Abstract
Background
Although human papillomavirus (HPV) vaccines are highly efficacious in protecting against HPV infections and related diseases, vaccination may trigger replacement by nontargeted genotypes if these compete with the vaccine-targeted types. HPV genotype replacement has been deemed unlikely, based on the lack of systematic increases in the prevalence of nonvaccine-type (NVT) infection in the first decade after vaccination, and on the presence of cross-protection for some NVTs.
Methods
To investigate whether type replacement can be inferred from early postvaccination surveillance, we constructed a transmission model in which a vaccine type and an NVT compete through infection-induced cross-immunity. We simulated scenarios of different levels of cross-immunity and vaccine-induced cross-protection to the NVT. We validated whether commonly used measures correctly indicate type replacement in the long run.
Results
Type replacement is a trade-off between cross-immunity and cross-protection; cross-immunity leads to type replacement unless cross-protection is strong enough. With weak cross-protection, NVT prevalence may initially decrease before rebounding into type replacement, exhibiting a honeymoon period. Importantly, vaccine effectiveness for NVTs is inadequate for indicating type replacement.
Conclusions
Although postvaccination surveillance thus far is reassuring, it is still too early to preclude type replacement. Monitoring of NVTs remains pivotal in gauging population-level impacts of HPV vaccination.
Keywords: competition, cross-immunity, cross-protection, honeymoon period, type replacement, vaccination, vaccine effectiveness
It is probably still too early to preclude human papillomavirus genotype replacement after a decade of postvaccine surveillance, because replacement could occur after a honeymoon period owing to the combination of infection-induced cross-immunity and vaccine-induced cross-protection for a nonvaccine type.
Papillomaviruses are ancient DNA viruses with high diversity in mammalian hosts. Human papillomaviruses (HPV) are classified into genotypes (generally termed “types”), defined by >10% DNA sequence divergence from one another in the L1 capsid gene [1]. Sexually transmitted HPV types are causally linked to anogenital and oropharyngeal cancers in men and women, which attribute to almost 5% of the 14 million new cancer cases occurring annually worldwide [2, 3]. HPV vaccination thus provides a great opportunity to largely reduce global cancer burden and may even eliminate cervical cancer as a public health problem [4].
Currently, 3 licensed HPV vaccines are available, constructed using L1 capsid antigens of 2, 4, or 9 HPV genotypes [2]. The 3 vaccines are highly efficacious in preventing HPV infections with the corresponding vaccine types (VTs), all including high-risk types HPV-16/18, which cause the majority of HPV-related cancers [5, 6]. The quadrivalent and nonavalent vaccines also target low-risk types HPV-6/11, primarily associated with anogenital warts. In addition, the nonavalent vaccine includes 5 other high-risk types, HPV-31/33/45/52/58, and the quadrivalent and bivalent vaccines reportedly provide partial cross-protection, predominantly against HPV-31 and HPV-31/33/45, respectively [5–10].
Because strain-specific vaccination against other multistrain pathogens, such as adenovirus and Streptococcus pneumoniae, has caused increased circulation of some non–vaccine-targeted strains, concerns about HPV type replacement have also been raised [11–15]. The prevailing assumption behind strain replacement is the competition between vaccine-targeted and nontargeted strains, so that vaccination releases nontargeted strains from competition as a side effect of eliminating the vaccine-targeted ones [12]. There are empirical findings supporting mutual suppression of viral loads between HPV types during coinfection within hosts and competitive exclusion within cells [16, 17]. An evoked immune response that clears infection with a given HPV type might also yield enhanced clearance of other types by activating antigen-presenting cells or cross-immunity. In contrast, multiple epidemiological studies on prevaccination HPV infection (or coinfection) data have found a lack of systematic negative associations between VTs and nonvaccine types (NVTs), presumed to be indicative of a lack of competitive interactions [18]. However, subsequent modeling studies demonstrated that positive associations might instead reflect the existence of natural cross-immunity, entailing a risk of type replacement [19, 20].
In the first decade after the introduction of HPV vaccination, surveillance data worldwide have shown a slight increase in the pooled prevalence of high-risk NVTs, not including HPV-31/33/45 [8]. This increase might reflect an increasing trend in sexual activities or an apparent increased detection of NVTs, which were previously masked by HPV-16/18 infections. Alternatively, NVTs could be occupying the ecological niche created by reducing HPV-16/18 infections. Type-specific trends of HPV NVTs, however, are more heterogeneous across settings, without consistent signals of type replacement. In addition, negative vaccine effectiveness (VE) has been reported for various NVTs (eg, HPV-40/42/43/54/56/59), but mostly without statistical significance, possibly owing to the limited power of monitoring studies [10, 21]. In a large-scale community-randomized study with 50% vaccination coverage, although no consistent patterns of type replacement were observed, the prevalence of HPV-39/51 did increase in HPV-vaccinated communities [22]. Taking these findings together, although approximately 10 years have elapsed since the wide-scale implementation of HPV vaccines, the likelihood of type replacement remains ambiguous.
In the current study , we investigated to what extent type replacement can be inferred from early postvaccination surveillance data. We constructed a transmission model in which 2 HPV types, 1 VT and 1 NVT, compete through naturally acquired (ie, infection-induced) cross-immunity. Short- and long-term trends of NVT prevalence were derived. From the short-term trends, we computed measures commonly used to evaluate type replacement in real-life surveillance, such as prevalence ratios relative to prevaccination periods and VE for NVTs. These were then compared with the long-term trends. We investigated how the occurrence and timing of type replacement depend on the model parameters, and we showed that, in some cases, even observed patterns of initial decreases in NVT prevalence and positive VE may not preclude type replacement in the long run.
METHODS
Model Structure
We constructed a heterosexual compartmental transmission model with 2 competing HPV types, 1 VT and 1 NVT. In the model, individuals of both sexes are divided into 2 risk groups, distinguished by the rates at which new sexual partners of the opposite sex are contacted (Supplementary Figure 1). Within each risk group, individuals have the same age-dependent contact rates and prefer partners from the same risk group and of a similar age. We assumed a constant influx of individuals entering the model population at age 10 years and leaving at age 70 years.
We assumed susceptible-infected-recovered-susceptible dynamics for both types (Supplementary Figure 2), in accordance with most heterosexual transmission models for studying the population impact of HPV vaccination [23]. Individuals enter the population being susceptible for both types and subsequently undergo events of acquisition, clearance, and waning of natural immunity.
Unvaccinated individuals acquire infection with type i (= vt, nvt) at a rate proportional to the rate of new contacts with individuals infected with type i. On established contact, the transmission probability is β i. If vaccinated, the infection acquisition rate is reduced by the type-specific vaccine efficacy θ i. The vaccine is assumed to be fully protective against the VT (θ vt = 100%) and may induce partial protection against the NVT (θ nvt < 100%). Acquired infections of type i are cleared at rate μ i. Clearance induces natural type-specific immunity, inhibiting reinfection with the same type. Immunity wanes at rates γ f and γ m in women and men, respectively, after which the individual again becomes susceptible to that type.
Between-type competition is modeled by natural cross-immunity. During the period of type-specific immunity, cross-immunity is assumed to accelerate the clearance of the other type by a factor h ≥ 1, without blocking infection. Cross-immunity is lost simultaneously with the waning type-specific immunity. For simplicity, we consider only the symmetric case in which h does not depend on the type. Modeled as such, the 2 types disadvantage one another through cross-immunity, whereby parameter h regulates the strength of between-type competition. See Supplementary Appendix A for a more detailed description of the model.
Parametrization and Scenarios
The sexual contact rates and mixing distribution were based on Dutch contact patterns (Supplementary Figures 3 and 4) [24]. The waning rates of natural immunity were γ f = 0.1 and γ m = 1 per year, corresponding to mean durations of 10 years in women and 1 year in men, respectively. These differences were chosen to reflect varying natural histories of HPV infection by sex and anatomic site; notably, cervicovaginal HPV infection likely induces some level of systemic immunity, whereas such protection is less obvious in men [25, 26]. To explore scenarios of different levels of competition, we used various prefixed values of h. For each value of h, other parameters were obtained by calibrating to data of age-specific prevaccination HPV prevalence of females in the Netherlands (Supplementary Figures 5-9) [27]. Data for the VT were the prevalence of HPV-16, which attains its maximal value of approximately 8% around age 23 years. For the NVT, we assumed a 4-time-lower prevalence.
We implemented a girls-only vaccination program targeting 12-year-olds with 95% uptake, complemented by a catch-up program up to age 18 years at the introduction of vaccination (Supplementary Figure 10). This setting roughly mimics HPV vaccination programs in most Western countries, albeit with an overoptimistic vaccine uptake to study the appearance of type replacement. For simplicity, we assumed no difference in uptake due to risk-group stratification. To explore scenarios of different levels of cross-protection for the NVT, we considered θ nvt = 0%, 10%, …, 50%; stronger cross-protection could probably prevent type replacement from occurring altogether. We performed sensitivity analyses regarding shorter natural immunity, lower vaccination coverage in girls, and higher coverage by adding boys’ vaccination (Supplementary Figure 11). See Supplementary Appendix B for the calibration process, parameter values used, and description of the sensitivity analyses.
Measures for Type Replacement
We defined type replacement as an increase in the prevalence of NVT infection in the postvaccination period as compared with the prevaccination equilibrium. Type replacement evaluated in the long run and short term were defined as follows.
Final Type Replacement
To evaluate the population impact of vaccination on NVT infection in the long run, we used PRfinal, which compares the NVT prevalence among all women in the postvaccination equilibrium to the prevaccination equilibrium; PRfinal > 1 denotes “final type replacement” and PRfinal < 1 an overall beneficial effect in the long run.
Short-term Type Replacement
We evaluated type replacement in the short term using PR(a, t), which compares the NVT prevalence among a-year-old (vaccinated and unvaccinated) women at t years after vaccination to the prevalence of the same age group in the prevaccination equilibrium.
Vaccine Effectiveness
Another short-term measure for type replacement was VE against NVT infection, here defined as the reduction in the risk of NVT infection in vaccinated relative to unvaccinated individuals, that it, VEnvt(a, t) = 1–RR(a, t), where RR(a, t) denotes the corresponding relative risk evaluated among a-year-old women at t years after vaccination. Positive VE, meaning less NVT infection among vaccinated than unvaccinated individuals, is commonly interpreted as evidence of a low risk of type replacement and negative VE a sign of type replacement. To scrutinize the ability of the 2 short-term measures to indicate final type replacement, we checked whether PR(a, t) > 1 and VEnvt(a, t) < 0 correctly indicate PRfinal for various choices of a and t.
RESULTS
Convergence to Postvaccination Equilibrium
We simulated scenarios for different levels of cross-immunity-mediated competition (h = 1, 1.33, …, 3) and vaccine-induced cross-protection (θ nvt = 0%, 10%, …, 50%). Throughout all scenarios in the base-case analysis, where high vaccination coverage was assumed, the VT was eliminated within 30–50 years (Supplementary Figures 15-23). By then, the NVT had also stabilized at the new postvaccination equilibrium (Figure 1 and Supplementary Figures 12–14 in Supplementary Appendix C).
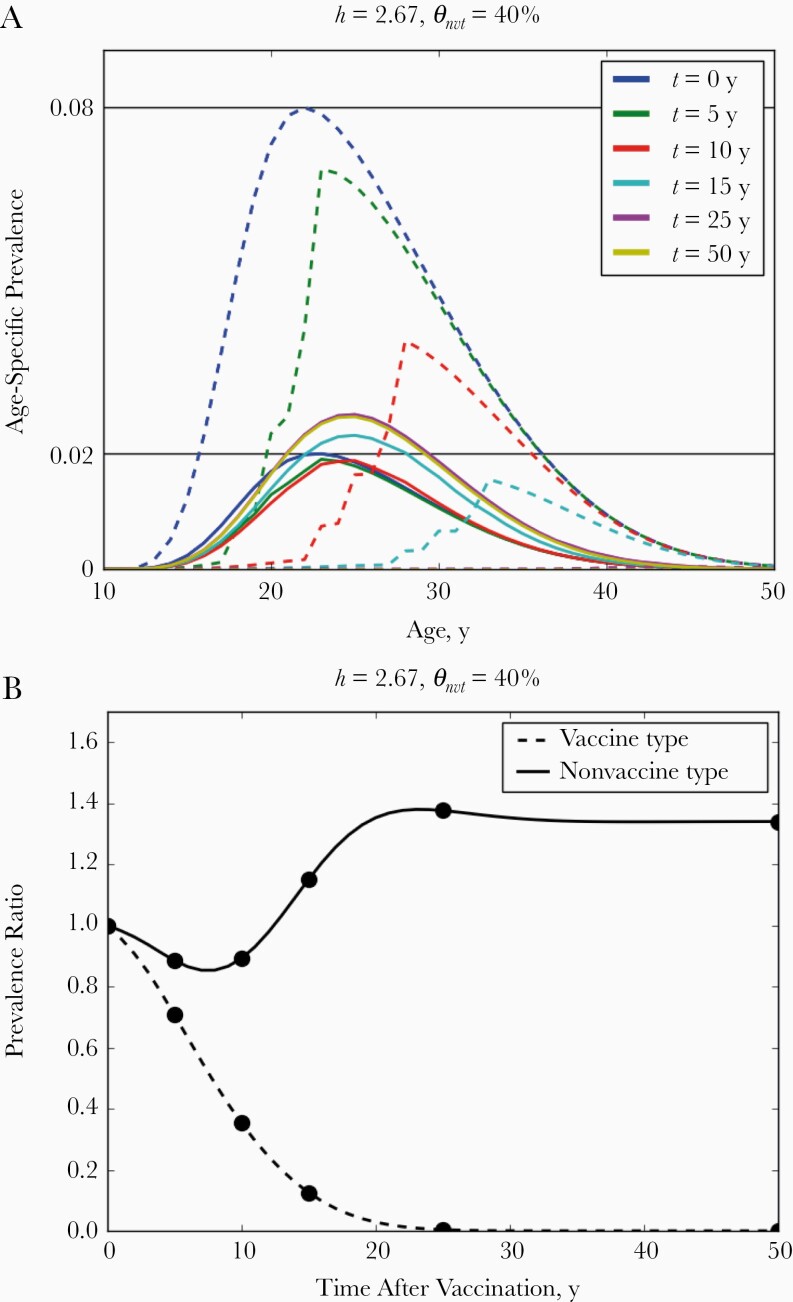
Figure 1.
A, Age-specific prevalence of vaccine-type (dashed lines) and nonvaccine-type (solid lines) infection in women after the introduction of vaccination, where t represents time after vaccination. B, Prevalence ratio comparing non–age-specific prevalence in all women with the corresponding prevaccination level. The competition parameter was set to h = 2.67 and the level of cross-protection to θ nvt = 40%. Black horizontal lines in A indicate the maximal prevaccination age-specific prevalence. Time points evaluated in A are depicted in B with black circles.
Final Type Replacement as a Trade-off Between Competition and Vaccine Cross-Protection
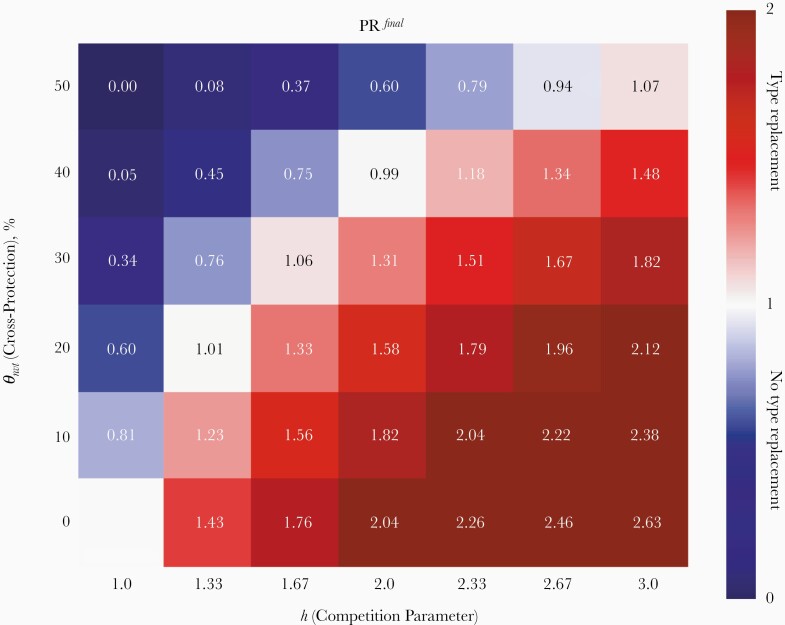
In the absence of both vaccine-induced cross-protection (θ nvt = 0%) and competition (h = 1), the NVT prevalence remained unchanged (lower left corner of Figure 2). When increasing the strength of competition in the absence of cross-protection, the extent of type replacement in the postvaccination equilibrium increased (bottom row in Figure 2). For a given level of competition, h > 1, increasing the level of vaccine-induced cross-protection attenuated the extent of final type replacement (follow each column upward in Figure 2). Type replacement could even be prevented if cross-protection were strong enough. Hence, the occurrence and extent of type replacement in the postvaccination equilibrium depend on a trade-off between the levels of competition and cross-protection.
Figure 2.
Outcomes regarding final type replacement expressed by prevalence ratios PRfinal, comparing the prevaccination and postvaccination equilibria among all women for different levels of competition h and cross-protection θ nvt. Red corresponds to occurrence of final type replacement and blue an overall beneficial effect.
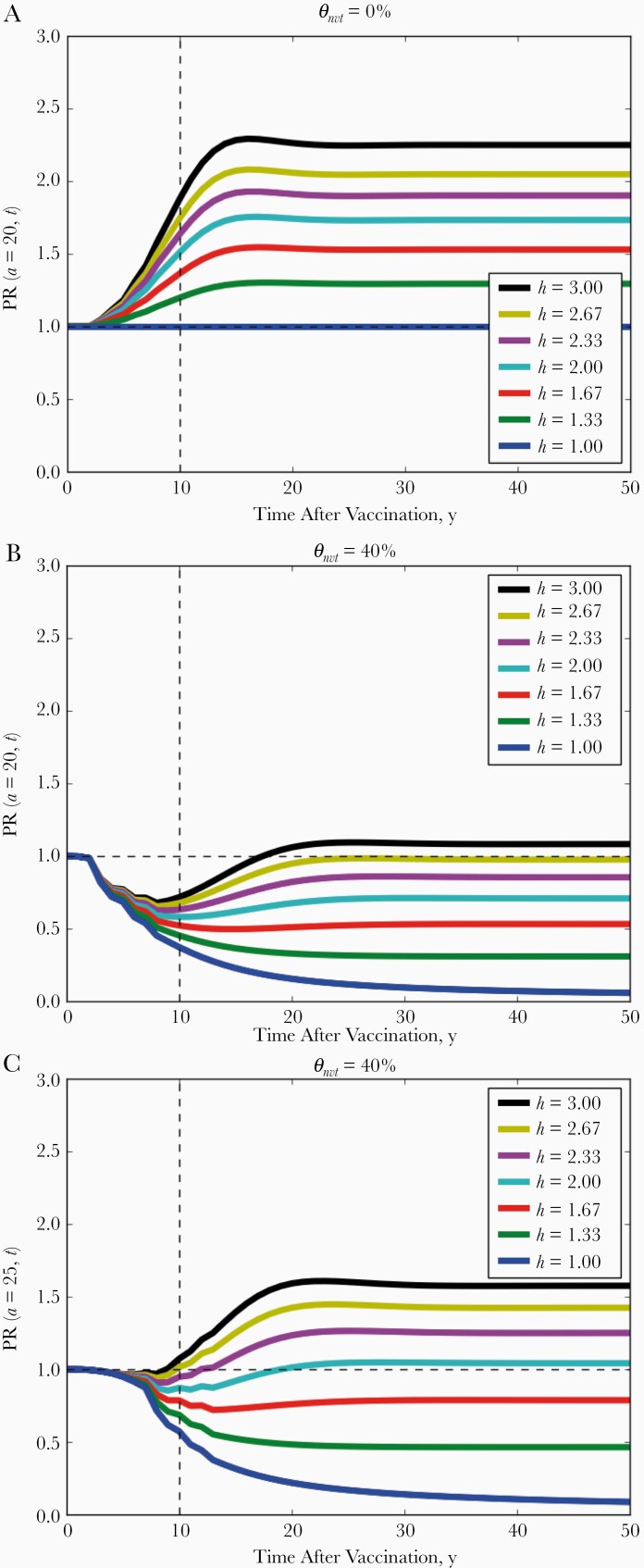
Figure 3.
Trends of the nonvaccine-type prevalence over time expressed by prevalence ratio PR(a,t) comparing the prevaccination equilibrium and t years after vaccination among 20-year-old (A, B) and 25-year-old (C) women. The level of vaccine-induced cross-protection to θ nvt is 0% in A and 40% in B and C. Different colors correspond to different levels of competition (h = 1, 1.33, …, 3).
Inferring Final Type Replacement Using Short-term Postvaccination Surveillance
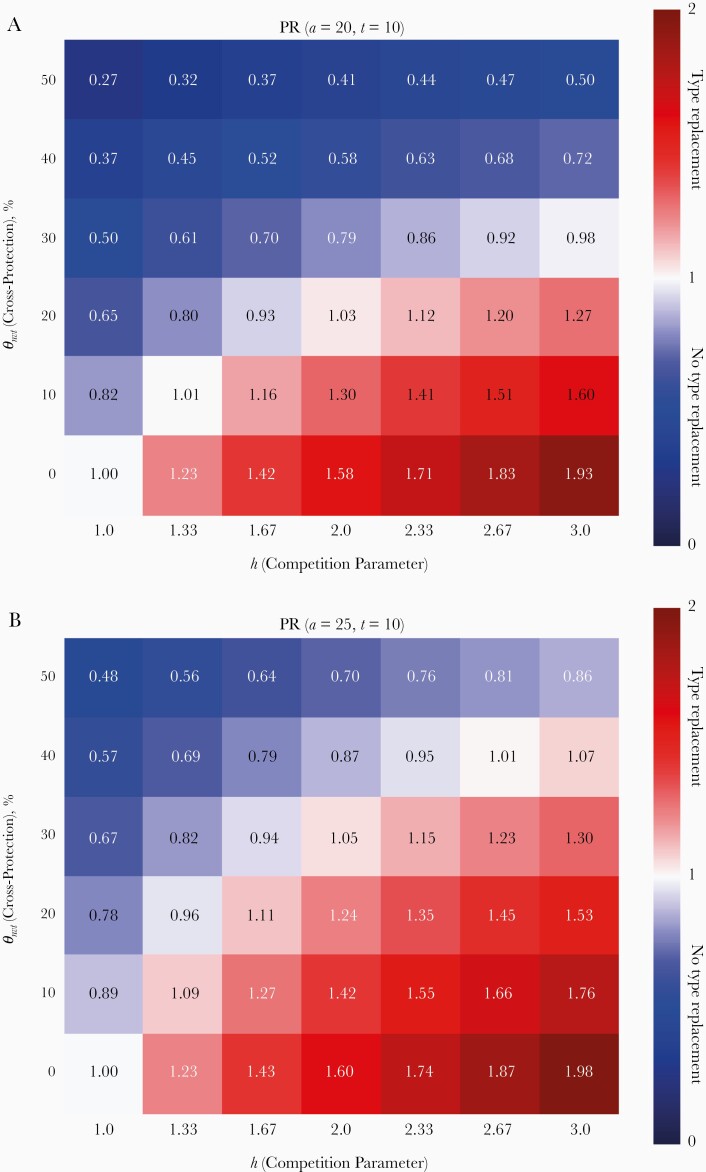
In the absence of cross-protection, short-term type replacement given by prevalence ratio PR(a, t) correctly indicated final type replacement among all ages and time points (see Figure 3A and Supplementary Figure 24 and compare the colors in the bottom rows of Figures 2 and 4A). Short-term type replacement is also a trade-off between competition and cross-protection (see Figure 4A for PR[a = 20, t = 10]). However, it did not always correctly indicate the occurrence of final type replacement (compare the colors in Figures 2 and 4A). Although final type replacement always occurred whenever short-term type replacement was observed, final type replacement sometimes occurred in absence of short-term type replacement. In the disagreeing scenarios, the NVT prevalence first decreased before rebounding into type replacement. In simulations, such initial decreases lasted up to 20 years (Figure 3B and Supplementary Figure 25).
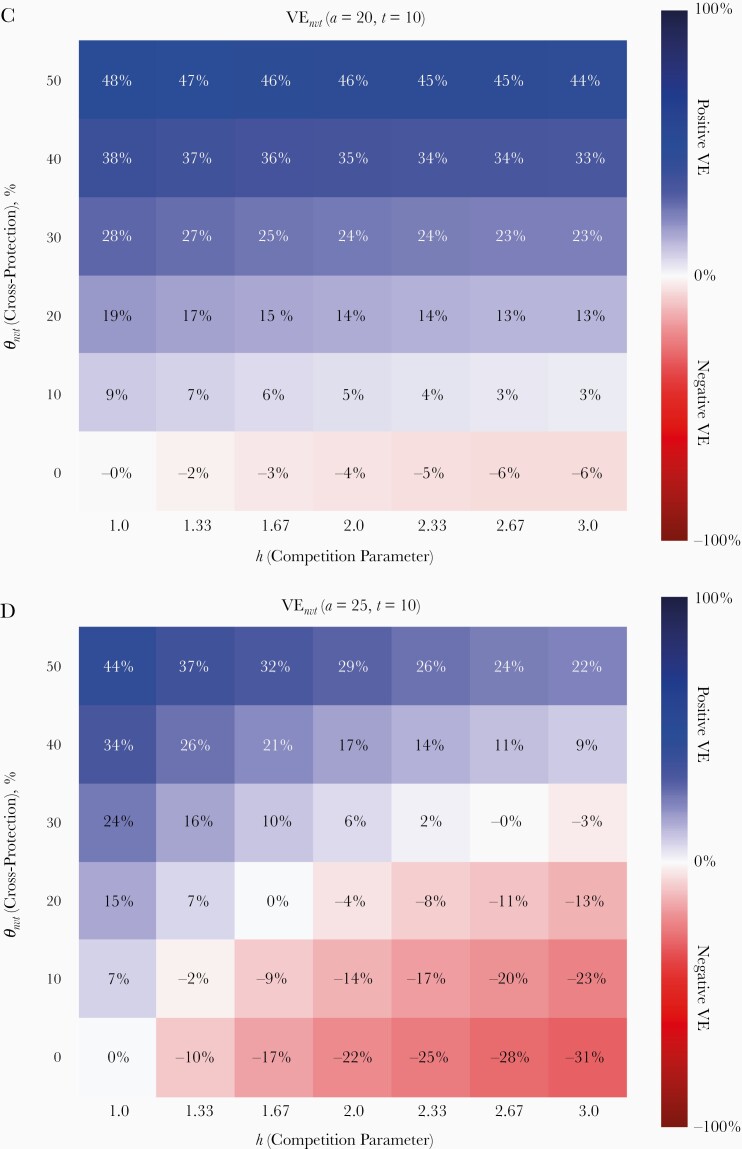
Figure 4.
Short-term evaluation of type replacement based on prevalence ratio PR(a, t = 10) and vaccine effectiveness VEnvt(a, t = 10), evaluated at 10 years after vaccination. A, B, PR(a = 20, t = 10) and PR(a = 25, t = 10) compare the prevalence of nonvaccine-type (NVT) infection between prevaccination equilibrium and t = 10 years after vaccination among 20- and 25-year-old women, respectively. C, D, VEnvt(a = 20, t = 10) and VEnvt(a = 25, t = 10) compare the risk of NVT infection between vaccinated and unvaccinated individuals at t = 10 years after vaccination among 20-year-old and 25-year-old women, respectively. Red corresponds to indication of type replacement based on the applied measure; blue, no indication.
Evaluation of short-term type replacement among older women corresponded better with final type replacement but was still not perfect (compare Figures 2 and 4B). In addition, owing to cross-protection, the NVT prevalence may undergo an age shift, whereby the NVT prevalence decreases in younger ages in the postvaccination equilibrium but increases in older ages (Figure 2). For instance, in the scenario with θ nvt = 40% and h = 2.67, where final type replacement did occur (Figure 2), the NVT prevalence among 20-year-old women stayed below the prevaccination level over all time t after vaccination (Figure 3B) but increased after 8 years among 25-year-old women (Figure 3B). Simulations showed that such sustained decreases in NVT prevalence occurred up to age 24 years in scenarios with final type replacement (Supplementary Figure 14, 24, 25). Owing to such age shifts, evaluating type replacement in younger age groups may fail to detect final type replacement.
Inferring Final Type Replacement Using VE for the NVT
In most simulations, VE, which compares the risk of NVT infection in vaccinated and unvaccinated individuals, performed poorly as an indicator of final type replacement. VE evaluated among 20-year-old women at 10 years after vaccination, VE(a = 20, t = 10), did not discern final type replacement except in the scenarios without cross-protection (compare Figures 2 and 4C). Because of cross-protection, the risk of NVT infection in vaccinated individuals may drop below the corresponding risk in unvaccinated individuals, whereas the risk in vaccinated as well as unvaccinated individuals may have increased relative to the prevaccination level. Hence, positive VE for NVT infection does not rule out type replacement.
It is interesting that VE evaluated among older age groups was more sensitive for detecting final type replacement. For example, VE evaluated after 10 years among the catch-up cohort vaccinated at age 25 years corresponded much better with final type replacement (compare Figures 2 and 4D). However, VE at any age lost its sensitivity for detecting type replacement as time went by; with the disappearance of the VT, vaccinated and unvaccinated individuals became identical with respect to the amount of naturally acquired cross-immunity, after which the difference in the risks for NVT infection between the 2 groups became dependent on cross-protection alone (Supplementary Figure 32). As a result, VE became immaterial for predicting type replacement.
Sensitivity Analyses
In the sensitivity analyses, we encountered the same qualitative results: initial decreases in NVT prevalence before rebounding into final type replacement; occurrence of final type replacement despite positive VE; sustained decreases in NVT prevalence in young age groups (Supplementary Figures 26–31). While retaining similar qualitative behaviors, the timing of type replacement varied among different analyses. Assuming shorter natural immunity or higher vaccination coverage accelerated type replacement. Lower coverage, as achieved in practice, delayed type replacement. Increasing the vaccination coverage beyond the critical level required for VT elimination seemed to reduce the scope of final type replacement by bolstering cross-protection to the NVT (compare Figure 2 and Supplementary Figure 31).
DISCUSSION
In the current study, we studied the potential emergence of genotype replacement after HPV vaccination, using a transmission model in which 2 HPV types compete through naturally acquired (ie, infection-induced) cross-immunity. We showed that the occurrence and timing of type replacement result from a balance between the levels of natural cross-immunity and vaccine-induced cross-protection. We found cross-protection attenuating or, if strong enough, preventing the occurrence of type replacement, in line with previous findings in modeling studies [28, 29]. However, we discovered that with weak cross-protection, the NVT prevalence may first decrease, typically for 5–20 years in the model, before rebounding into type replacement. Hence, 10 years of follow-up since the introduction of HPV vaccination may still be too short a period to detect type replacement.
We are the first to suggest the existence of a “type replacement–related” honeymoon period. This phenomenon is analogous to the general concept of a honeymoon period after vaccination, whereby the number of people who are susceptible to an infectious agent targeted by vaccination first decreases, owing to the combined impact of vaccine protection and preexisting natural immunity, but later rebounds [30–32]. In case of a type replacement–related honeymoon, the roles of vaccine protection and natural immunity are taken by cross-protection and natural cross-immunity, respectively. The initial decrease in the prevalence of a cross-protected type stems from the immediate cross-protection individuals receive from the vaccine, whereas the delayed occurrence of type replacement follows from a gradual loss of natural cross-immunity. Once the individuals who lack cross-immunity then acquire NVT infections, the infections would last longer, in turn amplifying the transmission of the NVT, triggering type replacement.
Detection of type replacement in postvaccination surveillance is complicated by several other factors, as demonstrated in our modeled evaluations. First, vaccine-induced cross-protection may lead to a lower risk of NVT infection in vaccinated individuals than in unvaccinated individuals, whereas the prevalence may increase in the general population. In other words, positive VE for NVTs does not necessarily preclude type replacement, and there are vaccine-specific differences. Second, the extent of type replacement may differ across age groups. In particular, evaluation of type replacement in young age groups may underestimate the risk in the general population. Third, the timing of type replacement depends on the vaccination coverage. With limited vaccination uptake, it may take longer to detect possible type replacement. Conversely, with ample vaccination uptake in 1 or both sexes, the VT would be cleared more rapidly, so that potential type replacement could be detected earlier.
In addition, our analysis highlights several less-addressed insights regarding type replacement. First, increasing vaccination coverage beyond the critical threshold, required for VT elimination, still increases the benefit of cross-protection for an NVT, while the extent of competitive release for that type is already saturated. The additional amount of cross-protection in the population may suppress or even prevent type replacement, which may not have been possible at a lower coverage. Second, our results suggest that those NVTs for which vaccine-induced cross-protection is weak in comparison to the strength of competition bear the highest risk for type replacement. The strength of natural immunity for HPV is generally believed to be weak [25, 33], although exact estimates are hard to obtain, partly because identification of individuals with preexisting immunity through serological measurements is complicated [19]. The tractability of vaccination status makes estimation of the level of cross-protection more permissible. In particular, type-specific efficacies for HPV-31/33/45 have been estimated to be >50% for the bivalent vaccine.
Findings of a 2019 study suggest that the level of cross-protection might be correlated with the phylogenetic distance from VTs [34]. Arguably, the level of natural cross-immunity follows a similar relation, meaning that closely related types would experience stronger competition and that occurrence of type replacement depends on an intricate balance with the level of vaccine-induced cross-protection. Consequently, though speculative, closely related HPV types against which the vaccine in use fails to evoke strong enough cross-protection are at increased risk for type replacement. The postvaccination increases of HPV-39 and HPV-51 in the Finnish community-randomized trial are interesting from this perspective [22], because these types have intermediate phylogenetic distance to HPV-16/18 relative to other high-risk types.
Eventually, the potential for type replacement has implications for decisions about the prevention of HPV-related diseases. If type replacement poses a real threat, its extent might be mitigated by increasing vaccination coverage or improving VE against the replacing genotypes. Because different countries currently use different vaccines and achieve different levels of vaccination coverage, the relative merits of different vaccination strategies may also differ. In addition, there are implications for national cervical screening programs, especially in the era of HPV DNA testing for detecting high-grade cervical lesions. Because the current HPV vaccines target the most oncogenic high-risk types, the residual cancer risk coming from the nontargeted types will determine the benefit of continued screening in vaccinated women [35]. Even if vaccination triggers type replacement, the benefit of eliminating the VTs would probably still surpass the disease burden caused by the less-carcinogenic replacing types. However, subtle increases in the occurrence of these nontargeted types could result in considerably greater risks than anticipated based on current genotype attribution to cervical lesions and cancer [36]. Hence, disregarding type replacement may possibly lead to overoptimistic down-scaling of cervical screening programs.
Our analysis has several strengths and weaknesses. We parametrized our model realistically based on surveys on sexual behavior and real-life vaccination schemes [24, 37]. These components are essential for generating realistic timescales in which vaccination effects propagate between cohorts. Nevertheless, our results are not meant for predicting actual levels of type replacement or HPV trends due to simplifying model assumptions, for example, regarding heterogeneity in sexual behavior, vaccination uptake across risk groups, and uncertainty in some poorly identified parameters, especially those concerning HPV transmissibility and immunity. For instance, we assumed relatively short durations of natural immunity of 1 and 10 years, on average, in men and women, respectively, whereas other modeling studies have tended to assume longer immunity [23].
Our sensitivity analyses showed that longer-lasting natural immunity leads to prolonged honeymoon periods, further complicating the detection of type replacement from early postvaccination surveillance. The assumed asymmetry in duration of natural immunity in men and women also differs from some other modeling studies, but we expect the duration of the honeymoon period to be mainly driven by the sex with the longer-lasting immunity. This remains to be determined. In addition, the risk of type replacement may be underestimated if cross-protection to NVTs lasts considerably shorter than VT protection.
Finally, the possibility of type replacement should not discourage efforts to further enhance HPV vaccination programs worldwide. Our analysis shows that the risk of type replacement may generally be diminished by increasing vaccination coverage and providing improved VE against possibly replacing oncogenic HPV types. These arguments plead in favor of sex-neutral HPV vaccination with broad-spectrum vaccines, adding another dimension to the construction of resilient vaccination programs [38]. Nevertheless, monitoring type replacement remains important, especially among HPV types and age groups that bear the highest risks for type replacement. Ultimately, improved assessment of type replacement may help efforts to optimize HPV control measures and eliminate cervical cancer as a public health problem.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Strategic Programme from the National Institute for Public Health and the Environment of the Netherlands (grant S/113005/01/PT [Prometheus project]).
Potential conflicts of interest. M. L. has received grants from Merck and the GlaxoSmithKline group of companies through his employers, the Karolinska Institute and the University of Tampere, for conducting human papillomavirus vaccination studies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: EUROGIN Congress, 2–5 December 2018, Lisbon, Portugal; and STI seminar at the National Institute for Public Health and the Environment of the Netherlands, 4 April 2019, Bilthoven, Netherlands.
References
- 1. de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology 2013; 445:2–10. [DOI] [PubMed] [Google Scholar]
- 2. Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer 2018; 18:240–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016; 4:e609–16. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO director-general calls for all countries to take action to help end the suffering caused by cervical caner. 2018. http://www.who.int/reproductivehealth/call-to-action-eliminationcervical-cancer/en/. Accessed 19 May 2018. [Google Scholar]
- 5. Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat Rev Clin Oncol 2013; 10:400–10. [DOI] [PubMed] [Google Scholar]
- 6. Joura EA, Giuliano AR, Iversen OE, et al. ; Broad Spectrum HPV Vaccine Study . A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711–23. [DOI] [PubMed] [Google Scholar]
- 7. Mesher D, Soldan K, Lehtinen M, et al. Population-level effects of human papillomavirus vaccination programs on infections with nonvaccine genotypes. Emerg Infect Dis 2016; 22:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drolet M, Benard E, Perez N, Brisson M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019; 394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lehtinen M, Luostarinen T, Vänskä S, et al. Gender-neutral vaccination provides improved control of human papillomavirus types 18/31/33/35 through herd immunity: results of a community randomized trial (III). Int J Cancer 2018; 143:2299–310. [DOI] [PubMed] [Google Scholar]
- 10. Woestenberg PJ, King AJ, van Benthem BHB, et al. ; Medical Microbiological Laboratories and the Public Health Services . Bivalent vaccine effectiveness against type-specific HPV positivity: evidence for cross-protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis 2018; 217:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stanley M, Villa LL. Monitoring HPV vaccination. Vaccine 2008; 26(suppl 1):A24–7. [DOI] [PubMed] [Google Scholar]
- 12. Lloyd-Smith JO. Vacated niches, competitive release and the community ecology of pathogen eradication. Philos Trans R Soc Lond B Biol Sci 2013; 368:20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gandon S, Day T. Evidences of parasite evolution after vaccination. Vaccine 2008; 26(suppl 3):C4–7. [DOI] [PubMed] [Google Scholar]
- 15. Lehtinen M, Paavonen J. Vaccination against human papillomaviruses shows great promise. Lancet 2004; 364:1731–2. [DOI] [PubMed] [Google Scholar]
- 16. Xi LF, Edelstein ZR, Meyers C, Ho J, Cherne SL, Schiffman M. Human papillomavirus types 16 and 18 DNA load in relation to coexistence of other types, particularly those in the same species. Cancer Epidemiol Biomarkers Prev 2009; 18:2507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biryukov J, Meyers C. Superinfection exclusion between two high-risk human papillomavirus (HPV) types during a co-infection. J Virol 2018; 92:e01993-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tota JE, Jiang M, Ramanakumar AV, et al. Epidemiologic evaluation of human papillomavirus type competition and the potential for type replacement post-vaccination. PLoS One 2016; 11:e0166329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Durham DP, Poolman EM, Ibuka Y, Townsend JP, Galvani AP. Reevaluation of epidemiological data demonstrates that it is consistent with cross-immunity among human papillomavirus types. J Infect Dis 2012; 206:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Man I, Wallinga J, Bogaards JA. Inferring pathogen type interactions using cross-sectional prevalence data: opportunities and pitfalls for predicting type replacement. Epidemiology 2018; 29:666–74. [DOI] [PubMed] [Google Scholar]
- 21. Tota JE, Struyf F, Merikukka M, et al. Evaluation of type replacement following HPV16/18 vaccination: pooled analysis of two randomized trials. J Natl Cancer Inst 2017; 109. doi: 10.1093/jnci/djw300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gray P, Palmroth J, Luostarinen T, et al. Evaluation of HPV type-replacement in unvaccinated and vaccinated adolescent females—post-hoc analysis of a community-randomized clinical trial (II). Int J Cancer 2018; 142:2491–500. [DOI] [PubMed] [Google Scholar]
- 23. Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health 2016; 1:e8–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Graaf H, Wijsen C.. Seksuele gezondheid in Nederland 2017. 2017. [Google Scholar]
- 25. Ranjeva SL, Baskerville EB, Dukic V, et al. Recurring infection with ecologically distinct HPV types can explain high prevalence and diversity. Proc Natl Acad Sci U S A 2017; 114:13573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giuliano AR, Nyitray AG, Kreimer AR, et al. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer 2015; 136:2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coupé VM, Berkhof J, Bulkmans NW, Snijders PJ, Meijer CJ. Age-dependent prevalence of 14 high-risk HPV types in the Netherlands: implications for prophylactic vaccination and screening. Br J Cancer 2008; 98:646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poolman EM, Elbasha EH, Galvani AP. Vaccination and the evolutionary ecology of human papillomavirus. Vaccine 2008; 26(suppl 3):C25–30. [DOI] [PubMed] [Google Scholar]
- 29. Murall CL, Bauch CT, Day T. Could the human papillomavirus vaccines drive virulence evolution? Proc Biol Sci 2015; 282:20141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brisson M, Edmunds WJ. Economic evaluation of vaccination programs: the impact of herd-immunity. Med Decis Making 2003; 23:76–82. [DOI] [PubMed] [Google Scholar]
- 31. Gandon S, Day T. The evolutionary epidemiology of vaccination. J R Soc Interface 2007; 4:803–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McLean AR. Vaccination, evolution and changes in the efficacy of vaccines: a theoretical framework. Proc Biol Sci 1995; 261:389–93. [DOI] [PubMed] [Google Scholar]
- 33. Thomas KK, Hughes JP, Kuypers JM, et al. Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis 2000; 182:1097–102. [DOI] [PubMed] [Google Scholar]
- 34. Bogaards JA, van der Weele P, Woestenberg PJ, van Benthem BH, King AJ. Bivalent HPV vaccine effectiveness correlates with phylogenetic distance from HPV vaccine types 16 and 18. J Infect Dis 2019; 220:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuschieri K, Ronco G, Lorincz A, et al. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer 2018; 143:735–45. [DOI] [PubMed] [Google Scholar]
- 36. Lissenberg-Witte BI, Bogaards JA, Quint WGV, Berkhof J. Estimating the human papillomavirus genotype attribution in screen-detected high-grade cervical lesions. Epidemiology 2019; 30:590–6. [DOI] [PubMed] [Google Scholar]
- 37. Brotherton JML, Bloem PN. Population-based HPV vaccination programmes are safe and effective: 2017 update and the impetus for achieving better global coverage. Best Pract Res Clin Obstet Gynaecol 2018; 47:42–58. [DOI] [PubMed] [Google Scholar]
- 38. Elfström KM, Lazzarato F, Franceschi S, Dillner J, Baussano I. Human papillomavirus vaccination of boys and extended catch-up vaccination: effects on the resilience of programs. J Infect Dis 2016; 213:199–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.