600E into ovarian surface epithelial cells had the same effect [13].
Although the tumourigenic effects of KRAS are well recognised, prior work has shown that the introduction of mutant KRAS into a variety of primary untransformed cells, including fibroblasts and pancreatic ductal epithelial cells, results in growth arrest and oncogene-induced senescence [22,23]. Endogenous tumour suppressive mechanisms, such as p21, are intact in normal cells, and it will take additional hits involving oncogenic and tumour suppressor pathways for these cells to undergo malignant transformation. These genetic and/or epigenetic events, that are yet to be elucidated for tubal epithelial cells, probably accompany the acquisition of morphological changes associated with progression to ovarian SBT or peritoneal implants.
The increased incidence of KRAS and BRAF mutations in endosalpingiosis associated with ovarian serous tumours than in those without is analogous to prior work analysing mutations in ovarian serous cystadenomas (which could be considered as a form of endosalpingiosis after clonal expansion in the ovary). Benign cystadenomatous epithelium adjacent to SBT frequently harbours the gene mutation found in the tumour [4]; however, no mutations were detected in cystadenomas that were not associated with SBT [11]. The inference that can be drawn from these observations is that in most cases, endosalpingiosis represents a normal physiological phenomenon of ectopically displaced cells (with no genetic alterations). The acquisition of an oncogenic driver mutation in a rare subset of lesions may lead unrelentingly to neoplastic transformation.
An alternative explanation is that endosalpingiosis associated with SBT or LGSC may, in fact, represent a very subtle early epithelial implant with deceptively bland morphology – a hypothesis supported by the finding of genetic alterations almost exclusively in those endosalpingiotic lesions associated with low-grade serous tumours. Although this possibility cannot be entirely excluded, we were very stringent in including only endosalpingiotic lesions that were morphologically indistinguishable from normal Fallopian tube and lacking the atypia seen in the associated tumour. Furthermore, the identification of KRAS mutations in endosalpingiosis from a patient with a wildtype LGSC (case #18) is definitive evidence that at least in a proportion of cases, endosalpingiosis arises independently from the tumour.
The entire morphological spectrum ranging from endosalpingiosis to florid SBT is not uncommonly encountered in lymph nodes from patients with SBT [8,24]. Based on histomorphology, investigators have suggested that the tumour foci probably arise independently from endosalpingiosis. Our observation of identical mutations across all epithelial lesions within the same lymph node is compatible with this impression. However, in some cases, no mutations were detected in endosalpingiosis located adjacent to tumour cells that were mutation-positive, suggesting that in these instances, their co-existence is merely coincidental.
McKenney et al [8] reported a trend for decreased survival in patients with lymph node involvement by SBT in the absence of nodal endosalpingiosis compared with cases in which SBT and endosalpingiosis co-exist within the same lymph node, suggesting thatlymph node involvement by SBT without endosalpingiosis represents either true metastasis or a more advanced stage of intranodal serous proliferation. Extrapolating from the situation of synchronous endometrioid tumours in uterus and ovary, the presence of associated ovarian endometriosis is often interpreted as evidence to suggest that the tumours represent independent primaries (both stage I) and are therefore associated with a better prognosis than a stage III endometrial cancer with adnexal metastasis [25].
Nonetheless, caution should be exercised when drawing parallels between endometriosis and endosalpingiosis. Oncogenic driver mutations were more commonly found in cases of endometriosis without associated malignancy (frequency of around 30%) [15,26] compared with endosalpingiosis without associated ovarian SBT or LGSC (1/13 [8%] cases). Given the limited sample size and only targeted analysis of BRAF and KRAS hotspot mutations in the present study, further work adopting an unbiased molecular profiling approach is needed to determine precisely mutational frequencies in incidental endosalpingiosis. This should serve as the basis for subsequent investigations exploring the fundamental similarities and differences in the pathogenesis of endometriosis versus endosalpingiosis.
When do KRAS or BRAF mutations arise? Recent work demonstrated the presence of mutations involving classical oncogenes and tumour suppressors in histologically normal endometrium, suggesting that mutations present in endometriosis may ultimately originate from the uterus [27]. Similarly, among the five Fallopian tubes analysed for mutations, one harboured low-frequency KRAS mutations. It should be noted that laser-capture microdissection may not be the ideal technique for addressing this issue, as it samples only a very focal area of the entire tubal mucosa. For the four remaining specimens, the presence of focal mutated clones in areas of unsampled epithelium cannot be ruled out. Our preliminary findings should prompt further in-depth studies to determine the mutation frequency in a larger cohort of histologically normal Fallopian tubes and those with papillary tubal hyperplasia. The possibility of a shared oncogenic stimulus, exerting a ‘field effect’ throughout the peritoneum, is another important consideration. In this scenario, endosalpingiosis may potentially acquire a mutation at any point in time, independent from the transformation event responsible for the development of the primary ovarian serous tumour.
Without dismissing the traditional view that implants are derived from the primary ovarian tumour, our cumulative evidence suggests an alternative theory for the pathogenesis of ovarian low-grade serous neoplasms and their associated extra-ovarian lesions, which accounts for frequent association of endosalpingiosis with low-grade serous neoplasia [1]. Initially, a single KRAS- or BRAF-mutated tubal epithelial cell (i.e. endosalpingiosis) undergoes a transient clonal expansion to form a glandular inclusion before oncogene-induced growth arrest. If located in ovarian tissue, which provides a fertile habitat enriched in hormones, growth factors and a blood supply, it can develop into a serous cystadenoma and, with accumulating molecular genetic and/or epigenetic alterations, eventually progress to SBT. On the other hand, if it resides outside the ovary, it typically remains ‘dormant’ as endosalpingiosis, but occasionally may undergo proliferation and develop into an epithelial ‘implant’. The higher prevalence of endosalpingiosis in patients with ovarian SBT with subsequent disease recurrence/progression compared with SBTs that do not recur [28], is consistent with a link between endosalpingiosis and the development of implants. Future studies using global genomic profiling approaches will be required to elucidate the phylogenetic relationships between endosalpingiosis, the primary ovarian low-grade serous tumour and associated extra-ovarian implants/metastatic lesions.
In summary, this study demonstrates that at least a subset of endosalpingiotic lesions are clonal and harbour the same oncogenic driver mutations shared with low-grade serous tumours. Our findings also provide a molecular foundation to study these inconspicuous, but biologically interesting, lesions and their relationship to ovarian low-grade serous tumours.
Supplementary Material
Figure S1. Morphological spectrum of endosalpingiosis and low-grade serous lesions
Figure S2. Case #1. Eutopic Fallopian tube epithelium, endosalpingiosis (Es #1 simple type, Es #2 florid type) and serous borderline tumour (SBT) were enriched by laser-capture microdissection and subjected to ddPCR to assess for the BRAFV600E mutation
Figure S3. Case #13. KRASG12D mutation in endosalpingiosis and serous borderline tumour, and low-frequency KRASG12D and KRASG12V mutations detected in eutopic Fallopian tube
Figure S4. Effects of KRASG12V in Fallopian tube epithelial cells in vitro
Acknowledgements
MHC was supported by a CIHR Postdoctoral Fellowship. This study was partially supported by the Richard W TeLinde endowment from the Department of Gynecology and Obstetrics, Johns Hopkins Medical Institutions.
Footnotes
No conflicts of interest were declared.
References
- 1.Esselen KM, Terry KL, Samuel A, et al. Endosalpingiosis: more than just an incidental finding at the time of gynecologic surgery? Gynecol Oncol 2016; 142: 255–260. [DOI] [PubMed] [Google Scholar]
- 2.Vang R, Shih I-M, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol 2009; 16: 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih I-M. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol 2016; 186:733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho CL, Kurman RJ, Dehari R, et al. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res 2004; 64: 6915–6918. [DOI] [PubMed] [Google Scholar]
- 5.Shih I-M, Kurman RJ. Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res 2005; 11:7273–7279. [DOI] [PubMed] [Google Scholar]
- 6.Ardighieri L, Zeppernick F, Hannibal CG, et al. Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J Pathol 2014; 232:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva EG, Tornos C, Zhuang Z, et al. Tumor recurrence in stage I ovarian serous neoplasms of low malignant potential. Int J Gynecol Pathol 1998; 17: 1–6. [DOI] [PubMed] [Google Scholar]
- 8.McKenney JK, Balzer BL, Longacre TA. Lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors): pathology, prognosis, and proposed classification. Am J Surg Pathol 2006; 30: 614–624. [DOI] [PubMed] [Google Scholar]
- 9.Zinsser KR, Wheeler JE. Endosalpingiosis in the omentum: a study of autopsy and surgical material. Am J Surg Pathol 1982; 6:109–117. [DOI] [PubMed] [Google Scholar]
- 10.Vang R, Shih I-M, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology 2013; 62:44–58. [DOI] [PubMed] [Google Scholar]
- 11.Cheng EJ, Kurman RJ, Wang M, et al. Molecular genetic analysis of ovarian serous cystadenomas. Lab Invest 2004; 84: 778–784. [DOI] [PubMed] [Google Scholar]
- 12.Wu RC, Wang P, Lin SF, et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol 2019; 248:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeppernick F, Ardighieri L, Hannibal CG, et al. BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. Am J Surg Pathol 2014; 38: 1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turashvili G, Grisham RN, Chiang S, et al. BRAFV600E mutations and immunohistochemical expression of VE1 protein in low-grade serous neoplasms of the ovary. Histopathology 2018; 73:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anglesio MS, Papadopoulos N, Ayhan A, et al. Cancer-associated mutations in endometriosis without cancer. N Engl J Med 2017; 376:1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurman RJ, Vang R, Junge J, et al. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol 2011; 35: 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn E, Kurman RJ, Vang R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma — evidence supporting the clonal relationship of the two lesions. J Pathol 2012; 226: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Nicholes K, Shih IM. The origin and pathogenesis of endometriosis. Annu Rev Pathol 2020; 15: 71–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diebold J, Seemuller F, Lohrs U. K-RAS mutations in ovarian and extraovarian lesions of serous tumors of borderline malignancy. Lab Invest 2003; 83: 251–258. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez AA, Moore WF, Robboy SJ, et al. K-ras mutations in Mullerian inclusion cysts associated with serous borderline tumors of the ovary. Gynecol Oncol 2001; 80: 201–206. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal Fallopian tube. J Pathol 2007; 211: 26–35. [DOI] [PubMed] [Google Scholar]
- 22.Morton JP, Timpson P, Karim SA, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A 2010; 107: 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res 2006; 66: 2881–2884. [DOI] [PubMed] [Google Scholar]
- 24.Djordjevic B, Clement-Kruzel S, Atkinson NE, et al. Nodal endosalpingiosis in ovarian serous tumors of low malignant potential with lymph node involvement: a case for a precursor lesion. Am J Surg Pathol 2010; 34: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 25.Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas—a prospective clinicopathologic study of 74 cases: a Gynecologic Oncology Group study. Gynecol Oncol 2001; 83: 355–362. [DOI] [PubMed] [Google Scholar]
- 26.Lac V, Verhoef L, Aguirre-Hernandez R, et al. Iatrogenic endometriosis harbors somatic cancer-driver mutations. Hum Reprod 2019; 34: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suda K, Nakaoka H, Yoshihara K, et al. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep 2018; 24:1777–1789. [DOI] [PubMed] [Google Scholar]
- 28.Chui MH, Xing D, Zeppernick F, et al. Clinicopathologic and molecular features of paired cases of metachronous ovarian serous borderline tumor and subsequent serous carcinoma. Am J Surg Pathol 2019; 43: 1462–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Morphological spectrum of endosalpingiosis and low-grade serous lesions
Figure S2. Case #1. Eutopic Fallopian tube epithelium, endosalpingiosis (Es #1 simple type, Es #2 florid type) and serous borderline tumour (SBT) were enriched by laser-capture microdissection and subjected to ddPCR to assess for the BRAFV600E mutation
Figure S3. Case #13. KRASG12D mutation in endosalpingiosis and serous borderline tumour, and low-frequency KRASG12D and KRASG12V mutations detected in eutopic Fallopian tube
Figure S4. Effects of KRASG12V in Fallopian tube epithelial cells in vitro