Abstract
In this phase 3 trial, older patients with acute myeloid leukemia ineligible for intensive chemotherapy were randomized 2:1 to receive the polo-like kinase inhibitor, volasertib (V; 350 mg intravenous on days 1 and 15 in 4-wk cycles), combined with low-dose cytarabine (LDAC; 20 mg subcutaneous, twice daily, days 1–10; n = 444), or LDAC plus placebo (P; n = 222). Primary endpoint was objective response rate (ORR); key secondary endpoint was overall survival (OS). Primary ORR analysis at recruitment completion included patients randomized ≥5 months beforehand; ORR was 25.2% for V+LDAC and 16.8% for P+LDAC (n = 371; odds ratio 1.66 [95% confidence interval (CI), 0.95–2.89]; P = 0.071). At final analysis (≥574 OS events), median OS was 5.6 months for V+LDAC and 6.5 months for P+LDAC (n = 666; hazard ratio 0.97 [95% CI, 0.8–1.2]; P = 0.757). The most common adverse events (AEs) were infections/infestations (grouped term; V+LDAC, 81.3%; P+LDAC, 63.5%) and febrile neutropenia (V+LDAC, 60.4%; P+LDAC, 29.3%). Fatal AEs occurred in 31.2% with V+LDAC versus 18.0% with P+LDAC, most commonly infections/infestations (V+LDAC, 17.1%; P+LDAC, 6.3%). Lack of OS benefit with V+LDAC versus P+LDAC may reflect increased early mortality with V+LDAC from myelosuppression and infections.
Introduction
While acute myeloid leukemia (AML) affects people of all ages, the majority of patients are of advanced age, with a median age at diagnosis of approximately 70 years in developed countries.1,2 Thus, the incidence of AML is rising, at least in part, as a result of the aging population.2
Older AML patients are less likely than younger patients to achieve a complete remission (CR) with standard therapy and tend to have comorbidities that prevent them from receiving intensive chemotherapy.3 For these patients, low-intensity therapies, such as subcutaneous administration of low-dose cytarabine (LDAC), are considered better options. As a result, LDAC has become a recommended therapy, and an established comparator and combination partner for investigational drugs, before the introduction of hypomethylating agents.4,5
Polo-like kinase 1 (Plk1) is a key regulator of mitosis, and its overexpression has been linked with poor prognosis in human cancer.6 Inhibition of Plk1 in vitro was found to block proliferation of leukemic cell lines, and to reduce the clonogenic potential of cell lines derived from patients with leukemia.7 Volasertib is a low-molecular-weight, adenosine triphosphate-competitive kinase inhibitor that potently inhibits Plk1, as well as the 2 closely related kinases, Plk2 and Plk3. In a previous study, volasertib treatment reduced tumor growth in colon and lung xenograft models, and increased apoptosis in samples derived from HCT 116 tumor-bearing nude mice.8 Volasertib has also shown robust antitumor activity in a xenograft model of AML; nude mice with established AML tumors treated with volasertib for 4 weeks experienced marked tumor regression and tolerated treatment well.9
In an open-label, randomized phase 2 trial, conducted in previously untreated AML patients aged ≥65 years who were ineligible for intensive therapy, objective response rates (ORRs; CR or CR with incomplete blood count recovery [CRi]) and overall survival (OS) favored volasertib in combination with LDAC (V+LDAC) over LDAC monotherapy (ORR: 31% versus 13%, odds ratio [OR] 2.91, P = 0.052; median OS 8.0 versus 5.2 months, hazard ratio [HR] 0.63 [95% confidence interval (CI), 0.40–1.00]; P = 0.047). There was an increase in nonhematologic adverse events (AEs) with V+LDAC compared with LDAC; the AEs with the most pronounced increase in frequency included gastrointestinal AEs grade 3 (21% versus 7%), febrile neutropenia grade 3 (38% versus 7%), and infections grade 3 (38% versus 7%). However, these AEs were clinically manageable.10 The current phase 3 study was conducted to confirm the results from the previous phase 2 study of the V+LDAC regimen for older AML patients who are unable to receive intensive therapies.
Materials and methods
Patients and study design
This was a prospective, randomized, double-blind, placebo-controlled study (NCT01721876) of V+LDAC compared with placebo + LDAC (P+LDAC). Eligible patients were aged ≥65 years, had previously untreated (except for hydroxyurea) AML (confirmed according to World Health Organization criteria11), and an Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≤2. Patients were required to be ineligible for intensive remission-induction therapy, based on documented disease and patient characteristics such as high-risk cytogenetics, secondary AML, and comorbidity. Exclusion criteria included: prior or concomitant treatment for AML (prior treatment for myelodysplastic syndrome was allowed); acute promyelocytic leukemia; clinical signs of leukemic central nervous system involvement; clinically relevant QT prolongation (>470 ms); and inadequate organ function (bilirubin >3× upper limit of normal and/or creatinine clearance <30 mL/min).
Eligible patients were randomized in a 2:1 ratio to receive V+LDAC or P+LDAC via an interactive voice/web response system, stratified according to ECOG PS (0–1 versus 2) and type of leukemia (de novo versus secondary). LDAC was administered subcutaneously at a dose of 20 mg twice daily on days 1–10 of each 4-week cycle, either at the investigative site or at the patient’s home, and either volasertib (350 mg) or placebo was added as a 1-hour intravenous infusion on days 1 and 15. Repeated cycles of treatment (with no limit to the number) were administered until disease progression or relapse, according to protocol-defined criteria for treatment continuation and unless the patient or investigator requested treatment discontinuation. If, at the end of each treatment cycle, criteria to continue treatment were not yet met, or if determined necessary by the investigator, subsequent cycles could be delayed for an unrestricted length of time. Dose reductions of volasertib or placebo were allowed in 50-mg decrements, to a minimum of 200 mg. Given the myelosuppressive effects of both volasertib and LDAC, anti-infective prophylaxis and/or growth factors such as granulocyte colony-stimulating factor could be administered according to local guidelines and standards.
The trial was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Guideline for Good Clinical Practice, and applicable specific requirements, and with the approval of the respective institutional review boards/independent ethics committees at each center. All patients provided written informed consent.
Study endpoints and assessments
The primary endpoint was ORR, as determined by the central, blinded review of bone marrow samples and the investigator’s assessment (evaluation of peripheral blood and physical examination). Bone marrow examination for response assessment was carried out at the end of every second cycle, or as soon as possible if disease progression was suspected. CR and CRi were defined according to European Leukemia Net (ELN) recommendations,5 and an additional criterion for CR was red blood cell transfusion independence within 7 days before response assessment. The key secondary endpoint was OS, defined as the time interval from the date of randomization to the date of death.
Two analyses were planned according to the study protocol. The primary analysis was performed shortly after completion of patient recruitment and assessed the primary efficacy endpoint, ORR, using efficacy data from the subset of patients randomized ≥5 months before the cutoff date, including those without response data. Analysis of OS at the primary analysis was descriptive and exploratory. The final analysis to assess the key secondary endpoint, OS, included all randomized patients and was carried out after at least 574 OS events had occurred.
Safety was assessed by determining the incidence and intensity of AEs, defined using the Common Terminology Criteria for Adverse Events (version 3.0), and changes in laboratory assessments and electrocardiograms. Safety evaluations of the treated populations (all randomized patients who received at least one dose of trial medication) were conducted at both the primary and final analyses.
An independent Data Monitoring Committee periodically reviewed unblinded results to monitor the conduct of the trial, ensure patient safety, and maintain the integrity of the data.
Statistical considerations
It was estimated that approximately 371 patients should be included in the primary analysis of ORR, providing 90% power to detect an OR of 2.85 (based on the phase 2 study10 and a phase 3 study of decitabine for elderly AML patients)12 using a 2-sided test and an alpha level of 0.05. A final planned sample size of 660 patients was selected to allow collection of an expected 574 OS events, assuming a dropout rate of ~10%.
The Cochran–Mantel–Haenszel test (adjusting for the 2 stratification factors used for randomization) was used to compare ORR between treatment groups, based on a 2-sided alpha-level of 0.05. Mantel–Haenszel estimates for OR and 95% CI were calculated.
For OS, Kaplan–Meier estimates were calculated for both arms. A log-rank test was carried out, stratified by the same 2 factors used for randomization. A stratified Cox proportional hazards model was used to estimate the HR between arms.
An unplanned, exploratory, post hoc analysis was conducted to better understand the difference between the phase 2 and phase 3 results, and to examine possible reasons for the different outcomes observed in this phase 3 trial (see Supplemental Digital Methods, http://links.lww.com/HS/A177).
Results
Patients and treatment
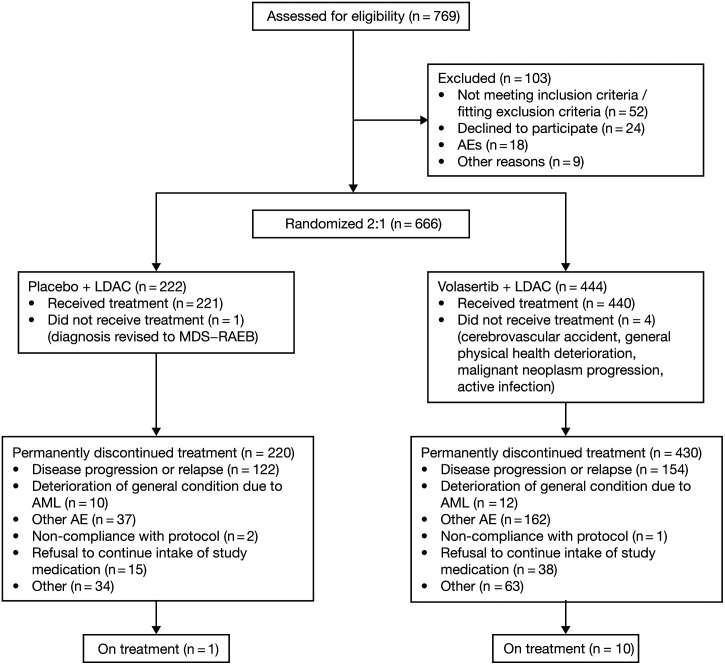
From February 25, 2013, to November 12, 2014, 769 patients were screened at 122 centers in 25 countries, and 666 patients were subsequently randomized (V+LDAC, n = 444; P+LDAC, n = 222). Of these, 661 patients received the study medication (V+LDAC, n = 440; P+LDAC, n = 221) (Figure 1). Patient demographics and baseline disease characteristics were generally balanced between treatment arms (Table 1). The most frequently documented medical reason for ineligibility for intensive remission-induction therapy was age (97.4%), followed by comorbidities (47.3%), most commonly cardiac disorders (20.7%).
Figure 1.
Disposition of patients included in the final analysis. AEs = adverse events; AML = acute myeloid leukemia; LDAC = low-dose cytarabine; MDS = myelodysplastic syndrome; RAEB = refractory anemia with excess blasts.
Table 1.
Baseline Patient Demographics and Disease Characteristics
| Characteristic | Primary Efficacy Analysis Set | Final Analysis Set | ||
|---|---|---|---|---|
| P+LDAC (n = 125) | V+LDAC (n = 246) | P+LDAC (n = 222) | V+LDAC (n = 444) | |
| Sex, n (%) | ||||
| Male | 75 (60.0) | 140 (56.9) | 135 (60.8) | 241 (54.3) |
| Female | 50 (40.0) | 106 (43.1) | 87 (39.2) | 203 (45.7) |
| Race, n (%) | ||||
| White | 88 (70.4) | 181 (73.6) | 158 (71.2) | 328 (73.9) |
| Asian | 21 (16.8) | 39 (15.9) | 39 (17.6) | 74 (16.7) |
| Other/missing | 16 (12.8) | 26 (10.6) | 25 (11.3) | 42 (9.5) |
| Age, median (min–max) | 75.0 (65–85) | 75.0 (65–93) | 76.0 (65–88) | 75.0 (65–93) |
| ECOG PS, n (%) | ||||
| 0 | 27 (21.6) | 48 (19.5) | 53 (23.9) | 100 (22.5) |
| 1 | 65 (52.0) | 136 (55.3) | 117 (52.7) | 241 (54.3) |
| 2 | 33 (26.4) | 62 (25.2) | 52 (23.4) | 103 (23.2) |
| WBC count/nL, n (%) | ||||
| <10/nL | 86 (68.8) | 173 (70.3) | 149 (67.1) | 310 (69.8) |
| ≥10/nL and <50/nL | 36 (28.8) | 52 (21.1) | 62 (27.9) | 104 (23.4) |
| ≥50/nL | 3 (2.4) | 21 (8.5) | 11 (5.0) | 30 (6.8) |
| Type of AML, n (%) | ||||
| De novo | 64 (51.2) | 130 (52.8) | 114 (51.4) | 230 (51.8) |
| Secondary AML | 61 (48.8) | 116 (47.2) | 108 (48.6) | 214 (48.2) |
| Preceding MDS | 45 (36.0) | 83 (33.7) | 77 (34.7) | 162 (36.5) |
| Preceding MPS | 8 (6.4) | 17 (6.9) | 18 (8.1) | 28 (6.3) |
| Therapy-relateda | 8 (6.4) | 16 (6.5) | 12 (5.4) | 24 (5.4) |
| Other | 3 (2.4) | 11 (4.5) | 10 (4.5) | 17 (3.8) |
| 2010 ELN genetic group, n (%) | ||||
| Favorable | 13 (10.4) | 28 (11.4) | 21 (9.5) | 47 (10.6) |
| Intermediate I | 38 (30.4) | 80 (32.5) | 71 (32.0) | 144 (32.4) |
| Intermediate II | 33 (26.4) | 42 (17.1) | 46 (20.7) | 75 (16.9) |
| Adverse | 36 (28.8) | 82 (33.3) | 70 (31.5) | 142 (32.0) |
| Missing | 5 (4.0) | 14 (5.7) | 14 (6.3) | 36 (8.1) |
| Mutation types, n (%) | ||||
| NPM1 | 16 (12.8) | 35 (14.2) | 29 (13.1) | 68 (15.3) |
| FLT3 ITD | 6 (4.8) | 13 (5.3) | 8 (3.6) | 22 (5.0) |
aPrior therapy with alkylating agents or topoisomerase II inhibitors.
AML = acute myeloid leukemia; ECOG PS = Eastern Cooperative Oncology Group Performance Status; ELN = European LeukemiaNet; ITD = internal tandem duplication; MDS = myelodysplastic syndrome; MPS = myeloproliferative syndrome; P+LDAC = placebo plus low-dose cytarabine; V+LDAC = volasertib plus low-dose cytarabine; WBC = white blood cell.
Data cutoff for the primary analysis was August 12, 2014; 371 patients had been assessed for the primary efficacy endpoint, ORR (randomized ≥5 mo before data cutoff; V+LDAC, n = 246; P+LDAC, n = 125), and 533 patients had been assessed for safety (received treatment; V+LDAC, n = 356; P+LDAC, n = 177). On December 18, 2014, based on the results of the primary analysis, blinding was suspended for all patients receiving ongoing treatment. Placebo administration was discontinued and the decision whether to continue patients on unblinded study treatment was taken by the investigators, based on individual benefit–risk evaluations and patient informed re-consent.
The subsequent final analysis (June 1, 2017) included all 666 randomized patients for efficacy analyses and all 661 treated patients for safety analyses. The final analysis was exploratory and descriptive, because potential bias was introduced by the unblinding after the primary analysis.
At both the primary and final analyses, the mean number of initiated treatment cycles was higher in the P+LDAC arm versus the V+LDAC arm (3.6 versus 2.8 and 5.1 versus 4.4 cycles, respectively). At both analyses, the median number of treatment cycles initiated was 2.0 for both the P+LDAC and V+LDAC arms (range 1–16 and 1–14 cycles, respectively, at the primary analysis; and 1–38 and 1–42 cycles, respectively, at the final analysis), and a higher percentage of patients in the P+LDAC arm received >6 cycles of treatment (15.7% versus 8.2% for V+LDAC in the primary analysis and 22.5% versus 16.2% in the final analysis).
Objective response
The primary analysis failed to show a statistically significant benefit of V+LDAC compared with P+LDAC in the primary endpoint; ORR was 25.2% in patients who received V+LDAC versus 16.8% in patients who received P+LDAC (OR 1.66 [95% CI, 0.95–2.89]; P = 0.071; Table 2). In the final analysis, the proportion of patients in the V+LDAC arm who achieved ORR was higher than in the P+LDAC arm (27.7% versus 17.1%; OR 1.88 [95% CI, 1.24–2.83]; P = 0.002; Table 2).
Table 2.
Response Rates by Treatment Arm: Primary and Final Analyses
| Primary Analysis | Final Analysis | |||
|---|---|---|---|---|
| P+LDAC (n = 125) | V+LDAC (n = 246) | P+LDAC (n = 222) | V+LDAC (n = 444) | |
| Patients who achieved CR, n (%) | 12 (9.6) | 23 (9.3) | 27 (12.2) | 67 (15.1) |
| Patients who achieved CRi, n (%) | 9 (7.2) | 39 (15.9) | 11 (5.0) | 56 (12.6) |
| Patients who achieved CR or CRi, n (%) | 21 (16.8) | 62 (25.2) | 38 (17.1) | 123 (27.7) |
| 95% CIa | 11.26–24.32 | 20.19–30.98 | 12.73–22.62 | 23.74–32.04 |
| OR V+LDAC vs P+LDACb | 1.66 | 1.88 | ||
| 95% CI | 0.95–2.89 | 1.24–2.83 | ||
| P | 0.071 | 0.002 | ||
| No response assessment/not evaluable for response, n (%) | 16 (12.8) | 95 (38.6) | 39 (17.6) | 158 (35.6) |
| Death ≤28 d after randomization, n (%) | 4 (3.2) | 27 (11.0) | 8 (3.6) | 52 (11.7) |
| Death >28 and ≤56 d after randomization, n (%) | 7 (5.6) | 30 (12.2) | 16 (7.2) | 50 (11.3) |
| Death >56 and ≤84 d after randomization, n (%) | 0 | 8 (3.3) | 2 (0.9) | 18 (4.1) |
| Median OS, mo (95% CI) | 6.5 (5.1–8.1) | 4.8 (3.8–6.4) | 6.5 (4.9–8.0) | 5.6 (4.5–6.8) |
| HR V+LDAC vs P+LDAC | 1.26 | 0.97 | ||
| 95% CI | (0.9–1.7) | (0.8–1.2) | ||
| P | 0.11 | 0.76 | ||
| Median EFS, mo (95% CI) | 3.1 (2.1–5.8) | 2.8 (2.3–3.8) | 2.8 (2.1–4.9) | 3.3 (2.6–4.2) |
| HR V+LDAC vs P+LDAC | 1.18 | 0.96 | ||
| 95% CI | (0.9, 1.6) | (0.8, 1.2) | ||
| P | 0.26 | 0.67 | ||
| Median RFS, mo (95% CI) | NE (3.7–NE) | 4.9 (3.6–13.4) | 18.7 (11.3–NE) | 13.1 (6.2–NE) |
| HR V+LDAC vs P+LDAC | 1.26 | 1.37 | ||
| 95% CI | (0.4–4.1) | (0.7–2.7) | ||
aWilson’s CI.
bOdds ratio derived from a Cochran–Mantel–Haenszel test stratified by baseline ECOG PS and type of AML. OR > 1 favors V+LDAC.
CI = confidence interval; CR = complete remission; CRi = complete remission with incomplete blood count recovery; EFS = event-free survival; HR = hazard ratio; NE = nonevaluable; OR = odds ratio; OS = overall survival; P+LDAC = placebo plus low-dose cytarabine; RFS = relapse-free survival; V+LDAC = volasertib plus low-dose cytarabine.
The proportion of patients who had no response assessment or were not evaluable for response was higher in the V+LDAC arm compared with P+LDAC (38.6% versus 12.8% in the primary analysis and 35.6% versus 17.6% in the final analysis). The majority of these cases were due to early death prior to the planned first response assessment at the end of treatment cycle 2 (Table 2). These patients were included in the primary efficacy analysis, although no response data were available.
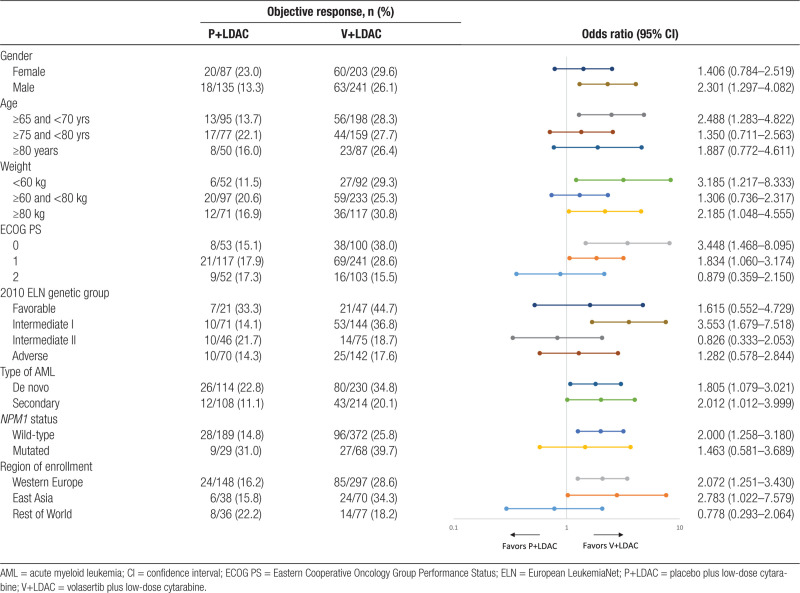
Subgroup analysis of ORR showed differences in response rates by gender, age, weight, ECOG PS, 2010 ELN genetic risk group,13 type of AML, NPM1 mutation status, and geographical region of enrollment, with a trend toward better ORR with V+LDAC compared with P+LDAC in most subgroups (Table 3). Notably, in the ECOG 2 subgroup, the addition of volasertib to LDAC seemed to negatively impact on the outcome, whereas in the ECOG 0 and 1 subgroups, respectively, the response analyses indicate a potential benefit with the addition of volasertib. Subgroup analysis of other genetic aberrations found in AML, such as mutations in FLT3 and CEBPA, was not conducted due to the small number of patients with these mutations in this trial.
Table 3.
Objective Response Rate by Treatment Arm and in Various Subgroups: Final Analysis
Overall survival
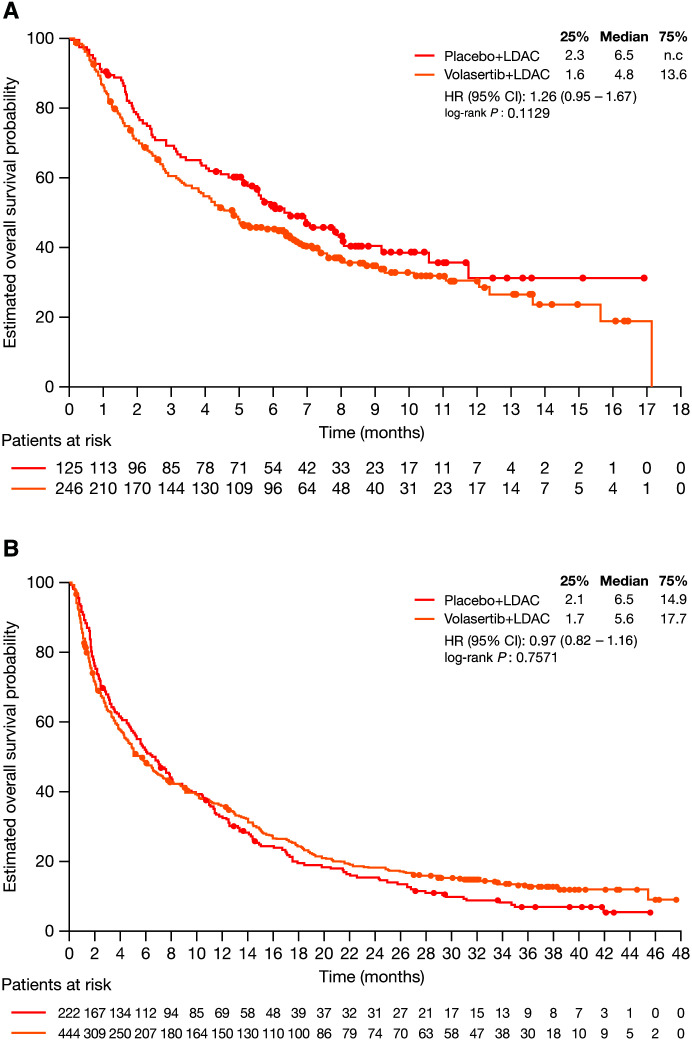
In the primary analysis, numerically shorter, but not statistically significant, OS was seen for the V+LDAC arm compared with the P+LDAC arm (median 4.8 versus 6.5 months; HR 1.26 [95% CI, 0.95–1.67]; P = 0.113; Figure 2). At the final analysis, survival probability over time was similar between the 2 treatment arms, with a median OS of 5.6 months on V+LDAC and 6.5 months on P+LDAC (HR 0.97 [95% CI, 0.82–1.16]; P = 0.757; Figure 2).
Figure 2.
Kaplan−Meier analysis of overall survival by treatment arm: primary analysis (A) and final analysis (B). CI = confidence interval; HR = hazard ratio; LDAC = low-dose cytarabine; n.c. = not calculable.
Subgroup analyses of OS based on baseline factors are provided in Supplemental Table 1, Figures 1 and 2, http://links.lww.com/HS/A177. Of note, in the respective ECOG 0 and 1 subgroups, the addition of volasertib to LDAC appeared to indicate a potential benefit. In the ECOG 2 subgroup, however, the addition of volasertib appeared to negatively impact OS.
Safety
Almost all patients experienced an on-treatment AE prior to final data cut-off (V+LDAC, 99.5%; P+LDAC, 97.7%; Supplemental Tables 2 and 3, http://links.lww.com/HS/A177). Across both arms, the most common AEs were infections/infestations (grouped term; V+LDAC, 81.3%; P+LDAC, 63.5%) and febrile neutropenia (V+LDAC, 60.4%; P+LDAC, 29.3%). The most commonly reported AEs in the individual arms were febrile neutropenia, thrombocytopenia, anemia, and neutropenia in the V+LDAC arm, and were nausea and pyrexia in the P+LDAC arm. The incidence of infections and infestations, and blood cytopenias were numerically higher in the V+LDAC arm than in the P+LDAC arm; Supplemental Table 3, http://links.lww.com/HS/A177). Patients in the V+LDAC arm had a higher incidence of grade ≥3 infections/infestations than patients in the P+LDAC arm (58.1% versus 38.3%; HR 1.77 [95% CI, 1.39–2.27]; P < 0.0001). Similarly, the incidence of febrile neutropenia was higher in patients receiving V+LDAC than in patients receiving P+LDAC (60.4% versus 29.3%; HR 2.84 [95% CI, 2.16–3.73]; P < 0.0001).
The incidence of grade ≥4 AEs was higher in the V+LDAC arm, compared with the P+LDAC arm. The most common grade 4 AEs in both arms were thrombocytopenia and neutropenia, and the difference in grade 4 AE frequency between treatment arms was driven by increased incidences of sepsis, febrile neutropenia, thrombocytopenia, anemia, neutropenia, and leukopenia in the V+LDAC arm. Importantly, AEs leading to death (grade 5) were reported with a higher frequency in the V+LDAC arm (31.2%) than in the P+LDAC arm (18.0%), potentially driven by a higher incidence of infections and infestations (17.1% versus 6.3%; Supplemental Table 3 and Figure 3, http://links.lww.com/HS/A177).
To further explore the difference in infectious complications between the treatment arms, we investigated the incidence, severity, and duration of neutropenia. Grades of neutropenia were similar between the treatment arms at baseline, with the lowest (grade 4) neutrophil levels reported in 39.1% and 46.4% of patients receiving P+LDAC and V+LDAC, respectively. However, over the course of treatment, more patients in the V+LDAC arm than in the P+LDAC arm experienced worsening of neutropenia, with grade 4 neutrophil values reported in 94.3% of patients receiving V+LDAC compared with 75.8% of patients receiving P+LDAC.
AEs in the grouped term mucositis were mostly of grade 1 or 2, but these AEs may have contributed to infectious complications. The frequency of any-grade mucositis (grouped term) was higher in patients receiving V+LDAC (33.3%) than in those receiving P+LDAC (12.6%). This difference in incidence between arms was driven by the most common AE terms in the grouped category, stomatitis and mucosal inflammation.
The majority of patients received treatment with antibiotics or antifungals during the study, and treatment with these was more frequent in the V+LDAC arm (antibiotics 95.2% and antifungals 76.1%) than in the P+LDAC arm (antibiotics 85.6% and antifungals 56.3%). The mean duration of antibiotic or antifungal use was similar between treatment arms.
Exploratory analyses
Subsequent to the primary analysis, ad hoc exploratory analyses were conducted to understand the difference in outcomes between the previous phase 2 study10 and the current phase 3 trial, and the possible reasons why this phase 3 trial did not meet its primary endpoint. One possible cause is differences in cycle 1 dose intensities; protocols for the phase 2 study and this phase 3 trial had similar rules to allow doses to be delayed or skipped if required, resulting in decreased dose intensities. In the majority of patients in the current study, lower dose intensities were caused by a delayed start of the subsequent treatment cycle, that is, length of treatment cycle >28 days. In the phase 2 trial, patients received a lower median dose intensity of volasertib (17.6 mg/d) than in this phase 3 trial (20.8 mg/d). Patients receiving lower V+LDAC dose intensities in this phase 3 trial had longer OS, longer time to fatal AEs and fatal infections, and a higher ORR than did patients receiving V+LDAC at a higher dose intensity (Supplemental Figures 3–5 and Table 4, http://links.lww.com/HS/A177).
To determine whether use of prophylactic antibiotics affected the incidence of fatal infections, an analysis of the time to fatal infection by extent of prophylactic antibiotic treatment was conducted. Patients in the V+LDAC arm who were not treated with prophylactic antibiotics had a higher risk of fatal infections than patients who received any prophylactic antibiotics (Supplemental Figure 6, http://links.lww.com/HS/A177).
A competing risk analysis was performed to explore separately the effect of volasertib on OS events resulting from lack of efficacy or nontolerability. A benefit was observed in the V+LDAC arm compared with the P+LDAC arm when AML-related deaths were considered by the investigator as potentially due to lack of efficacy, whilst a benefit was observed in the opposite direction for deaths considered by the investigator as potentially due to intolerability (Supplemental Figures 7 and 8, http://links.lww.com/HS/A177).
Discussion
The current randomized, double-blind, placebo-controlled, phase 3 trial was conducted to evaluate the efficacy and safety of volasertib, a highly potent and selective Plk inhibitor, combined with LDAC in previously untreated older patients with AML who were considered unsuitable for intensive chemotherapy, and aimed to confirm the encouraging results from the previous, randomized, open-label, phase 2 trial.10
The primary endpoint was not met; in the primary analysis, V+LDAC was not associated with significantly higher ORR compared with P+LDAC. In the final analysis, the proportion of patients who achieved an objective response was higher in the V+LDAC arm than in the P+LDAC arm; however, a substantially greater number of patients receiving V+LDAC had no response assessment or were not evaluable, primarily because of a higher death rate prior to the first response assessment at the end of cycle 2. In the subgroup analysis, the addition of volasertib to LDAC in patients in the ECOG 2 subgroup seemed to negatively impact the ORR.
In the primary analysis, the numerically shorter OS observed in the V+LDAC arm, in comparison with the P+LDAC arm, was likely due to a higher frequency of fatal infections in patients receiving V+LDAC. The study was subsequently unblinded, which may have influenced subsequent patient management, medical decision making, and, consequently, the outcomes seen in the trial. The final analysis, which demonstrated no difference in OS between treatment arms should, therefore, be considered exploratory and descriptive only. The competing risk modeling of survival endpoints indicated fewer deaths potentially due to lack of efficacy, but more deaths potentially due to intolerability, in the V+LDAC arm compared with P+LDAC arm. Such competing risk analyses are particularly important for oncology studies of elderly patients, since many older patients may die of non-cancer-related causes rather than from a lack of treatment efficacy.14 The particularly adverse OS of patients with ECOG PS 2 treated with V+LDAC contributed to the OS trend in the V+LDAC arm, most likely because frailer patients were at higher risk of severe AEs associated with volasertib treatment. Although previous studies have reported very low rates or absence of remissions with LDAC for patients with an adverse genetic profile,4,5 in the final analysis of this study, CR or CRi was reported in 14.3% of patients in the adverse genetic group who received P+LDAC, and in 17.6% of patients who received V+LDAC.
More grade ≥4 AEs and almost twice as many grade 5 AEs were reported in the V+LDAC arm compared with the P+LDAC arm. This was attributed to the more pronounced myelosuppression observed in the V+LDAC treatment group, in addition to the higher reported frequency of mucositis. These results were expected based on the mode of action of volasertib and on previous clinical studies;15–18 volasertib was expected to transiently inhibit the proliferation of normal dividing cells, leading to temporary myelosuppression, and increasing the risk of associated complications such as febrile neutropenia, infections, or thrombocytopenic bleeding.
The results of our exploratory analyses suggested that differences in dose intensity may have influenced outcomes. Median dose intensities resulted from medical assessment and decision-making by investigators; dose intensities were different between the previous phase 2 study and the current phase 3 study, although both studies had similar rules to adapt dosing. Additionally, the open-label nature of the phase 2 study versus the double-blind phase 3 design might have influenced medical assessment and decision making and, thus, dose intensity. Patients receiving a lower dose intensity of volasertib (and therefore also LDAC) in this phase 3 trial had a longer time to fatal AEs and fatal infections, which were some of the major factors contributing to the poorer OS in the V+LDAC arm compared to the P+LDAC arm.
Supportive care could potentially influence outcomes, and improvement of supportive care with the compulsory administration of prophylactic antibiotics/antifungals and blood transfusions may be advisable to proactively manage treatment-induced myelosuppression and avoid infections. Of note, the recommendations for supportive care were similar across the phase 2 and phase 3 studies, both of which allowed supportive care use at the investigator’s discretion. The results of our exploratory analyses suggest that prophylactic antibiotics may reduce the risk of fatal infections in patients treated with volasertib. Effective supportive care, along with reduction in dose intensity, may improve tolerability in patients receiving volasertib combination therapy, and ultimately improve OS.
A numerically higher ORR but no corresponding increase in survival for V+LDAC compared with P+LDAC is consistent with the results of trials testing other novel agents in older patients with AML who are ineligible for intensive chemotherapy. Clofarabine showed significantly superior ORRs compared with LDAC, but failed to show a survival benefit because the increased remission rate was obtained at a cost of greater toxicity.19 Addition of gemtuzumab ozogamicin to LDAC improved ORR but did not improve OS due to inferior survival after relapse. Additionally, in patients who did not achieve remission, survival was inferior in those who received the combination in comparison to those who received LDAC alone.20
As a result of the observed disparity between response rate and survival outcomes, there is ongoing debate as to whether the response rate is a good predictor of OS and whether it is suitable as a surrogate endpoint in trials of AML.21,22 A meta-analysis of 20 trials in AML showed a significant correlation between rates of CRi or better and median OS,23 supporting the use of CR plus CRi as the primary endpoint in this study.
At the time this study was designed, LDAC was considered the standard treatment for patients with AML who were ineligible for standard intensive chemotherapy. Since then, the hypomethylating agents azacitidine and decitabine have been introduced into therapy guidelines as recommended treatment for these patients.5 These agents may now be considered the preferred combination partners and comparators for clinical trials. Furthermore, in the phase 1b/2 M14-358 and phase 1b M14-387 studies, the BCL-2 inhibitor, venetoclax, in combination with azacitidine, decitabine, or LDAC, demonstrated encouraging CR rates and remission duration in AML patients of older age (≥60 yrs) or with comorbidities precluding the use of intensive induction chemotherapy. The pivotal phase 3 VIALE-A trial reported that, in patients with AML who were ineligible for intensive induction therapy due to comorbidities or age, treatment with venetoclax and azacitidine led to a significant improvement in OS (14.7 versus 9.6 mo, P < 0.001), composite complete remission (CR + CRi; 66.4% versus 28.3%, P < 0.001) and event-free survival (9.8 versus 7.0 months, P < 0.001), compared to treatment with placebo and azacytidine.24 Venetoclax in combination with a hypomethylating agent or LDAC therefore offer new therapy options for these patients.25–27
This randomized phase 3 trial did not meet its primary endpoint of ORR in the primary analysis, and did not confirm the survival benefits of volasertib in combination with LDAC seen in a previous randomized phase 2 study.10 There was a notably higher rate of fatal infections in patients who received V+LDAC, indicating that the volasertib dose and schedule used were not sufficiently tolerable. Development of volasertib was discontinued in 2018, following a strategic decision by the sponsor. Nevertheless, the results of this trial provide insight into the efficacy and tolerability of volasertib in older patients with AML, and may inform development of other Plk1 inhibitors.
Acknowledgments
The authors thank all participating patients and their families, the medical and nursing staff, and the other Study 1230.14 investigators for their contribution to this study. A complete list of investigators is provided in the Supplemental Digital Material, http://links.lww.com/HS/A177. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Hannah Simmons, MSc, of Ashfield MedComms, an Ashfield Health company.
Disclosures
HD reports receipt of consultancy fees from AbbVie, Agios, Amgen, Astellas Pharma, Astex Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Celgene, GEMoaB, Helsinn, Janssen, Jazz Pharmaceuticals, Novartis, Oxford BioMedica, and Roche; and receipt of grants or funds from Agios, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Jazz Pharmaceuticals, Novartis, and Pfizer. AS reports honoraria directly received from AbbVie, Amgen, Bristol-Myers Squibb, Gilead, Janssen, Novartis, Pfizer, Roche, Sanofi-Genzyme, and Takeda; receipt of consultancy fees from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Novartis, Pfizer, Roche, Sanofi-Genzyme, and Takeda; and receipt of grants or funds from AbbVie, Alexion, Amgen, Astellas, Bristol-Myers Squibb, DEMO Pharmaceuticals, Genesis, Gilead, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, raPHARM, Roche, Sanofi-Genzyme, Takeda, and WinMedica. WF reports participation on advisory boards for Morphosys, AbbVie, Pfizer, Amgen, Jazz Pharmaceuticals; support for meeting attendance from Amgen, Jazz Pharmaceuticals, Daiichi Sankyo Oncology, Bristol-Myers Squibb, and Servier; and receipt of support in medical writing from Amgen, Boehringer Ingelheim, Pfizer, and AbbVie. H-JK reports receipt of consultancy fees from Amgen, AML Global Portal, Astellas Pharma, Celgene, Novartis, SL VaxiGen, Yuhan Pharmaceuticals, Eutilex, and Otsuka Pharmaceuticals; and royalties of payments for lectures/manuscript preparations from Astella, AML Global Portal, Novartis, and AbbVie. J-HL reports receipt of grants or funds from Boehringer Ingelheim. KU reports honoraria directly received from Novartis, Astellas Pharma, Alexion, Eisai, Merck Sharp & Dohme, Otuska, Ono, Kyowa-Kirin, SymBio, Celgene, Daiichi Sankyo, Takeda, Nihon Shinyaku, PharmaEssentia, and Bristol-Myers Squibb; receipt of grants or funds from Astellas Pharma, AbbVie, Apelis, Otsuka Pharmaceutical, Daiichi Sankyo, Novartis, Merck Sharp & Dohme, Chugai, Pfizer, Bristol-Myers Squibb, Alexion, Ono, Janssen, SymBio, Celgene, Daiichi Sankyo, Sumitomo Dainippon, Takeda, Nihon Shinyaku, Novartis, Boehringer Ingelheim, Mundipharma, Chugai Pharma, Gilead, and Kyowa Kirin; receipt of grants/pending grants from Astellas Pharma, Otsuka Pharmaceutical, Apelis, SymBio, Takeda, Nihon Shinyaku, Novartis, Merck Sharp & Dohme, Alexion, Kyowa Kirin, Gilead, Chugai, Pfizer, Bristol-Myers Squibb, and Daiichi Sankyo; and receipt of royalties of payments for lectures/manuscript preparations from Novartis, Astellas Pharma, Alexion, Eisai, Merck Sharp & Dohme, Otuska, Ono, Kyowa-Kirin, Celgene, Daiichi Sankyo, Takeda, Nihon Shinyaku, PharmaEsseitia, Bristol-Myers Squibb, and Yakurt. H-AH reports receipt of consultancy fees from Amgen. CR reports receipt of grants/pending grants from AbbVie, Amgen, Novartis, Celgene, Jazz Pharmaceuticals, Agios, Daiichi Sankyo, Astellas Pharma, Sunesis, Roche, and MaaT Pharma. PR reports receipt of research grants from Incyte and Pfizer. IS reports honoraria received directly from Celgene, Bristol-Myers Squibb, Pfizer, Takeda, Janssen, and Amgen. FT reports participation on advisory boards for Celgene, Novartis, Jazz Pharmaceuticals, AbbVie, Astellas Pharma, and Pfizer. DJD reports receipt of consultancy fees from Amgen, Autolus, Agios, Blueprint Medicines, Forty-Seven, Incyte, Jazz Pharmaceuticals, Novartis, Pfizer, Servier, and Takeda; and research funding from Abbvie, GlycoMimetics, Novartis, and Blueprint Pharmaceuticals. MS reports participation on an advisory council or committee for Bristol-Myers Squibb, Celgene, Takeda, Millenium, and Pfizer. VB is an employee of SCS Boehringer Ingelheim Comm.V. MG is an employee of Boehringer Ingelheim Pharm. TT is an employee of Boehringer Ingelheim International GmbH. All the other authors have no conflicts of interest to disclose.
Sources of funding
This study was funded by Boehringer Ingelheim.
Study group members
Anagnostopoulos, Achilleas
Anagnostopoulos, Nikolaos
Ando, Kiyoshi
Aulitzky, Walter
Baltasar, Patricia
Bergeron, Julie
Bernard, Marc
Borbenyi, Zita
Briasoulis, Evangelos
Bulabois, Claude Eric
Cairoli, Roberto
Calbecka, Malgorzata
Capra, Marcelo
Chang, Cheng-Shyong
Choudhury, Ratnamala
Chromik, Jorg
Cortes, Jorge
Coutinho, Jorge
De Prijck, Bernard
Deeren, Dries
Delaunay, Jacques
Demeter, Judit
Döhner, Hartmut
Esteve, Jordi
Esteves, Graca
Fiedler, Walter
Fujisawa, Shin
Gasztonyi, Zoltan
Geissler, Klaus
Gjertsen, Bjorn Tore
Goetze, Katharina
Gomez, David
Goueli, Basem
Graux, Carlos
Guimaraes, Jose
Havelange, Violaine
Hogge, Donna
Horst, Heinz-August
Hou, Hsin-An
Iida, Hiroatsu
Jindra, Pavel
Jung, Chul Won
Kameoka, Yoshihiro
Kaporskaya, Tatiana
Kim, Hee-Je
Kim, Hyeoung Joon
Kindler, Thomas
Kobayashi, Yukio
Koh, Youngil
Kohler, Friedemann
Kraemer, Alwin
Krause, Stefan
Krauter, Juergen
Lee, Je Hwan
Maeda, Yoshinobu
Maertens, Johan
Mariz, Mario
Marmont, Filippo
Marolleau, Jean-Pierre
Martínez, Pilar
McDonald, Andrew
Minami, Hironobu
Miyamoto, Toshihiro
Miyazaki, Yasushi
Mohty, Mohamad
Mueller-Tidow, Carsten
Nakamae, Hirohisa
Neubauer, Andreas
Noppeney, Richard
Novak, Jan
Olga, Salamero
Onishi, Yasushi
Ossenkoppele, Gert
Pichler, Angelika
Pigneux, Arnaud
Pires, Andiara
Porkka, Kimmo
Recher, Christian
Rego, Eduardo
Reichle, Albrecht
Reman, Oumedaly
Riscala, Carlos
Robak, Tadeusz
Rossi, Giuseppe
Rousselot, Philippe
Salmenniemi, Urpu
Samoylova, Olga
Sandhu, Irwindeep
Sanz, Miguel Angel
Schiller, Gary
Schmid, Christoph
Selleslag, Dominik
Shneider, Tatiana
Sierra, Jorge
Sill, Heinz
Storring, John
Straetmans, Nicole
Strickland, Stephen
Susana, Vives
Symeonidis, Argiris
Theunissen, Koen
Thol, Felicitas
Thomas, Xavier
Turlure, Pascal
Uchida, Toshiki
Ueda, Yasunori
Usuki, Kensuke
Vey, Norbert
Vidriales, Belén
Waleed, Ghanima
Westermann, Joerg
Wiebe, Stefanie
Wulf, Gerald
Yamauchi, Takahiro
Yee, Karen
Zak, Pavel
Supplementary Material
Footnotes
Current address for Oliver G. Ottmann: Institute of Cancer & Genetics, Cardiff University School of Medicine, Cardiff, United Kingdom
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfill their role and obligations as authors under the ICMJE criteria. Furthermore, clinical study documents (eg, study report, study protocol, and statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the BI Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/transparency_policy.html. Before providing access, documents will be examined, and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants. Clinical Study Reports and Related Clinical Documents can be requested via this link: https://trials.boehringer-ingelheim.com/trial_results/clinical_submission_documents.html. All such requests will be governed by a Document Sharing Agreement. Bona fide, qualified scientific, and medical researchers may request access to de-identified, analyzable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request. Researchers should use https://trials.boehringer-ingelheim.com to request access to study data. Clinical trial registry: https://clinicaltrials.gov/. Clinical trial number: NCT01721876.
Supplemental digital content is available for this article.
References
- 1.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance Epidemiology and End Results (SEER) Program. SEER Cancer Statistics Review (CSR) 1975-2015. Available at: https://seer.cancer.gov/csr/1975_2015/. Accessed Apri 17, 2018.
- 3.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–1152. [DOI] [PubMed] [Google Scholar]
- 4.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. [DOI] [PubMed] [Google Scholar]
- 5.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Kim J. PLK-1 targeted inhibitors and their potential against tumorigenesis. Biomed Res Int. 2015;2015:705745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renner AG, Dos Santos C, Recher C, et al. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood. 2009;114:659–662. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph D, Steegmaier M, Hoffmann M, et al. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15:3094–3102. [DOI] [PubMed] [Google Scholar]
- 9.Gjertsen BT, Schöffski P. Discovery and development of the Polo-like kinase inhibitor volasertib in cancer therapy. Leukemia. 2015;29:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Döhner H, Lübbert M, Fiedler W, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 12.Malik P, Cashen AF. Decitabine in the treatment of acute myeloid leukemia in elderly patients. Cancer Manag Res. 2014;6:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. [DOI] [PubMed] [Google Scholar]
- 14.de Glas NA, Kiderlen M, Vandenbroucke JP, et al. Performing survival analyses in the presence of competing risks: a clinical example in older breast cancer patients. J Natl Cancer Inst. 2016;108:djv366. [DOI] [PubMed] [Google Scholar]
- 15.Ottmann OG, Müller-Tidow C, Krämer A, et al. Phase I dose-escalation trial investigating volasertib as monotherapy or in combination with cytarabine in patients with relapsed/refractory acute myeloid leukaemia. Br J Haematol. 2019;184:1018–1021. [DOI] [PubMed] [Google Scholar]
- 16.Schöffski P, Awada A, Dumez H, et al. A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer. 2012;48:179–186. [DOI] [PubMed] [Google Scholar]
- 17.Stadler WM, Vaughn DJ, Sonpavde G, et al. An open-label, single-arm, phase 2 trial of the Polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer. 2014;120:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nokihara H, Yamada Y, Fujiwara Y, et al. Phase I trial of volasertib, a Polo-like kinase inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2016;34:66–74. [DOI] [PubMed] [Google Scholar]
- 19.Burnett AK, Russell NH, Hunter AE, et al. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood. 2013;122:1384–1394. [DOI] [PubMed] [Google Scholar]
- 20.Burnett AK, Hills RK, Hunter AE, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27:75–81. [DOI] [PubMed] [Google Scholar]
- 21.Estey E, Othus M, Lee SJ, et al. New drug approvals in acute myeloid leukemia: what’s the best end point? Leukemia. 2016;30:521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medeiros BC. Interpretation of clinical endpoints in trials of acute myeloid leukemia. Leuk Res. 2018;68:32–39. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal SK, Mangal N, Menon RM, et al. Response rates as predictors of overall survival: a meta-analysis of acute myeloid leukemia trials. J Cancer. 2017;8:1562–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–629. [DOI] [PubMed] [Google Scholar]
- 25.Wei AH, Strickland SA, Jr, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37:1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. [DOI] [PubMed] [Google Scholar]
- 27.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.