Abstract
Objective:
The purpose of this study was to clarify the mediational pathways from genetic risk for alcohol use disorder (AUD) to AUD itself.
Method:
Using information on AUD status from first- through fourth-degree relatives obtained from national registries, we created a genetic risk score for AUD for the Swedish population. We first tested a simple mediational path model in males and females separately, with early onset externalizing psychopathology (EPP), internalizing psychopathology (IPP), and poor educational attainment (EA). We then tested a more complex model in a smaller, older sample of males that contained additional self-report measures from late adolescence.
Results:
In our basic model, the largest mediational pathway from AUD genetic risk to AUD in both sexes was via high EPP followed by low EA and high IPP. The EPP pathway was considerably stronger in males, the low EA pathway was modestly stronger in females, and the IPP pathway was identical in both sexes. Our more complex model replicated the strong externalizing pathway to AUD, showing that it connected to key downstream risk factors such as early drug and alcohol use and low resilience.
Conclusions
Our models concurred in showing that the strongest mediational pathway for genetic risk to AUD includes externalizing symptoms and disorders, which in turn predict further key downstream risk factors. Pathways through lower EA and IPP had smaller effects. IPP had mixed effects (partly predisposing and partly protective) on downstream risk factors. The largest sex difference was a stronger externalizing pathway to genetic risk to AUD in males than in females.
More than four decades of twin and adoption research has proven that aggregate genetic factors contribute substantially to the risk for alcohol use disorder (AUD; Verhulst et al., 2015). Efforts are now underway to identify and characterize the specific molecular genetic risk variants that underlie those aggregate effects (Kranzler et al., 2019; Lai et al., 2019; Sanchez-Roige et al., 2019). A major goal of this effort is to enable a tracing of the complex biological mediational pathways from risk variants to the clinical syndrome of AUD. Insights into these pathways are important because they can inform efforts at primary and secondary prevention and provide biological targets for the development of new pharmacologic agents to treat AUD.
The present study attempts a similar task based on an aggregate genetic risk score (GRS) calculated, in the general Swedish population, from the occurrence of AUD diagnoses in first- through fourth-degree relatives. Although such an approach cannot identify biological targets for drug development, it can provide further insight into etiological pathways and inform potential prevention methods. We asked the following three specific questions using structural equation modeling (SEM).
First, we applied, in a large general population sample of males (n = 487,142), a basic model in which we predicted risk for AUD from the subjects’ GRS and three mediational nodes—externalizing psychopathology (EPP), internalizing psychopathology (IPP), and low educational attainment (EA)—all obtained from population-wide registers.
Second, we applied the same model to females (n = 457,576) and examined similarities and differences between the sexes from the two patterns of results.
Third, in a considerably smaller and older sample of males with more detailed data (n = 43,431), we fit a model with a richer and more diverse set of mediational variables in an attempt to replicate and elaborate on the results of our simple model in males.
Method
We collected information on individuals from Swedish population-based registers with national coverage linking each person's unique personal identification number, which, to preserve confidentiality, was replaced with a serial number by Statistics Sweden (i.e., the Swedish Statistics Bureau). We secured ethical approval for this study from the Regional Ethical Review Board in Lund (No. 2008/409, 2012/795, and 2016/679).
We used three different data sets for the analyses: (1) all male individuals born in Sweden between 1975 and 1985, residing in Sweden at age 25 and with no AUD registration before age 25 (n = 487,142); (2) all female individuals using the same criteria as above (n = 457,576); and (3) male individuals conscripted into military service in Sweden in 1969 and 1970 residing in Sweden at age 25 and with no AUD registration before age 25 (n = 43,431). In all data sets we included a genetic risk score for alcohol use disorder (AUD GRS) based on information on AUD diagnoses in first- through fourth-degree relatives controlling for the effects of shared environment, registration for AUD after age 25, registration for externalizing disorder (particularly drug use disorder or criminal behavior) before age 25, registration for internalizing disorder (particularly major depression and anxiety disorders) before age 25, and highest achieved education at age 25 (i.e., educational attainment [EA]). We only examined individuals with AUD whose first registration occurred after age 25 and only studied externalizing disorders and internalizing disorders before age 25 so that these risk factors would precede AUD onset and thus plausibly mediate the impact of genetic vulnerability on AUD risk. Appendix Table 1 provides more detailed descriptions of these variables. (A supplemental appendix appears as an online-only addendum to this article on the journal's website.)
Table 1.
Descriptive statistics for our three samples
| Variable | Sample 1: Males | Sample 2: Females | Sample 3: Male conscripts |
|---|---|---|---|
| N | 487,142 | 457,576 | 43,431 |
| Year of birth | 1975–1985 | 1975–1985 | 1950–1951 |
| AUD | 4.68% | 1.90% | 12.1% |
| Externalizing PP | 15.48% | 6.92% | 5.16% |
| Internalizing PP | 2.83% | 5.66% | – |
| Educ. att., in years,a M (SD) | 12.9 (2.7) | 13.6 (2.7) | 11.9 (3.0) |
| Resilience,a M (SD) | – | – | 5.1 (1.9) |
| IQ,aM (SD) | – | – | 5.4 (2.0) |
Notes: AUD = alcohol use disorder; PP = psychopathology; educ. att. = educational attainment.
These variables are reversed in the analyses.
The birth cohorts used for Data Set 3 provide unique conscript material because more detailed information was collected at conscription in Sweden during these 2 years than at any other time. Information from the conscript register about the individuals was collected through questionnaires, with questions about medical symptoms, childhood and adolescent traits and behaviors, and alcohol and tobacco use. We included in Data Set 3 self-report data on externalizing behavior, internalizing behavior, drug use, and alcohol use, as well as test results on IQ and resilience from conscript examination. (See the Appendix for a description of all included variables.) For Data Set 3, we started with 44,684 individuals with information on the AUD GRS. A total of 1,160 individuals had a missing value on more than 10% of the questions, and 93 had an AUD registration before age 25 and were excluded. Of the remaining 43,431 individuals, 78% had no missing values; 15% had below 2%; and 7% had between 2% and 10%. To impute values, we used the predicted regression imputation method within specific groups of questions (i.e., using regression models to predict missing values based on similar covariates). Note that for IQ, resilience, and highest achieved education, we have reversed the scales in the analysis.
We used a structural equation model to investigate pathways from genetic risk to AUD registration after age 25. In Data Sets 1 and 2, we included three potential mediators: EPP before age 25, IPP before age 25, and EA. We placed EPP before IPP in these models because in both sexes, the mean (SD) age at first registration for EPP was 4 years earlier than the mean age at first registration for IPP: males 17.8 (2.5) versus 21.7 (2.1), and females 17.9 (2.5) versus 21.9 (2.0) years.
In Data Set 3, we also included self-report data at age on externalizing and internalizing traits and alcohol and drug use, as well as test results on IQ and resilience from conscript examination. For Data Set 3, the analysis consisted of a measurement model with factor loadings for the observed variables that index the four latent variables (externalizing behavior, internalizing behavior, drug use, alcohol use). It also had a structural model that consisted of paths connecting the four latent and the four observed variables (externalizing disorders before age 25 and EA, IQ, and resilience) of the model. Note that IPP before age 25 was not included in the analysis for Data Set 3 because of very low prevalence that produced unstable statistical estimates. For Data Sets 1 and 2, the analysis consisted of the structural model part with three observed variables (externalizing disorders before age 25, internalizing disorders before age 25 and EA).
We followed an approach developed in previous similar studies (Kendler et al., 2016a, 2017). We began with a fully saturated model and used a combination of three approaches to produce a model with the optimal balance of explanatory power and parsimony. In the first step, observing the significance levels of individual paths, we fixed sets of paths to zero when the associated z value was less than 1.96. Because some paths that remained significant were too small to be meaningful, the second step was to set all path estimates to zero with a value of less than .05, regardless of z value. Third, we added and subtracted paths that were marginal by significance and/or magnitude to see if we could arrive at a better overall fit, and indeed this produced a modest improvement in fit and explanatory power. We used two fit indices that reflect the success of the model in balancing explanatory power and parsimony: the Tucker–Lewis index (TLI) and the root mean square error of approximation (RMSEA). For the TLI and comparative fit index, values between .90 and .95 are considered acceptable and values of .95 or greater as good. For the RMSEA, “good” models have values of .05 or less. The fit function was weighted least squares. Mplus version 7.31 was used for model fitting (Muthén & Muthén, 2012). The raw correlation and path estimates for the three fitted models are seen in Tables 2–5 in the Appendix.
Results
Sample description
Table 1 provides descriptive statistics for our three study samples. Subjects from both Samples 1 and 2 were born between 1975 and 1985 (approximately 470,000 subjects each). As expected, compared with the female Sample 2, the male Sample 1 had higher rates of AUD and EPP and lower rates of IPP. Sample 3 (male) was much older and smaller in size.
Basic model in males
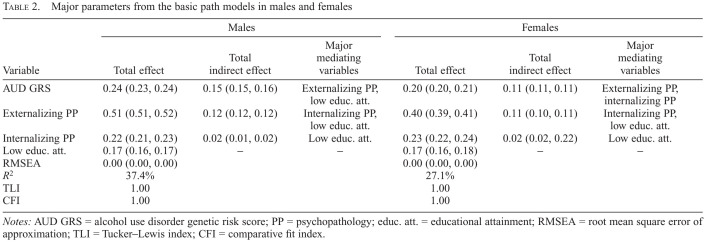
We fitted a basic path model in Sample 1, which begins with the AUD GRS and contains three potential mediators: EPP before age 25, IPP before age 25, and low EA. As seen in Table 2, this demonstrates an excellent fit to the data and explains 37.4% of the variance in AUD. The standardized GRS score has a total effect on AUD liability of +0.24, of which approximately 60% is mediated through our three variables and 40% is direct. As seen in Figure 1a, by far the largest mediational pathway from AUD GRS to AUD is via EPP, with smaller pathways mediated by EA and IPP. Of the four paths having an impact on AUD, the origin, in order of effect size, was EPP, IPP, low EA, and AUD GRS.
Table 2.
Major parameters from the basic path models in males and females
| Variable | Males | Females | ||||
|---|---|---|---|---|---|---|
| Total effect | Total indirect effect | Major mediating variables | Total effect | Total indirect effect | Major mediating variables | |
| AUD GRS | 0.24 (0.23, 0.24) | 0.15 (0.15, 0.16) | Externalizing PP, | 0.20 (0.20, 0.21) | 0.11 (0.11, 0.11) | Externalizing PP, |
| low educ. att. | internalizing PP | |||||
| Externalizing PP | 0.51 (0.51, 0.52) | 0.12 (0.12, 0.12) | Internalizing PP, | 0.40 (0.39, 0.41) | 0.11 (0.10, 0.11) | Internalizing PP, |
| low educ. att. | low educ. att. | |||||
| Internalizing PP | 0.22 (0.21, 0.23) | 0.02 (0.01, 0.02) | Low educ. att. | 0.23 (0.22, 0.24) | 0.02 (0.02, 0.22) | Low educ. att. |
| Low educ. att. | 0.17 (0.16, 0.17) | – | – | 0.17 (0.16, 0.18) | – | – |
| RMSEA | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | ||||
| R2 | 37.4% | 27.1% | ||||
| TLI | 1.00 | 1.00 | ||||
| CFI | 1.00 | 1.00 | ||||
Notes: AUD GRS = alcohol use disorder genetic risk score; PP = psychopathology; educ. att. = educational attainment; RMSEA = root mean square error of approximation; TLI = Tucker–Lewis index; CFI = comparative fit index.
Figure 1A.
Path estimates (standardized partial regression coefficients) for our basic model in males depicting the mediational pathways from the genetic risk score (GRS) for alcohol use disorder (AUD) to AUD vis three potential mediators: externalizing psychopathology (PP), internalizing PP, and low educational attainment (educ. att.).
Basic model in females
The results for the same model applied to females in Sample 2 are seen in Table 1 and Figure 1b. Our SEM model again achieved very good fit to the data and explained 27.1% of the variance in AUD, modestly lower than that seen in males. Constraining the path models to equality for males and females produced a highly significant deterioration in fit (p < .00001), indicating meaningful differences in path estimates across the sexes. The AUD GRS score had a total effect on AUD liability of +0.20, 55% of which was indirect. The largest direct mediation for the GRS was through a positive path through EPP, but it is noteworthy that both the path from AUD GRS to EPP and from EPP to AUD were smaller in females than in males. By contrast, the mediational pathway through low EA was modestly larger in females than males, whereas the pathway through IPP was identical.
Figure 1B.
Path estimates for our basic model in females depicting the mediational pathways from the genetic risk score (GRS) for alcohol use disorder (AUD) to AUD vis three potential mediators: externalizing psychopathology (PP), internalizing PP, and low educational attainment.
Enhanced model in males
A potential limitation of our simple models was that our measures of EPP and IPP relied on medical or legal registrations and might be insensitive to milder elevations in externalizing or internalizing traits that could have an impact on risk for AUD. Fortunately, in an older and much smaller cohort of Swedish males, self-report data at age 18 are available on externalizing and internalizing symptoms as well as alcohol and drug use in adolescence. We also used test results on IQ and resilience from conscript examinations and the same measures of EPP and IPP that we used with our basic model. This expanded set of variables permits us to examine a more complex mediational model from AUD GRS to AUD. Two limitations of this data were noteworthy: (a) GRS scores were less predictive in the older cohort in part because of less information being available in their older relatives and (b) rates of recorded diagnoses of IPP in that cohort were too rare to provide stable results.
The results are seen in Table 3 and Figure 2. The overall variance accounted for in AUD was 21.5% and the fit indices of the model were good. Four paths arose from the AUD GRS, the largest of which (+0.12) connected directly to AUD. The next three paths, in order of strength, were to (self-report) externalizing symptoms (+0.10), low IQ (+0.08), and EPP (obtained from registry diagnoses) (+0.05). No direct path connected AUD GRS to internalizing symptoms. Five variables directly predicted AUD risk, which in order of magnitude were EPP, alcohol use, AUD GRS, drug use, and low EA.
Table 3.
Major parameters from the enhanced path model in males
| Variable | Total effect | Total indirect effect | Major mediating variables |
|---|---|---|---|
| AUD GRS | 0.17 (0.16, 0.19) | 0.05 (0.05, 0.06) | Externalizing PP, |
| externalizing Sxs | |||
| Internalizing Sxs | 0.15 (0.15, 0.16) | 0.15 (0.15, 0.16) | Externalizing Sxs, |
| alcohol use | |||
| Externalizing Sxs | 0.36 (0.34, 0.37) | 0.36 (0.34, 0.37) | Externalizing PP, alcohol use |
| Drug use | 0.11 (0.08, 0.13) | 0.03 (0.02, 0.03) | Alcohol use |
| Alcohol use | 0.17 (0.014, 0.19) | 0.00 (0.00, 0.00) | – |
| Low IQ | 0.05 (0.04, 0.06) | 0.05 (0.04, 0.06) | Low educ. att., |
| externalizing PP | |||
| Low resilience | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) | Low educ. att. |
| Externalizing PP | 0.27 (0.24, 0.29) | 0.00 (0.00, 0.00) | – |
| Low educ. att. | 0.07 (0.06, 0.09) | – | |
| RMSEA | 0.042 (0.041, 0.04) | ||
| R2 | 21.5% | ||
| TLI | 0.963 | ||
| CFI | 0.966 |
Notes: AUD GRS = alcohol use disorder genetic risk score; PP = psychopathology; Sxs = symptoms; educ. att. = educational attainment; RMSEA = root mean square error of approximation; TLI = Tucker–Lewis index; CFI = comparative fit index.
Figure 2.
Path estimates for our enhanced basic model in males depicting the mediational pathways from the genetic risk score (GRS) for alcohol use disorder (AUD) to AUD. Paths are drawn in the color of the variable from which they originate. Sxs = symptoms; PP = psychopathology; educ. att. = educational attainment.
We are most interested in the mediational pathways from AUD GRS to AUD and point out three important results. First, externalizing behaviors constitute the most important mediational hub for AUD GRS effects, connecting to six downstream variables, all of which are associated with increased AUD risk: drug use, low IQ, EPP, low EA, alcohol use, and low resilience. Three of these paths (to drug use, alcohol use, and EP) are strong (i.e., > +0.50). That is, key mediators of genetic risk factors for AUD (e.g., high alcohol intake and drug use in adolescence, and low IQ and resilience) appear to arise from a direct effect of genetic risk factors on a generalized externalizing predisposition. Second, low IQ was a more modest mediational hub for GRS AUD, which in turn connected to both poor educational attainment, low resilience, and EPP. Third, the final mediational hub for AUD GRS was EPP.
In addition, this model illustrated the complex interrelationship between internalizing symptoms and AUD risk in males. Of the five paths from internalizing symptoms, two increased risk for AUD via relatively strong paths to externalizing behaviors and low resilience, whereas three were protective via weaker negative paths to alcohol use, EPP, and low EA.
Discussion
The goal of this report was to gain insight into the mediational pathways from AUD genetic risk to AUD onset and how these might differ in males and females. We did this in three stages, beginning with a basic model using the best available variables in a large general population of Swedish males. This model, which fit very well, found that slightly more than half of the impact of AUD GRS on AUD risk was indirect, with the remainder direct. The model contained three mediational pathways from AUD GRS to AUD, which, in order of strength, were via EPP, EA, and IPP. In this simple model, EPP was far and away the most important mediational hub for AUD GRS, not only acting directly on AUD risk by the strongest path in the model (+0.40), but also acting indirectly via influences on both IPP and low EA.
In the second stage, we compared the same simple model in an independent cohort of females where the best-fit model was significantly different from that seen in males. As in males, a bit more than half of the AUD GRS effect was indirect. Most importantly, the IPP mediational pathway was quite similar in the two sexes. However, the EPP pathway was substantially stronger in males than in females. Whereas the EA pathway was modestly stronger in females than in males. Restated, our results suggest that the most important difference in the pathways of the AUD GRS between the sexes is that genetic risk for AUD has an impact on externalizing behavior more strongly in males than in females. A more modest difference was seen in the pathway through low EA, which was more important in females than in males.
Our third stage used a unique cohort of Swedish males born in 1950–1951 for which self-report data at age 18 were available on four potentially important mediational variables: externalizing symptoms, internalizing symptoms, drug use, and alcohol consumption. Although the sample was much smaller than the cohorts used in our first two stages, it nonetheless permitted us to examine a much wider range of possible mediational mechanisms for AUD GRS. Three mediational variables were identified for the AUD GRS in this sample. By far the most important was externalizing symptoms self-reported at age 18, which in turn connected—quite strongly in some cases—to six key downstream risk factors: drug use, low IQ, EPP, low EA, alcohol use, and low resilience. These results replicated and extended our findings with the simple model in males demonstrating the central importance of externalizing traits or disorders as key pathways for genetic effects on AUD. Low IQ and EPP assessed up to age 25 were the second and third mediational hubs in the model. Last, this model shows the complexity of the internalizing pathway from AUD GRS to AUD in males, which included both predisposing and protective mediational paths.
It is also important to note that, in all of our models, a substantial proportion of the effects of AUD GRS on risk for AUD was direct and not mediated by the variables in the models. Counterintuitively, this direct effect was strongest in our most complex model. These findings can be interpreted two ways. First, other mediational paths might have been identified had we had access to more focused questionnaires, neuropsychologic measures, or imaging studies. Alternatively, some of the genetic effects are likely best understood at molecular levels that would include direct impact on the metabolism of ethanol and its actions on a wide range of brain mechanisms that likely mediate important aspects of vulnerability to AUD (Koob & Volkow, 2016).
Some aspects of our findings are consistent with classical AUD typologies (Babor et al., 1992; Cloninger et al., 1981, 1996; Del Boca & Hesselbrock, 1996; Jellinek, 1960; Leggio et al., 2009), whereas others are unexpected. Although terminology varies along with the number of typologies, most classification schemes include an early-onset AUD subtype that is considered strongly heritable, more typical among males, and correlated with externalizing behavior (e.g., other drug use, criminality) and a later-onset subtype, more common among females than the early-onset class, less strongly related to family history, and correlated with internalizing psychopathology. The former subtype is clearly supported by our findings, in that externalizing behavior—both at the level of psychopathology in males and females and at the level of symptoms in male conscripts—is a central hub in our models. These findings are also consistent with previous work demonstrating a genetic link between alcohol consumption (which is genetically correlated with alcohol problems; Sanchez-Roige, et al., 2019; Walters et al., 2018) and externalizing behavior in adolescence and young adulthood (Kendler et al., 2011).
In contrast, we did not observe a prominent “internalizing pathway” to AUD among females. The magnitude of the direct path from IPP to AUD was identical across the sexes in our basic models, and the total effect size differed little, which is inconsistent with typologies in which internalizing problems are more central to AUD among females. However, although the total effect of IPP on AUD did not differ substantively across sexes, the total effect of EPP was stronger among males. In addition, the total effect of the AUD GRS was lower among females, which is consistent with the putative lower heritability of the later onset, “internalizing” typology. Our findings suggest that the classical typologies, which were largely derived using small, selected samples, provide a useful framework but may be incomplete in their representation of classes of, and pathways to, AUD.
Limitations
These results should be interpreted in the context of two potentially important limitations. First, these results are dependent on the method of the diagnosis of AUD through Swedish registries. Although such administrative data has important advantages (e.g., no refusals or reporting biases), it would not be expected to identify cases in the same manner as interview-based methods. On average, our subjects with AUD are likely to have more severe symptoms than those meeting Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (American Psychiatric Association, 2013), criteria for AUD at interview. Therefore, our results may not extrapolate to AUD cohorts identified through population surveys, the majority of whom would likely be more mildly ill than those we studied here. However, the validity of our AUD definition is supported by the high rates of concordance observed across our ascertainment methods (the mean [SD] odds ratio is 24.5 [11.7]) (Kendler et al., 2018) and by the pattern of resemblance in relatives (Kendler et al., 2015, 2016b), which is similar to that found in studies based on personal interviews (Heath et al., 1997; Prescott & Kendler, 1999).
Second, our path model assumes a causal relationship between predictor and dependent variables that may not always be true. Some of the inter-variable relationships we assume to be A→B may be either A←B or A↔B. However, our simple models rely entirely on registry information and, therefore, are not susceptible to recall bias. Furthermore, we required that the onset of the EPP and IPP and the assessment of EA precede the first registration for AUD. Our more complex model did incorporate self-report measures available from the conscript registry, so the temporal ordering of variables is less certain. In our enhanced model, we drew the path from internalizing to externalizing symptoms from the clinical interpretation of the likely ordering. We reversed the path and re-ran the model with results nearly identical to the original—that is, this did not meaningfully affect other findings from our path models.
Conclusion
First, in both males and females, the most important mediational hub for the impact of AUD GRS on risk for AUD was generalized externalizing psychopathology assessed either by self-report behaviors and traits at age 18 and/or by prior registration for drug abuse or criminal behavior. Second, in our most complete model in males, a number of other well-studied risk factors, including low IQ, high alcohol or drug use, and predisposing personality traits, were connected into the pathway from genetic risk to AUD through a generalized vulnerability to externalizing disorders. Third, in males, low IQ constituted a second but weaker mediational hub for the effects of AUD GRS. Fourth, even in our more complete model, a substantial proportion of the effect of AUD GRS on risk for AUD was direct and not mediated through any of the variables in our model. Although speculative, it is likely that genetic risk factors that directly affected the metabolism of ethanol and its actions on a wide range of brain mechanisms would be captured in the model by these direct effects. Last, our results suggest that the mediational pathways for GRS for AUD operate in qualitatively different fashions in males and females. In particular, the mediational pathway of genetic effects through EPP is considerably stronger in males than in females, whereas the reverse effect is seen for the pathway through low EA. By contrast, the internalizing pathway is, in absolute terms, of similar strength in both sexes. However, in relative terms, the internalizing pathway is, as expected, more important for the AUD GRS to AUD pathway for females than for males. These results have obvious implications for attempts at the primary prevention of AUD, especially in those at high genetic risk.
Compliance With Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (No. 2008/409). As approved by Swedish ethical authorities, informed consent was not obtained from individual participants included in this study. All work was done at Lund University and Virginia Commonwealth University.
Footnotes
This project was supported by National Institute on Alcohol Abuse and Alcoholism Grant R01AA023534, grants from the Swedish Research Council (2016-01176), and Avtal om Läkarutbildning och Forskning (ALF) funding from Region Skåne.
References
- American Psychiatric Association. (5th ed.). Arlington, VA: Author; 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Babor T. F., Hofmann M., DelBoca F. K., Hesselbrock V., Meyer R. E., Dolinsky Z. S., Rounsaville B. Types of alcoholics, Evidence I. for an empirically derived typology based on indicators of vulnerability and severity. Archives of General Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. doi:10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Cloninger C. R., Bohman M., Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. doi:10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cloninger C. R., Sigvardsson S., Bohman M. Type I and Type II alcoholism: An update. Alcohol Health and Research World. 1996;20:18–23. [PMC free article] [PubMed] [Google Scholar]
- Del Boca F. K., Hesselbrock M. N. Gender and alcoholic sub-types. Alcohol Health and Research World. 1996;20:56–62. [PMC free article] [PubMed] [Google Scholar]
- Heath A. C., Bucholz K. K., Madden P. A., Dinwiddie S. H., Slutske W. S., Bierut L. J., Martin N. G. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. doi:10.1017/S0033291797005643. [DOI] [PubMed] [Google Scholar]
- Jellinek E. M. New Haven, CT: College and University Press; 1960. The disease concept of alcoholism. [Google Scholar]
- Kendler K. S., Gardner C., Dick D. M. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological Medicine. 2011;41:1507–1516. doi: 10.1017/S003329171000190X. doi:S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Ji J., Edwards A. C., Ohlsson H., Sundquist J., Sundquist K. An extended Swedish national adoption study of alcohol use disorder. JAMA Psychiatry. 2015;72:211–218. doi: 10.1001/jamapsychiatry.2014.2138. doi:10.1001/jamapsychiatry.2014.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Lönn S. L., Salvatore J., Sundquist J., Sundquist K. The origin of spousal resemblance for alcohol use disorder. JAMA Psychiatry. 2018;75:280–286. doi: 10.1001/jamapsychiatry.2017.4457. doi:10.1001/jamapsychiatry.2017.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Ohlsson H., Edwards A. C., Sundquist J., Sundquist K. A developmental model for alcohol use disorders in Swedish men. Psychological Medicine. 2016a;46:2759–2770. doi: 10.1017/S0033291716001409. doi:10.1017/S0033291716001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Ohlsson H., Edwards A. C., Sundquist J., Sundquist K. A developmental etiological model for drug abuse in men. Drug and Alcohol Dependence. 2017;179:220–228. doi: 10.1016/j.drugalcdep.2017.06.036. doi:10.1016/j.drugalcdep.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., PirouziFard M., Lonn S., Edwards A. C., Maes H. H., Lichtenstein P., Sundquist K. A national Swedish twinsibling study of alcohol use disorders. Twin Research and Human Genetics. 2016b;19:430–437. doi: 10.1017/thg.2016.62. doi:10.1017/thg.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Volkow N. D. Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. doi:10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler H. R., Zhou H., Kember R. L., Vickers Smith R., Justice A. C., Damrauer S., Gelernter J. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nature Communications. 2019;10:1499. doi: 10.1038/s41467-019-09480-8. doi:10.1038/s41467-019-09480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D., Wetherill L., Bertelsen S., Carey C. E., Kamarajan C., Kapoor M., Foroud T. Genome-wide association studies of alcohol dependence, DSM-IV criterion count and individual criteria. Genes, Brain and Behavior. 2019;18:e12579. doi: 10.1111/gbb.12579. doi:10.1111/gbb.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L., Kenna G. A., Fenton M., Bonenfant E., Swift R. M. Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychology Review. 2009;19:115–129. doi: 10.1007/s11065-008-9080-z. doi:10.1007/s11065-008-9080-z. [DOI] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. (7th ed.). Los Angeles, CA: Authors; 2012. Mplus user's guide: 1998–2012. [Google Scholar]
- Prescott C. A., Kendler K. S. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. doi:10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Sanchez-Roige S., Palmer A. A., Fontanillas P., Elson S. L., Adams M. J., Howard D. M., Clarke T.-K., the 23andMe Research Team, the Substance Use Disorder Working Group of the Psychiatric Genomics Consortium (2019Genome-wide association study meta-analysis of the Alcohol Use Disorders Identification Test (AUDIT) in two population-based cohorts American Journal of Psychiatry 176107–118.doi:10.1176/appi.ajp.2018.18040369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B., Neale M. C., Kendler K. S. The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychological Medicine. 2015;45:1061–1072. doi: 10.1017/S0033291714002165. doi:10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. K., Polimanti R., Johnson E. C., McClintick J. N., Adams M. J., Adkins A. E., Agrawal A., the 23andMe Research Team (2018Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders Nature Neuroscience 211656–1669.doi:10.1038/s41593-018-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]