Abstract
Malaria begins when Plasmodium-infected Anopheles mosquitoes take a blood meal on a vertebrate. During the initial probing process, mosquitoes inject saliva and sporozoites into the host skin. Components of mosquito saliva have the potential to influence sporozoite functionality. Sporozoite-associated mosquito saliva protein 1 (SAMSP1; AGAP013726) was among several proteins identified when sporozoites were isolated from saliva, suggesting it may have an effect on Plasmodium. Recombinant SAMSP1 enhanced sporozoite gliding and cell traversal activity in vitro. Moreover, SAMSP1 decreased neutrophil chemotaxis in vivo and in vitro, thereby also exerting an influence on the host environment in which the sporozoites reside. Active or passive immunization of mice with SAMSP1 or SAMSP1 antiserum diminished the initial Plasmodium burden after infection. Passive immunization of mice with SAMSP1 antiserum also added to the protective effect of a circumsporozoite protein monoclonal antibody. SAMSP1 is, therefore, a mosquito saliva protein that can influence sporozoite infectivity in the vertebrate host.
Keywords: mosquito, saliva protein, sporozoite, gliding, cell traversal
Sporozoite-associated mosquito saliva protein (SAMSP1; AGAP013726) is associated with sporozoites and SAMSP1 has positive impact on sporozoites. When SAMSP1 is blocked by active or passive immunization, there is significantly less Plasmodium infection in mice.
Malaria is a major infectious cause of disease and death in much of the world [1]. Infection begins when Plasmodium-infected Anopheles mosquitoes engorge on a vertebrate host. When probing for a blood meal, the infected mosquito injects saliva along with sporozoites into the skin. Sporozoites then migrate through the dermis and invade blood vessels, which transport the pathogen to the liver where systemic infection and replication begin. During the probing process, components of saliva induce vasodilatation, alter coagulation, and modulate other host responses to facilitate feeding [2–5]. In diverse arthropod-pathogen relationships, saliva can influence transmission to the vertebrate host, including sandflies and leishmania [6, 7], Aedes mosquitoes and flaviviruses [8, 9], and ticks and Borrelia [10]. Specific components in Anopheles gambiae saliva can also alter the efficiency of Plasmodium transmission. As an example, AgTRIO influences the host response during mosquito bite, and partially facilitates Plasmodium infection in mice [11]. In contrast, A. gambiae mosquito gamma interferon-inducible thiol reductase (mosGILT) diminishes the ability of Plasmodium to infect mice, demonstrating that different factors in saliva have diverse and competing effects [12, 13].
When sporozoites and mosquito saliva are inoculated into the skin, gliding motility and cell traversal activity help Plasmodium move through the dermis, penetrate blood vessels, and subsequently invade hepatocytes to establish the initial phase of systemic infection [14–17]. These processes are complex, and only partially understood. Expressed by sporozoites, the cell traversal for ookinetes and sporozoites protein facilitates sporozoite gliding and traversal [18]. Moreover, sporozoite proteins essential for cell traversal 1 and 2 [19, 20], and the gamete egress and sporozoite traversal protein [21] contribute to the traversal of host cells. In contrast, the mosquito saliva protein, mosGILT, has a negative impact on sporozoite cell traversal [12]. In the current study, we characterized another mosquito saliva protein, sporozoite-associated mosquito saliva protein 1 (SAMSP1; AGAP013726), that enhances Plasmodium traversal of host cells. Immunization of mice with SAMSP1 also decreases the initial liver burden of Plasmodium berghei infection in mice. Our data suggest that saliva protein, SAMSP1, helps facilitate Plasmodium infectivity in the vertebrate host and can serve as a target to help provide immunity against Plasmodium infection.
METHODS
Ethics Statement
All animal experiment protocols were approved by the Yale University Institutional Animal Care & Use Committee (protocol no. 2017–07941). All Plasmodium infections were performed in biosafety level 2 animal facilities. The Senegal National Ethics Committee (Senegal) and the Marseille-2 Ethical Committee (France) approved the ethical collection of human serum samples (approval no. 2006-A00581-50). The samples did not have any identifiable markers and had been used in the previous publications [22, 23].
Glutathione S-transferase–SAMSP1 Protein Expression
The SAMSP1 genetic sequence without the signal peptide region was amplified from the genome of A. gambiae and cloned into the pGEX-6-P2 vector by Gibson assembly (NEB). The primers are listed in Supplementary Table 1. The SAMSP1-expressing plasmid was transformed into BL21 chemically competent cells. Expression of the glutathione S-transferase (GST)–SAMSP1 fusion protein was induced with 0.1-mmol/L Isopropyl-β-D-Thiogalactopyranoside (IPTG) at 17°C for 24 hours. The cells were sonicated in phosphate-buffered saline (PBS), and soluble GST-SAMSP1 was purified using glutathione-sepharose 4B and the GST-tag was removed with PreScission Protease (GE Healthcare).
Generation of Polyclonal SAMSP1 Rabbit Antiserum
Recombinant SAMSP1 was emulsified in complete Freund’s adjuvant (CFA) and injected subcutaneously into a rabbit (200 μg per animal per injection). The rabbit was boosted twice at 2-week intervals with SAMSP1 and incomplete Freund’s adjuvant (IFA). Serum was collected 2 weeks after the last boost. Immunizations and serum collection was performed by Cocalico Biologicals. Antibody responses against SAMSP1 were determined using enzyme-linked immunosorbent assay (ELISA) and immunoblot (data not shown). Polyclonal rabbit immunoglobulin (Ig) G against SAMSP1 was purified using 1 mL of NAb Protein A/G Plus spin columns (Thermo Fisher Scientific).
SAMSP1 Protein Synthesis and Purification
To express SAMSP1 from insect cells, the SAMSP1 gene was subcloned from pGEX-SAMSP1 into pMT/Bip/V5-HisB. The primers used are listed in Supplementary Table 1. The plasmid was transfected into Drosophila S2 cells (American Type Culture Collection) with Cellfectin II (Thermo Fisher Scientific). The transfected S2 cells were grown in Schneider’s medium containing 10% fetal bovine serum, 1% penicillin-streptomycin, and 300-μg/mL hygromycin-B. To express SAMSP1 protein, the transfected S2 cells were induced with 500-μmol/L copper sulfate [12]. The SAMSP1 protein was purified from the medium using a cobalt resin column (Takara Bio). The purity was confirmed by means of sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (Supplementary Figure 1) and immunoblot (data not shown). Based on our group’s previous studies related to the concentration of mosquito saliva protein [11, 12], 1-µg/mL (0.05-mmol/L) or 10-µg/mL (0.5-mmol/L) SAMSP1 was used to study the effect of SAMSP1 in vitro and in vivo.
Animals
A. gambiae mosquitoes (4arr strain) were raised at 27°C and80% humidity, under a 12/12-hour light/dark cycle, and were maintained with 10% sucrose under standard laboratory conditions in the insectary at Yale University. Swiss Webster and C57BL/6 mice were purchased from Charles River Laboratories.
Mouse Active Immunization
Five-week-old female C57BL/6 mice were immunized with either 10 µg of SAMSP1 or ovalbumin (OVA) emulsified in CFA and then given 2 boosts of the respective antigen with IFA every 2 weeks. One week after the final boost, serum was collected from each mouse, and the titers for each antigen were tested with an ELISA to confirm antigen-specific antibodies.
P. berghei Infection
P. berghei (ANKA or NK65 RedStar; American Type Culture Collection) were maintained by serial passage in 6–8-week-old female Swiss Webster or C57BL/6 mice, as described elsewhere [22]. Briefly, Swiss Webster or C57BL/6 mice were challenged with P. berghei–infected red blood cells by intraperitoneal injection. A. gambiae mosquitoes then took a blood meal from the infected mice, when the parasitemia was approximately 5%. At 17–24 days after P. berghei infection, the mosquitoes were sorted using the fluorescent signal of the salivary glands. The salivary glands from infected mosquitoes were used to harvest the sporozoites.
Intradermal Injection of Sporozoites
The sporozoites were collected from salivary glands and then passed through 30 gauge syringes 10–15 times. The cell debris was removed by filtering through a 40-μm filter mesh. C57BL/6 mice were anesthetized with 100 mg/kg of ketamine and 10 mg/kg of xylazine. Next, 300 sporozoites were injected into the left ear of each mouse using glass micropipettes with a 80-µm-diameter beveled opening and a Nanoject II Auto-Nanoliter Injector (Drummond). To determine the burden of Plasmodium infection, the mice were euthanized 40 hours after injection, and their livers were collected for RNA extraction.
Gene Expression and Plasmodium Load
The expression of SAMSP1 in mosquito salivary glands and Plasmodium burden in livers were determined by means of reverse-transcription polymerase chain reaction (RT-PCR), which is described in the Supplementary Methods.
Cell Traversal Assay
The cell traversal assay is presented in detail in the Supplementary Methods.
Gliding Activity of Sporozoites In Vitro
The sporozoites were plated in a 96-well plate with Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum. The sporozoites were allowed to glide for 5 minutes at room temperature. The movement of sporozoites were recorded using the EVOS FL Auto cell imaging system (Thermo Fisher Scientific) and analyzed using IMARIS 9.5 software (Oxford Instruments).
Immunostaining of Sporozoites
Immunostaining was performed as described elsewhere [12]. Briefly, after fixed with 4% paraformaldehyde and then blocked with 1% bovine serum albumin (BSA) in PBS, the sporozoites were incubated with 100-µg/mL mouse anti-SAMSP1 IgG or anti-OVA IgG. The slides were then incubated with a goat anti-mouse Alexa Fluor 555 secondary antibody (Thermo Fisher Scientific; 1:500). Sporozoites were viewed using an EVOS cell imaging system.
Western Blotting Assay and ELISA
Western blotting assay and ELISA details are presented in the Supplementary Methods.
Analysis of Immune Cells by Flow Cytometry
The cell collection and the staining of cells are described in the Supplementary Methods. Samples were run on a Cytoflex flow cytometer and analyzed using FlowJo software.
Neutrophil Collection from Bone Marrow
Naive primary neutrophils were isolated from 8–10-week-old Swiss Webster mice, which is presented in detail in the Supplementary Methods.
Transwell Assay for Neutrophil Chemotaxis
A neutrophil chemotaxis Transwell assay was performed as described elsewhere [24]. Briefly, 106 neutrophils were put in the 3-μm pore size inserts (Costar 3421), and the inserts were put in 12-well plate with 500 μL of DMEM with 40-μg/mL f-Met-Leu-Phe (fMLP) and/or 3-μg/mL SAMSP1 protein for 2 hours in 5% carbon dioxide and 37°C incubator. After 1- and 2-hour incubations, the number of cells in each well was calculated using a hemocytometer.
Statistical Analysis
Data from ≥3 biological replicates were used to calculate means or medians for graphing purposes. The data was then assessed using a normality test, and when appropriate, the difference between the groups was examined using the unpaired Student t test, and the data were presented as means with standard deviations. When a normality test was not sufficient, statistical analyses used the Mann-Whitney test to determine the difference and the data were presented as median with interquartile range. The differences between >3 groups were compared using 1-way analysis of variance, and a post hoc analysis was used to determine whether there was significant difference between the control group and experimental group. Differences were considered statistically significant at P < .05. The analysis, graphs, and statistics of all comparisons were performed using Prism 9.0 software (GraphPad Software).
RESULTS
SAMSP1 Association With Sporozoites
Our group’s previously published data, using mass spectrometry to analyze sporozoites that were isolated from mosquito saliva, identified several saliva proteins that were associated with sporozoites, and SAMSP1 (AGAP013726) was one of the dominant proteins [12]. To evaluate the influence of sporozoites on the expression of SAMSP1 in salivary glands, we collected the salivary glands from female mosquitoes, with or without P. berghei infection. By RT-PCR, SAMSP1 gene expression was increased in the salivary glands after P. berghei invasion compared with the mosquitoes taking a sugar meal (Figure 1A). The presence of SAMSP1 protein in the salivary glands was confirmed by immunoblot (Figure 1B). Immunofluorescence staining using mouse anti-SAMSP1 IgG demonstrated that SAMSP1 was directly associated with 15% of the isolated sporozoites. Figure 1C shows a representative image of binding. These studies demonstrate that SAMSP1 interacts with sporozoites, which prompted further investigations on the impact of SAMSP1 on sporozoites.
Figure 1.
Sporozoite-associated mosquito saliva protein 1 (SAMSP1) is associated with sporozoites. A, The expression level of SAMSP1 by reverse-transcription polymerase chain reaction. The salivary glands were collected from individual mosquitoes. Mosquitoes with an uninfected blood meal or Plasmodium berghei–infected blood meal were examined 17–21 days after a blood meal. The additional group was mosquitoes given a sugar meal. Each point represents a single mosquito, and the bars represent median values with interquartile range (differences nonsignificant using the Kruskal-Wallis test). B, Immunoblots of salivary gland extract from 1 P. berghei–infected or uninfected female mosquito 17 days after a blood meal. C, Immunostaining with mouse anti-SAMSP1 antibody demonstrated that SAMSP1 is associated with the surface of P. berghei sporozoites. Murine anti-ovalbumin antibody (anti-OVA) was used as a control. All images are representative of >2 independent experiments. Abbreviations: CSP, circumsporozoite protein; SPZ, sporozoites.
SAMSP1 Enhancement of Cell Traversal by Sporozoites and Initial Plasmodium Liver Infection Level
To characterize the influence of SAMSP1 on sporozoite gliding activity and cell traversal, 2 processes associated with infectivity, we collected sporozoites from salivary glands and washed them with PBS once. The washed sporozoites were incubated with 10-µg/mL SAMSP1 or BSA for 1 hour. The sporozoites were then incubated in DMEM and the movement was recorded using an EVOS Imaging System for 5 minutes. There was a significant increase in movement speed when sporozoites were incubated with SAMSP1 compared with the control group (Figure 2A). Furthermore, there was significant enhancement of hepatocyte cell traversal when sporozoites were incubated with SAMSP1 (Figure 2B). In contrast, when sporozoites were incubated with 10-µg/mL anti-SAMSP1 IgG, there was significantly less traversal compared with controls (Figure 2C). To extend these findings to in vivo infection, we incubated sporozoites with either 10-µg/mL BSA or SAMSP1 and then injected the ears of C57B/L6 mice intradermally with 300 sporozoites. The group pretreated with SAMSP1 had significantly higher liver burdens than controls (Figure 2D).
Figure 2.
Sporozoite-associated mosquito saliva protein 1 (SAMSP1) augmented the infection of sporozoites in vitro and in vivo. A, The sporozoites (SPZ) were collected from Plasmodium berghei–infected mosquitoes and then incubated with 10-µg/mL SAMSP1 or bovine serum albumin (BSA). The movement of treated sporozoites was recorded with the EVOS FL Auto Cell Imaging System and analyzed using IMARIS software (medians shown with interquartile ranges [IQRs]; P = .003 with Mann-Whitney U test). B, C, After collection from salivary glands, the sporozoites were washed once with phosphate-buffered saline and then incubated with 10-µg/mL SAMSP1 or BSA for 1 hour (B). Alternatively, without washing, the sporozoites were incubated with 10-µg/mL mouse anti-SAMSP1 or anti-ovalbumin (anti-OVA) antibody for 1 hour (C). Sporozoites were then incubated with murine hepatocytes with fluorescently labeled dextran for 3 hours. The traversal of cells was determined by positive fluorescently labeled cells by means of fluorescence-activated cell sorting. Data are representative of >3 experiments (means shown with standard deviations; P < .05 with unpaired t test (B) or analysis of variance test followed by post hoc test (C)). D, First, 300 sporozoites, incubated with 10-µg/mL SAMSP1 or BSA for 1 hour, were intradermally injected into the left ears of individual C57BL/6 mice. Forty hours later, the livers were dissected, and the Plasmodium infection level was determined by means of reverse-transcription polymerase chain reaction (values shown as medians with IQRs; P < .05 with Mann-Whitney U test).
Effect of SAMSP1 on Neutrophil Chemotaxis In Vitro and In Vivo
Sporozoites stay in the dermis up to 3 hours after being delivered to skin by mosquito bite [25, 26]. While in the dermis, innate immune cells including macrophages and neutrophils influence the microenvironment at the bite site. Because SAMSP1 was initially identified by mass spectrometric analysis of sporozoites collected from mosquito saliva, we examined whether SAMSP1 could have an impact on the host environment [12]. To test whether SAMSP1 had an effect when sporozoites were delivered to the skin, we used mosquito salivary gland extract (SGE) to induce a local inflammatory response in the skin by intradermal injection, coinjected with 1-μg/mL SAMSP1 or BSA. Four hours later, the cell population at the injection site was determined using flow cytometry. There were significantly fewer neutrophils in the group administered SGE with the addition of SAMSP1 (Figure 3A). Macrophage (CD11b+CD11c−/CD45+Ly6G−), Langerhans cell (CD11b+CD11c+/CD45+Ly6G−) or dendritic cell populations (CD11b−CD11c+/CD45+Ly6G−) were similar in the experimental and control groups (data not shown). To further examine whether SAMSP1 affects neutrophil chemotaxis, we used bone marrow–derived neutrophils to perform a Transwell chemotaxis assay. fMLP (40 μg/mL) with or without SAMSP1 (3 μg/mL) was added to the lower chamber. SAMSP1 decreased neutrophil chemotaxis that is normally induced by N-Formyl-Met-Leu-Phe (Figure 3B).
Figure 3.
Sporozoite-associated mosquito saliva protein 1 (SAMSP1) affects neutrophil chemotaxis in vivo and in vitro. A, First, 300 sporozoites with salivary gland extract [SGE], mixed with 10-μg/mL SAMSP1 or bovine serum albumin (BSA), were injected intradermally into the ears of Swiss Webster mice. Four hours later, the injection sites were removed by punch biopsy. After staining, the cell population at the injection site was examined by means of fluorescence-activated cell sorting (values shown as medians with interquartile ranges; P < .05 with Mann-Whitney U test). B, The effect of SAMSP1 on neutrophil migration toward the chemoattractant f-Met-Leu-Phe (fMLP) (40 μg/mL) were evaluated using Transwell chambers. Bone marrow harvested neutrophils was added to a Transwell insert and incubated for 2 hours at 37°C to allow for migration to the lower wells containing fMLP, with or without 3-μg/mL SAMSP1. Cell numbers were determined using a hemocytometer (values shown as means with standard deviations; P < .05 with unpaired t test).
Effect of SAMSP1 Antiserum on P. berghei Liver Burden in Mice
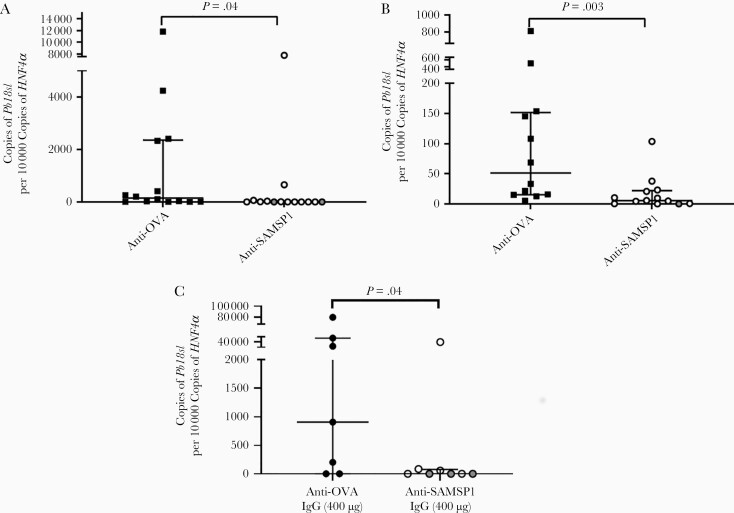
To further elucidate the role of SAMSP1 during the transmission of Plasmodium infection, we generated rabbit antiserum against SAMSP1 and determined whether SAMSP1 antiserum influences Plasmodium infection in mice administered sporozoites via intradermal inoculation. Mice that received SAMSP1 antiserum had a significantly lower parasite liver burden than control animals (Figure 4A). We then assessed whether SAMSP1 antiserum affected the transmission of Plasmodium by mosquito bite. Mice that received SAMSP1 antiserum also had a significantly lower liver burden after P. berghei–infected mosquito bite (Figure 4B). Identical experiments using IgG purified from SAMSP1 antiserum yielded similar results (P = .03 with the Mann-Whitney U test) (Figure 4C) and among the group administered 400 μg of anti-SAMSP1 IgG, 3 of 8 mice did not have detectable Plasmodium in the liver by RT-PCR. In the control group with anti-OVA IgG, all of the mice had Plasmodium infection in the liver.
Figure 4.
Passive immunization with sporozoite-associated mosquito saliva protein 1 (SAMSP1) antiserum reduces the initial Plasmodium berghei infection level in mice. A, C57BL/6 mice received 400 µL of SAMSP1 rabbit antiserum or control (ovalbumin [OVA]) rabbit antiserum 1 day before infection. First, 300 sporozoites collected from the salivary glands of P. berghei–infected mosquitoes were injected intradermally into the left ears of C57BL/6 mice. Then, 40 hours later, the livers were dissected and the Plasmodium infection level was determined using reverse-transcription polymerase chain reaction (RT-PCR). B, C57BL/6 mice received 400 µL of SAMSP1 rabbit antiserum or control (OVA) rabbit antiserum 1 day before infection. The next day, each mouse was exposed to 3 P. berghei–infected mosquitoes, and the liver burden was determined 40 hours later (values shown as medians with interquartile ranges [IQRs]; P < .05 with Mann-Whitney U test). C, To examine protection during mosquito-transmitted Plasmodium infection, C57BL/6 mice received 400 µg of anti-SAMSP1 rabbit immunoglobulin (Ig) G or 400 µg of anti-OVA rabbit IgG. The next day, mice were exposed to 3 P. berghei–infected mosquitoes. After 40 hours, the P. berghei liver burden was determined with RT-PCR. Two independent experiments were performed, and the pooled results are presented. Gray dots represent undetectable expression level of Pb18s in the liver (values shown as medians with IQRs; P < .05 with Mann-Whitney U test).
The Additive Protection of AntiSAMSP1 IgG with Anti-circumsporozoite Protein Antibody
Circumsporozoite protein (CSP) is one of the major pathogen-based vaccine targets against malaria and has undergone several clinical trials with moderate efficacy [27, 28]. We therefore determined whether passive immunization with anti-SAMSP1 IgG could work in combination with a CSP monoclonal antibody (mAb) (3D11 mAb) to enhance protective immunity against Plasmodium. We used a low dose of a CSP mAb, 30 µg, which does not fully prevent Plasmodium from causing a detectable infection [22] and a low, suboptimal dose of anti-SAMSP1 IgG. After passive immunization, there was no significant protection effect when the mice received either 100 µg of SAMSP1 IgG alone or 30 µg of 3D11 mAb alone. When mice received both anti-SAMSP1 IgG and 3D11 mAb, there was a significantly lower Plasmodium liver burden after the animals were fed upon by Plasmodium-infected mosquitoes, compared with controls (Figure 5).
Figure 5.
Passive immunization with sporozoite-associated mosquito saliva protein 1 (SAMSP1) immunoglobulin (Ig) G offers additive protection against Plasmodium infection, when combined with an anti-circumsporozoite protein (CSP) monoclonal antibody. C57BL/6 mice received 100 µg of anti-SAMSP1 IgG, 30 µg of anti-CSP monoclonal antibody (3D11), or both 1 day before infection. The control group of mice received 130 µg anti-ovalbumin (anti-OVA) IgG. The next day, mice were exposed to 3 Plasmodium berghei–infected mosquitoes. Forty hours later, the P. berghei liver burden was determined by means of reverse-transcription polymerase chain reaction. Two independent experiments were performed and the pooled results are presented (shown as medians with interquartile ranges; P < .05 with Kruskal-Wallis followed by post hoc test).
The Protection Effects of Active Immunization with SAMSP1 during P. berghei Infection in Mice
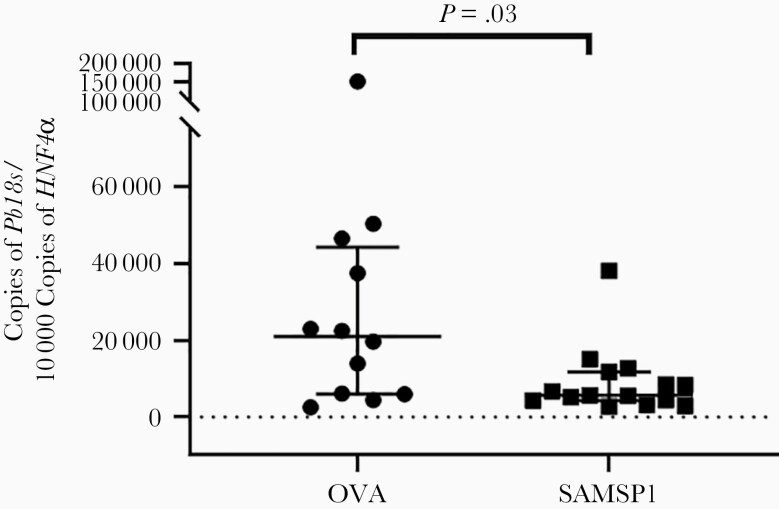
To further determine whether SAMSP1 can potentially be incorporated into a malaria vaccine strategy, we actively immunized C57BL/6 mice with 10 μg of SAMSP1 in CFA and boosted the animals twice using SAMSP1 with IFA. The control group was immunized with 10 μg of OVA, which has served as a control antigen in Plasmodium infection studies of mice [22]. Two weeks after the last vaccination, both groups had significant SAMSP1- or OVA-specific immunoglobulin by ELISA (Supplementary Figure 2). Each group of mice were exposed to P. berghei–infected mosquitoes. The group vaccinated with SAMSP1 had a significantly lower liver burden than the control animals (Figure 6). Therefore, active or passive immunization with SAMSP1 decreases the Plasmodium liver burden in the murine model.
Figure 6.
Active immunization against sporozoite-associated mosquito saliva protein 1 (SAMSP1) reduces the liver burden of Plasmodium infection in mice. C57BL/6 mice were immunized with 10 µg of SAMSP1 or ovalbumin (OVA) with complete Freund’s adjuvants and then boosted twice with incomplete Freund’s adjuvants. Three weeks after the last immunization, the mice were exposed to 3 Plasmodium berghei–infected mosquitoes. Forty hours later, the P. berghei liver burden was determined by means of reverse-transcription polymerase chain reaction. Two independent experiments were performed, and the pooled results are presented (shown as medians with interquartile ranges; P < .05 with Mann-Whitney U test).
SAMSP1 Antibody Responses in Malaria-Endemic Areas
The humoral responses to several mosquito saliva proteins has been examined as a correlate for mosquito exposure in malaria-endemic areas [23, 29, 30]. As SAMSP1 interacts with sporozoites [12], we used previously collected human serum samples from malaria-endemic (Senegal) and nonendemic (France) areas [23] to determine whether SAMSP1 is recognized by individuals in malaria-endemic areas. By ELISA, the SAMSP1 IgG titers were significantly increased in the malaria-endemic region compared with the nonendemic area (Figure 7). As SAMSP1 was initially identified using sporozoites collected directly from mosquito saliva [12], the humoral response to SAMSP1 also suggests a potential correlation between the presence of antibodies to SAMSP1 and exposure to Plasmodium in a malaria-endemic area. Further studies are needed to carefully examine whether the humoral response to SAMSP1 can be used, along with other markers, for exposure to Plasmodium-infected mosquito bite.
Figure 7.
Immunoglobulin (Ig) G response to sporozoite-associated mosquito saliva protein 1 (SAMSP1) is recognized in malaria-endemic area. Serum samples were collected from individuals from a malaria-endemic region (Senegal) and a nonendemic region (France) and have previously been examined for responses to other Anopheles gambiae salivary proteins [22, 23]. Enzyme-linked immunosorbent assay was performed using the serum samples from both locations (values shown as medians and interquartile ranges; P < .05 with Mann-Whitney U test).
DISCUSSION
Previous transcriptome studies have shown that both male and female mosquitoes express SAMSP1, and SAMSP1 is expressed in the midguts, salivary glands, and ovaries [31, 32]. There are similar proteins in other species of mosquitoes, including Aedes aegypti (AAEL003925) and Culex quinquefasciatus (CPIJ000829). There are also homologous proteins in Drosophila species. The function of SAMSP1 in these diverse species has not been elucidated. Our group’s previous and current studies show that SAMSP1 associates with sporozoites [12]. Because the expression of SAMSP1 is found in both male and female mosquitoes and the homologous protein is noted in Drosophila, SAMSP1 may also have a role that is not related to taking a blood meal or pathogen transmission. Further studies will be needed to characterize the pleiomorphic functions of this protein in mosquitoes or flies.
During the transmission of arthropod-borne pathogens, saliva proteins facilitate the ability of diverse microbes to infect the vertebrate host, including bacteria [10], viruses [8, 9, 33, 34] and protozoa [35, 36]. In our current study, we have shown that SAMSP1 can influence Plasmodium infectivity through enhancement of the gliding and traversal activities of sporozoites. Substrate-dependent gliding motility is used by sporozoites to help complete the transition from a mosquito to a vertebrate host [37]. Sporozoites with defects in gliding motility had a decreased ability to establish infection [15]. Another sporozoite activity required for dermal exit is cell traversal [14, 38], which allows the parasite to enter and exit host cells [19, 39]. It has been shown that anti-CSP antibody can significantly reduce the gliding of sporozoites in vitro and in vivo [40, 41], which may contribute to the protection induced by vaccination against CSP. Similarly, we show that adding SAMSP1 protein to sporozoites can facilitate gliding and cell traversal, thereby enhancing Plasmodium infectivity. SAMSP1 could potentially faciliate movement directly or indirectly by influencing sporozoite proteins that participate in movement.
During probing for a blood meal, mosquitoes inject saliva into the dermis, which can help in a successful blood meal by influencing coagulation, vasodilation, and immunomodulation [2–5]. Moreover, after a mosquito bite, neutrophils, monocytes, and other immune cells infiltrate the bite site and alter vascular permeability and inflammation [42]. Neutrophils migrate to the bite site and serve as an initial response to pathogens [42]. When intradermally inoculated with irradiated sporozoites, neutrophils can take up sporozoites [43]. Neutrophil depletion, however, did not affect the protection effects or tissue invasion of sporozoites after intradermal injection of 5 × 104 irradiated sporozoites [43]. It is also unknown whether neutrophil can help control mosquito-borne Plasmodium infection, during which only about 100 sporozoites are injected at the bite site. Based on our study, it is possible that SAMSP1 helps sporozoites avoid neutrophils, which may help to establish infection. Overall, these findings demonstrate that SAMSP1 has an effect on the host environment, and this may indirectly influence Plasmodium infectivity. Further studies are required to address whether neutrophil depletion affects mosquito-borne Plasmodium infection and whether SAMSP1 alters Plasmodium infection when neutrophils are depleted.
Currently, the most advanced malaria vaccine, RTS,S, is based on the Plasmodium CSP in conjunction with immunostimulatory epitopes of the hepatitis B surface antigen and AS01 adjuvant [27]. Overall, the CSP-based vaccine offers moderate protection with a 30%–50% reduction in infection rate in clinical trials, and wanes over time [27, 28]. Active or passive immunization, with SAMSP1 or SAMSP1 antiserum, reduced Plasmodium liver burden after either needle-delivered or mosquito-borne sporozoites infection. Furthermore, passive immunization with SAMSP1 antiserum afforded added protection to a CSP mAb. Based on all these data, immunization targeting SAMSP1 may be considered an important target for future malaria vaccines and can most likely be used to enhance the protection effects of current standard pathogen-based malaria vaccines that target the initial stage of infection.
After exposure to mosquitoes bites, there is a humoral immune response against some saliva proteins in human [30, 44–46], and it has been considered that the immune response to saliva protein may serve as a biomarker for a risk of malaria [29, 47–50]. As one of the example, the serum samples that we tested in this study had previously been used to identify 2 Anopheles salivary proteins, SG6 and 5’ nucleotidase, as potential biomarkers for mosquito bite exposure [23]. In the current study, we observed a significant higher humoral immune response to SAMSP1 in an malaria-endemic area. Because SAMSP1 is associated with sporozoites, it is possible that the IgG immune response to SAMSP1 may serve as a marker for exposure to Plasmodium-bearing mosquitoes. Futher longitudinal and cross-sectional studies in diverse malaria-endemic areas will be needed to determine whether SAMSP1 or other selected mosquito saliva proteins can be used in assays to determine malaria risk.
During the transmission of Plasmodium, mosquito saliva proteins can interact with both the vertebrate host and sporozoites. These interactions are all at the critical interface where sporozoites establish the initial stage of infection. SAMSP1 is a mosquito saliva protein that transiently interacts with sporozoites in saliva, influences sporozoite movement and the local host response. Interfering with SAMSP1, using specific antibodies can diminish the initial Plasmodium burden in mice, and may potentially be incorporated into future malaria vaccines, particularly in conjunction with pathogen-based antigens that target the initial stage of infection.The identification and characterization of additional mosquito saliva proteins that alter Plasmodium transmission will lead to a greater understanding of the initial stage of Plasmodium infection of a vertebrate host and enhance the potential for new vector-based vaccine strategies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Kathleen DePonte and Ming-Jie Wu for their technical assistance.
Financial support. These studies were supported by the National Institute of Allergy and Infectious Disease (R21 grant AI142708 and R41 grant AI145779 to E. F.), the National Institutes of Health (T32 grant AR007016-44 to M. F.), and the Dermatology Foundation (M. F.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report. Geneva, Switzerland:World Health Organization,2019. [Google Scholar]
- 2. Ribeiro JM. Vector salivation and parasite transmission. Mem Inst Oswaldo Cruz 1987; 82(suppl 3):1–3. [DOI] [PubMed] [Google Scholar]
- 3. Stark KR, James AA. Anticoagulants in vector arthropods. Parasitol Today 1996; 12:430–7. [DOI] [PubMed] [Google Scholar]
- 4. Watson EE, Liu X, Thompson RE, et al. Mosquito-derived anophelin sulfoproteins are potent antithrombotics. ACS Cent Sci 2018; 4:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Figueiredo AC, de Sanctis D, Gutiérrez-Gallego R, et al. Unique thrombin inhibition mechanism by anophelin, an anticoagulant from the malaria vector. Proc Natl Acad Sci U S A 2012; 109:E3649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soares MB, Titus RG, Shoemaker CB, David JR, Bozza M. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J Immunol 1998; 160:1811–6. [PubMed] [Google Scholar]
- 7. Qureshi AA, Asahina A, Ohnuma M, Tajima M, Granstein RD, Lerner EA. Immunomodulatory properties of maxadilan, the vasodilator peptide from sand fly salivary gland extracts. Am J Trop Med Hyg 1996; 54:665–71. [DOI] [PubMed] [Google Scholar]
- 8. Uraki R, Hastings AK, Marin-Lopez A, et al. Aedes aegypti AgBR1 antibodies modulate early Zika virus infection of mice. Nat Microbiol 2019; 4:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uraki R, Hastings AK, Brackney DE, Armstrong PM, Fikrig E. AgBR1 antibodies delay lethal Aedes aegypti-borne West Nile virus infection in mice. NPJ Vaccines 2019; 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramamoorthi N, Narasimhan S, Pal U, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 2005; 436:573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chuang YM, Freudzon M, Yang J, Dong Y, Dimopoulos G, Fikrig E. Anopheles gambiae lacking AgTRIO inefficiently transmits Plasmodium berghei to mice. Infect Immun 2019; 87:e00326-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schleicher TR, Yang J, Freudzon M, et al. A mosquito salivary gland protein partially inhibits Plasmodium sporozoite cell traversal and transmission. Nat Commun 2018; 9:2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Schleicher TR, Dong Y, et al. Disruption of mosGILT in Anopheles gambiae impairs ovarian development and Plasmodium infection. J Exp Med 2020; 217:e20190682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amino R, Giovannini D, Thiberge S, et al. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 2008; 3:88–96. [DOI] [PubMed] [Google Scholar]
- 15. Hopp CS, Chiou K, Ragheb DR, et al. Longitudinal analysis of Plasmodium sporozoite motility in the dermis reveals component of blood vessel recognition. Elife 2015; 4:e07789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ménard R. Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cell Microbiol 2001; 3:63–73. [DOI] [PubMed] [Google Scholar]
- 17. Montagna GN, Matuschewski K, Buscaglia CA. Plasmodium sporozoite motility: an update. Front Biosci (Landmark Ed) 2012; 17:726–44. [DOI] [PubMed] [Google Scholar]
- 18. Steel RWJ, Pei Y, Camargo N, et al. Plasmodium yoelii S4/CelTOS is important for sporozoite gliding motility and cell traversal. Cell Microbiol 2018; 20. Available at: https://pubmed.ncbi.nlm.nih.gov/29253313/. [DOI] [PubMed] [Google Scholar]
- 19. Ishino T, Chinzei Y, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol 2005; 7:199–208. [DOI] [PubMed] [Google Scholar]
- 20. Kaiser K, Camargo N, Coppens I, Morrisey JM, Vaidya AB, Kappe SH. A member of a conserved Plasmodium protein family with membrane-attack complex/perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol Biochem Parasitol 2004; 133:15–26. [DOI] [PubMed] [Google Scholar]
- 21. Talman AM, Lacroix C, Marques SR, et al. PbGEST mediates malaria transmission to both mosquito and vertebrate host. Mol Microbiol 2011; 82:462–74. [DOI] [PubMed] [Google Scholar]
- 22. Dragovic SM, Agunbiade TA, Freudzon M, et al. Immunization with AgTRIO, a protein in Anopheles saliva, contributes to protection against Plasmodium infection in mice. Cell Host Microbe 2018; 23:523–35.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali ZM, Bakli M, Fontaine A, et al. Assessment of Anopheles salivary antigens as individual exposure biomarkers to species-specific malaria vector bites. Malar J 2012; 11:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nuzzi PA, Lokuta MA, Huttenlocher A. Analysis of neutrophil chemotaxis. Methods Mol Biol 2007; 370:23–36. [DOI] [PubMed] [Google Scholar]
- 25. Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol 2007; 9:1215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amino R, Thiberge S, Martin B, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med 2006; 12:220–4. [DOI] [PubMed] [Google Scholar]
- 27. Agnandji ST, Lell B, Soulanoudjingar SS, et al. ; RTS,S Clinical Trials Partnership . First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863–75. [DOI] [PubMed] [Google Scholar]
- 28. RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rizzo C, Lombardo F, Ronca R, et al. Differential antibody response to the Anopheles gambiae gSG6 and cE5 salivary proteins in individuals naturally exposed to bites of malaria vectors. Parasit Vectors 2014; 7:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Remoue F, Cisse B, Ba F, et al. Evaluation of the antibody response to Anopheles salivary antigens as a potential marker of risk of malaria. Trans R Soc Trop Med Hyg 2006; 100:363–70. [DOI] [PubMed] [Google Scholar]
- 31. Papa F, Windbichler N, Waterhouse RM, et al. Rapid evolution of female-biased genes among four species of Anopheles malaria mosquitoes. Genome Res 2017; 27:1536–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baker DA, Nolan T, Fischer B, Pinder A, Crisanti A, Russell S. A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics 2011; 12:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conway MJ, Londono-Renteria B, Troupin A, et al. Aedes aegypti D7 saliva protein inhibits dengue virus infection. PLoS Negl Trop Dis 2016; 10:e0004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conway MJ, Watson AM, Colpitts TM, et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol 2014; 88:164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Titus RG, Ribeiro JM. The role of vector saliva in transmission of arthropod-borne disease. Parasitol Today 1990; 6:157–60. [DOI] [PubMed] [Google Scholar]
- 36. Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol 2001; 167:5226–30. [DOI] [PubMed] [Google Scholar]
- 37. Douglas RG, Amino R, Sinnis P, Frischknecht F. Active migration and passive transport of malaria parasites. Trends Parasitol 2015; 31:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coppi A, Tewari R, Bishop JR, et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe 2007; 2:316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mota MM, Pradel G, Vanderberg JP, et al. Migration of Plasmodium sporozoites through cells before infection. Science 2001; 291:141–4. [DOI] [PubMed] [Google Scholar]
- 40. Flores-Garcia Y, Nasir G, Hopp CS, et al. Antibody-mediated protection against Plasmodium sporozoites begins at the dermal inoculation site. mBio 2018; 9:e02194-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barry A, Behet MC, Nébié I, et al. Functional antibodies against Plasmodium falciparum sporozoites are associated with a longer time to qPCR-detected infection among schoolchildren in Burkina Faso. Wellcome Open Res 2018; 3:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pingen M, Bryden SR, Pondeville E, et al. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity 2016; 44:1455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mac-Daniel L, Buckwalter MR, Berthet M, et al. Local immune response to injection of Plasmodium sporozoites into the skin. J Immunol 2014; 193:1246–57. [DOI] [PubMed] [Google Scholar]
- 44. Waitayakul A, Somsri S, Sattabongkot J, Looareesuwan S, Cui L, Udomsangpetch R. Natural human humoral response to salivary gland proteins of Anopheles mosquitoes in Thailand. Acta Trop 2006; 98:66–73. [DOI] [PubMed] [Google Scholar]
- 45. Poinsignon A, Cornelie S, Mestres-Simon M, et al. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS One 2008; 3:e2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Penneys NS, Nayar JK, Bernstein H, Knight JW. Circulating antibody detection in human serum to mosquito salivary gland proteins by the avidin-biotin-peroxidase technique. J Am Acad Dermatol 1988; 18:87–92. [DOI] [PubMed] [Google Scholar]
- 47. Ya-Umphan P, Cerqueira D, Parker DM, et al. Use of an Anopheles salivary biomarker to assess malaria transmission risk along the Thailand-Myanmar border. J Infect Dis 2017; 215:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stone W, Bousema T, Jones S, et al. IgG responses to Anopheles gambiae salivary antigen gSG6 detect variation in exposure to malaria vectors and disease risk. PLoS One 2012; 7:e40170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rizzo C, Ronca R, Fiorentino G, et al. Wide cross-reactivity between Anopheles gambiae and Anopheles funestus SG6 salivary proteins supports exploitation of gSG6 as a marker of human exposure to major malaria vectors in tropical Africa. Malar J 2011; 10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Proietti C, Verra F, Bretscher MT, et al. Influence of infection on malaria-specific antibody dynamics in a cohort exposed to intense malaria transmission in northern Uganda. Parasite Immunol 2013; 35:164–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.