Abstract
Background
Memory impairment occurs in human immunodeficiency virus (HIV)-associated neurocognitive disorders (HAND) and amnestic mild cognitive impairment (aMCI), the precursor to Alzheimer disease (AD). Methods are needed to distinguish aMCI-associated from HAND-associated impairment in people with HIV (PWH). We developed a neuropsychological method of identifying aMCI in PWH and tested this by relating AD neuropathology (β-amyloid, phospho-Tau) to aMCI versus HAND classification.
Methods
Seventy-four HIV-positive cases (aged 50−68 years) from the National NeuroAIDS Tissue Consortium had neurocognitive data within 1 year of death and data on β-amyloid and phospho-Tau pathology in frontal brain tissue. High aMCI risk was defined as impairment (<1.0 SD below normative mean) on 2 of 4 delayed recall or recognition outcomes from a verbal and nonverbal memory test (at least 1 recognition impairment required). Differences in β-amyloid and phospho-Tau by aMCI and HAND classification were examined.
Results
High aMCI risk was more common in HAND (69.0%) versus no HAND (37.5%) group. β-amyloid pathology was 4.75 times more likely in high versus low aMCI risk group. Phospho-Tau pathology did not differ between aMCI groups. Neither neuropathological feature differed by HAND status.
Conclusions
Amnestic mild cognitive impairment criteria that include recognition impairment may help to detect AD-like cognitive/biomarker profiles among PWH.
Keywords: mild cognitive impairment, HAND, memory, β-amyloid, phospho-Tau
We developed a neuropsychological method of identifying HIV-positive individuals who have the Alzheimer precursor condition, amnestic mild cognitive impairment (aMCI), and provide support for this method by demonstrating an association between aMCI classification and β-amyloid neuropathology in postmortem HIV-positive cases.
Half of people with human immunodeficiency virus (PWH) in the United States are older than 50 years [1]. Even with effective antiretroviral therapy (ART), 45% of PWH experience neurocognitive complications, labeled HIV-associated neurocognitive disorders (HAND) [2]. Older PWH are also at risk for neurodegenerative disorders including Alzheimer disease (AD) and its precursor, amnestic mild cognitive impairment (aMCI). In fact, some evidence suggests a higher risk for aMCI/AD in PWH compared to the general population [3] possibly due to synergistic effects of HIV and aging on the brain [4, 5] and evidence of premature or accelerated aging phenotypes among PWH [6]. However, identifying aMCI among PWH is challenging given that episodic memory deficits are the defining feature of aMCI/AD and commonly characterize HAND. Methods of disentangling HAND from aMCI/AD are needed to provide early intervention options for the more progressive profile of aMCI/AD [7]. The annual rate of progression to AD dementia is 10%–15% among persons with aMCI in contrast to 1%–2% among cognitively normal older adults [8].
Differences in neurocognitive and neuropathological characteristics of HAND and aMCI/AD provide opportunities to differentiate the conditions. Whereas aMCI/AD is characterized by hippocampus-based encoding, storage, and retention deficits observed on recall and recognition tests [8], HAND is more dependent on frontal and subcortical structures with relatively normal memory retention but impaired memory retrieval (recall but not recognition deficits) [9–11]. The neuropathological hallmarks of AD, neuritic β-amyloid (Aβ)-based plaques and phospho-Tau (p-Tau)-based neurofibrillary tangles, are not commonly found in the neocortex of PWH [12–16] although diffuse Aβ plaques [12–14, 17, 18] and p-Tau neuropil threads [19] have been observed [13].
We aimed to develop a neuropsychological method of identifying PWH who are more likely to be on the AD trajectory and to validate this method by examining its relation to AD pathological markers. We exploited the differences between aMCI/AD and HAND neurocognitive profiles by adapting the actuarial Jak/Bondi criteria for aMCI [20] to include recognition impairment (more common in aMCI/AD) to identify aMCI risk among PWH. To test this approach, we leveraged a subsample of HIV-positive postmortem cases from the National NeuroAIDS Tissue Consortium (NNTC) that had an antemortem neuropsychological assessment within 1 year of death and had been previously evaluated for Aβ 42 and p-Tau pathology in frontal brain tissue. The frontal lobe is an early site of Aβ pathology in AD and a late site of p-Tau pathology after progressing from the medial temporal lobe [21]. We compared the prevalence of AD-associated neuropathological markers by antemortem aMCI risk and HAND status. We hypothesized that our results would provide validation for our neuropsychological method of detecting aMCI among PHW in that prevalence rates of AD-associated neuropathological markers would be higher in the high versus low aMCI risk group but would not differ by HAND status.
METHODS
Study Cohort
This study examined postmortem HIV-positive cases from the NNTC [22] (www.nntc.org) from 4 sites (University of Texas Medical Branch at Galveston, University of California San Diego, University of California Los Angeles, and Mount Sinai Medical Center in New York). Our inclusion criteria were: (1) having antemortem neuropsychological data within 1 year of death; (2) at least 50 years old at death; and (3) the presence (or absence) of frontal lobe Aβ and p-Tau pathology was measured in a prior study [23]. Eighty-three cases met inclusion criteria and, among these, we excluded 9 cases without relevant covariate data (eg, apolipoprotein E [APOE]-ɛ4 allele status, antemortem plasma HIV-1 RNA load). Our final sample comprised 74 cases with year of death ranging from 1999 to 2013. This study was approved by the Institutional Review Board at each NNTC site and written consent for participation in NNTC was obtained from all participants prior to death.
Neurocognitive Evaluation
A neuropsychological battery assessed 7 cognitive domains commonly affected by HIV: verbal fluency, working memory, processing speed, episodic memory for verbal and visual stimuli, executive function, and complex motor function. The specific cognitive tests are described elsewhere [24]. Raw test scores were transformed into age-, sex-, education-, and race-adjusted T-scores based on a normative sample of HIV-seronegative participants [25, 26]. Our criteria for HAND and aMCI classification were retroactively applied to participants’ neuropsychological data from the visit most proximal to death.
HAND Classification
HAND classification was based on one well-established approach [27] that required impairment in at least 2 cognitive domains, defined by performance of at least 1 standard deviation (SD) below the demographically adjusted normative mean on neuropsychological tests. HAND status was further categorized as asymptomatic neurocognitive impairment (no interference in everyday function), mild neurocognitive disorder (at least mild interference in everyday function), and HIV-associated dementia (marked interference in everyday function). A designation of “neuropsychological impairment due to other or undetermined causes” was provided when neurocognitive impairment was likely not HIV-related and was considered non-HAND. Due to low numbers within HAND subcategories, our comparisons were by overall HAND status although HAND subcategories were provided for descriptive purposes.
Adapted Jak/Bondi Classification for aMCI
The Jak/Bondi criteria are an established, neuropsychological method of classifying MCI in the general population [20]. As described in Bondi et al (2014), the Jak/Bondi criteria for MCI are an actuarial approach that requires 2 impaired neuropsychological tests (ie, >1 SD below demographically corrected normative mean) within a given domain [20, 28]. Classification of the aMCI subtype requires impairment on 2 memory-related outcomes including tests of free recall and/or recognition. Diagnosis of MCI using Jak/Bondi criteria produces greater diagnostic stability and stronger relationships between MCI status and biomarkers and rates of progression to AD than conventional criteria that typically include crude global cognitive screens and subjective cognitive complaints [20, 28]. To capitalize on the retention deficit that is unique to aMCI/AD rather than the retrieval deficit that is common to both aMCI/AD and HAND [8–11], we adapted the Jak/Bondi criteria for aMCI to require at least 1 of the 2 impaired memory outcomes be a recognition test. The Jak/Bondi criteria for general MCI was previously applied to an older cohort of PWH; however, it was not adapted to distinguish memory impairment that is more characteristic of aMCI versus HAND [3]. Application of our adapted Jak/Bondi criteria for aMCI requires 2 or more assessments of episodic memory with at least 1 being recognition. The episodic memory outcomes used in these criteria were the Hopkins Verbal Learning Test-Revised [29] and the Brief Visuospatial Memory Test-Revised [30] delayed free recall and recognition subtests. Participants were classified as high aMCI risk if they demonstrated impaired performance on at least 2 of the 4 measures with the conditional requirement that at least 1 of the impaired scores was a recognition measure. Impairment on memory outcomes was defined as >1 SD below the demographically corrected normative mean (T-score < 40). Participants that did not fulfill criteria for high aMCI risk were categorized as low aMCI risk.
More research is needed to identify differences in MCI- versus HAND-related profiles of deficits in other cognitive domains. We can leverage methods developed here to examine broader classifications of single-domain versus multidomain or nonamnestic MCI.
Neuromedical Evaluation
Participants completed a standardized neuromedical evaluation at the same visit as the neurocognitive evaluation. HIV disease characteristics were determined either by self-report or laboratory testing. Estimated duration of HIV disease was self-reported. Nadir CD4+ T-cell count was the lowest lifetime value among self-report and study-obtained CD4+ T-cell counts and released medical records. Antemortem CD4+ T-cell count was measured with flow cytometry. Antemortem plasma HIV-1 RNA level was measured by ultrasensitive polymerase chain reaction (Amplicor, Roche Diagnostic System; lower limit of detection <50 copies/mL) in a Clinical Laboratory Improvement Amendments-certified clinical laboratory. History of substance use disorders was assessed via the Composite International Diagnostic Interview [31], a computer-based, structured interview and diagnoses based on the DSM-IV. Genotyping of the APOE ɛ4 allele, the strongest genetic risk factor for sporadic AD, was described elsewhere [32]. History of antemortem medical comorbidities (eg, hypertension, cerebrovascular disease, non-AIDS–defining cancer, renal disease) were available for 53 participants and were determined by self-report or self-reported medication records.
Neuropathological Characterization
Brain tissue was sampled from the frontal lobe of cases as soon as possible after death (range, 0–120 hours postmortem delay) with 78% of cases autopsied within 24 hours. Five-μm-thick formalin-fixed paraffin-embedded tissue sections with no significant histopathologic changes were immunohistochemically stained with primary antibodies to Aβ (mouse monoclonal, clone 4G8, No. SIG-39220, Covance; 1:20 000 dilution) and p-Tau (mouse monoclonal, clone AT8, No. MN1020, Pierce Biotechnology; 1:1000). For antigen retrieval, the sections were incubated with 88% formic acid (5 minutes for Aβ staining) or 10 mM sodium citrate/0.05% Tween 20 buffer (pH 6) in 121°C autoclave (20 minutes for p-Tau staining). Immunohistochemical signals were developed using ImmPRESS anti-mouse IgG (peroxidase) polymer detection kit (Vector Laboratories) and diaminobenzidine (ImmPACT DAB peroxidase substrate; Vector Laboratories). The sections were counterstained with hematoxylin. Isocortex sections from AD cases were used as positive tissue controls. For negative reagent controls, the primary antibodies were omitted, as previously described [32].
On light microscopic examination, Aβ plaque pathology was considered present when extracellular Aβ-immunoreactive plaques were observed in the frontal cortex, regardless of their type (eg, diffuse plaques, cored plaques) or density (ie, focal, widespread). In cases showing Aβ plaque pathology, the majority of plaques were of diffuse type. Neuronal p-Tau pathology was considered present when there were p-Tau–immunoreactive neuropil threads, pretangle neuronal soma, neurofibrillary tangles, or their combinations in the frontal cortex, as previously described [23]. In cases showing p-Tau pathology, the density of p-Tau lesions was sparse (ie, barely present at ×100 magnification) except in 1 case in which the p-Tau lesions were moderate in density (ie, easily noted at ×100 magnification) [23].
Statistical Analyses
We examined differences in sample characteristics by HAND and aMCI risk status using χ 2 tests for categorical variables, t tests for normally distributed continuous variables, and Kruskal-Wallis H-tests for nonnormally distributed continuous variables as determined by the Shapiro-Wilk test. We used Fisher exact test to examine the frequency of high aMCI risk classification by HAND status.
Separate logistic regression models examined the odds of presence of Aβ plaque and p-Tau pathology by (1) aMCI risk group and (2) HAND groups. If a significant difference was detected between either grouping, this difference was further probed by examining differences among the 4-way categorization of aMCI risk status (+/−) by HAND status (+/−) with the aMCI−/HAND− group as the reference. Demographic characteristics (age, education, race/ethnicity, sex), HIV disease characteristics (nadir CD4+ T-cell count, duration of HIV infection, antemortem CD4+ T-cell count, log10 plasma HIV-1 RNA load, and ART status), APOE ɛ4 carrier status, and time between neurocognitive assessment and death were included as covariates in the models if they related to aMCI or HAND classification or presence of Aβ and p-Tau pathology at P ≤ .10 in univariate analyses. Covariates that were not significant in the multivariable model at P ≤ .10 were removed from the final model.
RESULTS
The sample was 81% male, 61% White with a mean age at death of 57 years (SD = 4.8). The time between neuropsychological assessment and death ranged from 1 to 12 months; however, this time span was less than 6 months for 70% of cases (mean = 4.3, SD = 3.8, median = 4.4). Forty-one cases (55%) were classified as high aMCI risk. Forty-two cases were classified as HAND (56.8%). As expected, high aMCI risk classification was more prevalent in the HAND (n = 29, 71.0%) versus non-HAND (n = 12, 37.5%) group (χ 2 = 7.32, P = .007; Table 1). Among HAND subcategories, all 15 HIV-associated dementia cases were in the high aMCI risk group (χ 2 = 15.14, P < .001), indicating greater cognitive impairment overall in the high versus low aMCI risk group. Although not significant, there was a higher proportion of cases deemed “neuropsychological impairment due to other or undetermined causes” in the high versus low aMCI risk group (χ 2 = 2.23, P = .13) suggesting that these “other/undetermined” causes of cognitive impairment may be AD related.
Table 1.
Proportion of aMCI Risk Groups as a Function of HAND Status
| HAND Status | High aMCI Risk | Low aMCI Risk |
|---|---|---|
| HAND | 29 (4 ANI, 10 MND, 15 HAD) | 13 (7 ANI, 6 MND, 0 HAD) |
| No HAND | 12 (3 normal, 9 NPI-O) | 20 (17 normal, 3 NPI-O) |
Abbreviations: aMCI, amnestic mild cognitive impairment; ANI, asymptomatic neurocognitive impairment; HAD, HIV-associated dementia; HAND, HIV-associated neurocognitive disorders; MND, mild neurocognitive disorder; NPI-O, neuropsychological impairment due to non-HIV–related or undetermined causes.
Table 2 displays sample characteristics by aMCI risk and HAND status. The proportion of men (χ 2 = 5.03, P = .03) and cases with detectable plasma HIV-1 RNA load prior to death (χ 2 = 8.38, P = .02) was higher in the high versus low aMCI risk group. The proportion of APOE ɛ4 carriers (χ 2 = 6.86, P = .009) and cases with detectable plasma viral load prior to death (χ 2 = 12.40, P = .002) was higher in the HAND versus non-HAND group. All other sample characteristics did not significantly differ between groups.
Table 2.
Sample Characteristics by aMCI Risk and HAND Classification
| Characteristics | High aMCI Risk (n = 41) | Low aMCI Risk (n = 33) | High vs Low aMCI Risk P Value | HAND (n = 42) | No HAND (n = 32) | HAND vs no HAND P Value |
|---|---|---|---|---|---|---|
| Demographic/clinical factors | ||||||
| Age, y, mean (SD) | 56.9 (4.2) | 56.5 (5.6) | .74 | 57.2 (4.3) | 56.2 (5.5) | .40 |
| Education years, mean (SD) | 12.6 (3.6) | 12.5 (2.5) | .92 | 12.4 (3.2) | 12.7 (3.1) | .75 |
| Sex, No. (% male) | 37 (90.2) | 23 (69.7) | .08 | 34 (80.9) | 26 (81.2) | .97 |
| Race | .50 | .32 | ||||
| White | 26 (63.4) | 19 (57.6) | 26 (61.9) | 19 (59.4) | ||
| Black | 11 (26.8) | 12 (36.4) | 12 (28.6) | 11 (34.4) | ||
| Other | 4 (9.8) | 2 (6.0) | 4 (9.5) | 2 (6.2) | ||
| Ethnicity | ||||||
| Hispanic | 4 (9.7) | 4 (12.1) | .75 | 6 (14.3) | 2 (6.3) | .27 |
| APOE ε4 carrier | 10 (24.4) | 5 (15.2) | .33 | 13 (30.9) | 2 (6.3) | .009 |
| Disease characteristics | ||||||
| Nadir CD4+ T-cell count, cells/μL, mean (SD) | 103.1 (133.0) | 63.4 (63.2) | .13 | 85.8 (122.3) | 85.0 (88.7) | .98 |
| Antemortem CD4+ T-cell count, cells/μL, mean (SD)a | 218.1 (238.4) | 182.3 (184.6) | .49 | 210.4 (233.7) | 190.7 (190.1) | .70 |
| Antemortem detectable plasma viral loadb | 30 (73.2) | 14 (42.4) | .02 | 31 (73.8) | 13 (40.6) | .002 |
| Duration of HIV disease, y, mean (SD) | 12.5 (7.7) | 15.2 (6.7) | .13 | 13.9 (6.6) | 13.5 (8.3) | .83 |
| Antemortem ART status, No. (% prescribed) | 30 (73.2) | 30 (90.9) | .053 | 33 (78.6) | 27 (84.4) | .53 |
| Antemortem clinical comorbiditiesc | ||||||
| History of a substance use disorder | 28 (75.7) | 28 (87.5) | .21 | 33 (82.5) | 23 (79.3) | .74 |
| Hypertension | 14 (46.7) | 7 (28.0) | .16 | 14 (40.0) | 7 (35.0) | .71 |
| Diabetes | 5 (17.2) | 3 (12.0) | .59 | 5 (14.7) | 3 (15.0) | .98 |
| Hyperlipidemia | 9 (32.1) | 3 (13.0) | .11 | 9 (28.1) | 3 (15.8) | .31 |
| Non-AIDS–defining cancer | 11 (40.7) | 8 (38.1) | .85 | 14 (46.7) | 5 (27.8) | .19 |
| Cerebrovascular disease | 5 (17.8) | 0 (0) | .06 | 3 (9.4) | 2 (14.3) | .62 |
| Chronic renal disease | 5 (17.8) | 3 (13.6) | .69 | 7 (21.2) | 1 (5.9) | .16 |
Data are No. (%) except where indicated. Differences in sample characteristics by HAND and aMCI risk status were tested using χ 2 tests for categorical variables, t tests for normally distributed continuous variables, and Kruskal-Wallis H-tests for nonnormally distributed continuous variables.
Abbreviations: aMCI, amnestic mild cognitive impairment; APOE ε4, apolipoprotein E ɛ4 allele; ART, antiretroviral therapy; HAND, HIV-associated neurocognitive disorders.
aData on antemortem CD4 T-cell count was missing in 2 of the 74 cases.
bData on antemortem viral load was missing in 6 of the 74 cases.
cData on antemortem comorbid conditions was available on 53 of the 74 cases.
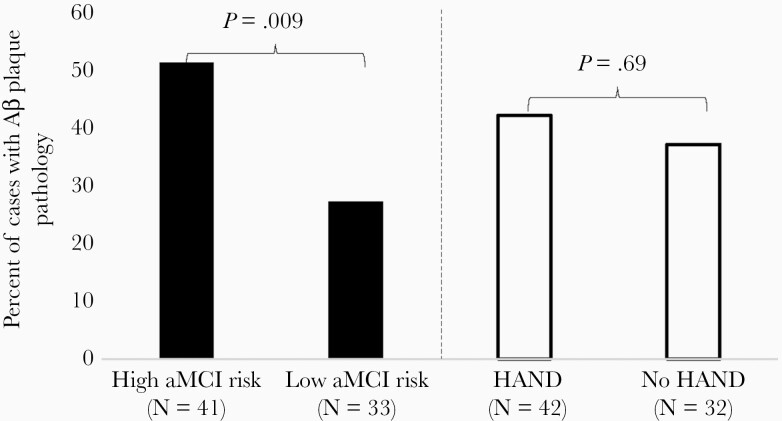
In the initial logistic regression modeling Aβ plaque pathology, covariates that met criteria for inclusion in the model included age at death, years of education, APOE ɛ4 status, nadir CD4+ T-cell count and antemortem ART status, log10 plasma HIV-1 RNA load, and CD4+ T-cell count. Due to their significance in the multivariable model, whereby greater years of education, use of ART, and APOE ɛ4 carrier status related to higher likelihood of Aβ plaques (P ≤ .10), these covariates were retained in the final model for Aβ plaque pathology. Table 3 displays statistical results. The likelihood of Aβ plaque pathology did not differ between HAND (43%) and non-HAND (38%) groups; however, Aβ plaque pathology was almost 5 times more likely in the high (51%) versus low (27%) aMCI risk group (Figure 1). When examining the 4-way categorization of aMCI+/− by HAND+/− status, Aβ plaque pathology was most prevalent in the aMCI+/HAND− group (58%) compared to the aMCI+/HAND+ (48%), aMCI−/HAND+ (30%), and aMCI−/HAND− (25%) groups with the difference being significant in the aMCI−/HAND− versus aMCI+/HAND+ comparison (odds ratio [OR], 5.31; 95% confidence interval [CI], 1.26–22.49; P = .02), and a trend in the aMCI−/HAND− versus aMCI+/HAND− comparison (OR, 4.35; 95% CI, .74–25.47; P = .10) likely due to the greater statistical power in comparisons of the larger aMCI+/HAND+ group.
Table 3.
Results of Logistic Regression Models Modelling the Likelihood of Aβ Plaque and p-Tau Pathology by aMCI Risk and HAND Groups and Relevant Covariates
| Outcomes | Odds Ratio (95% CI) | Standard Error | P Value |
|---|---|---|---|
| Presence of Aβ plaque pathology | |||
| Model 1 | |||
| Years of education (1-y increase) | 1.30 (1.06–1.58) | 0.10 | .01 |
| Antemortem ART use (vs no use) | 7.00 (1.24–37.9) | 0.87 | .03 |
| APOE ε4+ (vs APOE ε4−) | 2.37 (.59–9.45) | 0.71 | .22 |
| aMCI+ (vs aMCI−) | 4.75 (1.47–15.37) | 0.60 | .009 |
| Model 2 | |||
| Years of education (1-y increase) | 1.28 (1.05–1.55) | 0.10 | .01 |
| Antemortem ART use (vs no use) | 6.88 (1.25–37.8) | 0.87 | .03 |
| APOE ε4+ (vs APOE ε4−) | 2.47 (.62–9.85) | 0.71 | .19 |
| HAND+ (vs HAND−) | 1.26 (.41–3.89) | 0.57 | .69 |
| Presence of p-Tau pathology | |||
| Model 1 | |||
| Antemortem plasma viral load (1-unit increase) | 0.50 (.32–.77) | 0.02 | .002 |
| aMCI+ (vs aMCI−) | 0.95 (.32–2.79) | 0.55 | .93 |
| Model 2 | |||
| Antemortem plasma viral load (1-unit increase) | 0.50 (.32–.77) | 0.22 | .002 |
| HAND+ (vs HAND−) | 0.61 (.21–1.80) | 0.55 | .37 |
Logistic regression models estimated odds ratios and 95% CIs. Separate models were run adjusting for the significant covariates of years of education and antemortem ART use to examine aMCI risk status versus HAND as a predictor of presence (versus nonpresence) of Aβ pathology. Separate models were run adjusting for the significant covariates of antemortem plasma viral load to examine aMCI risk status versus HAND as a predictor of presence (versus nonpresence) of p-Tau pathology.
Abbreviations: Aβ, β-amyloid; aMCI, amnestic mild cognitive impairment; APOE ε4, apolipoprotein E ɛ4 allele; ART, antiretroviral therapy; CI, confidence interval; HAND, HIV-associated neurocognitive disorders; p-Tau, phospho-Tau.
Figure 1.
A comparison of the difference in the proportion of those with versus without Aβ plaque pathology between aMCI risk classification versus HAND classification. Abbreviations: Aβ, β-amyloid; aMCI, amnestic mild cognitive impairment; HAND, HIV-associated neurocognitive disorders.
In the initial logistic regression modeling p-Tau pathology, covariates that met criteria for inclusion in the model were age at death and antemortem log10 plasma HIV-1 RNA load, and CD4+ T-cell count. Higher plasma log10 HIV-1 RNA load remained significantly associated with a lower likelihood of p-Tau pathology (P = .002) in the multivariable model and so was retained in the final model for p-Tau pathology. The likelihood of p-Tau pathology did not differ between high (46%) versus low (54%) aMCI risk groups or between HAND (43%) versus non-HAND (59%) groups.
DISCUSSION
This study is an initial attempt to develop neuropsychological methods of distinguishing aMCI from HAND among PWH by leveraging differences in their cognitive profiles. We focused on neuropsychological methods because they represent the phenotypic manifestations of disease and are less costly and invasive than biomarker assessments. Specifically, we adapted neuropsychological criteria for aMCI classification to include recognition memory impairment that is more characteristic of aMCI/AD than HAND [9–11]. We tested this approach by comparing the AD neuropathological markers (Aβ and p-Tau) between aMCI risk and HAND groups. Using our adapted aMCI criteria, 55% of our sample was classified as high aMCI risk. This is higher than aMCI prevalence estimates in the general population, which range from 0.5% to 32% [33] depending on the diagnostic criteria. However, the current sample represents a high-risk group as it is an end-of-life, multimorbidity, HIV-seropositive sample. Additionally, some overlap or misclassification is possible until further research can advance our diagnostic methods.
When comparing the prevalence of AD neuropathology, frontal Aβ plaque pathology was significantly more likely in the high versus low aMCI risk group but did not differ between HAND groups, suggesting that the adapted aMCI criteria is detecting underlying AD neuropathology that the HAND classification is not. Conversely, the likelihood of frontal p-Tau pathology did not differ between aMCI risk or HAND groups, and we offer a few reasons why this may be. First, given regional and temporal differences in Aβ and p-Tau pathogenesis, with Aβ pathology typically initiating in the neocortex in AD while p-Tau pathology typically initiates in the medial temporal lobe [21], our frontal lobe measures of AD pathology may have allowed us to detect early stages of Aβ, but not p-Tau, pathology. A study is underway examining AD neuropathology in hippocampal tissue to better inform the ability of the adapted aMCI criteria to detect early-stage p-Tau pathology. Second, when examining covariate associations with neuropathological outcomes, we curiously found that HIV-1 RNA load was inversely related to the mostly sparse p-Tau, but not Aβ, pathology. We can only speculate as to why this may be, including an amplifying effect of ART, which typically lowers viral load, on p-Tau and/or a protective effect of an HIV-related viral mechanism on p-Tau, although it is unclear what that mechanism may be, and we are unaware of any studies examining these speculated links. A previous study reported lower levels of cerebrospinal fluid p-Tau in cognitively impaired (n = 49) or cognitively normal (n = 21) PWH (most on ART) compared to age-matched HIV-seronegative controls (n = 50) [34], which also hints at an inverse relationship between HIV-related disease factors and p-Tau pathology.
Another curious finding among covariates was the significant relationship between higher years of education and higher likelihood of Aβ plaque pathology. Given the end-of-life, multimorbidity nature of this sample, higher education may reflect improved morbidity/mortality by way of access to healthcare and health literacy. Indeed, higher years of education was significantly associated with older age at death (R = 0.26, P = .02). Given that Aβ plaque accumulation is strongly associated with older age [35], the association between education and Aβ plaques may be driven by age.
As expected, most (71%) of the high aMCI risk cases were also classified as HAND. Given the high prevalence of HAND among older PWH and similarities in biological mechanisms contributing to aMCI/AD and HAND (eg, low-grade inflammation, immune senescence, and compromised blood-brain barrier) [36–39], it is likely that a proportion of cases are on an AD trajectory either with or without comorbid HAND, but impairment has been solely attributed to HAND because of their HIV-seropositivity. Our results can inform and contribute to the urgent need for identifying methods to detect aMCI amid a background of HAND.
Our results indicate the existence of 4 distinct groups (aMCI−/HAND−, aMCI−/HAND+, aMCI+/HAND−, and aMCI+/HAND+) among PWH. The smallest group (n = 12) was the aMCI+/HAND− group and represented those that were only impaired in the memory domain (ie, single-domain aMCI) and, thus, did not meet HAND criteria (ie, impairment in ≥2 cognitive domains). It is possible that these cases are demonstrating the earliest signs of aMCI, as cognitive dysfunction in AD is typically observed in memory function initially followed by other cognitive domains [28, 29]. It is interesting that the aMCI+/HAND− group showed a larger proportion of Aβ plaque pathology (58%) compared to the aMCI+/HAND+ group (48%), although not significantly (P = .10), possibly due to the limited power in comparisons of the small aMCI+/HAND+ group (n = 12). Perhaps a memory deficit (inclusive of recognition impairment) in the absence of other cognitive deficits may particularly suggest risk of early-stage AD pathology in older PWH. A larger, longitudinal study is underway to compare the individual aMCI/HAND status groups by AD biomarkers and cognitive change.
Distinguishing between aMCI/AD and HAND is important for clinical and experimental reasons. Clinically, the more progressive profile of aMCI/AD requires different life planning and treatment options compared to HAND, which tends to be more stable [7]. A delayed aMCI/AD diagnosis in PWH would limit the opportunity to intervene early in the AD trajectory when interventions are most effective and life planning is better implemented. Clinicians could also assuage fears of pending AD in PWH that are not classified as high aMCI risk. Experimentally, the identification of aMCI/AD among PWH will improve studies of the prevalence and biopsychosocial determinants of HAND. Characterizing AD in the context of HIV will improve our understanding of the mechanisms linked to AD versus HAND and the potential for overlap that may increase risk for AD among PWH.
Our study has limitations. First, although 70% of cases died within 5 months of their neuropsychological battery, this time span was longer for other cases (6–12 months), suggesting that neurocognitive performance was less reflective of neuropathology in these cases. However, the inclusion of a time interval variable was not significant in statistical models and did not impact results. Second, neuropathological data was limited to the frontal lobe and cannot be generalized to other brain regions. The generalizability of our results is also limited in that our sample was predominantly male and white and a postmortem cohort, which tends to be sicker with more comorbidities than PWH in the general population. Third, the categorical characterization of Aβ and p-Tau pathology precluded us from examining the degree or deposition pattern of Aβ and p-Tau pathology as a function of classification group.
A major strength of our study was the ability to test our adapted aMCI criteria by relating it to postmortem neuropathology. Our initial findings give promise to the utility of recognition performance in aMCI criteria in detecting a higher likelihood of AD-related neuropathology and encourage further research to build on this work by identifying other clinical differences between aMCI and HAND, examining aMCI risk classification by cognitive trajectories and in-life cerebrospinal fluid biomarkers, and comparing aMCI risk groups in PWH to HIV-seronegative aMCI/AD and cognitively normal persons. Our hypothesis would be that PWH with high aMCI risk would demonstrate cognitive decline that is steeper than PWH classified as low aMCI risk but similar to HIV-seronegative persons with aMCI. Further research is also needed to identify and leverage cognitive characteristics that may help tease apart HAND from nonamnestic MCI.
Notes
Acknowledgments. The authors thank the participants and the staff of the National NeuroAIDS Tissue Consortium. The San Diego HIV Neurobehavioral Research Center group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, PhD, Codirector: Igor Grant, MD; Associate Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and Scott Letendre, MD; Center Manager: Thomas D. Marcotte, PhD; Jennifer Marquie-Beck, MPH; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, MD, PhD (PI), Scott Letendre, MD, J. Allen McCutchan, MD, Brookie Best, PharmD, Rachel Schrier, PhD, Debra Rosario, MPH; Neurobehavioral Component: Robert K. Heaton, PhD (PI), J. Hampton Atkinson, MD, Steven Paul Woods, PsyD, Thomas D. Marcotte, PhD, Mariana Cherner, PhD, David J. Moore, PhD, Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, PhD (PI), Monte S. Buchsbaum, MD, John Hesselink, MD, Sarah L. Archibald, MAGregory Brown, PhD, Richard Buxton, PhD, Anders Dale, PhD, Thomas Liu, PhD; Neurobiology Component: Eliezer Masliah, MD (PI), Cristian Achim, MD, PhD; Neurovirology Component: David M. Smith, MD (PI), Douglas Richman, MD; International Component: J. Allen McCutchan, MD, (PI), Mariana Cherner, PhD; Developmental Component: Cristian Achim, MD, PhD; (PI), Stuart Lipton, MD, PhD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI), Jennifer Marquie-Beck, MPH; Data Management and Information Systems Unit: Anthony C. Gamst, PhD (PI), Clint Cushman; Statistics Unit: Ian Abramson, PhD (PI), Florin Vaida, PhD (Co-PI), Anya Umlauf, MS.
Disclaimer . The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Financial support. This work was supported by the National Institutes of Health (NIH) (grant numbers RF1AG061070 [primary grant], PSTHN71, R01AG049810, R01 MH096648, P30MH062512, N01 MH22005, HHSN271201000036C, HHSN271201000030C, U24 MH100928, and R25MH081482). R. M. was supported by the NIH (grant numbers R01AG062387, K23MH107260, and K23MH107260 S1).
Potential conflicts of interest. M. W. B. is paid royalties from Oxford University Press and serves as a consultant for Eisai and Novartis. R. C. M. is a cofounder of KeyWise, Inc. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Annual Meeting of the International Society of Neurovirology, Chicago, IL, 10–14 April 2018; and Annual Meeting of the International Society of Neurovirology, Atlanta, GA, 12–19 November 2019.
References
- 1. Centers for Disease Control and Prevention. HIV Surveillance Report, 2016 vol 28. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 2 January 2018.
- 2. Heaton RK, Clifford DB, Franklin DR Jr, et al. ; CHARTER Group . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheppard DP, Iudicello JE, Bondi MW, et al. Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol 2015; 21:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Epps P, Kalayjian RC. Human immunodeficiency virus and aging in the era of effective antiretroviral therapy. Infect Dis Clin North Am 2017; 31:791–810. [DOI] [PubMed] [Google Scholar]
- 5. Hodes RJ, Sierra F, Austad SN, et al. Disease drivers of aging. Ann N Y Acad Sci 2016; 1386:45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stoff DM, Goodkin K, Jeste D, Marquine M. Redefining aging in HIV infection using phenotypes. Curr HIV/AIDS Rep 2017; 14:184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milanini B, Valcour V. Differentiating HIV-associated neurocognitive disorders from Alzheimer’s disease: an emerging issue in geriatric neuroHIV. Curr HIV/AIDS Rep 2017; 14:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–8. [DOI] [PubMed] [Google Scholar]
- 9. Becker JT, Caldararo R, Lopez OL, Dew MA, Dorst SK, Banks G. Qualitative features of the memory deficit associated with HIV infection and AIDS: cross-validation of a discriminant function classification scheme. J Clin Exp Neuropsychol 1995; 17:134–42. [DOI] [PubMed] [Google Scholar]
- 10. Peavy G, Jacobs D, Salmon DP, et al. Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction. The HNRC group. J Clin Exp Neuropsychol 1994; 16:508–23. [DOI] [PubMed] [Google Scholar]
- 11. White DA, Taylor MJ, Butters N, et al. Memory for verbal information in individuals with HIV-associated dementia complex. HNRC group. J Clin Exp Neuropsychol 1997; 19:357–66. [DOI] [PubMed] [Google Scholar]
- 12. Achim CL, Adame A, Dumaop W, Everall IP, Masliah E; Neurobehavioral Research Center . Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 2009; 4:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soontornniyomkij V, Moore DJ, Gouaux B, et al. Cerebral β-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE ε4 carriers. AIDS 2012; 26:2327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005; 19:407–11. [DOI] [PubMed] [Google Scholar]
- 15. Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol 2009; 4:163–74. [DOI] [PubMed] [Google Scholar]
- 16. Cooley SA, Strain JF, Beaumont H, et al. Tau positron emission tomography binding is not elevated in HIV-infected individuals. J Infect Dis 2019; 220:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry 1998; 65:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Everall I, Vaida F, Khanlou N, et al. ; National NeuroAIDS Tissue Consortium (NNTC) . Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol 2009; 15:360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 2006; 111:529–38. [DOI] [PubMed] [Google Scholar]
- 20. Bondi MW, Edmonds EC, Jak AJ, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 2014; 42:275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82:239–59. [DOI] [PubMed] [Google Scholar]
- 22. Morgello S, Gelman BB, Kozlowski PB, et al. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol 2001; 27:326–35. [DOI] [PubMed] [Google Scholar]
- 23. Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Sinsheimer JS, Levine AJ. Associations of regional amyloid-β plaque and phospho-tau pathology with biological factors and neuropsychological functioning among HIV-infected adults. J Neurovirol 2019; 25:741–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cysique LA, Franklin D Jr, Abramson I, et al. ; CHARTER Group; HNRC Group . Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol 2011; 33:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heaton R, Miller SW, Taylor MJ, Grant I.. Revised comprehensive norms for an expanded Halstead-Reitan Battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources, 2004. [Google Scholar]
- 26. Norman MA, Moore DJ, Taylor M, et al. ; HNRC Group . Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol 2011; 33:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009; 17:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brandt J, Benedict RH.. Hopkins verbal learning test-revised: professional manual. Odessa, FL: Psychological Assessment Resources, 2001. [Google Scholar]
- 30. Benedict RH. Brief visuospatial memory test-revised: professional manual. Lutz, FL: Psychological Assessment Resources, 1997. [Google Scholar]
- 31. World Health Organization. Composite international diagnostic interview (CIDI) version 2.1 [computer program]. Geneva, Switzerland: World Health Organization, 1997. [Google Scholar]
- 32. Umlauf A, Soontornniyomkij B, Sundermann EE, et al. Risk of developing cerebral β-amyloid plaques with posttranslational modification among HIV-infected adults. AIDS 2019; 33:2157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement 2012; 8:14–21. [DOI] [PubMed] [Google Scholar]
- 34. Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology 2009; 73:1982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015; 313:1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banks WA. Physiology and pathology of the blood-brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J Neurovirol 1999; 5:538–55. [DOI] [PubMed] [Google Scholar]
- 37. Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology 2007; 68:1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res 2007; 85:3036–40. [DOI] [PubMed] [Google Scholar]
- 39. Canet G, Dias C, Gabelle A, et al. HIV neuroinfection and Alzheimer’s disease: similarities and potential links? Front Cell Neurosci 2018; 12:307. [DOI] [PMC free article] [PubMed] [Google Scholar]