Abstract
Introduction:
The objective was to determine whether closer adherence to the alternative Mediterranean Diet (aMED) was associated with altered cognitive function.
Methods:
Observational analyses of participants (n = 7,756) enrolled in two randomized trials of nutritional supplements for age-related macular degeneration: Age-Related Eye Disease Study (AREDS) and AREDS2.
Results:
Odds ratios for cognitive impairment, in aMED tertile 3 (vs 1), were 0.36 (P = .0001) for Modified Mini-Mental State (<80) and 0.56 (P = .001) for composite score in AREDS, and 0.56 for Telephone Interview Cognitive Status-Modified (<30) and 0.48 for composite score (each P < .0001) in AREDS2. Fish intake was associated with higher cognitive function. In AREDS2, rate of cognitive decline over 5 to 10 years was not significantly different by aMED but was significantly slower (P = .019) with higher fish intake.
Discussion:
Closer Mediterranean diet adherence was associated with lower risk of cognitive impairment but not slower decline in cognitive function. Apolipoprotein E (APOE) haplotype did not influence these relationships.
Keywords: age-related macular degeneration, cognitive function, fish consumption, genetic interaction, Mediterranean diet
1 ⎹. INTRODUCTION
Dementia is a common and incurable disorder with major implications for individuals, families, and society. The worldwide prevalence of dementia was estimated in 2016 at 44 million and is projected to pass 115 million by 2050.1,2 Alzheimer’s disease (AD) is the most common form of dementia. Following the failure of many phase III trials, no medical treatment is currently available to prevent, delay, or modify the course of dementia.3 Thus modifying therapies and preventative approaches are important. Indeed, one third of AD cases worldwide have been attributed to potentially modifiable risk factors.4
Slow neurocognitive decline throughout life is an expected part of normal aging.5–7 However, some people experience accelerated cognitive decline, and may be at high risk of dementia.8,9 Altering the trajectory of cognitive decline through preventative approaches may be particularly fruitful in this population, that is, decreasing progression to mild cognitive impairment (MCI) and/or from MCI to dementia.
Diet may be an important factor in influencing progression to MCI and dementia. As a modifiable environmental factor, diet can exert profound effects on biological aging,10–14 and has been associated with age-related conditions linked to dementia, including cardiovascular disease and diabetes.15,16 Regarding food groups, fish intake has attracted attention for an association with slower decline in cognition and memory.17 The Mediterranean diet pattern has received interest.18,19 At least 14 systematic reviews have been conducted in this area,20 but the results of these and the constituent observational studies have been inconsistent.21 In addition, the question of potential interactions between diet and genotype in influencing cognitive decline remains controversial.17,22,23
The Age-Related Eye Disease Study (AREDS) and AREDS2 were multicenter phase III randomized clinical trials (RCT) designed to assess the effects of nutritional supplementation on progression to late age-related macular degeneration (AMD).24,25 According to some epidemiologic studies and a meta-analysis, AMD and dementia are associated at the population level.26,27 Indeed, AMD and AD are both neurodegenerative conditions of aging and share some environmental risk factors, including smoking and hypertension; however, their genetic risk profiles are distinct.28–30
The aims of this report were to use AREDS/AREDS2 for post hoc analyses to: (1) examine potential associations between Mediterranean diet adherence and both cross-sectional status and longitudinal changes in cognitive function, and (2) assess interactions between Mediterranean diet adherence and genotype in influencing cognitive function.
2 ⎹. METHODS
The AREDS/AREDS2 study designs, described previously,24,31,32 involved recruitment from U.S. retinal specialty clinics: AREDS, 4,757 participants (55–80 years) with no AMD to unilateral AMD, recruited (1992–1998) at 11 sites and AREDS2, 4,203 participants (50–85 years) with bilateral large drusen or unilateral late AMD, recruited (2006–2008) at 82 sites. Institutional review board approval was obtained at each site and written informed consent was obtained from all participants. The research was conducted under the Declaration of Helsinki and, for AREDS2, complied with the Health Insurance Portability and Accessibility Act.
2.1 ⎹. Study procedures
AREDS participants were randomly assigned to placebo, antioxidants, zinc, or the combination.24 AREDS2 participants were randomly assigned to receive the supplements that lowered risk of AMD progression in AREDS, either (1) alone, or with additional (2) lutein/zeaxanthin, (3) docosahexaenoic acid (DHA) plus eicosapentaenoic acid (EPA), or (4) the combination.25 Eligible participants had to provide informed consent and be free of conditions that would make follow-up or medication compliance difficult. This assessment was made by a physician. Hence, participants with dementia at baseline were effectively excluded.
2.2 ⎹. AREDS and AREDS2: Ancillary studies of cognitive function
An AREDS ancillary study examining cognitive function was added to the protocol31 and the tests were administered (25–30 minutes) in person by certified interviewers from 2000 to 2004. Of the 4,360 AREDS participants alive at ancillary study implementation, 3,070 (70%) consented and completed testing.31
An AREDS2 ancillary study examining cognitive function was pre-specified in the protocol.32 The tests were administered (30 minutes) over the telephone by certified interviewers. Justification for telephone testing had previously been demonstrated in AREDS data.33 The first testing administration was within 3 months of randomization. Repeat administrations occurred every 2 years, until close-out of the main study at 5 years. Of the 4,203 AREDS2 participants, 3,501 (83.3%) consented and completed testing.32 Following close-out at 5 years, 1,447 (41.3%) participants underwent repeat testing at 10 years in a follow-on study.
The batteries of cognitive function tests used have been described previously.31,32 The individual tests are listed in Table 1 and described in greater detail in the supporting information. These included the Modified Mini-Mental State Examination (3MS), for AREDS, and the Telephone Interview Cognitive Status-Modified (TICS-M, a version of the 3MS), for AREDS2. In addition, a composite score (representing an overall score for the whole battery) was calculated as the sum of the z-scores for each test within the battery.32,33
TABLE 1.
Cognitive function tests used, ranges in their scores (possible and observed within the study), and cut-off points used to define cognitive impairment
| Cognitive function test | Range possible | Range in study | Cut-off point | ||
|---|---|---|---|---|---|
| Min | Max | Min | Max | ||
| Age-Related Eye Disease Study | |||||
| 3MSa | 0 | 100 | 47 | 100 | ≤79 |
| Buschke immediate recall | 0 | 100 | 0 | 83.3 | ≤8.3 |
| Buschke overall word list | 0 | 12 | 0 | 11.2 | ≤3.3 |
| Logical memory part I | 0 | 75 | 0 | 69 | ≤22 |
| Logical memory part II | 0 | 50 | 0 | 45 | ≤9 |
| Animal category | 0 | - | 0 | 44 | ≤11 |
| Letter fluency | 0 | - | 1 | 91 | ≤21 |
| Logical memory part I | 0 | 75 | 0 | 69 | ≤22 |
| Logical memory part II | 0 | 50 | 0 | 45 | ≤9 |
| Digits backwardb | 0 | 12 | 0 | 12 | ≤3 |
| Composite scorec | - | - | −23.5 | 17.9 | ≤−7.0 |
| Age-Related Eye Disease Study 2 | |||||
| TICS-Ma | 0 | 41 | 12 | 41 | ≤29 |
| TICS-M recall | 0 | 10 | 0 | 10 | =0 |
| Animal category | 0 | - | 0 | 43 | ≤10 |
| Letter fluency | 0 | - | 0 | 118 | ≤19 |
| Alternating fluency | 0 | - | 0 | 14 | ≤1 |
| Logical memory part I | 0 | 75 | 0 | 69 | ≤22 |
| Logical memory part II | 0 | 50 | 0 | 46 | ≤9 |
| Digits backwardsb | 0 | 100 | 0 | 100 | ≤23 |
| Composite scorec | - | - | −21.1 | 17.6 | ≤−7.0 |
3MS, Modified Mini-Mental State Examination; TICS-M, Telephone Interview Cognitive Status-Modified.
The 3MS has a predefined cut-off point for cognitive impairment of 80 and the TICS-M has one of 30. For the other tests, cognitive impairment is defined as being in the lowest decile, except for the TICS-M recall, for which it is defined as being in the lowest quintile. In both study cohorts, the cut-off points were determined using only the participants included in the analyses; in AREDS2, the cut-off points were determined using all time points (though the same cut-off point was used for all study visits)
Different versions of the digits backward test were used in AREDS and AREDS2, hence the difference in the score ranges.
Composite score is the sum of Z-scores from each test within the battery.
2.3 ⎹. Outcome measurements
The primary outcome measurement was cognitive impairment, defined as (1) 3MS < 80 (AREDS) or TICS-M < 30 (AREDS2), and (2) composite score in the lowest decile (Table 1). The secondary outcome measurements were absolute scores of the (1) 3MS (AREDS) or TICS-M (AREDS2), and (2) composite.
2.4 ⎹. Modified Alternative Mediterranean diet index score
Validated food frequency questionnaires (FFQs) were administered to all participants at randomization: the AREDS FFQ, a 90-item, semi-quantitative modified Block FFQ,34 and the AREDS2 FFQ, a 131-item, semi-quantitative Harvard FFQ.35,36 In both FFQs, participants were asked how often, on average, they had consumed each food/beverage item during the preceding year.
The FFQs were used to determine the number of medium-sized servings of each food item consumed per week (or gram/day for alcohol). To calculate the modified Alternative Mediterranean Dietary Index (aMED) score, the foods were summed to obtain the intake for each of nine components: whole fruits, vegetables, whole grains, nuts, legumes, red meat, fish, monounsaturated fatty acid:saturated fatty acid ratio, and alcohol. For each component, sex-specific intake quartiles1–4 were calculated (separately for AREDS and AREDS2), with quartile 4 representing highest intake. Alcohol intake was converted into binary format: 4 for intake within the specified intervals (5–15 g/d [female] or 10–15 g/d [male]) and 1 for intake above or below the specified intervals.37 The quartiles for red meat were reversed (ie, quartile 4 with highest intake scored 1, as least aMED-adherent, and quartile 1 with lowest intake scored 4). The aMED score was calculated as the sum of quartile values for the nine components (range 9–36).
2.5 ⎹. Genotype analysis
As part of AREDS/AREDS2, 2,889 (AREDS) and 1,826 (AREDS2) participants consented to genotype analysis. Single nucleotide polymorphisms (SNPs) were analyzed using a custom Illumina Human-CoreExome array.28 APOE haplotypes were defined by rs429358 and rs7412.38 Following a previous report,39 three additional SNPs were analyzed: CR1 (rs3818361), CLU (rs11136000), and PICALM (rs3851179).
2.6 ⎹. Statistical analyses
For analyses of cognitive impairment, logistic regression was performed; for cognitive test scores, general linear models were used (mixed model regression for AREDS2). For AREDS2, the regression accounted for repeated measures, number of tests, and unequal time spacing by using a spatial power correlation; an autoregressive correlation structure was used for the repeated measures logistic regression. Significance was set at 0.013 (Bonferroni). For AREDS2, to compare rates of cognitive decline between aMED tertiles, data from participants with multiple testing were analyzed using a model that included an interaction term of time-point and aMED tertiles. Significance was set at 0.025 (Bonferroni).
The analyses of cognitive test scores were repeated, including an interaction term of aMED and genotype. Significance was set at 0.003 (Bonferroni). Further regression analyses were performed for the nine aMED components: separate models were made for each component (component i), adjusting for the modified aMED that did not include the respective component (modified aMED = total aMED – component i). Significance was set at 0.0014 (Bonferroni).
All models were adjusted for baseline age, sex, race, smoking, diabetes, hypertension, baseline depression score (Center for Epidemiological Studies-Depression Scale [CES-D] ≥16 or not), total calorie intake, and (AREDS2 only) years from baseline. Participants were excluded from an analysis if they had missing covariates or data for the relevant test. Analyses were conducted using SAS version 9.4 (SAS Institute Inc).
3 ⎹. RESULTS
3.1 ⎹. Participants
The AREDS enrolled 4,757 participants. Of the 4,360 participants alive at ancillary cognitive study implementation, 3,074 (70.5%) consented and completed one or more cognitive tests. Of these, 3,029 (98.5%) were included in one or more analyses in the current study. Similarly, AREDS2 enrolled 4,203 participants. Of these, 3,501 (83.3%) completed one or more cognitive tests, and 3,326 (95.0%) were included in one or more analyses. The characteristics of the participants are shown in Table 2, and their dietary intake in Tables S1 and S2 in supporting information. In the AREDS, the proportion of participants with cognitive impairment (3MS < 80) at the single time point of assessment was 3.9%. In the AREDS2, the proportion with cognitive impairment (TICSM < 30) at the study baseline was 14.8%. APOE risk haplotypes were significantly associated with increased risk of cognitive impairment and with lower test scores, in both AREDS and AREDS2 (Table S3 in supporting information).
TABLE 2.
The baseline demographic, clinical, and genetic characteristics of participants included in at least one analysis in the current study
| AREDS | AREDS2 | |
|---|---|---|
| Participants: n | 3029 | 3326 |
| Age (years): mean (SD) | 68.7 (4.9) | 72.9 (7.7) |
| Female: n (%) | 1721 (56.8) | 1924 (57.8) |
| Race: n (%) | ||
| White | 2912 (96.1) | 3231 (97.1) |
| Non-white | 117 (3.9) | 95 (2.9) |
| Education level: n (%) | ||
| High school or less | 935 (30.9) | 972 (29.2) |
| At least some college | 932 (30.8) | 1585 (47.7) |
| Postgraduate | 1160 (38.3) | 710 (21.3) |
| Unknown | 2 (0.1) | 59 (1.8) |
| Smoking status: n (%) | ||
| Never | 1435 (47.4) | 1419 (42.7) |
| Former | 1399 (46.2) | 1676 (50.4) |
| Current | 195 (6.4) | 231 (6.9) |
| History diabetes: n (%) | 231 (7.6) | 412 (12.4) |
| History hypertension: n (%) | 1046 (34.5) | 1938 (58.3) |
| 3MS score (AREDS) or TICS-M score (AREDS2): mean (SD) | 92.9 (6.3)a | 32.9 (3.5)a |
| Participants with cognitive impairment: n (%) | ||
| (3MS < 80 in AREDS; TICS-M < 30 in AREDS2) | 119 (3.9)a | 492 (14.8)a |
| Depression score CES-D > 16: n (%) | 374 (12.3) | 1411 (42.4) |
| Modified alternative Mediterranean diet index tertiles: n (%) | ||
| 1 | 901 (29.7) | 1096 (33.0) |
| 2 | 1031 (34.0) | 1010 (30.4) |
| 3 | 1097 (36.2) | 1220 (36.7) |
| Mean follow-up time (years): mean (SD) | ||
| To last cognitive test | - | 6.5 (3.8) |
| To end of study | 10.6 (1.4) | 8.6 (3.2) |
| Participants with genetic data: n | 2360 | 1525 |
| APOE risk alleles (rs429358): n (%) | ||
| 0 | 1877 (79.5) | 1213 (79.5) |
| 1 or 2 | 483 (20.5) | 312 (20.5) |
| APOE haplotype: n (%) | ||
| 0 (ε2/ε2 or ε2/ε3) | 382 (16.2) | 282 (18.5) |
| 1 (ε3/ε3, ε2/ε4, or ε1/ε3) | 1541 (65.3) | 959 (62.9) |
| 2 (ε3/ε4 or ε4/ε4) | 436 (18.5) | 284 (18.6) |
| CR1 rs3818361 | ||
| 0 (AA) | 103 (4.4) | 70 (4.6) |
| 1 (AG) | 780 (33.1) | 457 (30.0) |
| 2 (GG) | 1477 (62.6) | 998 (65.4) |
| CLU rs11136000 | ||
| 0 (TT) | 395 (16.7) | 233 (15.3) |
| 1 (TC) | 1132 (48.0) | 734 (48.1) |
| 2 (CC) | 833 (35.3) | 558 (36.6) |
| PICALM rs3851179 | ||
| 0 (TT) | 299 (12.7) | 207 (13.6) |
| 1 (TC) | 1096 (46.4) | 733 (48.1) |
| 2 (CC) | 965 (40.9) | 585 (38.4) |
3MS, Modified Mini-Mental State Examination; AREDS, Age-Related Eye Disease Study; CES-D, Center for Epidemiologic Studies-Depression Scale; SD, standard deviation; TICS-M, Telephone Interview Cognitive Status-Modified.
Cognitive function data are shown at baseline for AREDS2; for AREDS, they are shown at the single time point of assessment (during follow-up).
3.2 ⎹. Cross-sectional analysis of cognitive function by diet score
The results of cross-sectional analyses, according to aMED tertiles, are shown in Figure 1. In AREDS, the odds ratios for cognitive impairment, in aMED tertile 3 (vs 1), were 0.36 (P = .0001; 3MS) Figure 1A and 0.56 (P = .001; composite) Figure 1A. As regards absolute scores, the regression estimates, in aMED tertile 3 (vs 1), were +1.3 (P < .0001) and +1.0 (P < .0001), respectively Figure 1B. In AREDS2, the equivalent odds ratios were 0.56 (P < .0001; TICS-M) and 0.48 (P < .0001; composite) Figure 1C and equivalent linear regression estimates were +1.0 (P < .0001) and +1.5 (P < .0001) Figure 1D, respectively. Hence, higher aMED was associated with significantly lower risk of cognitive impairment and higher cognitive scores, with dose-response associations. The individual results for each test are shown in Figure S1 in supporting information, and the results of sensitivity analyses also in the supporting information.
FIGURE 1.
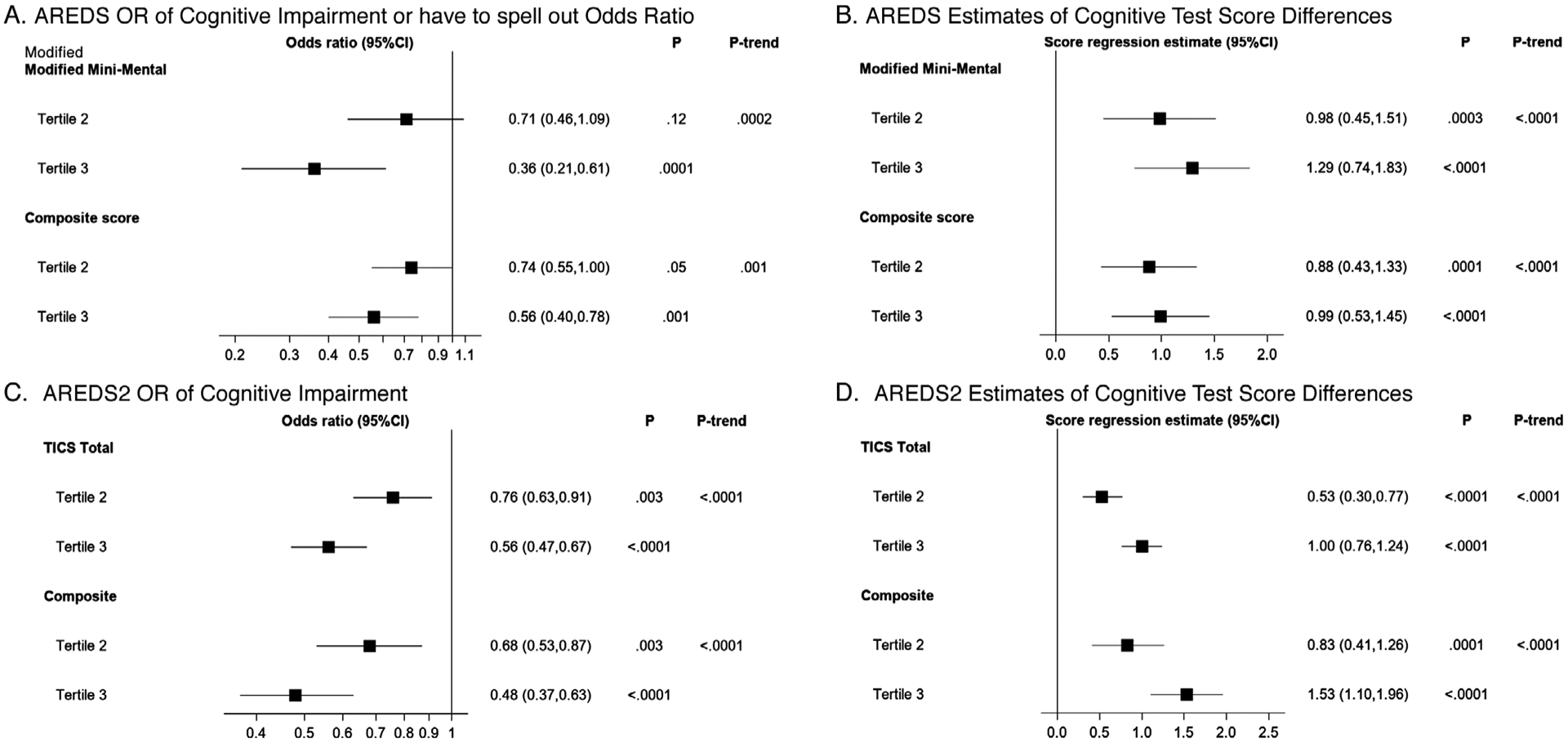
Odds ratios for cognitive impairment and estimates of cognitive test score differences (with 95% confidence intervals) by tertiles of the modified Alternative Mediterranean Diet. Results are shown in comparison to tertile 1 (reference), following adjustment for baseline age, sex, race, smoking, diabetes, hypertension, baseline depression score (Center for Epidemiologic Studies Depression Scale [CES-D] ≥16 or not), total calorie intake, and (AREDS2 only) years from baseline. Significance was set (by Bonferroni correction) at P = .013
The analyses were repeated with the inclusion of the interaction term between aMED and AREDS treatment assignment (by [1] treatment assignment, [2] antioxidant as main effect, and [3] zinc as main effect) or AREDS2 treatment assignment (by [1] treatment assignment, [2] DHA/EPA as main effect, and [3] lutein/zeaxanthin as main effect). In all cases, no significant interactions were observed (even at the nominal level).
In analyses of interactions between aMED and APOE haplotype, no significant interactions were observed in either AREDS or AREDS2 for the outcome of cognitive impairment. Similarly, in analyses of interactions between aMED and CR1, CLU, and PICALM genotypes, no significant interactions were observed in either cohort.
3.3 ⎹. ARESD2 longitudinal analysis of cognitive function, according to diet score
Longitudinal analyses were possible in AREDS2, owing to the repeated nature of the cognitive assessments (at baseline, 2, 4, and 10 years), but not in AREDS, where the cognitive assessments were not repeated. In AREDS2, the rate of change over time in cognitive function scores was not significantly different according to aMED tertiles: the P-interaction values between aMED tertile and study year were 0.22 (TICS-M) and 0.91 (composite). As shown in Figure 2, the proportion of participants with cognitive impairment changed over time in a similar way, irrespective of aMED tertile. The differences in mean TICS-M scores between participants in aMED tertiles 3 and 1 were: +0.8 (AREDS2 baseline), +1.0 (year 2), +0.9 (year 4), and +1.0 (year 10) Figure 2A. Similarly, the differences in mean composite scores were: +1.4, +1.6, +1.4, and +1.2, respectively Figure 2B.
FIGURE 2.
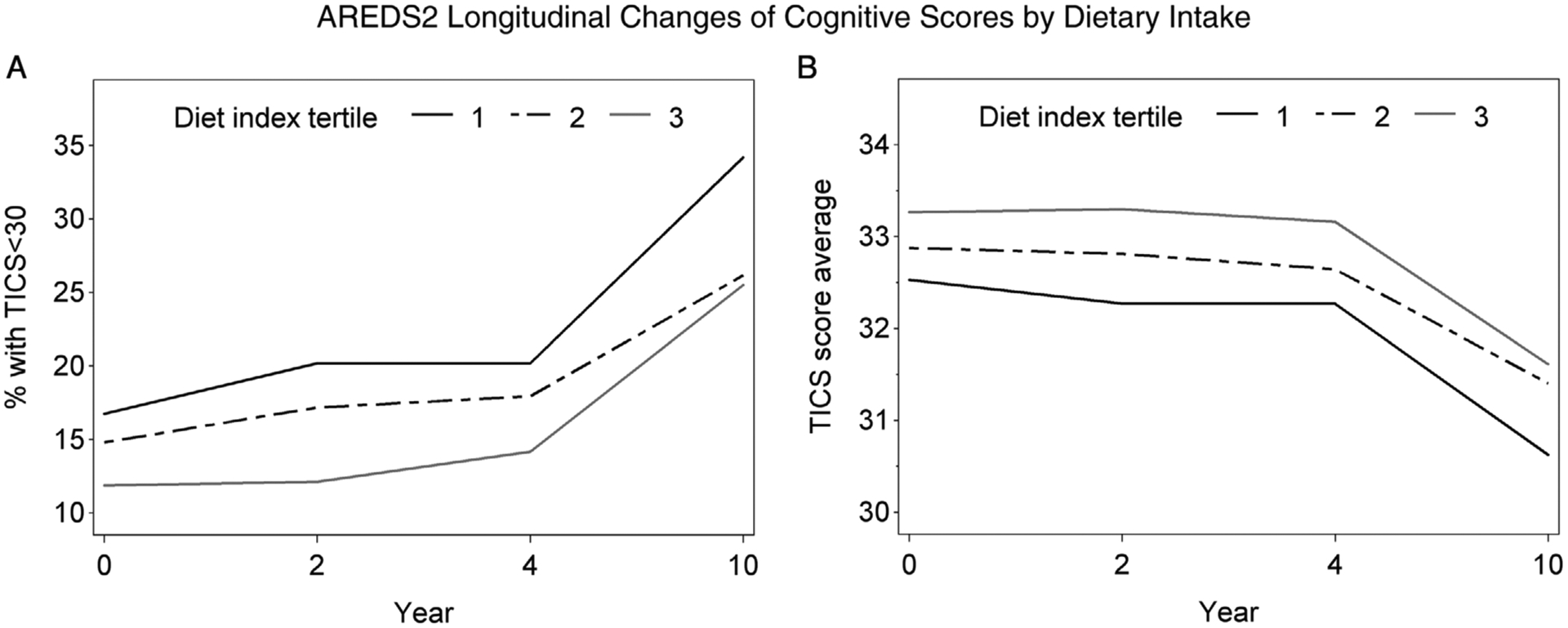
Participants with cognitive impairment (A) and estimates of cognitive test scores (B) from the Telephone Interview Cognitive Status-Modified, by tertiles of the modified Alternative Mediterranean Diet, in the Age-Related Eye Disease Study 2
APOE risk haplotypes were significantly associated with faster decline in cognitive function scores (P < .0001). However, no significant interactions were observed between aMED and APOE haplotype, in terms of rate of decline (P-value for interaction = 0.80 for TICS-M and P-value for interaction = 0.51 for the composite).
3.4 ⎹. Cross-sectional analysis of cognitive function, according to individual components
The results of cross-sectional analyses, according to quartiles of individual aMED components, are shown in Figure 3. Significant and consistent associations were observed for fish intake in both AREDS and AREDS2. In AREDS, the odds ratios for cognitive impairment, in quartile 4 (vs 1), were 0.36 (P = .001; 3MS) Figure 3A and 0.54 (P = .001; composite) Figure 3B. In terms of absolute scores, the regression estimates, in quartile 4 (vs 1), were +1.5 (P < .0001) Figure 3C and +1.2 (P < .0001) Figure 3D, respectively. In AREDS2, the equivalent odds ratios were 0.50 (P < .0001; TICS-M) Figure 3E and 0.54 (P = .001; composite) Figure 3F and estimates were +1.0 (P < .0001) Figure 3G and +1.5 (P < .0001) Figure 3H, respectively. In addition, significant protective associations were observed for vegetable intake in both AREDS and AREDS2, and for nut intake and moderate alcohol intake in AREDS2.
FIGURE 3.
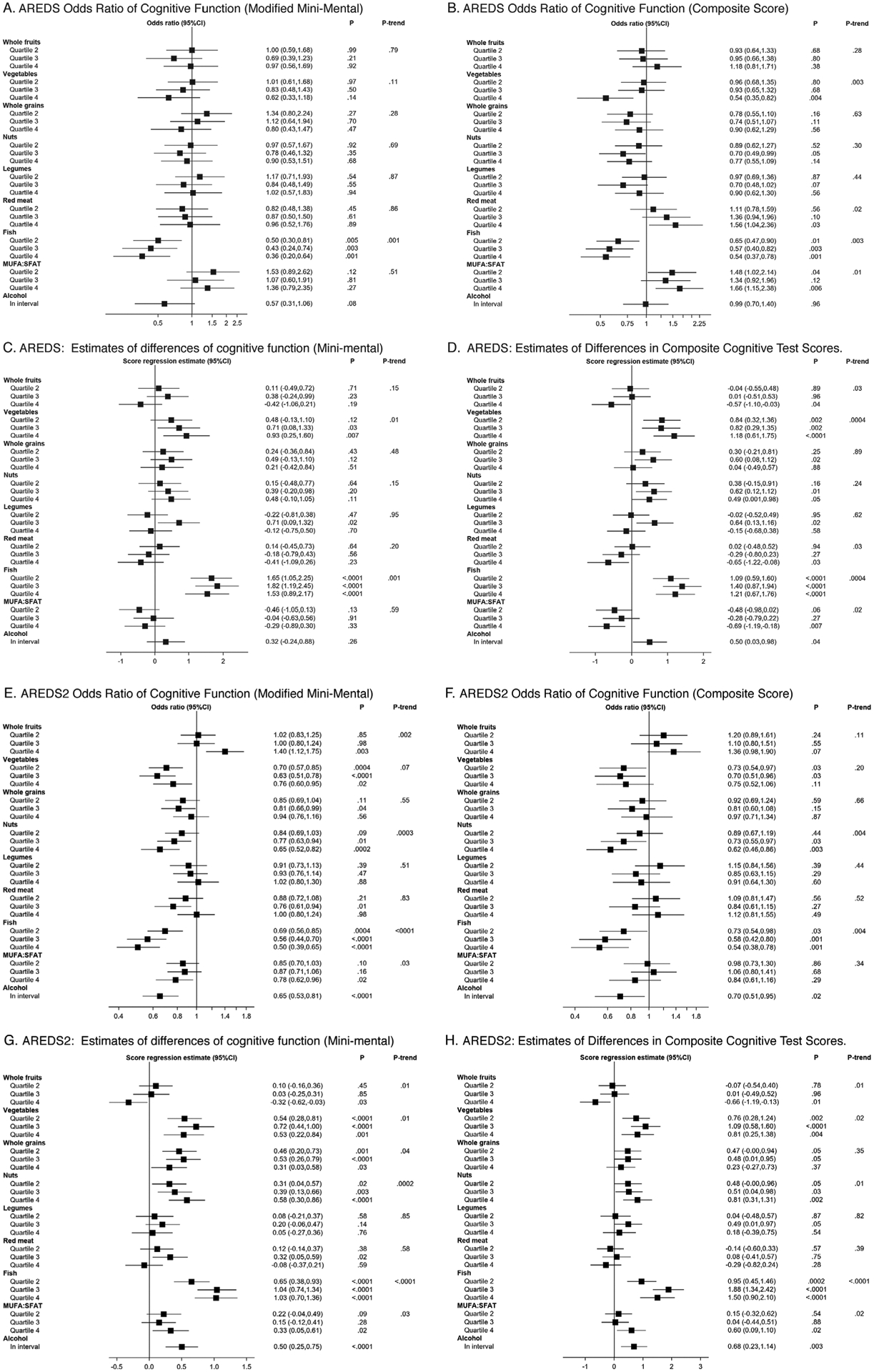
Odds ratios for cognitive impairment and estimates of cognitive test score differences (with 95% confidence intervals) by quartiles of the individual components of the modified Alternative Mediterranean Diet. Results are shown in comparison to quartile 1 (reference), following adjustment for baseline age, sex, race, smoking, diabetes, hypertension, baseline depression score (Center for Epidemiologic Studies Depression Scale [CES-D] ≥16 or not), total calorie intake, and (AREDS2 only) years from baseline. For all components except alcohol, higher quartiles refer to higher levels of intake of the component. For alcohol (considered in binary fashion), group 2 (“in interval”) refers to intake within the specified interval (ie, adherent to the modified alternative Mediterranean diet), while group 1 (reference) refers to intake above or below the specified interval. For red meat, higher quartiles refer to higher levels of intake, which is less adherent to the modified alternative Mediterranean diet. For monounsaturated fatty acid:saturated fatty acid ratio (MUFA:SFA), higher quartiles refer to higher ratios of MUFA:SFA intake, which is more adherent to the modified alternative Mediterranean diet. Significance was set (by Bonferroni correction) at P = .0014
In analyses of interactions between fish intake and APOE haplotype, for the outcome of cognitive impairment, no significant interactions were observed.
3.5 ⎹. Longitudinal analysis of cognitive function, according to fish intake
In AREDS2, the rate of change over time in cognitive function scores was different according to fish intake quartiles: the P-interaction values between quartile and study year were 0.019 (TICS-M) and 0.61 (composite). By regression analyses, the decline in TICS-M scores over time was numerically less steep for participants in fish intake quartile 4 (−0.19 [−0.23, −0.15] per year) versus 1 (−0.28 [−0.33, −0.22]).
4 ⎹. DISCUSSION
4.1 ⎹. Main findings, interpretation, and comparison with literature
Higher aMED adherence was associated with lower risk of cognitive impairment and higher cognitive function scores, with dose-response relationships. This finding was consistent between the two study populations of older people without frank dementia at baseline, one comprising individuals with a wide spectrum of AMD severity (including no disease) and the other comprising individuals with at least intermediate AMD. The strength of the negative association with cognitive impairment was relatively large, with odds ratios of ≈0.5–0.6 for aMED tertile 3 versus 1.
As regards individual components of the Mediterranean diet, the strongest and most consistent results were observed for fish intake, which was associated with lower risk of cognitive impairment and higher cognitive function scores. This finding was consistent between the two study populations. Again, the strength of the negative association was relatively large. It is therefore likely that fish intake contributed strongly to the findings observed for the Mediterranean diet.
The absolute differences in test scores by aMED tertile and fish intake were small so may not be clinically significant at the individual level, despite being highly statistically significant. However, the differences were sufficient to generate large differences in the risk of cognitive impairment, using the predefined cut-off criteria established in the literature. In addition, we consider that they are likely to be clinically meaningful at the population level.
In further analyses (see Table S4 in supporting information), we considered the possibility that the protective associations observed between aMED and cognitive function might be partially explained by education level. However, in both cohorts, after adjustment for education, the odds ratios for cognitive impairment and the estimates for cognitive function scores remained significant and were only slightly attenuated. In addition, mediation analyses40,41 were not consistent with the presence of education level as an important confounding variable.
In AREDS2 longitudinal analyses, higher aMED was not associated with slower decline in cognitive function, though higher fish intake did appear associated. Hence, in these studies, closer Mediterranean diet adherence and higher fish intake were associated with strong cross-sectional but absent or weaker longitudinal differences in cognitive function, over the 5- to 10-year time period studied.
These results are partially consistent with the results of previous prospective studies analyzed in at least 14 systematic reviews of the Mediterranean diet and cognitive function.20 Their constituent prospective studies may be considered in three categories: (1) no positive findings,42–45 (2) positive findings for cognitive level but not decline,46,47 and (3) positive findings for cognitive decline.48–56 The current study is therefore most consistent with the studies in category (2). In one of these, conducted on U.S. women (Nurses’ Health Study), higher aMED was associated with higher cognitive function but not slower decline in global cognition, TICS, or verbal memory.46 Consistent with our study, higher intakes of vegetables and fish were each independently associated with higher verbal memory scores. In the other previous study, higher aMED was also associated with differences in cognitive level but not decline;47 higher intake of legumes was independently associated with higher 3MS scores, while no signal was observed for fish or other components.
One recent systematic review confined itself to RCTs of the Mediterranean diet.20 Of the five RCTs examined, four contained findings that were mostly non-significant, with small effect sizes. However, one RCT conducted in Spain found that participants randomized to a Mediterranean diet had higher cognitive function scores after 6.5 years than those randomized to a low-fat control diet.57
Regarding fish intake, again, multiple systematic reviews have been conducted.17,58–62 In a recent meta-analysis of five cohort studies, higher fish intake was associated with slower decline in global cognition, particularly episodic memory.17 However, these significant relationships were generally not present in the individual studies and emerged only after pooling data. The current study adds strength to this potentially important association. Other meta-analyses have observed that higher fish intake is associated with decreased risk of dementia58,60 and/or AD.58–61
In this study, no interactions influencing cognitive function were observed between APOE and either aMED or fish intake. As regards fish intake, the recent meta-analysis mentioned above observed no evidence of effect modification by genotype at APOE or other AD-associated genes,17 while previous smaller studies reported interactions with APOE.22,23
Similarly, no interactions were observed between aMED and CR1, CLU, or PICALM genotype. This is in contrast to the Spanish RCT: in participants with T alleles at CLU, randomization to a Mediterranean diet led to significantly higher Mini-Mental State Examination scores; for participants without T alleles, no difference was found.39
4.2 ⎹. Interpretation and implications
The results demonstrated positive associations with higher adherence to a Mediterranean-type diet that were maintained but generally not substantially altered over follow-up of 10 years (with the possible exception of the findings for fish intake and cognitive decline). There are several potential explanations for differing associations with cognitive impairment versus cognitive decline. One possibility is that cognitive decline truly differs according to aMED, but the strength of the association was too small to be captured in this study; a larger sample size and longer follow-up period might be required. In addition, AREDS/AREDS2 participants likely had healthier diets and lifestyles than the U.S. population, and higher median education level, which may have led to narrower distributions of cognitive function and decline.
Alternatively, cognitive decline may not differ according to aMED. Differences in cognitive levels by diet might be explained by differences in peak cognitive function, much earlier in life, caused by differential aMED adherence, followed by relatively equal rates of decline. Previous authors have argued that the factors that influence neurocognitive development to peak cognitive function may not necessarily overlap with those that cause neurodegeneration.21 However, higher peak cognitive function associated with aMED adherence might, in this context, represent superior resilience to neurodegeneration in the form of greater brain reserve or cognitive reserve.63 Finally, confounding by unmeasured health or socioeconomic factors may have explained the protective association with cognitive level, though this is less consistent with the positive findings from the previous RCT.57
4.3 ⎹. Strengths and limitations
Strengths include the use of two datasets with large size, long follow-up time, and standardized collection of information (including genetic data in most participants). Limitations include post hoc hypothesis generation, likely exclusion of those AREDS/AREDS2 participants with substantial cognitive impairment, possible residual or unmeasured confounding (eg, physical activity), and differences in variables between the cohorts (eg, body mass index). Also, diet assessment by FFQ contains non-differential measurement error, though energy adjustment may partially address this.64,65 Because of inherent differences in AREDS and AREDS2 FFQs, differences exist in assignment of food items to components.
The FFQ was administered at baseline only in AREDS but at baseline and during follow-up in AREDS2. Because the dietary data at baseline were used as the predictor of interest in both cohorts, these analyses assume that diet did not change substantially during follow-up. However, this assumption is supported in AREDS2 by comparison of the baseline FFQ and the repeat FFQ (median interval of 5.4 years). At the cohort level (n = 2,970 participants), the median aMED was unchanged at 21.0 and the median intakes were essentially unchanged for each component. At the individual level, the median change in aMED was 0.0 and the median changes in intake were essentially zero for most components.
The study may have limited generalizability to populations with different dietary patterns. Because this study was conducted in cohorts in which most participants had some degree of AMD, complete generalizability to general populations (without AMD) is not certain. However, given that AMD and AD have distinct genetic risk profiles,28,29 without significant genetic pleiotropy,30 a high degree of generalizability might be expected. For example, the proportions of AREDS/AREDS2 participants with ε2/ε3/ε4 APOE haplotypes are as might be expected for a predominantly white population without frank dementia.
4.4 ⎹. CONCLUSIONS
In these two U.S. study populations, closer adherence to a Mediterranean-type diet was associated with lower risk of cognitive impairment and with higher cognitive function. The same applied to higher fish intake. APOE genotype did not significantly influence either relationship. However, closer Mediterranean diet adherence was not associated with decreased cognitive decline. These findings may help inform evidence-based dietary recommendations, adding strength to evidence that Mediterranean-type diet patterns may maximize cognitive reserve against impairment and dementia.
Supplementary Material
RESEARCH IN CONTEXT.
Systemic Review: Dementia, a leading cause of global disability in older people, has no available disease-modifying therapies. Observational data from two large clinical trials of nutritional supplements for the treatment of age-related macular degeneration (AMD) were analyzed to test whether closer adherence to a Mediterranean diet was associated with altered cognitive function.
Interpretation: Closer adherence to a Mediterranean diet was associated with lower risk of cognitive impairment but not cognitive decline. However, higher fish consumption was significantly associated with slower cognitive decline. APOE genotype did not influence these relationships.
Future Directions: These findings may help inform evidence-based dietary recommendations to maximize cognitive reserve against dementia. Using the same data, future analyses will evaluate individual nutrients that may be responsible for the inverse association between the diet and cognitive function found in these two studies.
HIGHLIGHTS.
Mediterranean diet adherence was associated with decreased risk of cognitive impairment
Higher fish consumption was associated with decreased risk of cognitive impairment and slower cognitive decline
APOE status did not influence these relationships
A Mediterranean diet may be recommended in dietary guidelines
ACKNOWLEDGMENT
We would like to thank Jill Reedy, PhD from the National Cancer Institute/National Institutes of Health for her advice on the analyses of the components of the alternative Mediterranean Diet (aMED).
FINANCIAL SUPPORT
This study is supported by the intramural program funds and contracts AREDS (Contract NOI-EY-0-2127) and AREDS2 (contract HHS-N-260-2005-00007-C; ADB contract N01-EY-5-0007) from the National Eye Institute/National Institutes of Health (NEI/NIH), Department of Health and Human Services, Bethesda, MD. Funds were generously contributed to these contracts by the following NIH institutes: Office of Dietary Supplements; National Center for Complementary and Alternative Medicine; National Institute on Aging; National Heart, Lung, and Blood Institute; and National Institute of Neurological Disorders and Stroke.
ROLE OF SPONSOR
With the exception of Drs. Traci Clemons and Julie Mares, the entire writing team was employed by the NIH, the sponsor of the study. In that capacity, the sponsoring organization was involved in each of the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding information
AREDS, Grant/Award Number: NOI-EY-0-2127; AREDS2, Grant/Award Numbers: HHS-N-260-2005-00007-C, N01-EY-5-0007; National Eye Institute/National Institutes of Health; Department of Health and Human Services
Footnotes
Appendix of AREDS and AREDS2 Research Group appear at the end of the manuscript
Trial Registration clinicaltrials.gov identifier: NCT00345176 for AREDS2 (none available for AREDS).
CONFLICTS OF INTEREST
T. Keenan, E. Agrón, J. Mares, T. Clemons, F. van Asten, A. Swaroop, E. Chew: no financial conflicts.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. e2. [DOI] [PubMed] [Google Scholar]
- 3.Buckley JS, Salpeter SR. A risk-benefit assessment of dementia medications: systematic review of the evidence. Drugs Aging. 2015;32(6):453–467. [DOI] [PubMed] [Google Scholar]
- 4.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. [DOI] [PubMed] [Google Scholar]
- 5.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. [DOI] [PubMed] [Google Scholar]
- 6.Casaletto KB, Umlauf A, Beaumont J, et al. Demographically Corrected Normative Standards for the English Version of the NIH Toolbox Cognition Battery. J Int Neuropsychol Soc. 2015;21(5):378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters R Ageing and the brain. Postgrad Med J. 2006;82(964):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li K, Chan W, Doody RS, Quinn J, Luo S, Alzheimer’s Disease Neuroimaging I. Prediction of conversion to Alzheimer’s disease with longitudinal measures and Time-To-Event data. J Alzheimers Dis. 2017;58(2):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Adherence to a Mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials. Front Nutr. 2016;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82(3):637–672. [DOI] [PubMed] [Google Scholar]
- 12.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1. BDNF and serotonin Ageing Res Rev. 2004;3(4):445–464. [DOI] [PubMed] [Google Scholar]
- 13.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. [DOI] [PubMed] [Google Scholar]
- 14.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, et al. Diet and overall survival in elderly people. BMJ. 1995;311(7018):1457–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement. 2017;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riederer P, Korczyn AD, Ali SS, et al. The diabetic brain and cognition. J Neural Transm. 2017;124(11):1431–1454. [DOI] [PubMed] [Google Scholar]
- 17.Samieri C, Morris MC, Bennett DA, et al. Fish intake, genetic predisposition to Alzheimer disease, and decline in global cognition and memory in 5 cohorts of older persons. Am J Epidemiol. 2018;187(5):933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006–1015. [DOI] [PubMed] [Google Scholar]
- 19.Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean diet; a literature review. Nutrients. 2015;7(11):9139–9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radd-Vagenas S, Duffy SL, Naismith SL, Brew BJ, Flood VM, Fiatarone Singh MA. Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr. 2018;107(3):389–404. [DOI] [PubMed] [Google Scholar]
- 21.Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Rest O, Wang Y, Barnes LL, Tangney C, Bennett DA, Morris MC. APOE epsilon4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology. 2016;86(22): 2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69(20):1921–1930. [DOI] [PubMed] [Google Scholar]
- 24.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials. 1999;20(6):573–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AREDS2 Research Group, Chew EY, Clemons T, SanGiovanni JP, et al. AREDS2 Research Group. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012;119(11):2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rong SS, Lee BY, Kuk AK, et al. Comorbidity of dementia and age-related macular degeneration calls for clinical awareness: a meta-analysis. Br J Ophthalmol. 2019;103(12):1777–1783. [DOI] [PubMed] [Google Scholar]
- 27.Lee CS, Larson EB, Gibbons LE, et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezazadeh M, Hosseinzadeh H, Moradi M, et al. Genetic discoveries and advances in late-onset Alzheimer’s disease. J Cell Physiol. 2019;234(10):16873–16884. [DOI] [PubMed] [Google Scholar]
- 30.Grassmann F, Kiel C, Zimmermann ME, et al. Genetic pleiotropy between age-related macular degeneration and 16 complex diseases and traits. Genome Med. 2017;9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clemons TE, Rankin MW, McBee WL, Age-Related Eye Disease Study Research G. Cognitive impairment in the Age-Related Eye Disease Study: aREDS report no. 16. Arch Ophthalmol. 2006;124(4):537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chew EY, Clemons TE, Agron E, et al. Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: the AREDS2 Randomized Clinical Trial. JAMA. 2015;314(8):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rankin MW, Clemons TE, McBee WL. Correlation analysis of the in-clinic and telephone batteries from the AREDS cognitive function ancillary study. AREDS Report No 15. Ophthalmic Epidemiol. 2005;12(4):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: aREDS Report No. 20. Arch Ophthalmol. 2007;125(5):671–679. [DOI] [PubMed] [Google Scholar]
- 35.Michaud DS, Giovannucci EL, Ascherio A, et al. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev. 1998;7(4):283–290. [PubMed] [Google Scholar]
- 36.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, et al. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr. 2014;99(1):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abondio P, Sazzini M, Garagnani P, et al. The genetic variability of APOE in different human populations and its implications for longevity. Genes (Basel). 2019;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Lapiscina EH, Galbete C, Corella D, et al. Genotype patterns at CLU, CR1, PICALM and APOE, cognition and Mediterranean diet: the PREDIMED-NAVARRA trial. Genes Nutr. 2014;9(3):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inc. SI. SAS/STAT Software: CAUSALMED Procedure [Available from: https://support.sas.com/rnd/app/stat/procedures/causalmed.html. The access date is 10/11/2019.
- 42.Olsson E, Karlstrom B, Kilander L, Byberg L, Cederholm T, Sjogren P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis. 2015;43(1):109–119. [DOI] [PubMed] [Google Scholar]
- 43.Samieri C, Grodstein F, Rosner BA, et al. Mediterranean diet and cognitive function in older age. Epidemiology. 2013;24(4):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vercambre MN, Grodstein F, Berr C, Kang JH. Mediterranean diet and cognitive decline in women with cardiovascular disease or risk factors. J Acad Nutr Diet. 2012;112(6):816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts RO, Geda YE, Cerhan JR, et al. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;29(5):413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samieri C, Okereke OI, E ED, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr. 2013;143(4):493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wengreen H, Munger RG, Cutler A, et al. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory. Health and Aging Am J Clin Nutr. 2013;98(5):1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trichopoulou A, Kyrozis A, Rossi M, et al. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr. 2015;54(8):1311–1321. [DOI] [PubMed] [Google Scholar]
- 51.Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koyama A, Houston DK, Simonsick EM, et al. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci. 2015;70(3):354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsivgoulis G, Judd S, Letter AJ, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology. 2013;80(18):1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93(3):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63(12):1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Lapiscina EH, Clavero P, Toledo E, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84(12):1318–1325. [DOI] [PubMed] [Google Scholar]
- 58.Bakre AT, Chen R, Khutan R, et al. Association between fish consumption and risk of dementia: a new study from China and a systematic literature review and meta-analysis. Public Health Nutr. 2018;21(10):1921–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng LF, Cao Y, Liang WX, et al. An exploration of the role of a fish-oriented diet in cognitive decline: a systematic review of the literature. Oncotarget. 2017;8(24):39877–39895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyun-saturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2016;103(2):330–340. [DOI] [PubMed] [Google Scholar]
- 61.Wu S, Ding Y, Wu F, Li R, Hou J, Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neurosci Biobehav Rev. 2015;48:1–9. [DOI] [PubMed] [Google Scholar]
- 62.Cao L, Tan L, Wang HF, et al. Dietary Patterns and Risk of Dementia: a Systematic Review and Meta-Analysis of Cohort Studies. Mol Neurobiol. 2016;53(9):6144–6154. [DOI] [PubMed] [Google Scholar]
- 63.Menardi A, Pascual-Leone A, Fried PJ, Santarnecchi E. The Role of Cognitive Reserve in Alzheimer’s Disease and Aging: a Multi-Modal Imaging Review. J Alzheimers Dis. 2018;66(4):1341–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kipnis V, Subar AF, Midthune D, et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158(1):14–21. discussion 2–6. [DOI] [PubMed] [Google Scholar]
- 65.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


