Abstract
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality for women in the United States and worldwide. There has been no American College of Cardiology (ACC)/American Heart Association guideline update specifically for the prevention of CVD in women since 2011. Since then, the body of sex-specific data has grown, in addition to updated hypertension, cholesterol, diabetes, atrial fibrillation, and primary prevention guidelines. The ACC CVD in Women Committee undertook a review of the recent guidelines and major studies to summarize recommendations pertinent to women. In this update, the authors address special topics, particularly the risk factors and treatments that have led to some controversies and confusion. Specifically, sex-related risk factors, hypertension, diabetes, hyperlipidemia, anticoagulation for atrial fibrillation, use of aspirin, perimenopausal hormone therapy, and psychosocial issues are highlighted.
Keywords: adverse pregnancy outcomes, aspirin, atrial fibrillation, cardiovascular disease, gestational diabetes
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality for women in the United States and worldwide (1). Overall, 1 in 3 women die from CVD, and 45% of women over age 20 years have some form of CVD (1).
There has been no American College of Cardiology (ACC)/American Heart Association (AHA) guideline update specifically for the prevention of CVD in women since 2011. The last statement to address this was the Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update published by the AHA (2). Since then, the body of sex-specific data has grown, in addition to updated hypertension guidelines in 2017 (3), updated cholesterol guidelines in 2018 (4), new atrial fibrillation (AF) guidelines in 2019 (5), and new 2019 ACC/AHA primary prevention of CVD guidelines (6). Although these guidelines provide an excellent review of the science and treatment pertaining to the whole population, women’s cardiovascular health can be optimized by focusing special attention to unique sex-specific aspects of care.
The ACC CVD in Women Committee undertook a review of the recent guidelines and major studies to summarize recommendations pertinent to women. In this update, we address special topics, particularly the risk factors and treatments that have led to some controversies and confusion. Specifically, we highlight sex-related risk factors, hypertension, diabetes, hyperlipidemia, anticoagulation for AF, use of aspirin, perimenopausal hormone therapy, and psychosocial issues. This update does not address nutrition, diet, exercise, and smoking cessation, which were well-covered in the 2019 primary prevention guideline (6), nor sudden cardiac death, which is beyond the scope of the primary prevention guidelines (Central Illustration).
CENTRAL ILLUSTRATION. Cardiovascular Disease Risk Factors in Women.
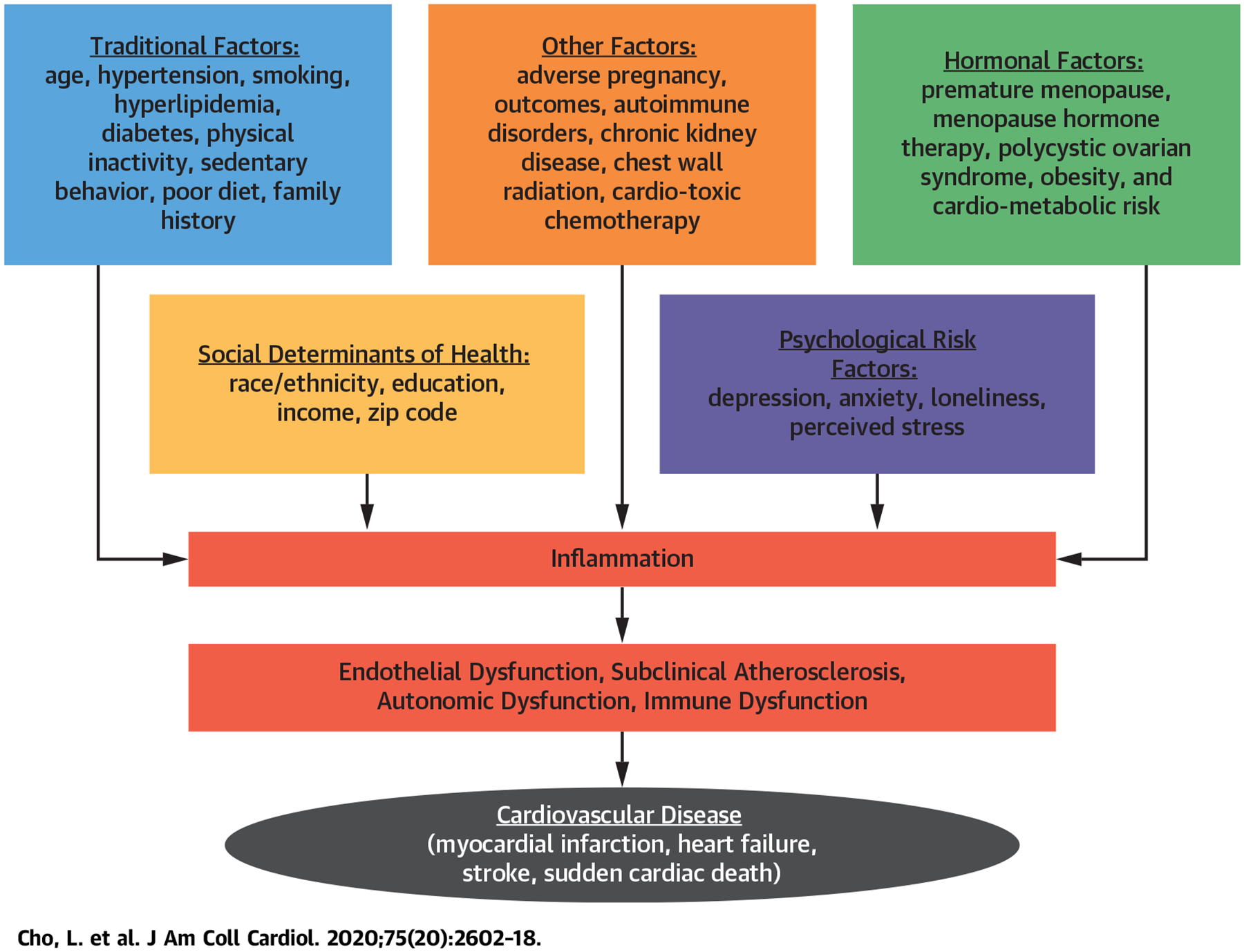
The factors shown in orange are incorporated in the atherosclerotic cardiovascular disease risk calculator. However, there are unique sex-specific factors as well as psychosocial factors that contribute to CVD risk and adverse outcomes.
CVD RISK FACTORS UNIQUE TO WOMEN
PREGNANCY-ASSOCIATED CONDITIONS THAT INCREASE FUTURE RISK OF CVD.
Adverse pregnancy outcomes (APO) occur in 10% to 20% of all pregnancies and are associated with a 1.8- to 4.0-fold risk of future CVD (7,8). Risk of CVD is higher with more severe forms of APO or more than 1 pregnancy complicated by an APO (9). Studies of vascular abnormalities in women with APO suggest placental dysfunction, and abnormal endothelial function may be a common pathway and early indicator of later cardiometabolic risk (10). The American College of Obstetrics and Gynecology recommends that women with APO and/or cardiovascular risk factors undergo cardiovascular risk screening within 3 months postpartum (11) (Figure 1, Table 1).
FIGURE 1. Recommendations for Cardiovascular Risk Screening After Adverse Pregnancy Outcomes.
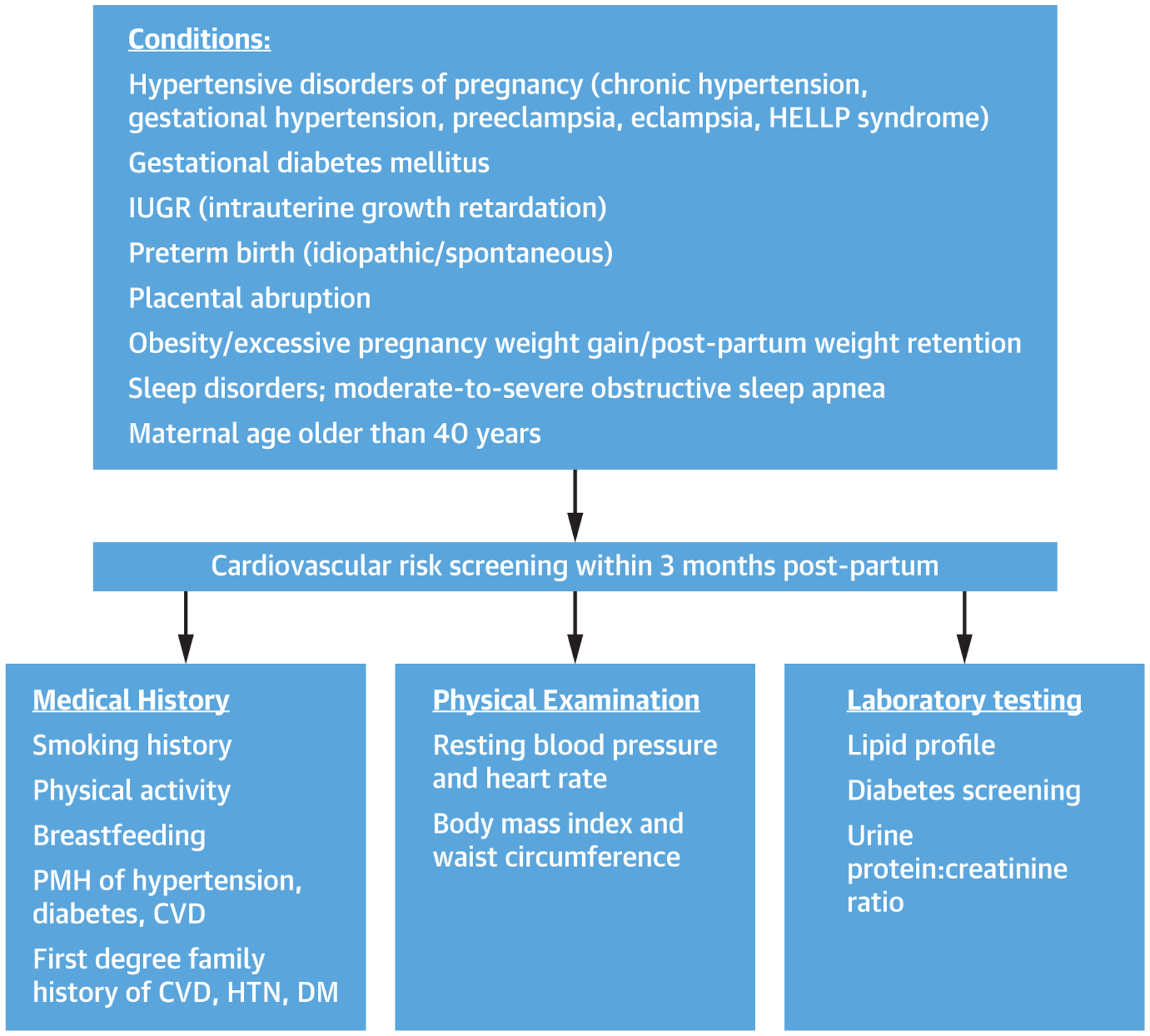
Adverse pregnancy conditions that require further cardiovascular screening within 3 months post-partum based on medical history, physical examination, and laboratory. CVD = cardiovascular disease; DM = diabetes mellitus; HELLP = hemolysis, elevated liver enzyme, low platelet count; HTN = hypertension; IUGR = intrauterine growth restriction; PMH = past medical history.
TABLE 1.
Complications During Pregnancy That Are Associated With Increased Cardiovascular Disease Risk
| Adverse Pregnancy Outcomes | Definition |
|---|---|
| Hypertensive disorders of pregnancy | This category includes gestational hypertension, chronic hypertension, and pre-eclampsia |
| Gestational hypertension | New-onset hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg) after 20 weeks gestation |
| Pre-eclampsia | New-onset hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg) after 20 weeks gestation with proteinuria or evidence of end-organ dysfunction |
| Chronic (pre-existing) hypertension | Hypertension present prior to 20 weeks gestation |
| Gestational diabetes | Glucose intolerance with onset or first recognition during pregnancy |
| Pre-term birth | Delivery before 37 weeks gestation |
| Early pre-term birth | Delivery before 34 weeks gestation |
| Pregnancy loss | Miscarriage or stillbirth |
| Intrauterine growth restriction | Fetal birthweight less than expected for the gestational age, ≤10th percentile |
DBP = diastolic blood pressure; SBP = systolic blood pressure.
HYPERTENSIVE DISORDERS OF PREGNANCY.
Hypertensive disorders of pregnancy are associated with development of incident hypertension after delivery and overall CVD. A meta-analysis of 3,488,160 women, including 198,252 with pre-eclampsia reported that after 10 to 15 years, women with pre-eclampsia had a 3.7-fold risk of hypertension, 2.2-fold risk of ischemic heart disease, 1.8-fold risk of stroke, and 1.5-fold risk of overall mortality (8). Pre-eclampsia was included as a “risk-enhancer” in the updated 2018 cholesterol guideline (4) and in the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease (6). In addition, all hypertensive disorders of pregnancy are associated with increased risk of chronic hypertension (12,13) as early as the first year after delivery (13), twice the risk of CVD-related hospitalizations within 3 years of delivery (14), and development of other classic CVD risk factors such as diabetes and hyperlipidemia (15). A 2019 study of the United Kingdom Biobank cohort found that hypertension during pregnancy was associated with increased risk of coronary disease (hazard ratio [HR]: 1.8; 95% confidence interval [CI]: 1.3 to 2.6; p < 0.001) as well as increased risk of heart failure and valvular heart disease (16). The American College of Obstetrics and Gynecology currently recommends initiation of low-dose aspirin in women with at least 1 high risk factor (history of pre-eclampsia, multifetal pregnancy, chronic hypertension, diabetes mellitus I or II, chronic kidney disease, or autoimmune disorder) or at least 2 moderate risk factors (nulliparity, obesity, family history of pre-eclampsia, socioeconomic factors, age >35 years, or personal history factors) to reduce the risk of pre-eclampsia (17).
GESTATIONAL DIABETES MELLITUS.
Women with a history of gestational diabetes mellitus are at increased risk of future CVD, including a 1.4- to 20-fold increased risk of type 2 diabetes mellitus, 2-fold risk of hypertension, 2-fold risk of stroke, and 2.8-fold risk of ischemic heart disease (18).
PRE-TERM BIRTH.
Pre-term birth is defined as delivery prior to 37 weeks gestation; idiopathic pre-term birth is associated with a 2-fold increased risk of CVD and deaths caused by coronary heart disease (19) even when adjusted for pre-pregnancy lifestyle and CVD risk factors (20). Risk of CVD is higher with more pre-term births and earlier pre-term birth (prior to 34 weeks).
PREGNANCY LOSS.
Women with prior pregnancy loss (miscarriage and stillbirth) are at approximately 2-fold increased risk of myocardial infarction (MI), cerebral infarction, and renovascular hypertension (21). In a meta-analysis of 10 studies, miscarriage was associated with a 1.45-fold increased risk of CVD, and more than 1 miscarriage was associated with a 2-fold risk of CVD (22).
Intrauterine growth restriction.
Intrauterine growth restriction (IUGR) is defined as an estimated fetal weight <10th percentile for the gestational age, often related to suboptimal uterine-placental perfusion (23). Several maternal factors are associated with fetal growth restriction, including hypertensive disorders and diabetes (23). Women with prior IUGR pregnancies are at increased risk for hyperlipidemia, hypertriglyceridemia, and insulin resistance (24). Additionally, echocardiographic cardiac changes have been observed in women during normotensive IUGR pregnancies, including higher prevalence of diastolic dysfunction and less cardiac reserve compared with control subjects (25). Low-dose aspirin started in early pregnancy may prevent IUGR in certain patients (26,27).
RISK PREDICTION MODELS.
Although adverse pregnancy outcomes are associated with later risk of CVD, the addition of pregnancy complications to standard cardiovascular risk prediction models have not significantly enhanced the predictive capabilities (28,29). Because adverse pregnancy outcomes are also associated with other conventional cardiovascular risk factors that are included in the standard risk models, the additive impact of pregnancy complications becomes less significant, particularly with increasing age. A history of adverse pregnancy outcomes may be most useful in younger women, prior to the development of conventional risk factors, and important for counseling of women about risk prevention.
PREMATURE MENOPAUSE.
Premature menopause (age <40 years) was considered a risk-enhancing factor in the 2018 cholesterol guideline (4). Menopause increases CVD risk because of the physiological responses to estrogen withdrawal, including changes in body fat distribution, reduced glucose tolerance, abnormal lipids, higher blood pressure (BP), increased sympathetic tone, endothelial dysfunction, and vascular inflammation (30). A 2019 pooled analysis from 15 observational studies including 301,438 women reported increased risk of nonfatal CVD in women with premature menopause (HR: 1.55; 95% CI: 1.38 to 1.73; p < 0.0001), early menopause (age 40 to 44 years; HR: 1.30; 95% CI: 1.22 to 1.39; p < 0.0001), and relatively early menopause (age 45 to 49 years; HR: 1.12; 95% CI: 1.07 to 1.18; p < 0.0001) (31). Recent data from the United Kingdom Biobank cohort reported premature menopause (before age 40 years) was associated with increased risk of CVD (HR: 1.36; 95% CI: 1.19 to 1.56; p < 0.001) after adjustment for conventional risk factors (32). The interaction between CVD and menopause is complex, and it may be that women at increased risk for CVD experience menopause at an earlier age.
POLYCYSTIC OVARIAN SYNDROME.
Polycystic Ovarian Syndrome (PCOS) is a common endocrine disorder that affects young women, and is characterized by ovulatory dysfunction (oligomenorrhea or amenorrhea), hyperandrogenism, infertility, and insulin resistance (33). Whether PCOS by itself confers high CVD risk, or whether the associated cardiometabolic features are the reason for increased risk is unclear (34). Women with PCOS are at an increased risk for development of metabolic syndrome features of abdominal obesity, diabetes, dyslipidemia, and hypertension (35). These factors contribute to endothelial dysfunction, which is a marker of CVD risk, and several studies have shown endothelial function abnormalities and subclinical atherosclerosis in PCOS (36). Ethnic variation in PCOS has also been reported, with East Asian women with PCOS having the highest prevalence of metabolic syndrome, despite a lower body mass index and less hyperandrogenic features (37). In addition to treatment of menstrual irregularities with oral contraceptives, metformin is recommended for patients who have cardiometabolic features such as abdominal obesity and insulin resistance (38). Although the 2018 cholesterol guideline did not include PCOS as a risk enhancer (4), the international guidelines for PCOS recommend that all women with PCOS should be screened for CVD risk, including close monitoring for weight changes every 6 to 12 months, at least annual BP check, fasting lipid panel, screen for glycemic control, and assessments for smoking and physical activity (39). Psychological factors, such as anxiety, depression, and eating disorders, are prevalent in PCOS, and guidelines recommend that health professionals take into consideration cultural sensitivities and weight-related stigma in women when addressing lifestyle-based interventions (38).
AUTOIMMUNE DISEASE.
Women are more likely to have underlying autoimmune and inflammatory conditions that contribute to increased CVD risk, beyond the traditional CVD risk factors. Conditions such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) are highly prevalent in women and are associated with accelerated atherosclerosis as well as coronary microvascular dysfunction (2,40). SLE is more prevalent in Asians, African Americans, African Caribbeans, Hispanic Americans compared with Caucasians. Black women are 2 to 4 times more likely to have SLE than white women (41). Ischemic heart disease is the number 1 cause of mortality in SLE. One study reported that young women with SLE (ages 35 to 44 years) were over 50 times more likely to have an MI compared with those of similar age in the Framingham Offspring study (42). There is a 50% increased risk of CVD mortality in RA compared with the general population (43). Furthermore, there is emerging data that the duration of time in RA flares is associated with increased risk of CVD events (44). A lipid paradox described in 1 study demonstrated that elevated erythrocyte sedimentation rate and low cholesterol levels were associated with CVD risk in RA patients (45). Higher levels of inflammation are associated with major adverse outcomes despite low to normal cholesterol levels in other studies (46,47). Treatment with anti-inflammatory agents such as statins, interleukin-1β receptor antagonist canakinumab, and colchicine improves CVD outcomes in various cohorts (47–49). The ACC/AHA risk score derived from pooled cohort equation to estimate atherosclerotic CVD risk does not incorporate these unique risk factors for women; however, the 2018 cholesterol guideline lists these factors as risk enhancing factors that should be taken into consideration when assessing a patient’s CVD risk (4).
TRADITIONAL RISK FACTORS
HYPERTENSION.
The 2017 ACC/AHA guidelines for the prevention, detection, evaluation, and management of high BP provides limited sex-specific guidance in the management of hypertension and focuses primarily on hypertension during pregnancy (3), which has been extensively reviewed in a paper discussing hypertension across a woman’s lifespan (50). However, there are certain unique aspects in women of the prevention, epidemiology, evaluation, and management of hypertension. Common risk factors for hypertension in women include: obesity, physical inactivity, increased salt intake, diabetes, and more than moderate alcohol consumption (i.e., >1 alcoholic drink/day). The combination of these risk factors is associated with a higher risk of hypertension, and obesity has the highest impact on the incidence of hypertension among women (51). Due to the up-regulation of renin-angiotensin receptors after menopause, salt restriction is beneficial in reducing the risk of hypertension. Indeed, reducing salt intake has been shown to reduce systolic BP in women with and without hypertension (52). The 2017 ACC/AHA guidelines recommend to ideally limit sodium intake to <1,500 mg/day or at least aim for a 1,000 mg/day reduction, and to enhance the intake of potassium from foods to at least 3,500 to 5,000 mg/day (3); however, there is no specific recommendations based on sex (Figure 2).
FIGURE 2. Hypertension in Women.
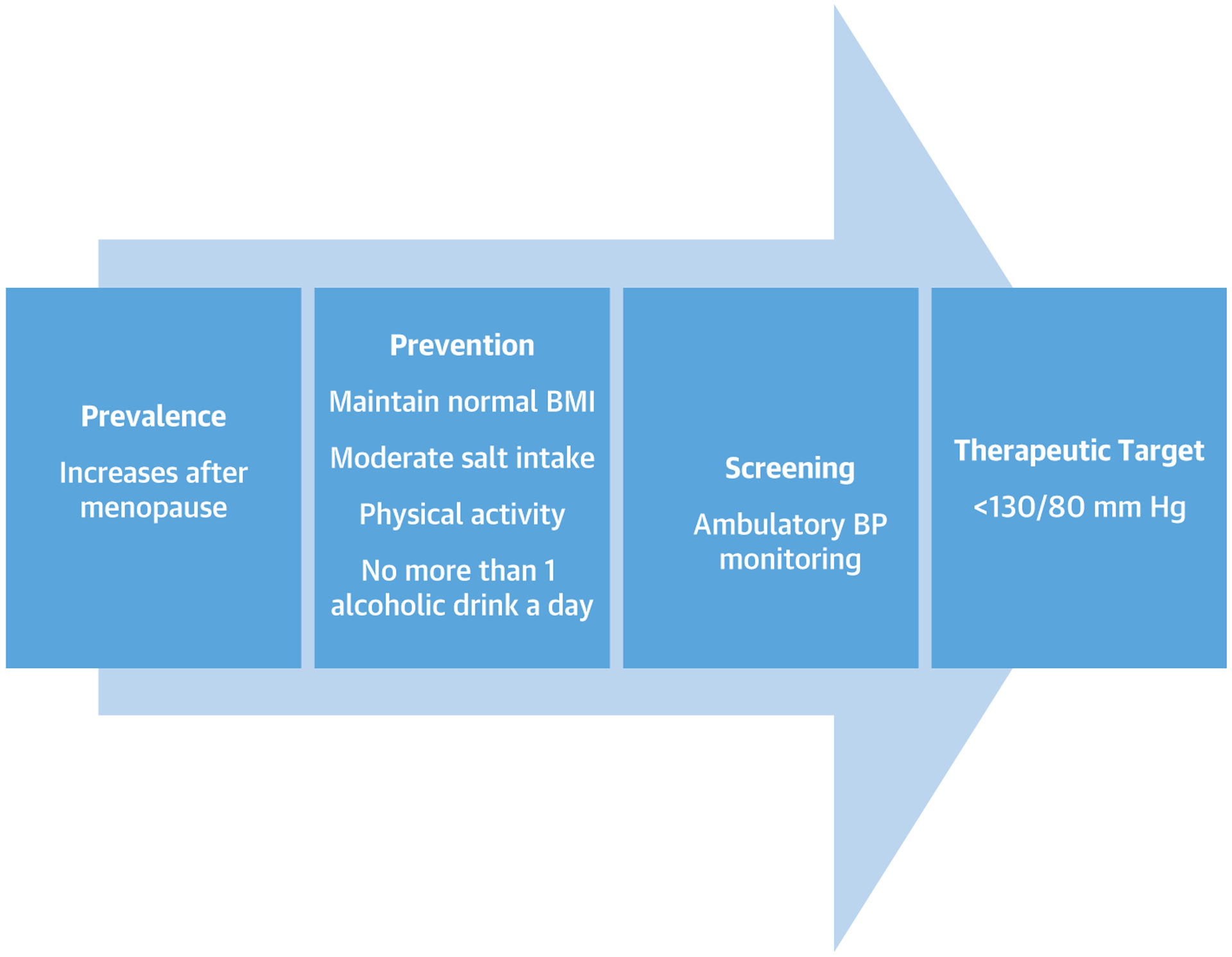
Progression of hypertension from prevalence to prevention, screening, and therapeutic target. BMI = body mass index; BP = blood pressure.
Attention needs to be given to the possible presence of secondary causes of hypertension among premenopausal women. In particular, women account for >90% of cases of fibromuscular dysplasia, a condition that affects 3.3% of the general population (53). Combined hormonal contraceptive can also result in an increase in BP, particularly among women with a pre-existing diagnosis of hypertension. Premenopausal women requiring antihypertensive therapy also require counseling on potential medication teratogenicity, particularly if receiving angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or aldosterone receptor antagonists (54).
The prevalence of hypertension among premenopausal women tends to be lower than men of similar age; however, hypertension becomes more prevalent in women after menopause (1).
In a recent sex-specific longitudinal BP analysis of >32,000 patients, women were found to have steeper increases in BP than men, which began as early as the third decade of life and persisted with aging, even after adjusting for the cardiometabolic risk factors. This is contrary to the beliefs that vascular diseases lag 10 years or more among women compared with men (55). Because BP represents a simple accessible measure of vascular aging and is a significant contributor to future cardiovascular events, these findings could help explain some of the differences in CVD presentations among women versus men, such as diastolic heart failure (55,56).
The 2017 ACC/AHA guideline recommends out-of-office monitoring of BP for confirmation and management of hypertension irrespective of sex (3). Notably, studies suggested that post-menopausal women are likely to experience a nondipping nighttime BP pattern (defined as <10% reduction in nighttime BP) (57). This phenomenon likely explains the higher incidence of cardiovascular events attributed to nighttime BP observed in women compared with men (58), suggesting that women might derive more benefit from BP control using ambulatory BP measurement as opposed to conventional BP monitoring.
Based on the findings of the SPRINT (Systolic Blood Pressure Intervention Trial), the 2017 ACC/AHA guideline recommends a therapeutic BP target of 130/80 mm Hg irrespective of sex (3,59). Despite the higher prevalence of hypertension among women, SPRINT only enrolled 36% women (59). Interestingly, women enrolled in SPRINT had lower cardiovascular risk than men (60,61). Two analyses for the sex-specific differences between an intensive BP-lowering strategy versus a standard BP-lowering strategy from SPRINT were conducted (60,61). One showed that women and men derive similar benefit from an intensive BP-lowering strategy (60), whereas the other analysis showed that women do not benefit from an intensive BP-lowering strategy after matching the baseline differences in both groups (61). Collectively, this suggests that the therapeutic BP target in women remains not well established even after the SPRINT trial results (62).
Randomized trial data suggest that there is no large difference between women and men in cardiovascular outcomes based on the antihypertensive regimen (63), but it appears that women might experience more side effects from antihypertensive medication (63,64). Perhaps thiazide diuretic agents are the only clearly beneficial agent in older women due to their effect in reducing calcium excretion and preventing osteoporosis (65).
DIABETES.
Diabetes mellitus (DM) is estimated to affect over 26 million people in the United States, of which 12.8 million are women, with the vast majority having type 2 DM (1). There are striking sex differences in prevalence of type 2 DM across the lifespan as well as sex differences in CVD outcomes.
Interestingly, there are differences in the type 2 DM incidence across the lifespan, with girls having higher rates of type 2 DM in youth, whereas men have higher rates during midlife, with similar incidence between men and women at later stages in life (66). The mechanism of sex difference may be due to sex differences in insulin resistance during adolescence and midlife, with female youth having higher insulin resistance during early childhood to puberty (66). These findings of early-onset of DM in female patients, which translates into longer duration of disease throughout their lifetime, should raise serious concerns given the recent Swedish Heart Registry finding that CVD mortality is significantly increased for people diagnosed with type 2 DM before the age of 40 years (67).
Diabetes increases the risk of having an MI or stroke by 2-fold (66). In the presence of type 2 DM, the absolute rate difference between the sexes is significantly diminished, although not fully eliminated (68,69). The cardioprotection that occurs in premenopausal women is thus reduced significantly with diabetes. A recent systematic review and meta-analysis of over 5 million patients found that the pooled relative risk for CVD mortality in patients with DM was 2.42 in women (95% CI: 2.10 to 2.78) and 1.86 (95% CI: 1.70 to 2.03) in men (70). There also appears to be greater excess risk of CVD mortality in women with DM compared with men (relative risk: 1.30; 95% CI: 1.13 to 1.49; p < 0.001) in pooled multiple adjusted analysis, although there was significant heterogeneity between the studies (70). Recently, the Atherosclerosis Risk In Communities study found DM was a stronger risk factor for CVD as well as CVD mortality among African-American women than among African-American men (71). These findings are similar to what has been seen in white male and female patients (71). In addition to atherosclerotic events, having DM increases the incidence of congestive heart failure. In the UK biobank study of 468,941 patients followed for 9.0 years, women with type 2 DM had significantly higher rates of incidence of heart failure (HR: 1.73; 95% CI: 1.34 to 2.24; p < 0.0001) as well as heart failure mortality (HR: 1.92; 95% CI: 1.25 to 2.94; p = 0.003) compared with men (72). Last, DM increases the risk of cancer mortality by 26% in women (95% CI: 1.16 to 1.36) and by 29% in men (95% CI: 1.18 to 1.42) (70). There was no sex difference in the association between diabetes and cancer mortality for diabetic patients (70).
There appears to be some sex-specific effects of pharmacotherapy for DM. For example, it has been reported that glucagon-like peptide-1 receptor agonists have better glycemic control among men than women; however, women had more weight loss (73). Thiazolidinediones appear to have better glycemic reduction in obese women, whereas nonobese men responded better with sulfonylureas (74,75). Reassuringly, the EMPA-REG (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) study, which showed reduction in cardiovascular mortality in diabetic patients treated with the sodium glucose cotransporter 2 inhibitor empagliflozin, showed no significant sex differences in benefit with the drug (76).
Given the increased cardiovascular risk, all patients with DM require aggressive risk factor reduction. However, studies have consistently shown that women are underdiagnosed and undertreated compared with men (77,78). Women with DM have poorer BP, lipid, and DM control compared with their male counterparts (66).
Table 2 lists BP, lipid, antiplatelet, and hemoglobin A1c goal for diabetic patients without established CVD. Although there are some differing targets among different societies (3,4,6,79–81) regarding BP target, the societies are consistent with aggressive lipid control for diabetic patients. There are no sex differences between the treatment and target recommendations. All societies agree that asymptomatic patients not be routinely screened for CAD.
TABLE 2.
DM Risk Management and Treatment Goals
| ADA (79,80) | ACC/AHA (3,4,6) | ESC (81) | |
|---|---|---|---|
| HgA1c | Goal <7.0% | <7.0% | <7.0% <6.5% if achievable without hypoglycemia Less stringent in elderly patients |
| HTN | Goal of <140/90 mm Hg Goal of <130/80 mm Hg if high risk of CVD |
BP goal of <130/80 mm Hg Initiate treatment if BP >130/80 mm Hg (specific DM recommendations) |
SBP target 130 mm Hg <130 mm Hg if tolerated but not <120 mm Hg In older patients (age >65 yrs) SBP goal 130–139 mm Hg DBP goal <80 mm Hg but not <70 mm Hg |
| LDL | <40 yrs no ASCVD risk factor—no statin <40 yrs ASCVD risk factors—high-intensity statin ≥40 yrs no ASCVD risk factor—moderate-intensity statin ≥40 yrs ASCVD risk factor—high-intensity statin |
>40 yrs of age, moderate-intensity statin regardless of 10-yr ASCVD risk DM patients with multiple ASCVD risk factors, it is reasonable to prescribe high-intensity statin therapy with the aim to reduce LDL-C levels by 50% or more Age 20 to 39 yrs of age with DM that is either of long duration (≥10 yrs of type 2 diabetes mellitus, ≥20 yrs of type 1 diabetes mellitus), albuminuria (≥30 μg of albumin/mg creatinine), estimated glomerular filtration rate <60 ml/min/1.73 m2, retinopathy, neuropathy, or ankle-brachial index (<0.9), it may be reasonable to initiate statin therapy |
Very high-risk LDL <55 mg/dl or LDL 50% reduction High risk <70 mg/dl or LDL 50% reduction Moderate risk <100 mg/dl |
| Aspirin | DM who are at increased risk of CVD | No specific DM recommendations | Only in very high risk/high risk |
ADA Definition: ASCVD risk factors—LDL ≥100 mg/dl, high blood pressure, smoking, chronic kidney disease, albuminuria, and family history of premature ASCVD. ESC Definition: Very high risk—target organ damage or 3 or more risk factors or type 2 DM duration >20 years. High risk—DM >10-yr duration without target organ damage, and any other risk factor. Moderate risk—type 1 DM and <35 years of age, type 2 DM <50 years of age with DM <10 yrs duration without risk factor.
ACC = American College of Cardiology; ADA = American Diabetes Association; AHA = American Heart Association; ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; CVD = cardiovascular disease; DBP = diastolic blood pressure; DM = diabetes mellitus; ESC = European Society of Cardiology; HgA1c = hemoglobin HgA1c; HTN = hypertension; LDL = low-density lipoprotein; SBP = systolic blood pressure.
BLOOD CHOLESTEROL MANAGEMENT IN WOMEN
Despite contemporary advancements in cholesterol-lowering therapy, women are less likely to receive guidelines-recommended statin therapy compared with men. They are also more likely to decline initial treatment and less likely to continue prescribed statin therapy (82). The 2018 AHA/ACC multisociety guideline on the management of blood cholesterol and the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease emphasize the importance of lipid management for reduction of atherosclerotic cardiovascular disease (ASCVD) risk and include some sex-specific risk-enhancing factors to help further identify women at increased ASCVD risk (4,6). In addition to lifestyle interventions with diet, exercise, and weight loss, the guidelines recommend statin therapy as the mainstay treatment in 4 groups of patients:
Clinical ASCVD;
Severe hypercholesterolemia (low-density lipoprotein [LDL] cholesterol ≥190 mg/dl);
Diabetes mellitus in adults (age 40 to 75 years);
Primary prevention in adults age 40 to 75 years at high risk (≥20%) and some adults at intermediate risk (≥7.5% to <20%) or borderline risk (5% to <7.5%) based on the presence of risk enhancers, the presence of an elevated coronary artery calcium score if measured, and clinician-patient risk discussion.
The benefit of statin therapy has been widely accepted for reduction of CVD events for secondary prevention in both sexes; however, the role of statin therapy for primary prevention in women has been debated over the past decade. This controversy stemmed in part from a lack of robust data on the efficacy of statins for primary prevention in women, as under-representation of women in randomized controlled trials left studies underpowered to adequately analyze outcomes by sex. In addition, early meta-analyses of statin therapy for primary prevention yielded conflicting data, with some studies showing no significant reduction in mortality or cardiovascular events in women (83). Since the Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update (2), 2 larger meta-analyses including over 40,000 women have demonstrated a similar benefit of statin therapy in women and men for both primary and secondary prevention, and this benefit was seen in both sexes across all levels of risk in primary prevention studies (84,85). Although no significant sex differences in adverse effects were identified in these meta-analyses, few statin trials reported adverse drug reactions by sex. Despite a paucity of randomized trial data, international consensus statements recognized female sex as a risk factor for statin-associated muscle symptoms (86,87). In patients with statin-associated muscle symptoms, careful review of concomitant medications and detailed history should be taken to understand factors that may contribute to statin side effects (86,87). Change in statin (hydrophilic vs. lipophilic) as well as intermittent statin dosing can be used to help overcome some of the muscle symptoms associated with statins (88).
There are currently no sex-specific guidelines for the management of blood cholesterol with statin therapy. Statins reduce cardiovascular events and all-cause mortality regardless of sex, and should be considered at recommended doses in women who meet criteria for 1 of the 4 guideline-recommended patient populations (Figure 3).
FIGURE 3. Recommendations for Statin for ASCVD Prevention in Women.
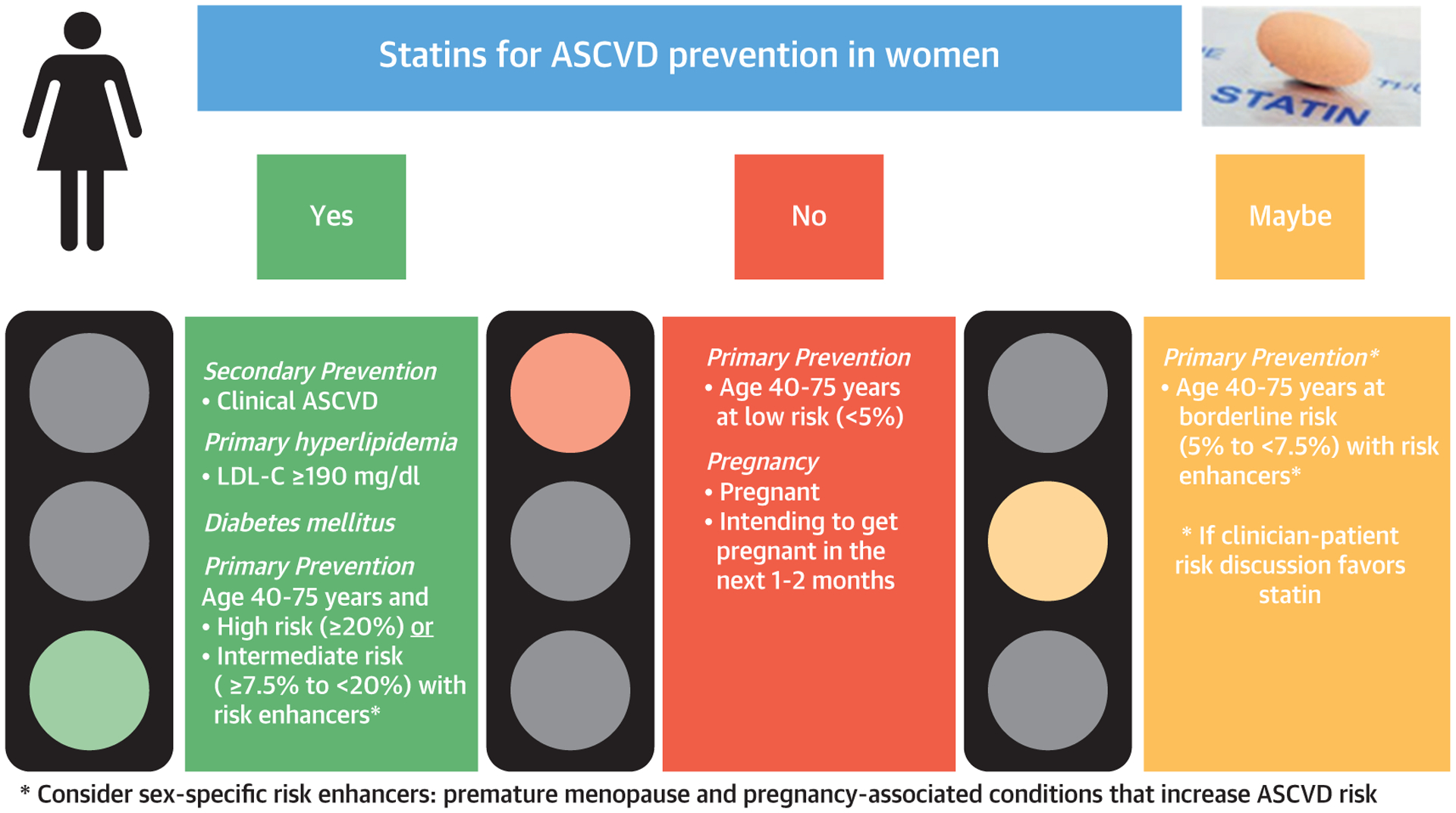
Statin therapy recommendations based on studies and guidelines. ASCVD = atherosclerotic cardiovascular disease; LDL-C = low-density lipoprotein cholesterol.
* Consider sex-specific risk enhancers: premature menopause and pregnancy-associated conditions that increase ASCVD risk
Reasons for sex differences in quality metrics on the patient and physician level need to be further investigated to ensure optimal primary and secondary prevention in women, given that sex differences in statin prescribing patterns and adherence continue to exist.
WOMEN WITH DYSLIPIDEMIA AND PREGNANCY
The guidelines recommend that premenopausal women on statin therapy need to stop the statin 1 to 2 months before attempting pregnancy (4). If the pregnancy is unplanned, the statin should be discontinued as soon as the pregnancy is known (4). Optimal management of cholesterol with healthy lifestyle habits should be discussed first in pregnant women with dyslipidemias (4). Bile acid sequestrants are approved for use during pregnancy.
FUTURE DIRECTIONS, STATINS, AND PREGNANCY.
The safety of pravastatin has been under study for the prevention of pre-eclampsia in high-risk pregnant women (89). Statins are known to have pleiotropic effects, which may diminish inflammation and oxidative stress, increase angiogenesis, inhibit the coagulation cascade, and protect the endothelium (90). Human clinical trials are now currently in progress to determine whether a hydrophilic statin may be used to prevent pre-eclampsia in high risk women.
NONSTATIN THERAPY IN WOMEN
Ezetimibe reduces cholesterol absorption in the small intestine and is a modest but effective lipid-lowering agent for both men and women. In particular, for women who experience statin-induced myalgias, ezetimibe is a nonstatin alternative for patients who are considered intolerant to statin therapy (defined as intolerant to 2 or more statins and failed alternate dosing therapy) or require additional LDL lowering in addition to maximum-tolerated statin. Monotherapy with ezetimibe will provide an 18% LDL reduction and add on therapy provides a 25% reduction (4). The IMPROVE IT (Ezetimibe added to Statin after Acute Coronary Syndrome) trial, which validated the effectiveness of ezetimibe in combination with simvastatin was conducted in a secondary prevention setting among post-acute coronary syndrome patients, average age over 60 and were predominantly men. Therefore, the effectiveness of ezetimibe in women (in particular midlife women) in the primary prevention setting is less understood (91).
Proprotein convertase subtilisin/kexin type 9 (PCSK9) are monoclonal antibodies with 2 U.S. Food and Drug Administration approved injectables currently available on the market. Cardiovascular outcome studies of PCSK9 inhibitors using alirocumab (ODYSSEY Outcomes [Alirocumab and Cardiovascular Outcome after Acute Coronary Syndrome]) and evolocumab (FOURIER [Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease]), demonstrated that PCSK9 inhibition added to maximum-tolerated statin significantly reduced LDL cholesterol levels and the rate of major adverse cardiovascular events (92,93). Both studies had smaller numbers of women who participated in their clinical trials; however, subgroup analysis found no treatment heterogeneity by sex (92,93). The OSLER-1 (Open Label Study of Long-Term Evaluation Against LDL-C Trial) evaluated longer-term effects of evolocumab during open-label hypercholesterolemia treatment for up to 5 years in over 1,000 patients who tolerated evolocumab up to 4 years (94). Women accounted for 53% of the cohort and demonstrated excellent tolerability to evolocumab with an annual 1.4% discontinuation rate. Although there has not been a primary prevention trial of PCSK9 inhibitors, they seem to be well tolerated and effective at lowering LDL in both men and women.
ASPIRIN THERAPY.
Among women with established ASCVD, the role of aspirin is well-established; aspirin reduces subsequent vascular events by approximately 25% (95). Aspirin reduces the risk of athero-thrombosis by irreversibly inhibiting platelet function, but this same mechanism comes at a trade-off of increased risk of bleeding, especially in the gastrointestinal tract. In primary prevention, the role of aspirin has been controversial and net benefit less certain for most healthy women. This is because in primary prevention, the absolute risk of vascular events is lower than in secondary prevention, but the complication rates (bleeding) are comparable.
The 2005 WHS (Women’s Health Study), the largest aspirin primary prevention trial, evaluated low-dose aspirin (100 mg every other day) versus placebo in nearly 40,000 women ≥45 years that were free of ASCVD at baseline. The WHS found that low-dose aspirin reduced the risk of stroke over a 10-year follow-up without reducing the risk of MI; however, among women age ≥65 years, aspirin significantly reduced risk of major cardiovascular events including both ischemic stroke and MI (96). Longer (15-year) follow-up suggested that low-dose aspirin was ineffective or harmful for most healthy women, but there may be benefit for women over age 65 years when considering both colorectal cancer and ASCVD events (97).
However, 3 more recent randomized clinical trials, ASCEND (Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus), ARRIVE (Use of Aspirin to Reduce Risk of Initial Vascular Events in Patients at Moderate risk of Cardiovascular Disease), and ASPREE (Effect of Aspirin of All-Cause Mortality in the Healthy Elderly), published in 2018, found a lack of net benefit, suggesting that prophylactic aspirin should not be used in the routine primary prevention of ASCVD (98–100). The ASCEND trial evaluated low dose aspirin versus placebo in over 15,000 adults who had diabetes but no ASCVD and found that the absolute benefit for reduction in serious vascular events conferred by aspirin were largely counterbalanced by the increased risk of bleeding (98). The ARRIVE trial, evaluating over 12,000 adults at intermediate estimated ASCVD risk, found no benefit of aspirin for reducing vascular events but increased risk of gastrointestinal bleeding (99). Finally, the ASPREE trial of over 19,000 adults age >65 years (including 56% women) found no reduction in cardiovascular events with aspirin, but there was an increased risk of bleeding and risk of death (100,101). Finally, an updated 2019 meta-analysis found that the number needed to treat to cause major bleeding was lower than the number needed to treat to prevent an ASCVD event (210 vs. 265), suggesting more harm than benefit (102).
These findings guided the updated aspirin recommendations in the 2019 ACC/AHA Guideline on the Primary Prevention of CVD (6). The 2019 guidelines state that most healthy people do not need to take aspirin, and there were no sex-specific recommendations. These recommendations differ from prior AHA guidelines, which recommended that aspirin could be considered for patients with 10-year ASCVD risk ≥10%. There may still be select patients age 40 to 70 years who have a high ASCVD risk who may benefit from aspirin if they are at low risk for bleeding. One might consider low-dose aspirin (75 to 100 mg/day) among current smokers, those with a strong family history of premature ASCVD, those with very elevated cholesterol suboptimally treated with statins, those with subclinical atherosclerosis such as a coronary artery calcium (CAC) scores ≥100, and other select patients at high ASCVD risk. However, these decisions are needed in the context of a clinician-patient risk discussion. Clinicians should qualitatively evaluate for bleeding risk and withhold aspirin in primary prevention patients with prior gastrointestinal bleeding, known bleeding disorder, severe liver disease, thrombocytopenia, concurrent anticoagulation or NSAID use, or uncontrolled hypertension.
The more recent trials differ than prior trials, since in the modern era, smoking rates are lower and there is more contemporary preventive therapy, including greater prevalence of statin use and BP control. The percent of patients taking statins in ASPREE, ARRIVE, and ASCEND was 34%, 43%, and 75%, respectively (98–100). Population-specific modeling might help identify those anticipated to derive a net benefit of aspirin for primary prevention, but most primary prevention patients are unlikely to benefit (Figure 4) (103).
FIGURE 4. Recommendation for Aspirin for ASCVD Prevention in Women.
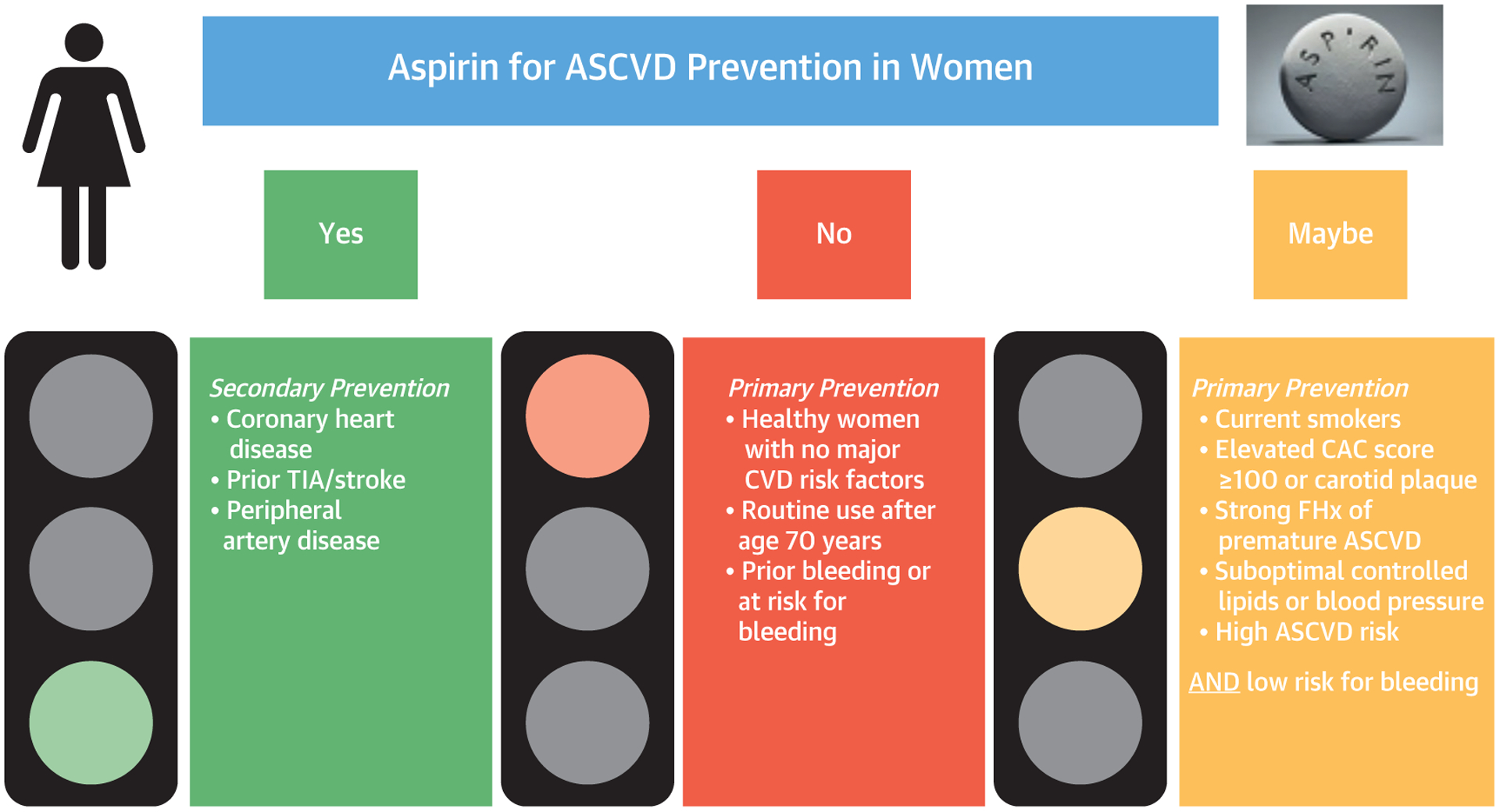
Aspirin therapy recommendation based on studies and guidelines. ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CVD = cardiovascular disease; FHx = family history; TIA = transient ischemic attack.
STROKE PREVENTION FOR AF.
Many studies have shown that women are at greater risk for AF-related stroke than men. The reason for this higher risk is unclear. Even after adjusting for differences in stroke risk factors and stroke prevention treatment with oral anticoagulants, women have about a 20% to 30% higher risk of stroke than men with AF (104,105). As a result of this higher risk, female sex was incorporated into the commonly used algorithm, CHA2DS2-VASc score to predict the risk of stroke in patients with nonvalvular AF (106,107).
In 2018, a consensus statement regarding sex differences in arrhythmias was published by the European Heart Rhythm Association and endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society (108). The statement emphasized the residual stroke risk in women compared with men using vitamin K antagonists and recommended the use of the novel anticoagulants as the first choice (109,110). Compared with men with AF, women with AF had worse stroke severity and more permanent disability after a stroke (111). The statement also highlighted the lower risk of bleeding seen in women compared with men with the use of the novel anticoagulants (108). The statement noted that since a meta-analysis of all 4 novel anticoagulants showed no significant difference with regard to their safety and efficacy in women compared with dose-adjusted warfarin, the novel anticoagulant can be used interchangeably in women depending on personalized needs (112).
The 2019 AHA/ACC/Heart Rhythm Society update on AF guidelines changed the Class I recommendation for anticoagulation, increasing the CHA2DS2-VASc score from ≥2 to ≥3 for women and no change in recommendation for men (CHA2DS2-VASc scores of ≥2) (5). Table 3 is a comparison of the recommendations of the American and European guidelines as well as the updated European recommendations (5,113). Table 4 is key points in atrial fibrillation and women.
TABLE 3.
Comparison and Summary of the Recommendations for Stroke Prevention for Patients With Nonvalvular AF
| Recommendations for Stroke Prevention for Patients With Nonvalvular Atrial Fibrillation | |||
|---|---|---|---|
| CHA2DS2-VASc Score | ACC/AHA/HRS (5) | ESC (113) | EHRA/HRS/AP HRS (108) |
| 0 | No anticoagulant | No antithrombotic | No antithrombotic |
| 1 | OAC or ASA or no antithrombotic (IIb) | OAC for men (IIa) | OAC for men (IIa) |
| 2 | OAC for men (I) | OAC for men (I) | OAC for men (I) |
| ≥3 | OAC for men and women (I) | OAC for men and women (I) | OAC for men and women (I) |
AF = atrial fibrillation; AP HRS = Asia Pacific Heart Rhythm Society; ASA = acetylsalicylic acid; EHRA = European Heart Rhythm Association, HRS = Heart Rhythm Society; OAC = oral anticoagulant; other abbreviations as in Table 2.
TABLE 4.
Key Points in AF and Women
| Stroke Prevention in AF in Women | (Ref. #) | First Author (Year) |
|---|---|---|
| Female sex is a risk modifier and adding female sex to the CHA2DS2-VASc score matters for age >65 yrs or >2 non-sex-related stroke risk factors. | (107) | Nielsen et al. (2018) |
| Women with AF have a greater stroke severity and worse long-term outcome in terms of permanent disability, compared with men with AF. | (108,111) | Linde et al. (2018); Martin et al. (2017) |
| Women with AF have a greater residual stroke risk even with well-controlled VKAs, which was not seen in randomized controlled trials of the novel anticoagulants. | (109,110) | Sullivan et al. (2012); Pancholy et al. (2014) |
| Women taking novel anticoagulants have lower major bleeding rates compared with men. | (108) | Linde et al. (2018) |
| There were no significant differences among the novel anticoagulants in terms of safety and efficacy for women. | (112) | Moseley et al. (2017) |
AF = atrial fibrillation; VKA = vitamin K antagonist.
There are no sex-specific recommendations for left atrial appendage closure devices or surgical occlusion of the left atrial appendage orifice. However, in a pooled patient-level analysis of the PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients With Atrial Fibrillation) and PREVAIL (Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy), in women, LAA closure significantly reduced bleeding compared with patients treated with warfarin (HR: 0.17; 95% CI: 0.074 to 0.369; p < 0.001) (114).
MENOPAUSAL HORMONE THERAPY.
At this time, there is no role for menopausal hormone therapy (MHT) for CVD prevention. This recommendation is consistent with the American College of Obstetrics and Gynecology statement published in 2013 and reaffirmed in 2018 (115). Since the publication of the HERS (Heart and Estrogen/progestin Replacement Study) (116) secondary prevention trial of MHT, and WHI (Women’s Health Initiative) Study (117), a primary prevention trial of MHT for CVD, long-term use of MHT for CVD prevention is not recommended, as both trials failed to demonstrate cardiovascular benefit and suggested potential harm.
However, there has been much discussion regarding the “timing hypothesis” of MHT. In a combined analysis of the 2 WHI trials, estrogen + progesterone and unopposed estrogen alone, women who started MHT closer to menopausal onset appeared to have lower risk developing subclinical atherosclerosis (118,119) and lower risk of developing CVD (118). However, these findings have not been seen consistently in other trials (120,121).
The most recent meta-analysis in 2017 combining similar long-term MHT studies showed that increased risk of MHT outweighs any benefit in regard to prevention of CVD (122). An increased risk of venous thromboembolism with hormone therapy has been shown with all forms of hormone therapy except for transdermal estrogen (122). Thus, it is imperative that even younger patients who are being considered for treatment for post-menopausal vasomotor symptoms with MHT be assessed for personal and familial risk of venous thromboembolism.
DEPRESSION AND PSYCHOLOGICAL ISSUES IN WOMEN
A large body of epidemiological, experimental, and clinical observations have long linked acute and chronic emotional stress and psychological disturbances, such as depression, to physiological perturbations of the cardiovascular system and the risk of CVD (123,124). Psychosocial stress tends to be a more important risk factor for cardiometabolic diseases in women than in men, not only because women in general have higher exposures to psychosocial stress and adversity than men, but also because they may be more vulnerable to the effects of such exposures (125). In particular, depression, early-life adversities, socioeconomic deprivation, and post-traumatic stress disorder (PTSD) are more prevalent in women than in men and tend to show more robust associations with cardiometabolic risk in women than in men, especially in younger populations or with early exposure (125).
Depression affects approximately 7% of the population each year, and is about 2-fold more common in women than in men (126). Depression is a recognized risk factor for incident MI and cardiac death (127). Among women, a clinical diagnosis of depression is associated with a doubling of risk of CVD even over a period of decades (128,129). Although few studies have examined sex-related differences, available data suggest that depression may be an especially strong risk factor for early-onset CVD in women (130,131).
Compared with men, women have a higher exposure to severe childhood adversities, such as physical and sexual abuse and child neglect, which are increasingly recognized as risk factors for CVD (132). Similar to depression, exposure to adversity in early life appears to be a stronger predictor of CVD in women than it is in men (133). These early exposures are also predisposing factors for depression and PTSD, as well as strong correlates of adverse lifestyle behaviors.
Although general symptoms of anxiety, measured with a variety of scales, have been associated with incident CVD in a number of studies, individual study results are heterogeneous and the effect sizes are in general modest (134). In contrast, symptoms of PTSD, a condition previously classified among anxiety disorders, have been consistently related to increased risk of CVD (135). In the United States, PTSD affects 9.7% of women (past year prevalence) versus 3.6% of men (136). In a prospective study of women, those with ≥5 PTSD symptoms had an over 3-fold higher risk of ischemic heart disease compared with those without PTSD symptoms, independent of CVD risk factors and depression (137). In the Nurses’ Health Study II, women who reported ≥4 PTSD symptoms had a 60% higher risk of CVD; those with a history of trauma but no PTSD symptoms also showed an elevated CVD risk (45% higher) (138).
There are multiple possible mechanisms linking depression, PTSD, psychological stress, and trauma to CVD. All of these conditions and exposures are associated with poor health behaviors, such as smoking, poor dietary habits, and physical inactivity. Alterations in neurobiological stress response pathways can also play a role, leading to increased inflammation, chronic autonomic dysregulation, endothelial dysfunction, and hypercoagulability (122). Therefore, recognition and management of psychosocial stressors should be useful in promoting a healthy lifestyle and preventing cardiometabolic risk. Currently there are no national guidelines or recommendations on the assessment of these factors in preventive cardiology care. Although there is currently limited understanding of whether interventions addressing psychosocial and emotional disturbances prevent progression to cardiometabolic diseases, recognition and management of these factors should help the quality of life of patients with these conditions, many of whom are women.
CONCLUSIONS
Women have different manifestations of CVD, and studies have shown sex differences in their response to risk factors and treatments. In addition, unique aspects that pertain to women, such as pregnancy-associated conditions that increase future risk, PCOS, and treatment-related issues specific to women, need to be considered when treating women. Knowledge of updated guideline recommendations are critical in shared decision-making plans to treat women and men to improve CVD outcomes.
HIGHLIGHTS.
CVD remains the leading cause of morbidity and mortality in women.
Women have unique risk factors for CVD—such as PCOS and pregnancy-associated conditions that increase future risk of CVD.
Women also have different manifestations of CVD, and studies have shown sex differences in their response to risk factors and treatments.
Knowledge of unique risk factors in women as well as treatment gap is critical in lowering cardiovascular risk in women.
Acknowledgments
Dr. Cho has received research support from Novartis; has received research support from and served as a consultant to Esperion and Amgen; and has served as a consultant to AstraZeneca. Dr. Minissian has served as a consultant for Amgen, Medtelligence, and the North American Center for Continuing Medical Education; and has received research support from the National Institutes of Health and NIHF. Dr. Pepine has received support from the NIH/NHLBI (WISE HFpEF, HL146158), NIH/NCATS (University of Florida Clinical and Translational Science, UL1TR001427), the Gatorade Trust through the University of Florida Department of Medicine, the McJunkin Family Foundation, and the U.S. Department of Defense (WARRIOR, PR161603). Dr. Vogelman has served as a consultant for the American Heart Association; and has served as a speaker for Aptus Health. All other authors have reported that they have no relationships relevant to the contents of this paper.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- APO
adverse pregnancy outcomes
- ASCVD
atherosclerotic cardiovascular disease
- BP
blood pressure
- CVD
cardiovascular disease
- DM
diabetes mellitus
- HR
hazard ratio
- IUGR
intrauterine growth restriction
- PCOS
polycystic ovarian syndrome
- PTSD
post-traumatic stress disorder
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC author instructions page.
REFERENCES
- 1.Benjamin EJ, Muntner, Alonso A, et al. , American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics -2019 update; a report from the American Heart Association. Circulation 2019;139: e56–528. [DOI] [PubMed] [Google Scholar]
- 2.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71: 2199–269.29146533 [Google Scholar]
- 4.Grundy SM, Stone JN, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–350. [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. [DOI] [PubMed] [Google Scholar]
- 6.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74: 1376–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minissian MB, Kilpatrick S, Eastwood J-A, et al. Association of spontaneous preterm delivery and future maternal cardiovascular disease. Circulation 2018;137:865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas DM, Parker CB, Marsh DJ, et al. Association of adverse pregnancy outcomes with hypertension 2 to 7 years postpartum. J Am Heart Assoc 2019;8:e013092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane-Cordova AD, Khan SS, Grobman WA, et al. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC Review topic of the week. J Am Coll Cardiol 2019;73: 2106–16. [DOI] [PubMed] [Google Scholar]
- 11.ACOG practice bulletin no. 212: pregnancy and heart disease. Obstet Gynecol 2019;133:e320–56. [DOI] [PubMed] [Google Scholar]
- 12.Tooher J, Thornton C, Makris A, et al. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension 2017;70:798–803. [DOI] [PubMed] [Google Scholar]
- 13.Black MH, Zhou H, Sacks DA, et al. Hypertensive disorders first identified in pregnancy increase risk for incident prehypertension and hypertension in the year after delivery. J Hypertens 2016;34: 728–35. [DOI] [PubMed] [Google Scholar]
- 14.Jarvie JL, Metz TD, Davis MB, et al. Short-term risk of cardiovascular readmission following a hypertensive disorder of pregnancy. Heart 2018;104: 1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development. Ann Intern Med 2018;169:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honigberg MC, Zekavat SM, Aragam K, et al. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol 2019;74:2743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol 2018;132:e44–52. [DOI] [PubMed] [Google Scholar]
- 18.Daly B, Toulis KA, Thomas N, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study. PLOS Medicine 2018;15:e1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu P, Gulati M, Kwok CS, et al. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc 2018;7:e007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women: clinical perspective. Circulation 2017;135:578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranthe MF, Andersen EAW, Wohlfahrt J, et al. Pregnancy loss and later risk of atherosclerotic disease. Circulation 2013;127:1775–82. [DOI] [PubMed] [Google Scholar]
- 22.Oliver-Williams CT, Heydon EE, Smith GCS, et al. Miscarriages and future maternal CVD: a systematic review and meta-analysis. Heart 2013; 99:1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ACOG practice bulletin no. 204: fetal growth restriction. Obstet Gynecol 2019;133:e97–109. [DOI] [PubMed] [Google Scholar]
- 24.Manten GT, Sikkema MJ, Voorbij HA, et al. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy 2007;26:39–50. [DOI] [PubMed] [Google Scholar]
- 25.Melchiorre K, Sutherland GR, Liberati M, et al. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension 2012;60:437–43. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet 2020;395: 285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loussert L, Vidal F, Parant O, et al. Aspirin for prevention of preeclampsia and fetal growth restriction. Prenat Diagn 2020;40:519–27. [DOI] [PubMed] [Google Scholar]
- 28.Markovitz AR, Stuart JJ, Horn J, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J 2019;40: 1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart JJ, Tanz LJ, Cook NR, et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol 2018;72: 1252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosano GM, Vitale C, Marazzi G, et al. Menopause and cardiovascular disease: the evidence. Climacteric 2007;10 Suppl 1:19–24. [DOI] [PubMed] [Google Scholar]
- 31.Zhu D, Chung HF, Dobson AJ, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 2019;11: e553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honigberg MC, Zekavat SM, Aragam K, et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA 2019. November 18 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018;110:364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med 2019. September 4 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Daan NM, Louwers YV, Koster MP, et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil Steril 2014;102: 1444–51.e1443. [DOI] [PubMed] [Google Scholar]
- 36.Carmina E, Orio F, Palomba S, et al. Endothelial dysfunction in PCOS: role of obesity and adipose hormones. Am J Med 2006;119:356.e351–6. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Qiao J. Ethnic differences in the phenotypic expression of polycystic ovary syndrome. Steroids 2013;78:755–60. [DOI] [PubMed] [Google Scholar]
- 38.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2018;89:251–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J 2016;37: 1799–806. [DOI] [PubMed] [Google Scholar]
- 41.Feldman CH, Hiraki L, Liu J, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Art and Rheum 2013;65:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMahon M, Hahn BH, Skaggs BJ. Systemic lupus erythematosus and cardiovascular disease: prediction and potential for therapeutic intervention. Expert Rev Clin Immunol 2011;7:227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59: 1690–7. [DOI] [PubMed] [Google Scholar]
- 44.Myasoedova E, Chandran A, Ilhan B, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis 2016;75:560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 2011;70:482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 2018;39: 3499–507. [DOI] [PubMed] [Google Scholar]
- 49.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–505. [DOI] [PubMed] [Google Scholar]
- 50.Wenger NK, Arnold A, Bairey Merz CN, et al. Hypertension across a woman’s life cycle. J Am Coll Cardiol 2018;71:1797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA 2009;302:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacks FM, Svetkey LP, Vollmer WM, et al. , for the DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 53.Brinza EK, Gornik HL. Fibromuscular dysplasia: advances in understanding and management. Cleve Clin J Med 2016;83:S45–51. [DOI] [PubMed] [Google Scholar]
- 54.Vest AR, Cho LS. Hypertension in pregnancy. Cardiol Clin 2012;30:407–23. [DOI] [PubMed] [Google Scholar]
- 55.Ji H, Kim A, Ebinger JE, et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 2020;5:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wenger NK Adverse cardiovascular outcomes for women—biology, bias, or both? JAMA Cardiol 2020. January 15 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 57.Routledge FS, McFetridge-Durdle JA, Dean CR. Stress, menopausal status and nocturnal blood pressure dipping patterns among hypertensive women. Can J Cardiol 2009;25:e157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boggia J, Thijs L, Hansen TW, et al. Ambulatory blood pressure monitoring in 9357 subjects from 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension 2011;57:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright JT Jr., Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foy CG, Lovato LC, Vitolins MZ, et al. Gender, blood pressure, and cardiovascular and renal outcomes in adults with hypertension from the Systolic Blood Pressure Intervention Trial. J Hypertens 2018;36:904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ochoa-Jimenez R, Viquez-Beita K, Daluwatte C, et al. Sex differences of patients with systemic hypertension (from the Analysis of the Systolic Blood Pressure Intervention Trial [SPRINT]). Am J Cardiol 2018;122:985–93. [DOI] [PubMed] [Google Scholar]
- 62.Wenger NK, Ferdinand KC, Bairey Merz CN, et al. Women, hypertension, and the systolic blood pressure intervention trial. Am J Med 2016;129: 1030–6. [DOI] [PubMed] [Google Scholar]
- 63.Turnbull F, Woodward M, Neal B, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J 2008;29:2669–80. [DOI] [PubMed] [Google Scholar]
- 64.Os I, Franco V, Kjeldsen SE, et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension 2008;51:1103–8. [DOI] [PubMed] [Google Scholar]
- 65.Puttnam R, Davis BR, Pressel SL, et al. Association of 3 different antihypertensive medications with hip and pelvic fracture risk in older adults: secondary analysis of a randomized clinical trial. JAMA Intern Med 2017;177:67–76. [DOI] [PubMed] [Google Scholar]
- 66.Huebschmann AG, Huxley RR, Kohrt WM, et al. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia 2019;62:1761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sattar N, Rawshani A, Franzen S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risk: Findings from the Swedish National Diabetes Registry. Circ 2019;139:2228–37. [DOI] [PubMed] [Google Scholar]
- 68.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohort, including 775, 385 individuals and 12,539 strokes. Lancet 2014;383:1973–80. [DOI] [PubMed] [Google Scholar]
- 69.Prospective Studies Collaboration, Asia Pacific Cohort Studies Collaboration. Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980,793 adults from 68 prospective studies. Lancet Diabetes Endocrinol 2018;6:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, O’Neil A, Jiao Y, et al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med 2019;17:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.George KM, Selvin E, Pankow JS, et al. Sex differences in the associations of diabetes with cardiovascular disease outcomes among African-American and white participants in the Atherosclerosis Risk In Communities Study. Am J Epidemiol 2018;187:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sillars A, Ho FK, Pell GP, et al. Sex differences in the association of risk factors for heart failure incidence and mortality. Heart 2020;106:203–12. [DOI] [PubMed] [Google Scholar]
- 73.Anichini R, Cosimi S, Di Carlo A, et al. Gender difference in response predictors after 1-year exenatide therapy twice daily in type 2 diabetic patients: a real world experience. Diabetes Metab Syndr Obes 2013;6:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dennis JM, Henley WE, Weedon MN, et al. MASTERMIND Consortium Sex and BMI alter the benefits and risks of sulfonylureas and thiazolidinediones in type 2 diabetes: a framework for evaluating stratification using routine clinical and individual trial data. Diabetes Care 2018;41: 1844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Today Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zinman B, Inzucchi SE, Wanner C, et al. , for the EMPA-REG OUTCOME Investigators. Empagliflozin in women with type 2 diabetes and cardiovascular disease—an analysis of EMPA-REG OUTCOME. Diabetologia 2018;61:1522–7. [DOI] [PubMed] [Google Scholar]
- 77.Peters SAE, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatments, and control in the United States, 2001–2016. Circulation 2019;139:1025–35. [DOI] [PubMed] [Google Scholar]
- 78.Wright AK, Kontopantelis E, Emsley R, et al. Cardiovascular risk and risk factor management in type 2 diabetes:a population-based cohort study assessing sex disparities. Circulation 2019;139: 2742–53. [DOI] [PubMed] [Google Scholar]
- 79.American Diabetes Association. Glycemic targets: standards of medical care in diabetes–2018. Diabetes Care 2018;41 Suppl 1:S55–64. [DOI] [PubMed] [Google Scholar]
- 80.American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes–2018. Diabetes Care 2018;41: S86–104. [DOI] [PubMed] [Google Scholar]
- 81.Cosentino F, Grant PJ, Aboyans V, et al. , for the ESC Scientific Document Group. 2019 ESC guideline on diabetes, pre-diabetes, and cardiovascular disease developed in collaboration with the EASD: The Task Force for Diabetes, Pre-Diabetes, and Cardiovascular Disease of the ESC and EASD. Eur Heart J 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 82.Nanna MG, Wang TY, Xiang Q, et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes 2019;12: e005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bukkapatnam RN, Gabler NB, Lewis WR. Statins for primary prevention of cardiovascular mortality in women: a systematic review and meta-analysis. Prev Cardiol 2010;13:84–90. [DOI] [PubMed] [Google Scholar]
- 84.Kostis WJ, Cheng JQ, Dobrzynski JM, et al. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012;59:572–82. [DOI] [PubMed] [Google Scholar]
- 85.Fulcher J, O’Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397–405. [DOI] [PubMed] [Google Scholar]
- 86.Stroes E, Thompson P, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel statement on assessment, aetiology and management. Eur Heart J 2015;36: 1012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mancini GB, Baker S, Bergeron J. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol 2016; 32 Suppl 7:S35–65. [DOI] [PubMed] [Google Scholar]
- 88.Mampuya WM, Frid D, Rocco M, Huang J, et al. Treatment strategies in patients with statin intolerance: The Cleveland Clinic Experience. Am Heart J 2013;166:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Costantine MM, Cleary K, Hebert MF, et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016;214. 720.e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liao JK, Lauf U. Pleotrophic effects of statins. Annu Rev Pharmcol Toxicol 2005;45:89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cannon CP, Blazing MA, Giugliano RP, et al. , for the IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz GG, Steg PG, Szarek M, et al. , for the ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–107. [DOI] [PubMed] [Google Scholar]
- 93.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376: 1713–22. [DOI] [PubMed] [Google Scholar]
- 94.Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term low-density lipoprotein cholesterol–lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia. JAMA Cardiol 2017;2:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 96.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–304. [DOI] [PubMed] [Google Scholar]
- 97.Van Kruijsdijk RC, Visseren FL, Ridker PM, et al. Individualised prediction of alternate-day aspirin treatment effects on the combined risk of cancer, cardiovascular disease and gastrointestinal bleeding in healthy women. Heart 2015;101: 369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Group ASC, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379: 1529–39. [DOI] [PubMed] [Google Scholar]
- 99.Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018;392: 1036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379: 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med 2018;379:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA 2019;321:277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Selak V, Jackson R, Poppe K, et al. Predicting bleeding risk to guide aspirin use for the primary prevention of cardiovascular disease: a cohort study. Ann Int Med 2019;170:357–68. [DOI] [PubMed] [Google Scholar]
- 104.Marzona I, Proietti M, Farcomeni A, et al. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: a systematic review and meta-analysis of 993,600 patients. Int J Cardiol 2018;269:182–91. [DOI] [PubMed] [Google Scholar]
- 105.Bassand JP, Accetta G, Al Mahmeed W, et al. Risk factors for death, stroke, and bleeding in 28, 628 patients from the GARFIELD-AF registry: rationale for comprehensive management of atrial fibrillation. PLoS One 2018;13:e0191592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137: 263–72. [DOI] [PubMed] [Google Scholar]
- 107.Nielsen PB, Skjoth F, Overvad TF, et al. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: Should we use a CHAD2DS2-VA score rather than CHAD2DS2-VASc? Circulation 2018;137:832–40. [DOI] [PubMed] [Google Scholar]
- 108.Linde C, Bongiorni MG, Birgersdotter-Green U, et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018;20. 1565–1565ao. [DOI] [PubMed] [Google Scholar]
- 109.Sullivan RM, Zhang J, Zamba G, et al. Relation of gender-specific risk of ischemic stroke in patients with atrial fibrillation to differences in warfarin anticoagulation control (from AFFIRM). Am J Cardiol 2012;110:1799–802. [DOI] [PubMed] [Google Scholar]
- 110.Pancholy SB, Sharma PS, Pancholy DS, et al. Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol 2014;113:485–90. [DOI] [PubMed] [Google Scholar]
- 111.Martin RC, Burgin WS, Schabath MB, et al. Gender-specific differences for risk of disability and death in atrial fibrillation-related stroke. Am J Cardiol 2017;119:256–61. [DOI] [PubMed] [Google Scholar]
- 112.Moseley A, Doukky R, Williams KA, et al. Indirect comparison of novel oral anticoagulants in women with nonvalvular atrial fibrillation. J Women’s Health 2017;26:214–21. [DOI] [PubMed] [Google Scholar]
- 113.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Euro Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 114.Price MJ, Reddy VY, Valderrabano M, et al. Bleeding outcomes after left atrial appendage closure compared with long-term warfarin: a pooled, patient-level analysis of the WATCHMAN randomized trial experience. J Am Coll Cardiol Intv 2015;8:1925–32. [DOI] [PubMed] [Google Scholar]
- 115.Committee on Gynecologic Practice. ACOG Committee Opinion No. 565: hormone therapy and heart disease. Obstet Gynecol 2013;121:1407–10. [DOI] [PubMed] [Google Scholar]
- 116.Hulley S, Grady D, Bush T, et al. , for the Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280:605–13. [DOI] [PubMed] [Google Scholar]
- 117.Rossouw JE, Anderson GL, Prentice RL, et al. , for the Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–33. [DOI] [PubMed] [Google Scholar]
- 118.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297:1465–77. [DOI] [PubMed] [Google Scholar]
- 119.Manson JE, Allison MA, Rossouw JE, et al. , for the WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med 2007;356:2591–602. [DOI] [PubMed] [Google Scholar]
- 120.Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med 2014;161:249–60. [DOI] [PubMed] [Google Scholar]
- 121.Hodis HN, Mack WJ, Henderson VW, et al. , for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 2016;374:1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marjoribanks J, Farquhar C, Roberts H, et al. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev 2017;1:CD004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vaccarino V, Bremner JD. Psychiatric and behavioral aspects of cardiovascular disease. In: Zipes DP, Libby P, Bonow RO, Mann DL, Tomaselli GF, editors. Braunwald’s Heart Disease–A Textbook of Cardiovascular Medicine. 11th edition. Philadelphia, PA: Elsevier-Saunders, 2019: 1879–89. [Google Scholar]
- 124.Kivimaki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 2018;15:215–29. [DOI] [PubMed] [Google Scholar]
- 125.Vaccarino V, Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev 2017;74:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). 2015. Available at: http://www.samhsa.gov/data/. Accessed December 1, 2019.
- 127.Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol 2017;14: 145–55. [DOI] [PubMed] [Google Scholar]
- 128.O’Neil A, Fisher AJ, Kibbey KJ, et al. Depression is a risk factor for incident coronary heart disease in women: an 18-year longitudinal study. J Affect Disord 2016;196:117–24. [DOI] [PubMed] [Google Scholar]
- 129.Whang W, Kubzansky LD, Kawachi I, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol 2009;53: 950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wyman L, Crum RM, Celentano D. Depressed mood and cause-specific mortality: a 40-year general community assessment. Ann Epidemiol 2012;22:638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suglia SF, Demmer RT, Wahi R, Keyes KM, Koenen KC. Depressive symptoms during adolescence and young adulthood and the development of type 2 diabetes mellitus. Am J Epidemiol 2016;183:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suglia SF, Koenen KC, Boynton-Jarrett R, et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation 2018;137:e15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Korkeila J, Vahtera J, Korkeila K, et al. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart 2010;96:298–303. [DOI] [PubMed] [Google Scholar]
- 134.Roest AM, Martens EJ, de Jonge P, et al. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol 2010; 56:38–46. [DOI] [PubMed] [Google Scholar]
- 135.Edmondson D, von Kanel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry 2017;4:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kessler RC, Berglund PA, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Arch Gen Psychiatry 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
- 137.Kubzansky LD, Koenen KC, Jones C, et al. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol 2009;28:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sumner JA, Kubzansky LD, Elkind MS, et al. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation 2015;132:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


