Abstract
Converging evidence from neuroimaging studies has revealed altered connectivity in cortical–subcortical networks in youth and adults with autism spectrum disorder (ASD). Comparatively little is known about the development of cortical–subcortical connectivity in infancy, before the emergence of overt ASD symptomatology. Here, we examined early functional and structural connectivity of thalamocortical networks in infants at high familial risk for ASD (HR) and low-risk controls (LR). Resting-state functional connectivity and diffusion tensor imaging data were acquired in 52 6-week-old infants. Functional connectivity was examined between 6 cortical seeds—prefrontal, motor, somatosensory, temporal, parietal, and occipital regions—and bilateral thalamus. We found significant thalamic-prefrontal underconnectivity, as well as thalamic-occipital and thalamic-motor overconnectivity in HR infants, relative to LR infants. Subsequent structural connectivity analyses also revealed atypical white matter integrity in thalamic-occipital tracts in HR infants, compared with LR infants. Notably, aberrant connectivity indices at 6 weeks predicted atypical social development between 9 and 36 months of age, as assessed with eye-tracking and diagnostic measures. These findings indicate that thalamocortical connectivity is disrupted at both the functional and structural level in HR infants as early as 6 weeks of age, providing a possible early marker of risk for ASD.
Keywords: ASD, functional connectivity, high-risk infants, structural connectivity, thalamus
Introduction
Autism spectrum disorder (ASD) is a highly heritable neurodevelopmental condition associated with altered brain development (Ecker 2017). While the etiology of ASD remains poorly understood (Chen et al. 2015; Lyall et al. 2017; Constantino 2018), a large body of neuroimaging studies have found abundant evidence implicating both functional and structural connectivity abnormalities in ASD (Ecker et al. 2015; Hernandez et al. 2015; Rane et al. 2015). Resting-state functional connectivity (rs-fcMRI) studies have suggested that ASD is characterized by disrupted connectivity across several brain regions, including atypical connectivity within the default mode (Uddin, Supekar and Menon 2013; Washington et al. 2014; Hull et al. 2017; Padmanabhan et al. 2017), salience (Uddin, Supekar, Lynch, et al. 2013; Abbott et al. 2016; Green et al. 2016; Margolis et al. 2019), central executive (Abbott et al. 2016; Menon 2018; Lawrence et al. 2019), attention (Farrant and Uddin 2016; Duan et al. 2017), social cognition (von dem Hagen et al. 2013; Olivito et al. 2017; Chen et al. 2018; Muller and Fishman 2018), visual (Keown et al. 2013; Chen et al. 2018), and sensorimotor networks (Nebel et al. 2014; Cerliani et al. 2015; Green et al. 2017). Collectively, while these studies have suggested that functional brain connectivity is disrupted in ASD, there is little consensus on a uniform explanatory model of functional brain atypicalities in ASD with age, gender, comorbidities, and various methodological choices likely affecting directionality (i.e., hypo- vs. hyper-connectivity) of the observed atypicalities (Linke et al. 2017; Reiter et al. 2019; Olson et al. 2020).
Additionally, a growing body of work using diffusion tensor imaging (DTI) has also identified atypical structural connectivity in youth with ASD (Di et al. 2018). Indeed, white matter tract integrity has been found to be compromised in individuals with ASD within the corpus callosum, cingulum, projections fibers within subcortical regions (internal/external capsule, anterior thalamic radiation), cerebellum, and associations fibers within the frontal, parietal, temporal, and occipital lobes, such as the uncinate fasciculus, arcuate fasciculus, superior longitudinal fasciculus, inferior longitudinal fasciculus, and fronto-occipital fasciculus in children and adults with ASD (Rane et al. 2015; Ismail et al. 2016; Yamasaki et al. 2017). Recent studies have also shown altered white matter integrity in females with ASD relative to neurotypical females (Rane et al. 2015). Despite little consistency in the localization of the reported atypicalities across studies, the existing literature supports the hypothesis of abnormal development of both functional networks and white matter tracts in ASD.
Neuroimaging studies to date have largely focused on children and adolescents or adults with ASD. While ASD symptomatology typically emerges in the first years of life, there have been comparatively fewer neuroimaging studies with toddlers and younger children with ASD, likely due to the challenges in conducting MRI studies in this age group. In recent years, however, several neuroimaging studies have been undertaken in infants at high familial risk for ASD (i.e., siblings of children with ASD) who have a much higher occurrence rate than the general population (Ozonoff et al. 2011). Prospectively, following these high-risk (HR) infants provides the unique opportunity to study the neural antecedents of risk for developing ASD prior to the onset of overt symptoms. Thus, examining brain connectivity in these young HR infants may help identify early biomarkers of ASD and, relatedly, the deployment of earlier and more effective interventions. Many studies in HR infants have focused on structural brain development; this work has identified enlarged postnatal brain volume, including greater gray matter, white matter, as well as cerebellar and subcortical volume; (Hazlett et al. 2017; Swanson et al. 2017; Wolff et al. 2018; Pote et al. 2019), and increased extra-axial fluid (Shen et al. 2017; Shen and Piven 2017) starting as early as 6 months of age. Studies using DTI in HR infants have identified alterations in white matter tracts connecting frontal, temporal, and subcortical regions, as well as reduced local and global efficiency in occipital, parietal, and temporal lobe tracts between ages 6 and 36 months (Lewis et al. 2014; Conti et al. 2015; Lewis et al. 2017; Shen and Piven 2017; Wolff et al. 2018). Few studies have also found that white matter tract trajectories have a unique pattern of increased fractional anisotropy (FA) at age 6 months followed by slower subsequent growth in HR infants who later developed ASD compared with their non-ASD peers (Wolff et al. 2012; Solso et al. 2016). Interestingly, atypical lateralization of dorsal language tracts has also been observed in HR infants as young as 6 weeks of age, which predicted later language development as well as ASD symptomatology (Liu et al. 2019).
In recent years, studies using rs-fcMRI in HR infants have also began to emerge linking early alterations in functional connectivity to subsequent ASD symptomatology. Notably, Emerson et al. (2017) found that whole-brain rs-fcMRI metrics obtained at 6 months of age and their association with behavioral measures could distinguish between HR infants who were later diagnosed with ASD at age 24 months from those who did not. Other rs-fcMRI studies have implicated specific networks. For instance, atypical functional connectivity in the default mode, dorsal attention, and visual networks has been linked to poor initiation of social attention in HR infants between ages 12 and 24 months (Eggebrecht et al. 2017). In addition, altered patterns of connectivity in these networks, as well as in control and subcortical networks, have been shown to be associated with stereotyped and restricted behaviors in HR infants between 12 and 24 months of age (McKinnon et al. 2019). Of note, two recent rs-fcMRI studies reported atypical longitudinal development of language-related networks from 1.5 to 9 months of age in HR infants (Liu et al. 2020), as well as altered functional connectivity in social brain regions in HR newborns (Ciarrusta et al. 2019), suggesting that the neural underpinnings of atypical language and social development in HR infants may be detectable at birth, or in the first few weeks of life. Some studies have also examined local versus long-range functional connectivity in infants reporting increased local functional connectivity in HR infants (Ciarrusta et al. 2020) and reduced longitudinal development of long-range connectivity from the first few weeks of life to 9 months (Liu et al. 2020).
In parallel to the neuroimaging studies described above, recent work using eye-tracking methods to assess social visual engagement in HR infants has greatly contributed to the search for early neuroendophenotypes of ASD (Klin, Schultz and Jones 2015). HR infants have been shown to exhibit atypical gaze patterns while viewing social stimuli in the first year of life (Elsabbagh et al. 2012, 2013; Chawarska et al. 2013), as well as enhanced visual search abilities and a preference for geometric figures over social scenarios (Gliga et al. 2015; Pierce et al. 2016). Importantly, this aberrant processing of socially relevant information as measured by eye-tracking techniques has been found to be strongly influenced by genetic factors as well as to predict later diagnosis of ASD (Jones and Klin 2013; Constantino et al. 2017). In particular, diminished attention to faces on free-viewing tasks of social scenes (Elsabbagh et al. 2012; Chawarska et al. 2013, 2016; Jones and Klin 2013; Constantino et al. 2017) has been observed in HR infants and toddlers who eventually receive an ASD diagnosis. Less attention to speaking versus non-speaking faces, as well as more attention paid to the mouth versus eyes, has also been associated with familial risk status (Elsabbagh et al. 2014; Shic et al. 2014). A recent study using a free-viewing eye-tracking paradigm involving animated social scene found that while attention to faces increased in the first year of life for both HR infants and control participants, greater attention to faces was particularly associated with better social communicative skills in HR infants (Tsang et al. 2019). To our knowledge, only one study to date has examined the neural correlates of social-visual engagement in HR infants combining both neuroimaging and eye-tracking techniques (Tsang T, Green S, Liu J, Lawrence K, Jeste S, Bookheimer S, Dapretto M. under review. Altered salience network connectivity in 6-week-old infants at risk for autism.). Interestingly, greater connectivity between salience network hubs and prefrontal regions at 6 weeks of age was associated with increased attention to faces in the first year of life only; in contrast, higher salience network connectivity with sensorimotor regions in HR infants was associated with later atypicalities in social and sensorimotor development. Similar to prior functional imaging studies in HR infants, this study focused primarily on cortical network connectivity patterns. However, theoretical models have posited a switch from more reflexive, externally driven sensory responses under subcortical control to experience-dependent responses (e.g., social engagement) under cortical control around 6–8 weeks of age (Johnson and Mareschal 2001; Klin, Shultz, et al. 2015; Shultz et al. 2018). Hence, examining cortical–subcortical connectivity and its relationships to behavioral phenotypes of early social development during this crucial age window is essential to foster our understanding of the pathogenesis of ASD risk.
Of particular interest is the thalamus, a critical subcortical structure involved in the integration of visual, auditory, and somatomotor functions. During development, thalamocortical pathways provide crucial input for the specialization of the neocortex (Schlaggar and O'Leary 1991; Stojic et al. 1998; O'Leary and Nakagawa 2002). Normative patterns of functional thalamocortical connectivity have been identified in neurotypical individuals using rs-fcMRI (Zhang et al. 2008, 2010; Fair et al. 2010). Studies on children and adolescents with ASD have demonstrated both reduced functional thalamocortical connectivity, especially for thalamic-prefrontal networks, and overconnectivity within thalamic-temporal networks (Nair et al. 2013, 2015). In addition to reports of aberrant functional thalamocortical connectivity, there is also evidence of alterations in structural thalamocortical connectivity in ASD, with higher mean diffusivity (MD) indices for motor and somatosensory tracts in individuals with ASD as compared with neurotypical peers (Nair et al. 2013). In another study by Nair et al. (2015), diffusivity indices between thalamus and several prefrontal, parietal, and temporal regions were also impacted with reduced FA within thalamic-prefrontal tracts and higher MD within tracts connecting the thalamus and these regions. Notably, these aberrant patterns of functional and structural thalamocortical connectivity in ASD individuals were found to be associated with poor social interaction skills, executive functioning difficulties, restricted and repetitive behaviors, and sensorimotor atypicalities.
Despite the critical role of the thalamus for the early specialization of the neocortex and evidence implicating altered thalamocortical connectivity in ASD symptomatology (Schlaggar and O'Leary 1991; Stojic et al. 1998; O'Leary and Nakagawa 2002; Nair et al. 2013, 2015; Chen et al. 2016; Green et al. 2017; Woodward et al. 2017; Linke et al. 2018; Iidaka et al. 2019), relevant evidence about the thalamus and its functional and structural connections in HR infants remains limited. Only one study thus far has examined the relationship between structural measures of thalamic functioning and both verbal and nonverbal skill acquisition between 6 and 24 months of age in HR infants, reporting that atypical patterns of language development were associated with thalamic volume in this group (Swanson et al. 2017). Thus, our objective for the current study was to examine both functional and structural thalamocortical connectivity in HR infants during this pivotal developmental transition and to examine whether early atypicalities would predict risk for later developing overt symptoms of ASD. Our hypotheses were that both functional and structural thalamocortical networks would be impacted in HR infants as early as 6 weeks of age. Similarly to what previously observed in older children and adolescents with ASD (Nair et al. 2013, 2015), we expected underconnectivity within thalamic-prefrontal networks and overconnectivity with thalamic-temporal networks along with accompanying atypical diffusivity indices within these networks. We also predicted that these network alterations would be associated with later behavioral phenotypes of ASD such as decreased preferential viewing of social stimuli using eye-tracking, and social interaction deficits on diagnostic measures in the first 3 years of life. One of the primary challenges of studying a small group of infants at high familial risk for ASD is the potential lack of generalizability of our findings to a broader set of ASD individuals with no prior familial risk for ASD. A limited sample may also not allow us to examine differences between HR infants who do eventually receive an ASD diagnosis compared with those who do not. However, our multimodal approach can provide important insights into the early neural mechanisms associated with both familial risk for ASD as well as later social development across both groups.
Materials and Methods
Participants
Infants were recruited as part of a longitudinal developmental project at the University of California, Los Angeles (NIH ACE-II) examining early signs of ASD risk from birth to age 36 months. Our sample consisted of 52 6-week-old infants who successfully underwent an rs-fcMRI scan during natural sleep, including 24 HR infant siblings of children diagnosed with ASD and 28 low-risk (LR) infants with no family history of first or second degree relatives with ASD. DTI data were also collected for a subset of 47 infants (HR = 24 HR; LR = 23); of these, 17 HR and 15 LR infants provided data that met the quality control threshold for DTI analyses (see DTI processing section below). See Table 1 for demographic details of each group. Exclusionary criteria for both groups included prematurity (birth before 36 weeks of gestation), as well as serious medical and neurological conditions (i.e., perinatal stroke, hydrocephalus). Age was corrected to 40 weeks of gestation for infants born between 36 and 38 weeks to account for differences in development within this gestational period. All the HR participants were later-born in their families (i.e., they were not the first-born child), since their higher risk of developing ASD derived from having an older sibling with ASD. In comparison, only 12 out of 28 LR infants (42.8%; between-group P < 0.001) in the rs-fcMRI subset and nine out of 15 LR infants (60%; between-group P < 0.01) in the DTI subset were later-born infants; hence, birth order was also included as a covariate in the imaging analyses to account for these group differences. Written informed consent was obtained from all participants’ caregivers. The study protocol was approved by the UCLA Institutional Review Board.
Table 1.
Demographic information for HR and LR groups for rs-fcMRI and DTI data
| Rs-fcMRI | DTI | |||||
|---|---|---|---|---|---|---|
|
LR
N = 28 |
HR
N = 24 |
P |
LR
N = 15 |
HR
N = 17 |
P | |
| Age at scan (Weeks) | 6.65 ± (1.20) | 6.34 ± (1.21) | .22 | 6.43 ± (1.27) | 6.42 ± (1.08) | .96 |
| Gestation length (Weeks) | 37.99 ± (0.29) | 37.70 ± (0.82) | .52 | 38.82 ± (1.51) | 39.73 ± (0.88) | .07 |
| Sex | .21 | .31 | ||||
| Female | 12 (43%) | 11 (46%) | 4 (27%) | 7 (41%) | ||
| Male | 16 (57%) | 13 (54%) | 11 (73%) | 10 (59%) | ||
| Race | .43 | .36 | ||||
| White | 22 (79%) | 13 (54%) | 12 (80%) | 10 (59%) | ||
| Non-white | 6 (21%) | 11 (46%) | 3 (20%) | 7 (41%) | ||
| Maternal Education | .86 | .89 | ||||
| No college | 1 (4%) | 0 | 0 | 0 | ||
| College degree | 9 (32%) | 17 (71%) | 5 (33%) | 12 (71%) | ||
| Graduate Degree | 18 (64%) | 7 (29%) | 10 (67%) | 5 (29%) | ||
| Family income | .18 | .82 | ||||
| Not answered | 1 (4%) | 2 (8%) | 0 | 2 (12%) | ||
| <50 K | 3 (11%) | 5 (21%) | 2 (13%) | 3 (18%) | ||
| 50–75 K | 5 (18%) | 4 (17%) | 2 (13%) | 2 (12%) | ||
| 75–100 K | 6 (21%) | 5 (21%) | 4 (27%) | 4 (23%) | ||
| 100–125 K | 4 (14%) | 2 (8%) | 2 (13%) | 2 (12%) | ||
| >125 K | 9 (32%) | 6 (25%) | 5 (33%) | 4 (23%) | ||
Study Design
As part of the longitudinal study, both HR and LR participant groups underwent MRI scanning (including rs-fcMRI and DTI) at corrected age 6 weeks in order to examine the connectivity between six cortical regions of interest (ROIs)—prefrontal, parietal, occipital, motor, somatosensory, and temporal—and bilateral thalamus. For all imaging analyses, gestational age, birth order, and head motion (in the case of rs-fcMRI) were modeled as covariates and cluster-corrected to control for multiple comparisons at P < 0.05. To elucidate the relationship between these thalamocortical networks and early social correlates of risk for ASD, we also examined behavioral measures that were collected across multiple timepoints as part of the broader longitudinal project protocol. These measures involved a free-view eye-tracking session at ages 6, 9, and 12 months, involving animated social interactions where our metric of interest was attention to faces. Additionally, diagnostic measures of risk for ASD symptoms (including clinician administered measures and caregiver reports) were collected at different time points between 12 and 36 months of age in both HR and LR infants. The eye-tracking metric and diagnostic measures used in our analyses were selected a priori to examine the relationship between imaging indices of thalamocortical connectivity and both early social functioning and risk for ASD. For all correlations between imaging indices and eye-tracking metric, head motion and language development scores were included as covariates. For correlations between imaging indices and diagnostic measures of risk for ASD, head motion and overall cognitive development scores were included as covariates. All the analyses correlating imaging indices and behavioral measures were Bonferroni-corrected to control for multiple comparisons at P < 0.05. Details about the imaging analyses and behavioral measures used are provided below.
Imaging Data Acquisition
Participants underwent MRI at age 6 weeks on a 3 T Siemens Trio scanner using a 12-channel head coil during natural sleep. Parents were instructed to put their infant to sleep using their typical routine before transferring the infant to the scanner bed. Infants were placed on a custom-made bed situated inside the head coil and secured with a Velcro strap. Pliable and soft silicone earplugs and MiniMuffs Neonatal Noise Attentuators (Natus Medical Inc.) were used as hearing protection. A weighted blanket and foam pads were used to minimize motion. Additionally, a research staff member remained in the scanner room during the scan to monitor infants for waking, movement, or signs of distress. A scout localizer was used for alignment. The rs-fcMRI scan was acquired using an EPI gradient-echo acquisition lasting 8 min, covering the whole cerebral volume [TR = 2000 ms, TE = 28 ms, field of view (FOV) = 192 mm, 34 slices, voxel size = 3 × 3 × 4mm]. A high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR = 5000 ms, TE = 34 ms, FOV = 192 mm, 34 slices, voxel size = 1.5 × 1.5 × 4mm) was acquired coplanar with the functional scans to ensure identical distortion characteristics of the images. The DTI scans (TR = 9500 ms, TE = 87 ms, FOV = 256 mm, 75 slices, voxel size = 2 × 2 × 2mm) consisted of 32 diffusion-weighted directions (b = 1000), three scans without any diffusion sensitization (b = 0), and six additional directions (b = 50).
Behavioral Measures
For the eye-tracking task, video stimuli were a 2-min free-viewing segment of “A Charlie Brown Christmas” used in prior eye-tracking studies administered at ages 6, 9, and 12 months of age using a Tobii T60XL system (Frank et al. 2014; Tsang et al. 2019). The video stimuli (See Supplementary Fig. 1) consisted of animated characters conversing (~80 s), cheering and dancing in a group (~20 s), and speaking to a group of characters (~20 s). One animated character was an anthropomorphized dog, which was present in ~20 cumulative, but nonconsecutive, seconds of the clip; all other animated characters were depictions of human children. Caregivers held their infant on their lap for the duration of the video, ~60 cm from the computer monitor (65-cm screen; 720 × 480 pixels resolution; <0.05o visual angle; 60-Hz temporal resolution).
At 12 months, participants completed the Autism Observation Scale for Infants (AOSI; Bryson et al. 2008)—an 18-item observational measure used to identify and track early signs of ASD administered by expert clinicians. At 24 months, caregiver ratings were obtained on the Social Responsiveness Scale, Second Edition–Preschool version (SRS-P; Constantino and Gruber 2012)—a 65-item checklist of ASD symptomatology with ratings ranging from 0 (not true) to 3 (almost always true). At 36 months, the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al. 2012)—a semi-structured standardized assessment of ASD symptoms was administered by expert clinicians who were research-reliable on this measure. Finally, at 36 months, the Mullen Scales of Early Learning (Mullen 1995) was also administered to both HR and LR participants, to obtain an estimate of early language development (Mullen Verbal Development Quotient; VDQ) and overall cognitive development (Mullen Cognitive Development scores; see Table 2 for group means for each measure).
Table 2.
Descriptive statistics for imaging indices, eye-tracking metrics, and behavioral outcomes measures for HR and LR groups
| LR | HR | ||||
|---|---|---|---|---|---|
| Mean (SD) | Range (Min-Max) | Mean (SD) | Range (Min-Max) | P | |
| Rs-fcMRI | N = 28 | N = 24 | |||
| Prefrontal | 0.093 (.128) | (0.132–0.332) | −0.121 (0.112) | (−0.342–0.056) | <0.01 |
| Parietal | 0.134 (0.179) | (−0.201–0.402) | 0.151 (0.162) | (−0.224–0.426) | 0.73 |
| Occipital | 0.014 (0.148) | (−0.176–0.417) | 0.091 (0.145) | (−0.135–0.354) | 0.05 |
| Motor | 0.083 (0.150) | (−0.226–0.462) | 0.164 (0.126) | (−0.120–0.398) | 0.03 |
| Somatosensory | 0.119 (0.211) | (−0.465–0.512) | 0.076 (0.153) | (−0.170–0.398) | 0.42 |
| Temporal | 0.224 (0.112) | (0.033–0.541) | 0.245 (0.158) | (−0.063–0.492) | 0.61 |
| DTI | N = 15 | N = 17 | |||
| Prefrontal FA | 0.165 (0.038) | (0.135–0.299) | 0.211 (0.018) | (0.137–0.211) | 0.55 |
| Prefrontal MD | 0.001 (0.0004) | (0.0012–0.0013) | 0.001 (0.0007) | (0.0011–0.0014) | 0.48 |
| Parietal FA | 0.151 (0.040) | (0.125–0.288) | 0.158 (0.013) | (0.120–0.158) | 0.31 |
| Parietal MD | 0.001 (0.0005) | (0.0011–0.0012) | 0.001 (0.0005) | (0.0011–0.0013) | 0.09 |
| Occipital FA | 0.144 (0.041) | (0.117–0.283) | 0.143 (0.008) | (0.115–0.143) | 0.41 |
| Occipital MD | 0.001 (0.0004) | (0.0011–0.0012) | 0.001 (0.0003) | (0.0011–0.0012) | 0.03 |
| Motor FA | 0.318 (0.045) | (0.127–0.318) | 0.191 (0.018) | (0.128–0.191) | 0.51 |
| Motor MD | 0.001 (0.0007) | (0.0012–0.0013) | 0.001 (0.0001) | (0.0011–0.0014) | 0.17 |
| Somatosensory FA | 0.342 (0.048) | (0.143–0.342) | 0.166 (0.019) | (0.139–0.203) | 0.43 |
| Somatosensory MD | 0.001 (0.0005) | (0.0010–0.0011) | 0.001 (0.0007) | (0.0010–0.0012) | 0.43 |
| Temporal FA | 0.123 (0.030) | (0.123–0.251) | 0.137 (0.008) | (0.124–0.157) | 0.47 |
| Temporal MD | 0.001 (0.0003) | (0.0012–0.0013) | 0.001 (0.0005) | (0.0012–0.0014) | 0.34 |
| Eye-tracking (6 months) | N = 21 | N = 23 | |||
| Fixation on faces (%) | 66.3 (20.0) | (29.0–97.4) | 63.4 (22.4) | (25.9–98.4) | 0.42 |
| Eye-tracking (9 months) | N = 24 | N = 23 | |||
| Fixation on faces (%) | 66.7 (24.0) | (30.4–98.4) | 63.4 (17.6) | (39.8–93.5) | 0.39 |
| Eye-tracking (12 months) | N = 20 | N = 20 | |||
| Fixation on faces (%) | 72.8 (18.8) | (43.2–98.9) | 66.5 (22.0) | (27.3–97.4) | 0.18 |
| a AOSI (12 months) | N = 24 | N = 25 | |||
| Total score | 4.2 (1.8) | (1.0–9.0) | 4.8 (3.2) | (1.0–15.0) | 0.42 |
| b SRS-P (24 months) | N = 24 | N = 17 | |||
| Total score | 43.2 (10.4) | (8.0–57.0) | 53.8 (17.5) | (8.0–72.0) | <0.01 |
| c ADOS-2 (36 months) | N = 25 | N = 22 | |||
| Comparison score | 2.7 (2.5) | (1.0–10.0) | 3.5 (2.4) | (1.0–10.0) | 0.25 |
| d Mullen (36 months) | N = 24 | N = 21 | |||
| VDQ | 59.31 (10.09) | (23.0–73.0) | 49.52 (13.63) | (19.0–63.5) | 0.05 |
| cognitive development | 236.75 (40.59) | (86.0–294.0) | 201.19 (54.80) | (78.0–273.0) | 0.06 |
aAOSI yields a Total max score of 44 and each item is rated on a scale of 0–3; AOSI scores of 0 indicate typical function, scores of 2 or 3 indicate atypical function with increasing severity of impairment.
bSRS-P: Social Responsiveness Scale, Second Edition—Preschool version; T-scores have a mean of 50 and SD of 10.
cADOS-2: Autism Diagnostic Observation Schedule, Second Edition; ADOS-2 Comparison scores range from 1 to 10, scores ≤2 indicate minimal-to-low evidence of autism-related symptoms, and scores of 3 indicate low level evidence of autism-related symptoms.
dMullen VDQ: Mullen Verbal Development Quotient; an average of Expressive and Receptive Language subscale T-scores; T-scores have a mean of 50 and SD of 10.
dMullen Cognitive Development: Global Cognitive Development T-score is a sum of Visual Reception, Fine Motor, Receptive Language, and Expressive Language subscale T-scores; Cognitive Development T-scores have a mean of 200 and SD of 30.
Data Analysis
Regions of Interest
The automated anatomical labeling atlas normalized to the UNC neonate template (Shi et al. 2011) was used to obtain bilateral masks for our six cortical seeds: motor, occipital, parietal, prefrontal, somatosensory, and temporal regions (Fig. 1A). The masks for these cortical ROIs were created by combining anatomical parcellations from this atlas (see Supplementary Table 1 for a complete listing of parcellations comprising each cortical ROI). For DTI analyses, white matter tracts connecting only those thalamocortical regions, which demonstrated impacted functional connectivity findings, were examined.
Figure 1 .
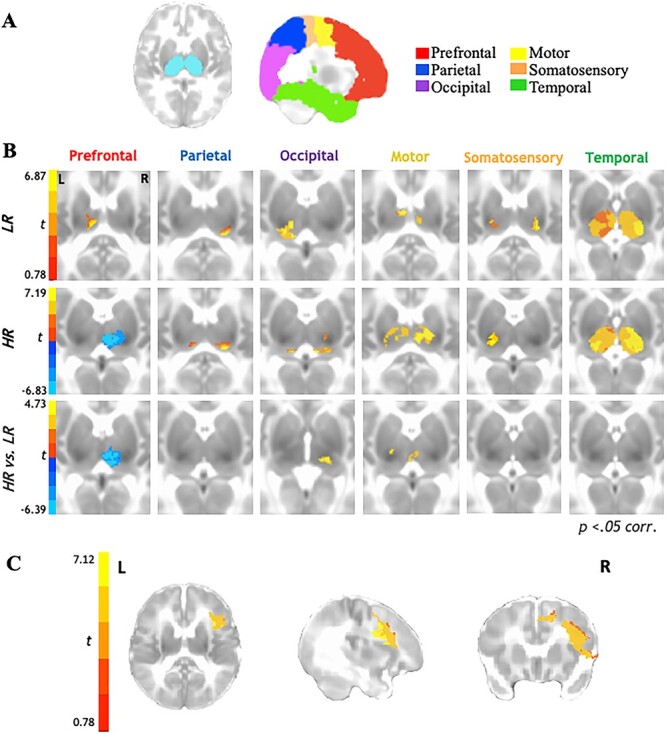
Functional thalamocortical connectivity results for 6-week-old infants at higher-risk (HR) for developing ASD compared with their LR peers. (A) Bilateral thalamic masks and cortical seeds of interest. (B) Partial correlation analysis results indicate thalamocortical connectivity for the LR group (top row) consistent with prior literature with typically developing children and adults. In contrast, HR group shows marked underconnectivity for prefrontal-thalamic networks (middle row). Between-samples paired t-test results indicate that HR group shows additional overconnectivity effects for occipital-thalamic and motor-thalamic networks as compared with LR group (bottom row). (C) Reverse connectivity analysis using thalamic clusters of underconnectivity with prefrontal ROI for HR group implicates that the right inferior frontal gyrus as driving the prefrontal-thalamic underconnectivity findings.
Rs-fcMRI Data Processing
Functional images were processed using the Analysis of Functional NeuroImages (Cox 1996) and FMRIB Software Library suites (Smith et al. 2004). Skull-stripping was done using AFNI’s 3dskullstrip for the structural images, and AFNI’s 3dautomask was used for the functional images. The functional images were then slice-time and head motion corrected registering each functional volume to the average functional volume using FSL’s FLIRT. For group comparisons, images were standardized to the UNC neonate template using FSL’s nonlinear registration tool. In order to reduce motion artifacts, six rigid-body motion parameters were modeled as nuisance variables and removed with regression from all analysis. Motion for each time point, which is defined as root mean square of the sum displacement (RMSD) of all six translational and rotational axes, was determined for each participant. For any instance of RMSD >0.5 mm, the time point was censored, and only participants with ≥80% (≥192) time points remaining were included. No significant group differences were found for the total number of volumes scrubbed per group; an average of 7.12 volumes were removed for HR group (11 participants) and 6.76 for LR group (8 participants) (t(50) = 0.06, P = 0.95). RMSD was significantly higher in the HR group prior to censoring [F(2,50) = 6.27, P = 0.02], but there were no group differences after censoring [F(2,50) = 2.27, P = 0.11]. Postcensoring RMSD was used as a co-variate in all group-level analyses. In order to isolate spontaneous low-frequency BOLD fluctuations (Cordes et al. 2001), rs-fcMRI time series were bandpass filtered (0.008 < f < 0.08 Hz), using a second-order Butterworth filter, which was also applied to all nuisance regressors described below. Images were spatially smoothed to a Gaussian full width at half-maximum (FWHM) of 5 mm, using AFNI’s 3dBlurToFWHM.
Similar to prior thalamocortical studies in healthy individuals and youth with ASD, partial correlation analyses were performed in AFNI to obtain the unique connectivity pattern between each cortical ROI and thalamus bilaterally (Zhang et al. 2008; Fair et al. 2010; Nair et al. 2013). The average BOLD time series was extracted from each cortical ROI and FOV was restricted to bilateral thalamus. Partial correlations were computed between each bilateral cortical ROI and each voxel within bilateral thalamus, eliminating the shared variance by regressing out the time series from the other cortical ROIs. Two-sample, two-tailed t-tests were performed for between-group comparisons, and all statistical maps were adjusted for multiple comparisons to a corrected P < 0.05 using AFNI’s 3dClustSim (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). For all resulting significant clusters, parameter estimates of connectivity strength were extracted in AFNI and then correlated with behavioral measures. For ROIs demonstrating atypical connectivity patterns with bilateral thalamus in the HR group, a reverse connectivity approach was also implemented wherein the timeseries were extracted from clusters resulting from the main partial correlation analyses and then correlated with all voxels within the corresponding cortical ROI (using simple correlations) in order to determine whether specific regions within the larger cortical ROIs were driving the observed connectivity patterns.
DTI Data Processing
The diffusion-weighted imaging data were checked for data quality using DTIPrep (Liu et al. 2010), which automatically detects and tracks bad gradient diffusion-weighted images. Volumes were also visually inspected, and if the presence of residual artifacts was detected, these were then removed. Datasets including less than 23 (72%) gradient diffusion-weighted images were excluded due to low signal-to-noise ratio (Wolff et al. 2012). Thirteen datasets did not meet this threshold and were removed, resulting in a final sample of 34 infants (HR = 19, LR = 15) for these analyses. Groups did not differ significantly on average number of gradients remaining following quality control (gradients remaining for HR: M = 30.80, SD = 1.90; gradients remaining for LR: M = 30.68, SD = 1.67; P = 0.85). DTI data were then preprocessed and analyzed using FSL’s FDT—FMRIB’s Diffusion Toolbox version 3.0 (Behrens et al. 2003, 2007). FSL’s FDT includes motion correction, eddy current correction, and brain extraction. DTIFit was used to fit the diffusion tensor model at each voxel for fiber tracking. FSL was used to preprocess the T2 structural scan that was used to register diffusion data. At last, the DTI data were registered to a neonate template brain in standard space (Shi et al. 2011) using a 12-parameter affine transformation.
Probabilistic fiber tracking was performed using FDT to derive white matter tracts originating from each cortical seed and terminating at the thalamus. Unique exclusion masks were created for each target ROI (whole cortex minus specific target ROI), and the bilateral thalamus was selected as the termination mask. FSL’s BEDPOSTX was used to generate a Bayesian estimate of the probability distribution of different directions at each voxel. For each subject, all tractography outputs were first thresholded to exclude voxels with connectivity values less than 10 000 tracts. These tracts were then normalized to standard space and summed to create a normalized tract, which was thresholded to included voxels that belonged to a particular tract in at least 50% of the subjects within each group. Two additional HR participants were excluded during fiber tracking as their target tracts did not meet these thresholding standards resulting in a final sample of 17 HR and 15 LR infants. The diffusion tensor was calculated at each voxel, and indices of white matter connectivity strength, including FA and MD, were generated for tracts between each cortical ROI and thalamus. A one-way analysis of variance (ANOVA) was performed in SPSS software version 25.0 (IBM) for between group comparisons for these DTI indices within each cortical ROI-thalamic tract.
Eye-tracking Data Processing
For the “Charlie Brown” eye-tracking task, a 9-point calibration scheme was repeatedly used prior to data collection until the infant’s point of gaze (PoG) was within 1o of the center of the target. The video stimuli were presented only after the calibration criterion had been reached to ensure accuracy in PoG data to be included in the analyses. Eye-tracking data were not available for 1 HR and 3 LR participants at age 6-months; 1 HR and 2 LR participants at age 9 months, and for 1 HR and 4 LR participants at age 12 months. Additionally, infants were excluded due to a failure to complete the eye-tracking calibration procedure described (6-month: 0 HR; 2 LR; 9-month: 0 HR; 1 LR; 12-month: 1 HR; 1 LR). An additional 2 (0 HR, 2 LR) 6-month-old, 1 (0 HR, 1 LR) 9-month-old, and 5 (2 HR, 3 LR) 12-month-old infants were excluded due to excessive fussiness (e.g., crying and/or excessive movement), which compromised data quality (PoG <30% of viewing time). Importantly, the number of trials provided by each infant did not differ by risk status; from 6 to 12 months, HR and LR infants contributed an average of 5.15 ± 1.76 and 5.46 ± 1.86 trials (P = 0.42), respectively, and participated an average of 2.65 ± 0.13 and 2.28 ± 0.71 sessions (P = 0.14). On-screen valid looking time also did not differ by risk status at 6-month (HR: M = 65.61%, LR: M = 68.37%; P = 0.56), 9-month (HR: M = 68.52%, LR: M = 72.19%; P = 0.13), and 12-month (HR: M = 67.43%, LR: M = 70.21%; P = 0.28) timepoints. Our metric of interest was percent fixation on any face during the depicted social interactions. The areas of interest including each character’s face (anthropomorphized dog, human children) were hand-traced similar to prior research with the same stimuli (Frank et al. 2014; Tsang et al. 2019) using software written in MATLAB (MathWorks, Inc.). On average, the pixels that included faces on average comprised 37.42 ± 16.49% of the screen. PoGs that fell within a 30-pixel radius of our face area of interest for a minimum of 100 ms were classified as visual fixations. Valid on-screen viewing time was operationalized as the percent of total fixations directed to any part of the display relative to the total duration of the video stimuli. This measure of valid looking time was also used as a measure of overall task engagement. For data obtained at age 6, 9, and 12 months, we calculated the percentage of time fixating on faces relative to the total duration of the video stimuli, respectively (see Table 2 for N’s and group means at each time point).
Correlations with Behavioral Indices
Since the Mullen VDQ scores were correlated with percentage of total fixations on faces for both the LR (r = 0.76, P < 0.01) and HR (r = 0.84, P < 0.01) groups, partial correlations controlling for language development (Mullen VDQ scores) were conducted in SPSS between these eye-tracking metrics (collected at 6, 9, and 12 months of age) and both rs-fcMRI and DTI indices of connectivity strength for regions where significant group differences were observed. Additionally, postcensoring RMSD was controlled for in these partial correlations between rs-fcMRI indices and eye-tracking metrics. In the HR group only, these functional and structural connectivity indices were also correlated with the total scores on the AOSI, SRS-P, and ADOS-2. Partial correlations were conducted in SPSS between the imaging indices (controlling for postcensoring RMSD and Mullen Cognitive Development scores at age 36 months) and these diagnostic measures.
Results
Thalamocortical Connectivity
Rs-fcMRI results (Fig. 1B; Table 2) between the six cortical ROIs and bilateral thalamus indicated that in both LR and HR groups (Fig. 1B; top panel, middle panel), parietal and occipital cortices were largely connected with the pulvinar, motor cortices were connected with the anterior ventral and mediodorsal nuclei, and somatosensory cortices were connected with the posterior ventral nuclei; temporal cortices showed robust connectivity with most thalamic nuclei at this age across both groups. Prefrontal cortices showed positive connectivity with anterior/mediodorsal nuclei of the thalamus in the LR group (Fig. 1B; top panel); in contrast, the HR group displayed negative connectivity between prefrontal cortices and the anterior and mediodorsal (extending to lateral dorsal) nuclei of the thalamus (Fig. 1B; middle panel). Direct between-group comparisons confirmed these differences to be statistically significant (Fig. 1B; bottom panel), with prefrontal-thalamic networks showing significant underconnectivity in the HR group compared with LR group. Additionally, between-group comparisons also revealed that the HR group showed overconnectivity in thalamic-occipital and thalamic-motor networks as compared with the LR group (Fig. 1B; bottom panel).
Given that the prefrontal cortex comprises a relatively large ROI, we further examined which specific functional subregions of the prefrontal cortex might be driving the thalamic-prefrontal underconnectivity observed in the HR group. For this reverse functional connectivity analysis, we extracted the timeseries from the thalamic cluster showing significant underconnectivity between the prefrontal cortical ROI and bilateral thalamus and then correlated this timeseries with each voxel within the prefrontal ROI. Results from this correlation analysis (Fig. 1C) indicated significant connectivity between this thalamic cluster and the right inferior frontal gyrus (see Supplementary Fig. 2 for an additional partial correlation analysis between right inferior frontal gyrus and bilateral thalamus revealing significant negative thalamic connectivity within the same thalamic cluster previously identified using the larger prefrontal ROI).
Next, we examined whether white matter structural connectivity was impacted within the thalamocortical networks where we observed atypical connectivity in our rs-fcMRI analyses. That is, we examined group differences in DTI indices within the thalamic-prefrontal, thalamic-occipital, and thalamic-motor white matter tracts. Results from the ANOVA analyses run with all DTI indices for each thalamocortical tract revealed that MD (P = 0.03) was significantly higher in the HR group compared with the LR group (Fig. 2; Table 2), though FA values did not differ significantly between the two groups (P = 0.41). No other significant group differences were observed across any DTI indices for the other thalamocortical tracts.
Figure 2 .
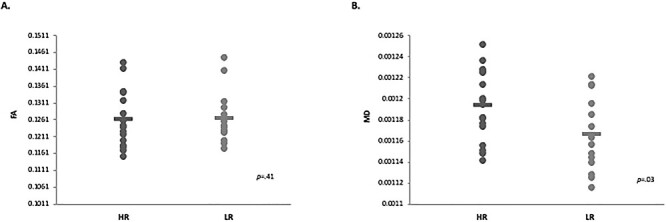
Diffusion tensor imaging thalamic-occipital connectivity results for 6-week-old HR infants compared with their LR peers. (A) Between-group results indicate no significant differences between groups for FA for thalamic-occipital tracts. (B) Between-group results indicate that MD was significantly higher for HR group compared with LR group for thalamic-occipital tracts.
Brain-Behavior Relationships
Partial correlations controlling for head motion and language development were conducted between eye-tracking metrics and brain-based indices of functional and structural connectivity extracted from regions showing significant between-group differences (i.e., rs-fcMRI connectivity strength for thalamic-prefrontal, thalamic-occipital, and thalamic-motor networks; DTI diffusivity indices for thalamic-occipital network). In the LR group, greater thalamic-prefrontal rs-fcMRI connectivity strength was associated with higher percent time spent looking at faces at age 9 months (r = −0.57, P = 0.01; Fig. 3A); similar patterns of correlations were observed in this group at the 6- and 12-month timepoints, but these did not survive correction for multiple comparisons. No other significant correlations between brain-based measures and eye-tracking metrics survived correction for multiple comparisons in either group.
Figure 3 .
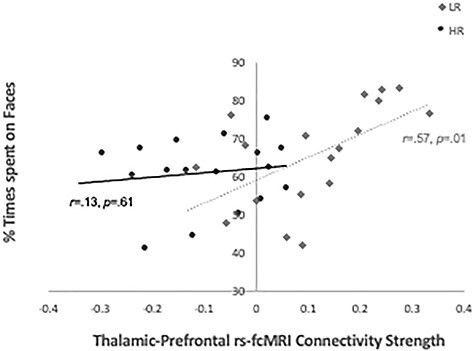
Partial correlations (controlling for postcensoring RMSD, and Mullen VDQ scores at 36 months) with eye-tracking indices on the “Charlie Brown” paradigm between percent time spent looking at faces during the eye-tracking task and thalamic-prefrontal rs-fcMRI connectivity strength in HR and LR groups at age 9 months.
At last, for the HR group only, we examined the relationship between the same indices of functional and structural connectivity with caregiver ratings and clinician-administered measures of ASD risk. Partial correlation results after controlling for head motion and overall cognitive development showed that higher thalamic-occipital rs-fcMRI connectivity strength was associated higher scores on the SRS-P obtained at age 24 months (r = 0.56, P = 0.03; Fig. 4A) and higher comparison scores on the ADOS-2 obtained at age 36 months (r = 0.55, P = 0.02; Fig. 4B). No other significant correlations were observed between connectivity indices and both caregiver ratings and clinician-administered measures.
Figure 4 .
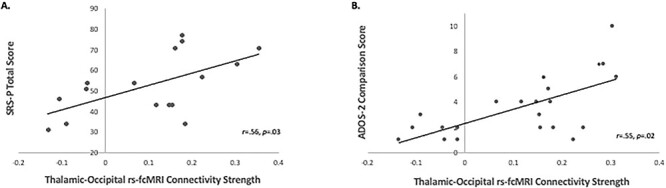
Partial correlations with diagnostic measures of ASD risk controlling for head motion and overall cognitive development. (A) Partial correlation (controlling for postcensoring RMSD, and Mullen Cognitive Development score at age 36 months) between rs-fcMRI connectivity strength for thalamic-occipital network and SRS-P total T-score at 24 months for the HR group. (B) Partial correlations (controlling for RMSD postcensoring, and Mullen Cognitive Development score at age 36 months) between rs-fcMRI connectivity strength for thalamic-occipital network and ADOS-2 comparison score at 36 months for the HR group.
Notably, five out of 24 HR infants (~21%) met criteria for ASD diagnosis using the ADOS-2 at age 36 months and expert clinical judgment, along with two out of 28 LR infants (~7%). However, LR infants who met the criteria for ASD diagnosis at age 36 months were not excluded from the imaging analyses since group assignment was based on familial risk, rather than diagnostic outcome. Additionally, the LR infants who eventually received an ASD diagnosis were not outliers on the imaging indices within the LR sample; hence, we opted to retain them in the current study (see Supplementary Figs 3 and 4 for plots of imaging indices with participants meeting criteria for ASD highlighted for both groups).
Discussion
This is the first multimodal study to examine both the functional and structural thalamocortical connectivity in infants at high (HR) and low (LR) familial risk for ASD as early as 6-week postbirth. Overall, our findings indicate that thalamocortical connectivity patterns in LR infants were similar to what observed in prior studies in older neurotypical individuals (Zhang et al. 2008, 2010; Fair et al. 2010; Nair et al. 2013, 2015). In contrast, connectivity of thalamocortical networks in the HR group was atypical with significant functional underconnectivity observed in thalamic-prefrontal networks, accompanied by functional overconnectivity in thalamic-motor and thalamic-occipital networks. Structural connectivity of thalamocortical networks was also impacted with higher white matter diffusivity observed in thalamic-occipital tracts in HR infants as compared with LR infants. Interestingly, indices of both functional and structural thalamocortical connectivity at 6 weeks of age were predictive of later social development and risk for ASD. More specifically, stronger thalamic-prefrontal functional connectivity in LR infants was associated with greater attention to faces (vs. non-social but salient visual information) as assessed with eye-tracking from 6 to 12 months of age; in contrast, aberrant connectivity in thalamic-occipital networks in HR infants was associated with increased ASD symptomatology from 24 to 36 months, as indexed by both caregivers’ report and clinician administered measures.
Aberrant Connectivity Patterns
In LR infants, thalamocortical connectivity patterns generally mirrored those reported in prior studies in older neurotypical individuals (Zhang et al. 2008, 2010; Fair et al. 2010; Nair et al. 2013, 2015) whereby prefrontal cortices show strongest connections with anterior and medial nuclei of the thalamus, motor cortices with ventral anterior and lateral nuclei, somatosensory cortices with ventral posterior nuclei, parietal and occipital cortices with lateral pulvinar, and temporal cortices with medial pulvinar. While it is hard to accurately identify thalamic nuclei in such young infants, it appears that our findings in LR infants closely match this normative pattern observed in adolescents and adults, except for temporal cortices which showed significant functional connectivity with almost all thalamic nuclei. Our results are also consistent with prior studies on thalamocortical functional connectivity in full-term new-born infants (Alcauter et al. 2014; Toulmin et al. 2015; Cai et al. 2017) demonstrating thalamocortical network organization similar to what observed in older individuals, despite the use of slightly different ROIs. Interestingly, preterm infants (gestational age = 24–32 weeks) instead showed thalamic underconnectivity with prefrontal regions and overconnectivity with postcentral gyri compared with full-term infants (Toulmin et al. 2015). Additionally, prior research has suggested that the first 2 years of life are marked by rapid myelination (indexed by increasing FA and decreasing MD) of crucial white matter tracts in the developing neonate brain (Geng et al. 2012; Cancelliere et al. 2013; Yu et al. 2020). However, our current findings of increased diffusivity in thalamic-occipital tracts are more consistent with recent studies that have specifically found increased diffusivity in white matter tracts of preterm compared with full-term neonates including occipital white matter (Li et al. 2016; Knight et al. 2018). Thus, the patterns of thalamocortical connectivity observed in HR infants in the current study are similar to those seen in preterm infants in that thalamic-prefrontal networks were underconnected, whereas thalamic connectivity with primary sensory regions were overconnected, along with possible delays in myelination of crucial white matter thalamocortical tracts.
The observed pattern of reduced connectivity between thalamus and supramodal cortical regions (prefrontal), and increased connectivity between thalamus and primary cortical regions (visuo-motor) in HR infants in this study also align well with the altered thalamocortical connectivity previously observed in youth with ASD (Nair et al. 2013, 2015). In these prior thalamocortical studies, functional underconnectivity was reported with prefrontal, parieto-occipital, motor, and somatosensory cortices, along with overconnectivity with temporal and limbic cortices. However, DTI indices in the underlying thalamocortical white matters tracts in these prior studies were found to be mostly impacted in frontal, temporal, and parietal regions, in contrast to our current findings of impacted white matter connectivity in thalamic-occipital tracts in HR infants. Interestingly, a study by (Fair et al. 2010) examining the typical maturational trajectories of these thalamocortical networks from childhood to adulthood suggested an increase in connectivity within thalamic-prefrontal, thalamic-motor, and thalamic-somatosensory networks with age, whereas thalamic-temporal connectivity typically appeared to weaken with increasing age. Hence, our prior findings on thalamocortical connectivity in youth with ASD as well as the current findings in HR infants suggest that both of these groups may be on a delayed and/or differential maturational trajectory for subcortical–cortical connectivity compared with normative peers. Notably, however, thalamic-occipital networks showed a quadratic developmental trajectory in healthy controls with connectivity peaking in adolescent years and weakening with age (Fair et al. 2010); thus, the early thalamic-occipital overconnectivity in HR infants and subsequent underconnectivity in ASD youth observed in the current and prior studies suggest a more complicated disruption in the maturation of this network that may be linked to distinct behavioral symptoms at each developmental period.
Associations with Social Attention and Risk for ASD
The atypicalities in early brain connectivity we observed in the present study, and their relationships with later eye-tracking and behavioral measures, suggest disruptions in specific thalamocortical networks implicated in social attention and engagement in HR infants even after controlling for group differences in language and overall cognitive development. In LR infants, thalamic-prefrontal functional connectivity was associated with greater attention to social stimuli such as faces in the first year of life. In contrast, this relationship was not observed in HR infants who instead showed thalamic underconnectivity with prefrontal cortex, particularly the right inferior frontal gyrus. Notably, this region has been implicated in endogenous as well as exogenous attentional shifts (Peelen et al. 2004; de Fockert and Theeuwes 2012; Caruana et al. 2015; Koike et al. 2016), as well as social cognition processes (Hartwigsen et al. 2019; Sato and Uono 2019). Accordingly, atypical thalamic connectivity with this region may underlie later difficulties in orienting attention to socially relevant information in HR infants. Additionally, aberrant functional connectivity in thalamic-occipital networks in HR infants was associated with an increased ASD symptoms as observed by caregivers and clinicians from 24 to 36 months. As thalamic-occipital networks are involved in modulation and orientation of visual engagement (Carrera and Bogousslavsky 2006; Arend et al. 2008; Snow et al. 2009; Li et al. 2018), early disruptions in the neural development of these networks may contribute to atypicalities in the processing of visual information. The group differences we observed in the relationships between thalamocortical connectivity and behavioral and diagnostic measures during the first year of life could possibly be a very early biomarker for subsequent difficulties with socio-visual engagement and risk for the ASD phenotype.
A number of eye-tracking studies in HR infants have previously highlighted that disrupted attention to and visual processing of social information may be a precursor to eventual diagnosis of ASD. Collectively, this body of work has demonstrated diminished attention to faces (Elsabbagh et al. 2012; Chawarska et al. 2013, 2016; Jones and Klin 2013; Constantino et al. 2017) and biological motion (Klin et al. 2009; Klin, Klaiman, et al. 2015) in infants and toddlers who eventually receive an ASD diagnosis. Importantly, neuroimaging findings have also shown that network atypicalities in frontal language regions and white matter tracts involved in low-level sensory processing in HR infants as young as 6 months of age are predictive of an eventual ASD diagnosis (Lewis et al. 2014, 2017). The current study extends these prior findings and highlights the role of early thalamic-prefrontal connectivity in scaffolding normative social attention as well as the involvement of altered thalamic-occipital connectivity for increased risk for ASD symptoms. Taken together, the present findings suggest that atypical subcortical connectivity with prefrontal and occipital regions might provide one of the earliest indicators of aberrant brain development in HR infants.
Atypical social attention has also been demonstrated in prior research with older youth with ASD (Klin et al. 2002; Caron et al. 2006; McPartland et al. 2011; Samson et al. 2012; Guillon et al. 2014; Chita-Tegmark 2016), together with findings of reduced functional connectivity in social cognition networks involving prefrontal, limbic, and posterior temporal brain regions in ASD individuals (Pelphrey et al. 2011; Gotts et al. 2012; von dem Hagen et al. 2013; Cheng et al. 2015; Linke et al. 2018; Muller and Fishman 2018; Delbruck et al. 2019; Maximo and Kana 2019; Odriozola et al. 2019; Sato and Uono 2019). Importantly, key ASD symptomatology including atypical social attention and repetitive stereotyped behaviors has also been previously linked to thalamocortical network disruptions in youth with ASD (Nair et al. 2013, 2015). Our current findings thus suggest that very early disruptions in key thalamocortical circuits involved in social-visual engagement—such as thalamic-prefrontal and thalamic-occipital networks—may underlie risk for atypical social development in HR infants, preceding and perhaps contributing to the overt manifestation of overt ASD symptoms later in development.
While the expected recurrence rate of ASD diagnosis in our HR sample (~21%) is consistent with prior reports in this population (Ozonoff et al. 2011), given the small number of HR infants who were diagnosed with ASD at 36 month of age, it is important to note that the observed differences in brain connectivity patterns, and related associations with deficits in social attention, can only be taken to reflect familial risk for ASD rather than ASD diagnostic outcome. Interestingly, the patterns of aberrant thalamocortical connectivity observed in our HR group as a whole are similar to those seen in very preterm infants (Toulmin et al. 2015) even though gestation length within our sample (36–40 weeks) was not associated with our reported group differences (Supplementary Figs 5–7). The atypical thalamocortical connectivity found in the current study is thus likely to underlie risk for a broader spectrum of neurodevelopmental challenges in HR infants paralleling the poorer cognitive, socio-communicative, and psychiatric; outcomes often associated with preterm birth (Johnson and Marlow 2011; Maxwell et al. 2017; Zmyj et al. 2017; Stalnacke et al. 2019). Indeed, our HR sample demonstrated significantly lower verbal abilities, global cognitive development, and socio-communicative skills than their LR peers during their toddler years (Table 2), consistent with prior studies highlighting suboptimal cognitive development, socio-communicative deficits, and psychiatric symptomatology (e.g., ADHD, anxiety disorders) in this group regardless of ASD diagnosis (Schwichtenberg et al. 2013; Ozonoff et al. 2014; Miller et al. 2015, 2016; Shephard et al. 2017). Future investigations with larger samples of HR infants, as well as broader longitudinal assessment of cognitive and psychiatric outcomes over protracted time periods, will be critical to fully elucidate the relationship between early atypicalities in thalamocortical connectivity and risk for the ASD phenotype versus other suboptimal developmental trajectories.
Limitations and Conclusions
This study has several limitations. As mentioned above, the overall limited sample size only allowed us to identify atypicalities associated with increased risk for ASD-like social difficulties, as opposed to those predictive of a subsequent ASD diagnosis. The sample size for our structural connectivity analyses was further reduced, relative to the sample used in our analyses of functional connectivity, as infants often woke up more before or during the DTI sequence which was acquired in the MRI session. Reduced power for these analyses could partly explain the limited between-group differences observed in structural connectivity (after correction for multiple comparisons). Additionally, several participants had missing or unusable eye-tracking data across the three different timepoints (6, 9, and 12 months of age); this may have impacted the outcomes of our correlational analyses with eye-tracking metrics, resulting in trend-level associations with atypical connectivity indices for some of the timepoints that did not survive correction for multiple comparisons.
To the best of our knowledge, this is the first study to examine thalamocortical functional and structural connectivity in new-born infant siblings at high familial risk for ASD. Our results indicate disruptions in functional and structural thalamocortical connectivity occurring very early in life in infants at high familial risk for ASD and implicate early thalamocortical connectivity in both typical and atypical social development. Our findings further our understanding of the early neural mechanisms underlying risk for the ASD and have implications for identifying earlier time frames for screening HR infants as well as informing targeted interventions that may help steer brain development toward more normative pathways.
Funding
National Institute of Child Health and Human Development (NICHD P50 HD055784); Autism Science Foundation Postdoctoral Fellowship (16-004 to A.N.); Brain Mapping Medical Research Organization; Brain Mapping Support Foundation; Pierson-Lovelace Foundation; The Ahmanson Foundation; Capital Group Companies Charitable Foundation; William M. and Linda R. Dietel Philanthropic Fund; Northstar Fund; National Center for Research Resources; Office of the Director of the National Institutes of Health (award numbers C06RR012169, C06RR015431, and S10OD011939). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Notes
Human subjects’ oversight and approval was provided by UCLA’s Institutional Review Board. Special thanks to the participants and their families, and staff and volunteers who contributed toward imaging data collection. Conflict of Interests: The authors declare that they have no competing interests.
Supplementary Material
References
- Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, Muller RA. 2016. Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cereb Cortex. 26:4034–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W. 2014. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci. 34:9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend I, Machado L, Ward R, McGrath M, Ro T, Rafal RD. 2008. The role of the human pulvinar in visual attention and action: evidence from temporal-order judgment, saccade decision, and antisaccade tasks. Prog Brain Res. 171:475–483. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. 2007. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli Oet al. 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 6:750–757. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. 2008. The autism observation scale for infants: scale development and reliability data. J Autism Dev Disord. 38:731–738. [DOI] [PubMed] [Google Scholar]
- Cai Y, Wu X, Su Z, Shi Y, Gao JH. 2017. Functional thalamocortical connectivity development and alterations in preterm infants during the neonatal period. Neuroscience. 356:22–34. [DOI] [PubMed] [Google Scholar]
- Cancelliere A, Mangano FT, Air EL, Jones BV, Altaye M, Rajagopal A, Holland SK, Hertzler DA 2nd, Yuan W. 2013. DTI values in key white matter tracts from infancy through adolescence. AJNR Am J Neuroradiol. 34:1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron MJ, Mottron L, Berthiaume C, Dawson M. 2006. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 129:1789–1802. [DOI] [PubMed] [Google Scholar]
- Carrera E, Bogousslavsky J. 2006. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 66:1817–1823. [DOI] [PubMed] [Google Scholar]
- Caruana N, Brock J, Woolgar A. 2015. A frontotemporoparietal network common to initiating and responding to joint attention bids. NeuroImage. 108:34–46. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. 2015. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiat. 72:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Powell K, DiNicola L, Shic F. 2016. Enhanced social attention in female infant siblings at risk for autism. J Am Acad Child Adolesc Psychiatry. 55:188–195.e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Shic F. 2013. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol Psychiatry. 74:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Uddin LQ, Zhang Y, Duan X, Chen H. 2016. Atypical effective connectivity of thalamo-cortical circuits in autism spectrum disorder. Autism Res. 9:1183–1190. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang J, Uddin LQ, Wang X, Guo X, Lu F, Duan X, Wu L, Chen H. 2018. Aberrant functional connectivity of neural circuits associated with social and sensorimotor deficits in young children with autism spectrum disorder. Autism Res. 11:1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JA, Penagarikano O, Belgard TG, Swarup V, Geschwind DH. 2015. The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol. 10:111–144. [DOI] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Gu H, Zhang J, Feng J. 2015. Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain J Neurol. 138:1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chita-Tegmark M. 2016. Social attention in ASD: a review and meta-analysis of eye-tracking studies. Res Dev Disabil. 48:79–93. [DOI] [PubMed] [Google Scholar]
- Ciarrusta J, Dimitrova R, Batalle D, O'Muircheartaigh J, Cordero-Grande L, Price A, Hughes E, Kangas J, Perry E, Javed Aet al. 2020. Emerging functional connectivity differences in newborn infants vulnerable to autism spectrum disorders. Transl Psychiatry. 10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrusta J, O'Muircheartaigh J, Dimitrova R, Batalle D, Cordero-Grande L, Price A, Hughes E, Steinweg JK, Kangas J, Perry Eet al. 2019. Social brain functional maturation in newborn infants with and without a family history of autism spectrum disorder. JAMA Netw Open. 2:e191868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. 2018. Deconstructing autism: from unitary syndrome to contributory developmental endophenotypes. Int Rev Psychiatry. 30:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. 2012. Social responsiveness scale. 2nd ed. Los Angeles (CA): Western Psychological Services. [Google Scholar]
- Constantino JN, Kennon-McGill S, Weichselbaum C, Marrus N, Haider A, Glowinski AL, Gillespie S, Klaiman C, Klin A, Jones W. 2017. Infant viewing of social scenes is under genetic control and is atypical in autism. Nature. 547:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Calderoni S, Marchi V, Muratori F, Cioni G, Guzzetta A. 2015. The first 1000 days of the autistic brain: a systematic review of diffusion imaging studies. Front Hum Neurosci. 9:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. 2001. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol. 22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Fockert JW, Theeuwes J. 2012. Role of frontal cortex in attentional capture by singleton distractors. Brain Cogn. 80:367–373. [DOI] [PubMed] [Google Scholar]
- Delbruck E, Yang M, Yassine A, Grossman ED. 2019. Functional connectivity in ASD: atypical pathways in brain networks supporting action observation and joint attention. Brain Res. 1706:157–165. [DOI] [PubMed] [Google Scholar]
- Di X, Azeez A, Li X, Haque E, Biswal BB. 2018. Disrupted focal white matter integrity in autism spectrum disorder: a voxel-based meta-analysis of diffusion tensor imaging studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 82:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chen H, He C, Long Z, Guo X, Zhou Y, Uddin LQ, Chen H. 2017. Resting-state functional under-connectivity within and between large-scale cortical networks across three low-frequency bands in adolescents with autism. Prog Neuro-Psychopharmacol Biol Psychiatry. 79:434–441. [DOI] [PubMed] [Google Scholar]
- Ecker C. 2017. The neuroanatomy of autism spectrum disorder: an overview of structural neuroimaging findings and their translatability to the clinical setting. Autism. 21:18–28. [DOI] [PubMed] [Google Scholar]
- Ecker C, Bookheimer SY, Murphy DG. 2015. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol. 14:1121–1134. [DOI] [PubMed] [Google Scholar]
- Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, Adams CM, Snyder AZ, Lewis JD, Estes AMet al. 2017. Joint attention and brain functional connectivity in infants and toddlers. Cereb Cortex. 27:1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Bedford R, Senju A, Charman T, Pickles A, Johnson MH, Team B. 2014. What you see is what you get: contextual modulation of face scanning in typical and atypical development. Soc Cogn Affect Neurosci. 9:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH, Team B. 2013. The development of face orienting mechanisms in infants at-risk for autism. Behav Brain Res. 251:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, Pickles A, Baron-Cohen S, Bolton P, Johnson MHet al. 2012. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Curr Biol. 22:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RW, Adams C, Nishino T, Hazlett HC, Wolff JJ, Zwaigenbaum L, Constantino JN, Shen MD, Swanson MR, Elison JTet al. 2017. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 9:eaag2882. doi: 10.1126/scitranslmed.aag2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, Snyder AZ, Raichle ME, Stevens AA, Nigg JTet al. 2010. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant K, Uddin LQ. 2016. Atypical developmental of dorsal and ventral attention networks in autism. Dev Sci. 19:550–563. [DOI] [PubMed] [Google Scholar]
- Frank MC, Amso D, Johnson SP. 2014. Visual search and attention to faces during early infancy. J Exp Child Psychol. 118:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Gouttard S, Sharma A, Gu H, Styner M, Lin W, Gerig G, Gilmore JH. 2012. Quantitative tract-based white matter development from birth to age 2years. NeuroImage. 61:542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga T, Bedford R, Charman T, Johnson MH, Team B. 2015. Enhanced visual search in infancy predicts emerging autism symptoms. Curr Biol. 25:1727–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. 2012. Fractionation of social brain circuits in autism spectrum disorders. Brain J Neurol. 135:2711–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, Dapretto M. 2016. Salience network connectivity in autism is related to brain and behavioral markers of sensory overresponsivity. J Am Acad Child Adolesc Psychiatry. 55:618–626.e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, Dapretto M. 2017. Reduced modulation of thalamocortical connectivity during exposure to sensory stimuli in ASD. Autism Res. 10:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon Q, Hadjikhani N, Baduel S, Roge B. 2014. Visual social attention in autism spectrum disorder: insights from eye tracking studies. Neurosci Biobehav Rev. 42:279–297. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Neef NE, Camilleri JA, Margulies DS, Eickhoff SB. 2019. Functional segregation of the right inferior frontal gyrus: evidence from coactivation-based parcellation. Cereb Cortex. 29:1532–1546. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KNet al. 2017. Early brain development in infants at high risk for autism spectrum disorder. Nature. 542:348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Rudie JD, Green SA, Bookheimer S, Dapretto M. 2015. Neural signatures of autism spectrum disorders: insights into brain network dynamics. Neuropsychopharmacology: official publication of the American college of. Neuropsychopharmacology. 40:171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull JV, Dokovna LB, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD. 2017. Resting-state functional connectivity in autism spectrum disorders: a review. Front Psych. 7:205. doi: 10.3389/fpsyt.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Kogata T, Mano Y, Komeda H. 2019. Thalamocortical Hyperconnectivity and amygdala-cortical hypoconnectivity in male patients with autism spectrum disorder. Front Psych. 10:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail MM, Keynton RS, Mostapha MM, ElTanboly AH, Casanova MF, Gimel'farb GL, El-Baz A. 2016. Studying autism spectrum disorder with structural and diffusion magnetic resonance imaging: a survey. Front Hum Neurosci. 10:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Mareschal D. 2001. Cognitive and perceptual development during infancy. Curr Opin Neurobiol. 11:213–218. [DOI] [PubMed] [Google Scholar]
- Johnson S, Marlow N. 2011. Preterm birth and childhood psychiatric disorders. Pediatr Res. 69:11R–18R. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. 2013. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 504:427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Muller RA. 2013. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep. 5:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. 2002. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 59:809–816. [DOI] [PubMed] [Google Scholar]
- Klin A, Klaiman C, Jones W. 2015. Reducing age of autism diagnosis: developmental social neuroscience meets public health challenge. Rev Neurol. 60(Suppl 1):S3–S11. [PMC free article] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. 2009. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 459:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Shultz S, Jones W. 2015. Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neurosci Biobehav Rev. 50:189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MJ, Smith-Collins A, Newell S, Denbow M, Kauppinen RA. 2018. Cerebral white matter maturation patterns in preterm infants: an MRI T2 relaxation anisotropy and diffusion tensor imaging study. J Neuroimaging. 28:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T, Tanabe HC, Okazaki S, Nakagawa E, Sasaki AT, Shimada K, Sugawara SK, Takahashi HK, Yoshihara K, Bosch-Bayard Jet al. 2016. Neural substrates of shared attention as social memory: a hyperscanning functional magnetic resonance imaging study. NeuroImage. 125:401–412. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Hernandez LM, Bookheimer SY, Dapretto M. 2019. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 12:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Evans AC, Pruett JR, Botteron K, Zwaigenbaum L, Estes A, Gerig G, Collins L, Kostopoulos P, McKinstry Ret al. 2014. Network inefficiencies in autism spectrum disorder at 24 months. Transl Psychiatry. 4:e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Evans AC, Pruett JR Jr, Botteron KN, McKinstry RC, Zwaigenbaum L, Estes AM, Collins DL, Kostopoulos P, Gerig Get al. 2017. The emergence of network inefficiencies in infants with autism spectrum disorder. Biol Psychiatry. 82:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BX, Liu GS, Ling XY, Chen HF, Luo XQ. 2016. Evaluation of white matter myelination in preterm infants using DTI and MRI. Zhongguo Dang Dai Er Ke Za Zhi. 18:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kumar Y, Gupta N, Abdelbaki A, Sahwney H, Kumar A, Mangla M, Mangla R. 2018. Clinical and neuroimaging findings in thalamic territory infarctions: a review. J Neuroimaging. 28:343–349. [DOI] [PubMed] [Google Scholar]
- Linke AC, Jao Keehn RJ, Pueschel EB, Fishman I, Muller RA. 2018. Children with ASD show links between aberrant sound processing, social symptoms, and atypical auditory interhemispheric and thalamocortical functional connectivity. Dev Cogn Neurosci. 29:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke AC, Olson L, Gao Y, Fishman I, Muller RA. 2017. Psychotropic medication use in autism spectrum disorders may affect functional brain connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Okada NJ, Cummings KK, Jung J, Patterson G, Bookheimer SY, Jeste SS, Dapretto M. 2020. Emerging atypicalities in functional connectivity of language-related networks in young infants at high familial risk for ASD. Dev Cogn Neurosci. 45:100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tsang T, Jackson L, Ponting C, Jeste SS, Bookheimer SY, Dapretto M. 2019. Altered lateralization of dorsal language tracts in 6-week-old infants at risk for autism. Dev Sci. 22:e12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, Styner M. 2010. Quality control of diffusion weighted images. Proc SPIE Int Soc Opt Eng. 7628: 76280J--. doi: 10.1117/12.844748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. 2012. Autism diagnostic observation schedule. 2nd ed. Los Angeles (CA): Western Psychological Services. [Google Scholar]
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, Park BY, Snyder NW, Schendel D, Volk Het al. 2017. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. 38:81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Pagliaccio D, Thomas L, Banker S, Marsh R. 2019. Salience network connectivity and social processing in children with nonverbal learning disability or autism spectrum disorder. Neuropsychology. 33:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximo JO, Kana RK. 2019. Aberrant "deep connectivity" in autism: a cortico-subcortical functional connectivity magnetic resonance imaging study. Autism Res. 12:384–400. [DOI] [PubMed] [Google Scholar]
- Maxwell JR, Yellowhair TR, Oppong AY, Camacho JE, Lowe JR, Jantzie LL, Ohls RK. 2017. Cognitive development in preterm infants: multifaceted deficits reflect vulnerability of rigorous neurodevelopmental pathways. Minerva Pediatr. 69:298–313. [DOI] [PubMed] [Google Scholar]
- McKinnon CJ, Eggebrecht AT, Todorov A, Wolff JJ, Elison JT, Adams CM, Snyder AZ, Estes AM, Zwaigenbaum L, Botteron KNet al. 2019. Restricted and repetitive behavior and brain functional connectivity in infants at risk for developing autism spectrum disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 4:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Webb SJ, Keehn B, Dawson G. 2011. Patterns of visual attention to faces and objects in autism spectrum disorder. J Autism Dev Disord. 41:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. 2018. The triple network model, insight, and large-scale brain organization in Autism. Biol Psychiatry. 84:236–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Iosif AM, Young GS, Hill M, Phelps Hanzel E, Hutman T, Johnson S, Ozonoff S. 2016. School-age outcomes of infants at risk for autism spectrum disorder. Autism Res. 9:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Young GS, Hutman T, Johnson S, Schwichtenberg AJ, Ozonoff S. 2015. Early pragmatic language difficulties in siblings of children with autism: implications for DSM-5 social communication disorder? J Child Psychol Psychiatry. 56:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. 1995. Mullen scales of early learning. AGS ed. Circle Pines (MN): American Guidance Service Inc. [Google Scholar]
- Muller RA, Fishman I. 2018. Brain connectivity and neuroimaging of social networks in autism. Trends Cogn Sci. 22:1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Carper RA, Abbott AE, Chen CP, Solders S, Nakutin S, Datko MC, Fishman I, Muller RA. 2015. Regional specificity of aberrant thalamocortical connectivity in autism. Hum Brain Mapp. 36:4497–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller RA. 2013. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain J Neurol. 136:1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Joel SE, Muschelli J, Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. 2014. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp. 35:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DD, Nakagawa Y. 2002. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr Opin Neurobiol. 12:14–25. [DOI] [PubMed] [Google Scholar]
- Odriozola P, Dajani DR, Burrows CA, Gabard-Durnam LJ, Goodman E, Baez AC, Tottenham N, Uddin LQ, Gee DG. 2019. Atypical frontoamygdala functional connectivity in youth with autism. Dev Cogn Neurosci. 37:100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivito G, Clausi S, Laghi F, Tedesco AM, Baiocco R, Mastropasqua C, Molinari M, Cercignani M, Bozzali M, Leggio M. 2017. Resting-state functional connectivity changes between dentate nucleus and cortical social brain regions in autism spectrum disorders. Cerebellum. 16:283–292. [DOI] [PubMed] [Google Scholar]
- Olson LA, Mash LE, Linke A, Fong CH, Muller RA, Fishman I. 2020. Sex-related patterns of intrinsic functional connectivity in children and adolescents with autism spectrum disorders. Autism. 24:2190–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, Johnson S, Miller M, Rogers SJ, Schwichtenberg AJet al. 2014. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry. 53:398–407.e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins Ket al. 2011. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 128:e488–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Lynch CJ, Schaer M, Menon V. 2017. The default mode network in autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Heslenfeld DJ, Theeuwes J. 2004. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. NeuroImage. 22:822–830. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. 2011. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry. 52:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Marinero S, Hazin R, McKenna B, Barnes CC, Malige A. 2016. Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biol Psychiatry. 79:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pote I, Wang S, Sethna V, Blasi A, Daly E, Kuklisova-Murgasova M, Lloyd-Fox S, Mercure E, Busuulwa P, Stoencheva Vet al. 2019. Familial risk of autism alters subcortical and cerebellar brain anatomy in infants and predicts the emergence of repetitive behaviors in early childhood. Autism Res. 12:614–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane P, Cochran D, Hodge SM, Haselgrove C, Kennedy DN, Frazier JA. 2015. Connectivity in autism: a review of MRI connectivity studies. Harv Rev Psychiatry. 23:223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter MA, Mash LE, Linke AC, Fong CH, Fishman I, Muller RA. 2019. Distinct patterns of atypical functional connectivity in lower-functioning autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 4:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Mottron L, Soulieres I, Zeffiro TA. 2012. Enhanced visual functioning in autism: an ALE meta-analysis. Hum Brain Mapp. 33:1553–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Uono S. 2019. The atypical social brain network in autism: advances in structural and functional MRI studies. Curr Opin Neurol. 32:617–621. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, O'Leary DD. 1991. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science. 252:1556–1560. [DOI] [PubMed] [Google Scholar]
- Schwichtenberg AJ, Young GS, Hutman T, Iosif AM, Sigman M, Rogers SJ, Ozonoff S. 2013. Behavior and sleep problems in children with a family history of autism. Autism Res. 6:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, Emerson RW, Shaw D, Elison JT, Swanson MRet al. 2017. Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biol Psychiatry. 82:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Piven J. 2017. Brain and behavior development in autism from birth through infancy. Dialogues Clin Neurosci. 19:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E, Milosavljevic B, Pasco G, Jones EJ, Gliga T, Happe F, Johnson MH, Charman T, Team B. 2017. Mid-childhood outcomes of infant siblings at familial high-risk of autism spectrum disorder. Autism Res. 10:546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, Shen D. 2011. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One. 6:e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shic F, Macari S, Chawarska K. 2014. Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biol Psychiatry. 75:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Klin A, Jones W. 2018. Neonatal transitions in social behavior and their implications for autism. Trends Cogn Sci. 22:452–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DEet al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Snow JC, Allen HA, Rafal RD, Humphreys GW. 2009. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc Natl Acad Sci U S A. 106:4054–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solso S, Xu R, Proudfoot J, Hagler DJ Jr, Campbell K, Venkatraman V, Carter Barnes C, Ahrens-Barbeau C, Pierce K, Dale Aet al. 2016. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol Psychiatry. 79:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnacke SR, Tessma M, Bohm B, Herlenius E. 2019. Cognitive development trajectories in preterm children with very low birth weight longitudinally followed until 11 years of age. Front Physiol. 10:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojic AS, Lane RD, Killackey HP, Qadri BA, Rhoades RW. 1998. Thalamocortical and intracortical projections to the forelimb-stump SI representation of rats that sustained neonatal forelimb removal. J Comp Neurol.. 401:187–204. [PubMed] [Google Scholar]
- Swanson MR, Shen MD, Wolff JJ, Elison JT, Emerson RW, Styner MA, Hazlett HC, Truong K, Watson LR, Paterson Set al. 2017. Subcortical brain and behavior phenotypes differentiate infants with autism versus language delay. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmin H, Beckmann CF, O'Muircheartaigh J, Ball G, Nongena P, Makropoulos A, Ederies A, Counsell SJ, Kennea N, Arichi Tet al. 2015. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc Natl Acad Sci U S A. 112:6485–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T, Johnson S, Jeste S, Dapretto M. 2019. Social complexity and the early social environment affect visual social attention to faces. Autism Res. 12:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, Ryali S, Menon V. 2013. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiat. 70:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. 2013. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ. 2013. Reduced functional connectivity within and between 'social' resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 8:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, Warburton S, Sawyer AT, Wolfe A, Mease-Ference ER, Girton L, Hailu A, Mbwana Jet al. 2014. Dysmaturation of the default mode network in autism. Hum Brain Mapp. 35:1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AMet al. 2012. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 169:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Jacob S, Elison JT. 2018. The journey to autism: insights from neuroimaging studies of infants and toddlers. Dev Psychopathol. 30:479–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Giraldo-Chica M, Rogers B, Cascio CJ. 2017. Thalamocortical dysconnectivity in autism spectrum disorder: an analysis of the autism brain imaging data exchange. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Maekawa T, Fujita T, Tobimatsu S. 2017. Connectopathy in autism spectrum disorders: a review of evidence from visual evoked potentials and diffusion magnetic resonance imaging. Front Neurosci. 11:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Peng Y, Kang H, Peng Q, Ouyang M, Slinger M, Hu D, Shou H, Fang F, Huang H. 2020. Differential white matter maturation from birth to 8 years of age. Cereb Cortex. 30:2673–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. 2008. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 100:1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. 2010. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 20:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmyj N, Witt S, Weitkamper A, Neumann H, Lucke T. 2017. Social cognition in children born preterm: a perspective on future research directions. Front Psychol. 8:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


