Abstract
Rationale:
Work with α-pyrrolidinopentiophenone (α-PVP), a second-generation synthetic cathinone, has been generally limited to the racemate. Given that with other synthetic cathinones there are behavioral and neurochemical differences between their enantiomers, differences may also be seen with α-PVP.
Objectives:
The present study assessed the relative contribution of each enantiomer to the aversive effects of racemic-α-PVP by comparing their ability to induce a conditioned taste avoidance.
Methods:
Adult male Sprague-Dawley rats were exposed every other day for four exposures to a novel saccharin solution followed immediately by an injection of 0 (saline vehicle) or 1.5, 3 or 6 mg/kg of S-, R- or racemic-α-PVP (IP). On alternating days, all subjects were given access to water to assess any unconditioned effects of α-PVP on general fluid consumption.
Results:
Rats injected with the racemate and S-isomer of α-PVP displayed avoidance of the drug-associated saccharin solution, although this avoidance was dose-dependent only for the subjects injected with the racemate. There was no evidence of taste avoidance in animals injected with the R-enantiomer at any dose tested. Animals injected with 3 mg/kg racemic-α-PVP did not differ in avoidance from those treated with 1.5 mg/kg of the S-enantiomer, but subjects treated with 6 mg/kg racemic-α-PVP displayed a significantly stronger avoidance than those treated with 3 mg/kg S-α-PVP.
Conclusions:
The present work suggests that the aversive effects of racemic α-PVP are mediated primarily by its S-isomer. The fact that at the highest dose tested (6 mg/kg), the racemate induces an avoidance greater than the simple additive effects of the S- and R-isomers (at 3 mg/kg) suggests that while the R-isomer may not induce taste avoidance at this dose it may interact synergistically with the S-isomer in mediating the effects of the racemic mixture. These results were discussed in terms of similar effects with other behavioral and physiological endpoints reported with a number of psychostimulants and suggest that the enantiomers of α-PVP are an important variable in characterizing its behavioral effects.
Keywords: α-PVP, Enantiomers, Conditioned taste avoidance, Synthetic cathinones, “Bath Salts”
1. Introduction
Since their debut to the recreational drug market around 2010, the synthetic cathinones (commonly known as “bath salts”) have taken on a multitude of forms mostly in an effort to evade law enforcement. As the first-generation synthetic cathinones became illegal (Council Decision EU, 2010; DEA, 2011, 2013), a second generation emerged whose behavioral and biological effects have not yet been fully investigated. One such compound is α-pyrrolidinopentiophenone (α-PVP), also known as “flakka”, which has both structural and pharmacological similarities to the first-generation synthetic cathinone MDPV (3,4-methylenedioxypyrovalerone) (for a review, see Glennon and Young, 2016). Regarding its mechanism of action, α-PVP is a potent reuptake inhibitor of dopamine (DA) and norepinephrine (NE) with substantially weaker action at the serotonin transporter (Baumann et al., 2016; Glennon and Young, 2016; Marusich et al., 2014; Meltzer et al., 2006). Like other psychostimulants (including the synthetic cathinones), α-PVP has been reported to support self-administration in a dose-dependent manner (Aarde et al., 2015; Gannon et al., 2017; Huskinson et al., 2017; Javadi-Paydar et al., 2017; for a review, see Riley et al., 2018).
Consistent with its ability to support IVSA, α-PVP has been reported to be rewarding in a variety of behavioral preparations used to assess such effects, e.g., place preference conditioning (Gatch et al., 2015; Marusich et al., 2016; Nelson et al., 2017) and intracranial self-stimulation (Watterson et al., 2014). In this context, it is important to note that most drugs of abuse are also reported to have aversive effects as assessed through taste avoidance conditioning (see Hunt and Amit 1987; Goudie, 1979; Verendeev and Riley, 2011; Wise et al., 1976) and the likelihood of their use and abuse is thought to be a balance of these aversive and rewarding effects (Cunningham, 1979; Lin et al., 2017; Riley, 2011; Stolerman and D’Mello, 1981; Verendeev and Riley, 2012). In the conditioned taste avoidance (CTA) procedure, animals are given access to a novel tasting solution and then injected with a drug, and following several such pairings the animal reduces or avoids consumption of the drug-paired taste, presumably due to its association with the drug’s aversive effects (Garcia and Ervin, 1968; Riley and Tuck, 1985; Rozin and Kalat, 1971; see www.CTAlearning.com). Although well documented with a wide variety of drugs of abuse, such assessments of conditioned taste avoidance with the synthetic cathinones are relatively limited (for MDPV, see King et al., 2014, 2015; Merluzzi et al., 2014; Woloshchuk et al., 2016; for demonstrations with related psychostimulants, see Cappell and LeBlanc 1971; Cobuzzi et al., 2014; Ferrari et al., 1991). Recent work with α-PVP has demonstrated that it, too, has aversive effects as indexed by taste avoidance conditioning. For example, Nelson et al. (2017) reported that male Sprague-Dawley rats injected with various doses of α-PVP following consumption of a novel saccharin solution displayed dose-dependent avoidance of the α-PVP-associated taste. Specifically, 3 mg/kg α-PVP induced significant avoidance, while animals injected with 0.3 and 1 mg/kg did not differ significantly from saline controls.
Although α-PVP appears to have both rewarding and aversive effects, one should consider the fact that it has a chiral center and exists as a racemate of two enantiomers, S-α-PVP and R-α-PVP (see Figure 1). Based on work with other psychostimulants, including a variety of cathinones, this different stereochemistry may produce different behavioral, addictive, therapeutic and/or pharmacological effects. For example, Glennon et al. (1984) demonstrated that rats trained to discriminate S(+)-amphetamine sulfate (1 mg/kg) from saline displayed different discriminative control to racemic, S- and R- amphetamine in terms of dose sensitivity, e.g., (±)-amphetamine, ED50 = 0.62 mg/kg; S(+)-amphetamine, ED50 = 0.42 mg/kg; R(−)-amphetamine, ED50 = 1.23 mg/kg, suggesting greater potencies for the S-, than the R-, enantiomer (for an assessment of d-amphetamine, l-amphetamine and methamphetamine self-administration in rhesus monkeys, see Balster and Schuster, 1973). Fantegrossi and colleagues (2009) reported that mice trained to discriminate 3 mg/kg of racemic 3,4-methylenedioxymethamphetamine (MDMA) from saline displayed dose-dependent and full generalization to S-MDMA at 1 mg/kg, but they failed to display any generalization to R-MDMA at any dose tested. Interestingly, both enantiomers (1.5 mg/kg) were effective as training drugs in establishing discriminative control (with full substitution of the racemate and other isomer; for other studies exploring the effects of the enantiomers of MDMA on a variety of behavioral and physiological measures, see Curry et al., 2018; Fantegrossi et al., 2002, 2003; Frau et al., 2013; McClung et al., 2010; Wang and Woolverton, 2007).
Figure 1.
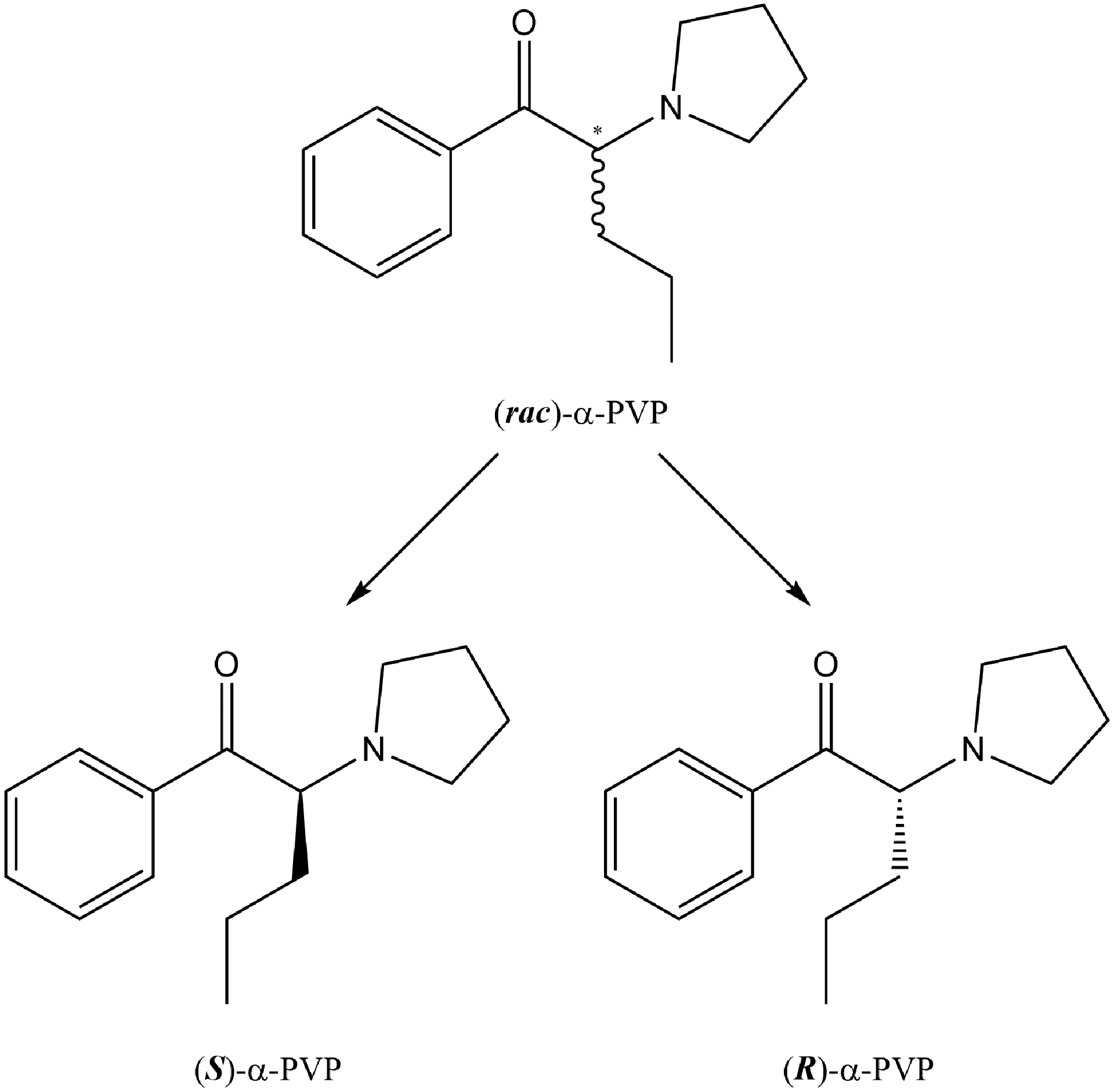
Chemical structure of racemic α-pyrrolidinopentiophenone (α-PVP) and its enantiomers
Cathinone (the root compound from which the synthetic cathinones are derived; see Banks et al., 2014) has also been found to have enantiomer-specific effects. For example, Hutsell et al. (2016) reported that both enantiomers of cathinone facilitated intracranial self-stimulation (ICSS; percent of maximum control rate), but that the S-enantiomer was 10-fold more potent in this facilitation (for a comparison of the releasing effect of (+) and (−)cathinone at central and peripheral catecholamine storage sites, see Kalix et al., 1986; for an assessment of the discriminative stimulus effects of l-cathinone and a comparison of dose-response and time-course effects of l-cathinone compared to the racemate and d-cathinone, see Schechter, 1986). Other assessments demonstrating differences with enantiomers have also been reported for the synthetic cathinones. For example, Gannon and colleagues (2017) described differences in progressive ratio self-administration of MDPV and its enantiomers. Specifically, although all compounds supported self-administration, Emax for R-MDPV was significantly less than that for the racemate and S-MDPV, which were not statistically different (for other work assessing the contribution of each enantiomer of MDPV in drug discrimination, locomotor activity and thermoregulation in mice, see Gannon et al., 2016; for a comparison of MDPV enantiomers and their actions on neurotransmitter reuptake and behavioral effects in an ICSS assay in rats, see Kolanos et al., 2015). Interestingly, Gregg et al. (2015) reported that R-mephedrone induced significant place preference conditioning, whereas S-mephedrone did not. Further, R-mephedrone facilitated ICSS to a greater extent than S-mephedrone. These findings with mephedrone relative to those with other synthetic cathinones illustrate the effects with the enantiomers are drug specific.
Although the behavioral and physiological effects of α-PVP (as well as their neurochemical mediation) are becoming better characterized, little is known about the relative contributions of the S and R isomers to these effects. To date, there is only one study (Gannon et al., 2017) assessing each enantiomer of α-PVP, in this case their IVSA. Although racemic α-PVP and both enantiomers were self-administered (with no differences in Emax), the racemate and enantiomers did differ in their relative potencies with S-α-PVP > racemate > R-α-PVP (ED50 = 0.008, 0.015 and 0.39, respectively). Given the overall findings of differential effects of specific enantiomers for a range of synthetic cathinones and the role of aversion in abuse vulnerability (see above), a better understanding of the stereospecificity of the aversive properties of α-PVP might provide further insight into its use and abuse. Accordingly, the goal of the present study was to determine the relative contribution of each enantiomer to the aversive effects of racemic α-PVP.
2. Methods
2.1. Subjects
The subjects were 80 experimentally naïve male Sprague-Dawley rats (Envigo, Indianapolis, IN). They entered the animal research facility at American University on postnatal day (PND) 21 and were allowed to mature undisturbed. Beginning on PND 83, all animals were weighed daily to index health status and to reduce handling stress during the following experimental procedures. These procedures began on PND 90 at which point subjects weighed between 323 and 444 grams. All procedures adhered to the Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003) and were approved by the Institutional Animal Care and Use Committee at American University.
2.2. Drugs and solutions
Racemic α-pyrrolidinopentiophenone HBr (α-PVP), S-(−) α-pyrrolidinopentiophenone fumarate 0.25 H2O and R-(+) α-pyrrolidinopentiophenone fumarate 0.25 H2O (all synthesized and generously provided by the Drug Design and Synthesis Section, MTMDB, NIDA and NIAAA) were each dissolved in isotonic saline (0.9%) and injected intraperitoneally (IP) at 1.5, 3 or 6 mg/kg. Equivolume isotonic saline (vehicle) was administered to controls. Each drug (and vehicle) solution was prepared daily and passed through a 0.2 um filter prior to injection to remove any potential particulates. Saccharin (sodium saccharin, Sigma) was prepared as a 1g/l (0.1%) solution in tap water.
2.3. Apparatus
Subjects were housed two per home-cage (38.9 × 56.9 × 26.2 cm; 1181 cm2). The room in which the cages were located was maintained on a 12-h light/dark cycle (0800 – 2000h) at 23 °C. Training and testing took place during the lights-on phase of the light cycle. Unless stated otherwise, food and water were available ad libitum. During taste avoidance conditioning, animals were transferred to a separate testing room and placed in individual hanging, stainless-steel wire mesh test cages (24.3 × 19 × 18 cm) on the front of which 50-ml graduated tubes were placed for fluid presentation.
2.4. Procedure
2.4.1. Habituation
On PND 90, subjects were deprived of water and 24 h later (PND 91) were given 20-min access to tap water in the test cages. Following this access, the animals were returned to their home cages. This limited access procedure was repeated for 10 days to allow water consumption to stabilize (with animals approaching the drinking tube within 2 sec with the average volume of water consumed not increasing or decreasing by more than 2 ml for 3 consecutive days). Water intake was evaluated by the difference between pre- and post-consumption volumes.
2.4.2. Conditioned Taste Avoidance
On Day 1 of this phase (PND 102), subjects were placed in their test cages and permitted 20-min access to a novel saccharin solution. Subjects were then assigned to one of 10 drug groups such that overall saccharin consumption was similar among groups and injected with either the saline vehicle or 1.5, 3 or 6 mg/kg of racemic, S- or R-α-PVP (n = 8 per group). In previous work (Nelson et al., 2017), racemic α-PVP at 3 mg/kg induced a significant, but intermediate, suppression of saccharin consumption. Doses of 1.5 and 6 mg/kg were expected to allow for assessments of the relative strength of conditioning by racemic α-PVP and its isomers at doses likely to produce weak (1.5) and complete (6 mg/kg) avoidance. Assessments with this dose range resulted in Groups Vehicle, Rac1.5, Rac3, Rac6, R1.5, R3, R6, S1.5, S3 and S6. On the next day (Day 2), all subjects were given 20-min access to water in the test cages and injected with vehicle to assess any residual effects of α-PVP on general fluid consumption. This two-day cycle was repeated for a total of four times.
On the day following the fourth cycle (PND 111), animals were placed in the test cages and given 20-min access to both saccharin and tap water in a two-bottle avoidance test with no subsequent injections. In this test, one bottle was offered (saccharin or water) on either the left or the right front of the test cage. Immediately after the first bottle was sampled, it was removed and the second bottle was presented on the opposite side. After that bottle was sampled, it was removed and both bottles were then placed simultaneously on their respective sides of the front of the cage. The order of presentation and side placement were counterbalanced across animals, and consumption of both saccharin and water was recorded after 20 min had elapsed. Animals were then returned to their home cages with ad libitum water access.
2.5. Statistical Analysis
Saccharin consumption on conditioning trials was analyzed using a 10 × 4 mixed model ANOVA with the between-subjects factor of Group (Vehicle, Rac1.5, Rac3, Rac6, R1.5, R3, R6, S1.5, S3 and S6) and the within-subjects factor of Trial (1–4). In the instance of a significant two-way interaction, simple effects of Group at each Trial (multivariate analysis) and Trial for each Group (multivariate analysis) were assessed, followed by Bonferroni-adjusted multiple comparisons. To assess any residual unconditioned effects of drug injection, water consumption averaged across intervening recovery days was analyzed using a one-way ANOVA with the between-subject factor of Group (Vehicle, Rac1.5, Rac3, Rac6, R1.5, R3, R6, S1.5, S3 and S6) and Bonferroni-adjusted multiple comparisons.
Percent saccharin of total fluid consumption on the final two-bottle test was analyzed using a one-way ANOVA with the between-subject factor of Group (Vehicle, Rac1.5, Rac3, Rac6, R1.5, R3, R6, S1.5, S3 and S6) and Bonferroni-adjusted multiple comparisons. Saccharin preference was calculated as a percentage, where the volume of saccharin consumed was divided by total volume consumed (water + saccharin). A one-way ANOVA was also conducted on total fluid consumed (water + saccharin) during the final two-bottle test, with the between-subject factor of Group (see above) followed by Bonferroni-adjusted multiple comparisons.
Statistical significance was set at p ≤ 0.05.
3. Results
3.1. Acquisition of Conditioned Taste Avoidance
The 10 × 4 mixed model ANOVA on saccharin consumption revealed a significant main effect of Group [F(9, 70) = 18.04, p < 0.0001] as well as a significant Group × Trial interaction [F(27, 210) = 11.39, p < 0.0001]. There was no significant main effect of Trial [F(3, 210) = 0.7812, p = 0.506].
Racemic Mixture.
Subjects injected with the racemic α-PVP displayed dose-dependent decreases in saccharin consumption over repeated conditioning trials. Bonferroni-corrected multiple comparisons indicated that on Trial 1 there were no significant differences among any groups with subjects drinking approximately 10 ml of saccharin (Figure 2, Panel A). On Trial 2, significant differences in saccharin consumption emerged with animals injected with 6 mg/kg of racemic α-PVP drinking significantly less saccharin than those injected with either vehicle, 1.5 or 3 mg/kg (p < 0.001), and animals injected with 3 mg/kg drinking less than those injected with vehicle (p < 0.01). Subjects injected with 1.5 and 3 mg/kg did not differ in consumption on this trial. These differences were maintained on Trial 3. On this trial, subjects injected with 1.5 mg/kg α-PVP also differed from vehicle controls (p’s < 0.05). On the final conditioning trial (Trial 4), subjects injected with 6 mg/kg of the racemate continued to drink less saccharin than subjects injected with either 1.5 mg/kg or vehicle (p < 0.001); animals injected with 3 mg/kg drank less than vehicle but did not differ from those injected with either 1.5 or 6 mg/kg.
Figure 2.
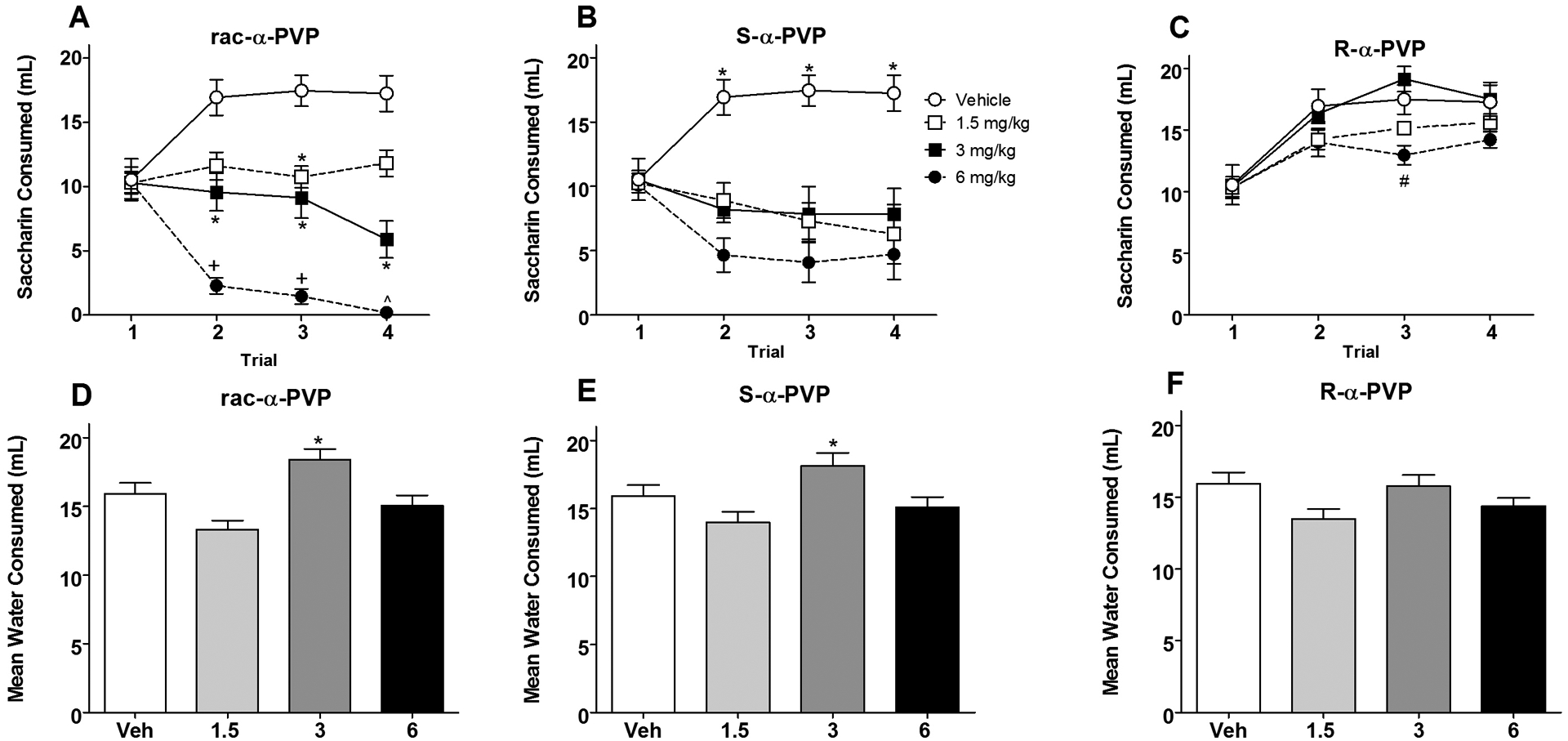
Mean (+/− SEM) saccharin consumption (ml) over Trials 1–4 for Groups rac-α-PVP (Panel A), S-α-PVP (Panel B) and R-α-PVP (Panel C). Panel A: *significantly different from vehicle injected groups. +significantly different from all other groups. ^significantly different from vehicle and 1.5 mg/kg. Panel B: *significantly different from drug-injected groups. Panel C: #significantly different from 3 mg/kg. Mean (+/− SEM) water consumption averaged over Recovery Days 1–4 for Groups rac-α-PVP (Panel C), S-α-PVP (Panel D) and R-α-PVP (Panel E). Panels D and E: *significantly different from 1.5 and 6 mg/kg.
S-α-PVP.
Subjects injected with the S-isomer of α-PVP displayed significant avoidance of the saccharin solution, although not in a dose-dependent manner. Bonferroni-corrected multiple comparisons indicated that on Trial 1 there were no significant differences among any groups with subjects drinking approximately 10.5 ml of saccharin (Figure 2, Panels B). On each of the remaining trials, all groups injected with S-α-PVP consumed significantly less saccharin then those injected with vehicle, although there were no significant differences in consumption among the S-α-PVP-injected groups.
R-α-PVP.
Subjects injected with the R-isomer of α-PVP did not display significant avoidance of the saccharin solution at any dose. Bonferroni-corrected multiple comparisons indicated that on Trial 1 there were no significant differences among any groups with subjects drinking approximately 10.5 ml of saccharin (Figure 2, Panels C). Saccharin consumption increased for each group (in a manner similar to that for subjects injected with vehicle), and at no point did any R-α-PVP-injected group differ from vehicle-injected controls. On Trial 3, animals treated with 6 mg/kg R-α-PVP drank significantly less saccharin than those injected with 3 mg/kg R-α-PVP (p < 0.05).
Water consumption on intervening recovery sessions remained elevated throughout conditioning for all groups (14–18 ml; see Figure 2, Panels D, E and F). The one-way ANOVA on water consumption (averaged over the four recovery days) revealed a significant main effect of Group [F (9,70) = 8.709, p < 0.001]. Animals injected with 3 mg/kg in both the racemic and S-isomer groups drank significantly more than those injected with either 1.5 or 6 mg/kg (p < 0.05). Subjects injected with the R-isomer did not differ among themselves or from those injected with the vehicle.
3.2. Drug Comparisons
Given that the racemate is composed of 50% of each isomer, it would be expected that the strength of the avoidance induced by the racemate at dose X would be similar to that based on the additive effects of the S- and R-isomers at half of that dose (1/2 X). Accordingly, using the data from the fourth and final one-bottle exposure to saccharin, consumption was compared between the racemic group and those injected with the R and S isomers of α-PVP. These comparisons were made between Rac3 and R1.5/S1.5 and between Rac6 and R3/S3 using Bonferroni-corrected pairwise comparisons from the previously described 10 × 4 mixed model ANOVA on saccharin consumption (see Results 3.1).
On the fourth trial, there were no significant differences in consumption between Groups Vehicle and R1.5 (see Figure 3, Panel A), i.e., relative to vehicle-treated subjects, there was no evidence of any avoidance in subjects injected with the R-isomer with both groups consuming approximately 17 ml of saccharin. Subjects in both Groups Rac3 and S1.5 consumed significantly less saccharin than subjects in Groups Vehicle and R1.5 (ps < 0.001), but Groups Rac3 and S1.5 did not differ significantly from each other. For this dose comparison, the degree of avoidance with the racemate (at 3 mg/kg) was functionally identical to the additive effects of the S- and R-isomers (at 1.5 mg/kg). On this same trial, there was again no significant difference in saccharin consumption between subjects in Groups Vehicle and R3. Both groups consumed approximately 17 ml of saccharin, indicative of an absence of an avoidance in Group R3. On this comparison, both Groups Rac6 and S3 drank significantly less than subjects in Groups Vehicle and R3 (ps < 0.01); however, subjects in Group Rac6 drank significantly less saccharin than those in Group S3 (ps < 0.05) (Figure 3, Panel B), i.e., the avoidance for Group Rac6 was greater than the additive effects of the S- and R-isomers (at 3 mg/kg).
Figure 3.
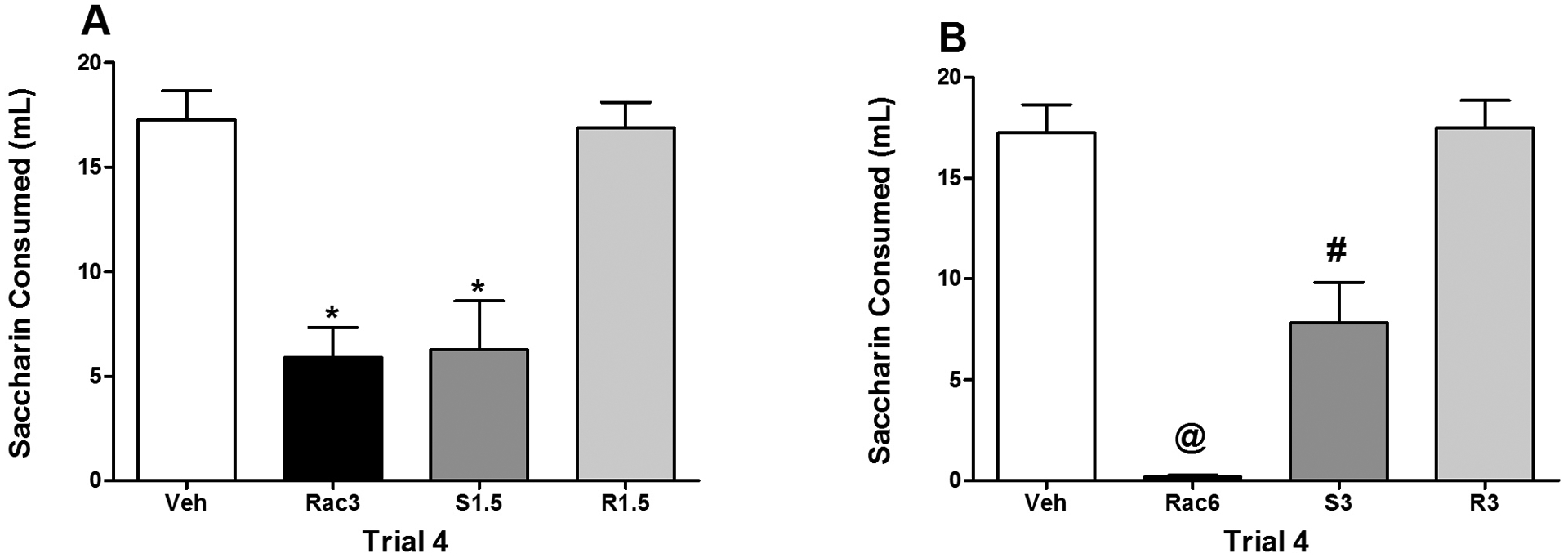
Mean (+/− SEM) saccharin consumption (ml) on Trial 4 for Groups Veh, Rac3, S1.5 and R1.5 (Panel A) and Groups Veh, Rac6, S3 and R3 (Panel B). Panel A: *significantly different from Groups Veh and R1.5. Panel B: @significantly different from all other groups. #significantly different from Groups Veh and R3.
3.3. Two-Bottle Test
On the final two-bottle avoidance test, subjects injected with the racemic mixture or the S-isomer consumed a smaller percent of saccharin relative to those injected with vehicle; subjects injected with the R-isomer maintained a high percentage of saccharin consumption at all doses (and comparable to vehicle-injected subjects). The one-way ANOVA on the percent of saccharin consumed revealed a significant effect of Group [F(9,70) = 20.52, p < 0.001]. Specifically, animals injected with either the racemate or the S-enantiomer consumed 25% or less saccharin on this test relative to approximately 80% by vehicle controls (all p’s < 0.001) (see Figure 4, Panels A and B). This was evident at all doses with no significant dose-dependent differences. Subjects injected with the R-enantiomer did not differ in the percent saccharin consumed from vehicle-injected animals at any dose, nor were there significant differences in consumption among rats injected with the R-enantiomer (Figure 4, Panel C).
Figure 4.
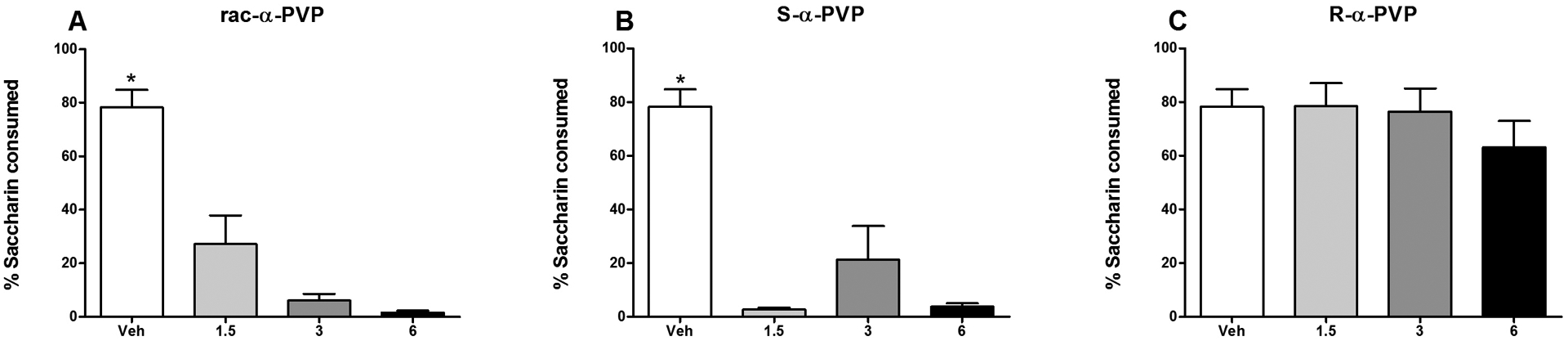
Mean (+/− SEM) percent saccharin consumed during the final two-bottle avoidance test for Groups rac-α-PVP (Panel A), S-α-PVP (Panel B) and R-α-PVP (Panel C). *significantly different from 1.5, 3 and 5
The one-way ANOVA on total fluid consumed (water + saccharin) during the final-two bottle test revealed a significant main effect of Group [F (9, 70) = 5.595, p < 0.001]. Bonferroni-corrected pairwise comparisons showed that animals injected with 1.5 mg/kg of racemic-α-PVP drank significantly less total fluid than those injected with 3 mg/kg of the racemate (p < 0.01). Rats injected with 1.5 mg/kg of S-α-PVP consumed significantly less total fluid than those injected with either vehicle or 3 mg/kg of S-α-PVP (p’s < 0.05). There were no significant differences in total fluid consumed on the final two-bottle test between groups injected with R-α-PVP, nor were there any differences in consumption between R-α-PVP-treated animals and those injected with the vehicle (data not shown).
4. Discussion
Recently, we have reported that α-PVP is aversive as indexed in the taste avoidance procedure (see Nelson et al., 2017). Although α-PVP (like other psychostimulants, including MDPV) induces taste avoidance (see above), it remains unknown to what extent its S- and R-enantiomers mediate the aversive effects of the racemate. Given that abuse vulnerability is thought to be a function of the balance of the rewarding and aversive effects of a drug, understanding the basis of the aversive effects of α-PVP may provide insight into its use and abuse potential. Accordingly, the present study assessed the relative contribution of the S- and R-enantiomers of α-PVP to the aversive effects of the racemic mixture (at 1.5, 3 and 6 mg/kg) as indexed by conditioned taste avoidance.
As described, subjects injected with the racemic mixture or S-isomer of α-PVP displayed significant taste avoidance, drinking less than subjects injected with the vehicle. Interestingly, only animals injected with the racemate displayed dose-dependent avoidance that is characteristic of a host of compounds previously reported to induce taste avoidance (see Klosterhalfen and Klosetrhalfen, 1985; Riley and Freeman, 2004), including MDMA (Cobuzzi et al., 2014), MDPV (Merluzzi et al., 2014) and α-PVP (Nelson et al., 2017). While significant avoidance was induced by both the racemate and the S-isomer, there was no evidence of such conditioning in subjects injected with R-α-PVP. Animals in this group never differed from those injected with vehicle at any dose or on any conditioning trial, suggesting that at these doses the R-isomer was not aversive. The fact that on intervening Recovery Days during which only water was available, consumption remained elevated for all groups with no consistent differences among them, suggesting that the avoidance of saccharin by subjects injected with the racemate and S-isomer was not a function of general suppression of fluid consumption produced by α-PVP. Similar to the significant suppression of consumption over repeated conditioning trials, animals injected with the racemate and S-isomer displayed almost exclusive preference for water in the two-bottle avoidance test, while animals injected with the vehicle and R-isomer preferred saccharin, indicative of the absence of any aversive effects in these two latter two groups. As previously reported for a range of behavioral and neurochemical endpoints, the two isomers contributed differentially to the effect produced by the racemate (see Fantegrossi et al., 2009; Gannon et al., 2016; Hutsell et al., 2016; Kolanos et al., 2015).
The main objective of the present work was to assess the relative contribution of the two isomers to the aversive effects of the racemate. As noted above, the racemate is composed of 50% of each isomer and as such it would be expected that the strength of the avoidance induced by the racemate at dose X would be comparable to the combined effects produced by the S- and R-isomers at half of that dose (1/2 X). To address this possibility, on the final one-bottle avoidance test animals injected with the racemate at 3 mg/kg were compared to those injected with 1.5 mg/kg of the S- and R-isomers and animals injected with the racemate at 6 mg/kg were compared to those injected with the S- and R-isomers at 3 mg/kg. Given that animals injected with the R-isomer never differed from controls (and displayed no avoidance of the saccharin solution), these comparisons were functionally between animals injected with the racemate and those injected with the S-isomer.
As described, animals injected with either the racemate (3 mg/kg) or the S-isomer (1.5 mg/kg) drank significantly less saccharin than animals injected with either the vehicle or R-isomer and the avoidance induced by the racemate and the S-isomer did not significantly differ. At least for these dose comparisons, avoidance induced by 3 mg/kg was comparable to the additive effects of the two isomers, as predicted. This was not evident at the higher dose of the racemate. Animals injected with 6 mg/kg of the racemic mixture and those injected with 3 mg/kg of the S-isomer again drank significantly less than the vehicle and R-isomer injected groups; however, at these dose comparisons, the racemate group displayed almost compete suppression of saccharin consumption, while animals injected with the 3 mg/kg of the S-isomer consumed approximately 7 ml, a significant difference between these latter two groups (again with no avoidance by animals injected with the R-isomer).
The fact that the effects produced by the racemate were not comparable to the combined effects of the two enantiomers (at the 6 mg/kg to 3 mg/kg comparison) is not consistent with simple additivity of the effects of the two isomers, but it is consistent with other work making related comparisons. For example, in an examination of the effects of MDMA and its isomers on social interactions in Swiss Webster and C57BL/mice, Curry et al. (2018) reported that the racemate was more potent than expected from the effects of the S- and R-isomers. Similarly, Fantegrossi et al. (2002) noted that the racemate and R- and S-isomers of MDMA all maintained IVSA with no consistent potency order (see also Fantegrossi et al., 2003 for a similar analysis with the locomotor stimulatory effects of MDMA). In an analysis of the effect of MDPV on hyperthermia, Gannon et al. (2016) reported that neither the S- or R-enantiomer produced effects comparable to the racemate (for other work reporting additivity, see Frau et al., 2013; Gannon et al., 2017). The basis for the fact that for some measures the effects of the racemate are greater than those produced by the addition of the effects of the two isomers has been suggested to be a function of the ability of the two enantiomers to interact synergistically, although the mechanisms underlying this synergism is not known (see Gannon et al., 2016; Curry et al., 2018; Fantegrossi et al., 2002, 2003). That in the present experiment this synergism was evident at the high dose comparisons, but not at the lower, suggest further that whatever may be mediating these synergistic interactions are dose-dependent. Independent of the mechanism involved, it appears that while the S-isomer contributes primarily to the aversive effects of racemic α-PVP, the R-isomer may be contributing in some as yet determined synergistic manner.
The doses in the present experiment ranged from 1.5 to 6 mg/kg and are limited in terms of the doses that could have been examined, and given the abovementioned dose-dependency, it would be interesting to assess the effects of other doses of the isomers in this preparation. However, the doses chosen were based on the specific question addressed, i.e., the relative contribution of the two isomers to the effects of the racemic mixture. Earlier, Nelson et al. (2017) reported that doses of the racemate lower than 1.5 (specifically 0.3 and 1 mg/kg) did not support taste avoidance conditioning. Like this early work, the dose of 1.5 of the racemate in the present experiment was not consistently aversive (only different in one of the multiple comparisons examined). Lower doses of the racemate (as reported in Nelson et al.), would not be expected to produce an aversion with which to compare the contribution of lower doses of the S- and R- isomers. Of course, lower doses of the isomers might induce an avoidance, but again their relative contribution to the effects of the racemate could not be determined. The highest dose assessed in the present study was 6 mg/kg, and as we report this dose induced near complete suppression of consumption (average consumption on the last conditioning trial for this group was less than 1 ml). This degree of suppression was expected given that significant avoidance at 3 mg/kg was reported by Nelson et al. (2017). If higher doses of the enantiomers had been assessed, they would be examined in relation to the racemate at doses higher than the one at which we have already seen such strong suppression. If the enantiomers (especially the R) produced avoidance at high doses, it would be impossible to assess its contribution given the floor effect with the racemate. Of course, a demonstration of the aversive effects at the higher doses of the isomers would be interesting in terms of any behavioral activity in this design and assessments of a broader dose range with α-PVP (and other synthetic cathinones) will be important to characterizing the behavioral effects of these drugs and their enantiomers.
Demonstrating differences between the S- and R-enantiomers in the aversive effects of α-PVP does provide some insight into the basis of its action at least in terms of which isomer mediates the aversive effects of the racemate. What such a demonstration does not provide is which neurochemical system might be mediating these effects. As noted above, α-PVP works primarily as a reuptake inhibitor of both DA and NE (with weaker actions on the 5-HT transporter). Accordingly, α-PVP might be expected to induce taste avoidance via increasing levels of the brain amines. Although little is known about the nature of the aversive effects of the vast majority of drugs effective in inducing taste avoidance (and nothing on the synthetic cathinones; for a general review, see Verendeev and Riley, 2012), some work with the monoamine reuptake inhibitor cocaine may be relevant to the neurochemical mediation of α-PVP’s aversive effects (given that both α-PVP and cocaine are monoamine reuptake inhibitors). Prior work from our laboratory and others has shown that selective DA antagonists (e.g., pimozide, haloperidol) block the acquisition of cocaine-induced taste avoidance in rats (Hunt et al., 1985; Serafine et al. 2011; though see Gale, 1984), while the NE antagonists propranolol and prazosin potentiate (Freeman et al., 2008) and the 5-HT3 antagonist tropisetron has no effect on (Briscione et al., 2013) cocaine-induced avoidance. Further, a history with the DAT inhibitor GBR 12909 weakens taste avoidance induced by cocaine in rats (presumably through the habituation of the aversive effects of elevated DA levels; Serafine et al., 2012), while the SERT inhibitor fluoxetine has no effect on cocaine-induced taste avoidance (Serafine and Riley, 2010) and cocaine potentiates avoidance induced by the NET inhibitor (Serafine and Riley, 2009). Although these data suggest that DA may mediate the aversive effects of cocaine in rats (see Serafine and Riley, 2013 for a review), other work has demonstrated that SERT and NET knock-out (KO) mice both display significantly weaker cocaine-induced taste avoidance than wild type mice (with only a minimal attenuation in DAT KO mice). Further, and again in mice, a history of the NET inhibitor nisoxetine or the SERT inhibitor fluoxetine significantly attenuates cocaine-induced taste avoidance, while a history with the DAT inhibitor vanoxerine has minimal effects. Little work has been done examining the effects of either pharmacological or genetic manipulations on α-PVP-induced taste avoidance, although it is interesting to note that a history of MDPV weakens taste avoidance induced by cocaine (see Woloschchuk et al., 2016), suggesting a role of DA in MDPV-induced avoidance.
As described above, abuse vulnerability is thought to be a function of the balance between the drug’s rewarding and aversive effects and understanding this relative balance and the myriad of subject (sex, age, strain, species) and experiential (dose of drug, drug history, route of administration, drug frequency) factors known to impact it (Cunningham et al., 2009; Freeman and Riley, 2009; King and Riley, 2017; Riley et al., 2009) may be critical to predicting and treating drug abuse. This study suggests that the relative contribution of the enantiomers to the racemic mixture is an additional factor to consider when evaluating the balance of these affective properties and ultimately the risk involved with potential abuse liability.
Acknowledgements
This research was supported in part by a grant from the Mellon Foundation to A.L.R. The Mellon Foundation had no further role in the study design, data collection, analysis and interpretation, the writing of the report or the decision to submit the manuscript for publication. The work of the Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch (MTMDB), National Institute on Drug Abuse (NIDA), and National Institute of Alcohol Abuse and Alcoholism (NIAAA) was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA). No conflict declared.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA (2015) In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: Self-administration and locomotor stimulation in male rats. Psychopharmacology 232:3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Schuster CR (1973) A comparison of d-amphetamine, l-amphetamine, and methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav 1:67–71. [DOI] [PubMed] [Google Scholar]
- Banks ML, Worst TJ, Rusyniak DE, Sprague JE (2014) Synthetic cathinones (“bath salts”). J Emerg Med 46:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Huestis MA (2016) Neuropharmacology of 3,4-methylenedioxypyrovalerone (MDPV), its metabolites, and related analogs. Curr Topics Behav Neurosci 32:93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscione MA, Serafine KM, Merluzzi AP, Rice KC, Riley AL (2013) The effects of the 5-HT3 receptor antagonist tropisetron on cocaine-induced conditioned taste aversions. Pharmacol Biochem Behav 105:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE (1971) Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacologia 22:352–356. [DOI] [PubMed] [Google Scholar]
- Cobuzzi JL, Siletti KA, Hurwitz ZE, Wetzell B, Baumann MH, Riley AL (2014) Age differences in (±)3,4-methylenedioxymethamphetamine (MDMA)-induced conditioned taste aversions and monoaminergic levels. Dev Psychobiol 56:635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council Decision EU (2010) 2010/759/EU: Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures. Official Journal L 322:4–45. [Google Scholar]
- Cunningham CL (1979) Flavor and location aversions produced by ethanol. Behav Neural Biol 27:362–367. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA (2009) Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TR (eds) Conditioned Taste Aversions: Behavioral and Neural Processes. Oxford University Press, New York, pp 387–421. [Google Scholar]
- Curry DW, Young MB, Tran AN, Daoud GE, Howell LL (2018) Separating the agony from ecstasy: R(−)-3,4-methylenedioxymethamphetamine has prosocial and therapeutic-like effects without signs of neurotoxicity in mice. Neuropharmacology 128:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, United States Department of Justice (2011) Schedules of controlled substances: Temporary placement of three synthetic cathinones into schedule I. Federal Register 76:65371–65375. [PubMed] [Google Scholar]
- Drug Enforcement Administration, United States Department of Justice (2013) Establishment of drug codes for 26 substances. Federal Register 78:664–666. [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH (2003) Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology 166:202–211. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murai N, Mathuna BO, Pizarro N, de la Torre R (2009) Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine and its enantiomers in mice: Pharmacokinetic considerations. J Pharmacol Exp Ther 329:1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G (2002) 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology 161:356–364. [DOI] [PubMed] [Google Scholar]
- Ferrari CM, O’Connor DA, Riley AL (1991) Cocaine-induced taste aversions: Effect of route of administration. Pharmacol Biochem Behav 38:267–271. [DOI] [PubMed] [Google Scholar]
- Frau L, Simola N, Plumitallo A, Morelli M (2013) Microglial and astroglial activation by 3,4-methylenedioxymethamphetamine (MDMA) in mice depends on S(+) enantiomer and is associated with an increase in body temperature and motility. J Neurochem 124: 69–78. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Riley AL (2009) The origins of conditioned taste aversion learning: An historical analysis, in: Reilly S, Schachtman T (eds) Conditioned Taste Aversion: Behavioral and Neural Processes. Academic Press, New York, pp 9–33. [Google Scholar]
- Freeman KB, Verendeev A, Riley AL (2008) Noradrenergic antagonism enhances the conditioned aversive effects of cocaine. Pharmacol Biochem Behav 88:523–532. [DOI] [PubMed] [Google Scholar]
- Gale K (1984) Catecholamine-independent behavioral and neurochemical effects of cocaine in rats. NIDA Res Monogr 54:323–332. [PubMed] [Google Scholar]
- Garcia J, Ervin FR (1968) Gustatory-visceral and telereceptor-cutaneous conditioning—adaptation in internal and external milieus. Commun in Behav Biol 1:389–415. [Google Scholar]
- Gannon BM, Rice KC, Collins GT (2017) Reinforcing effects of abused ‘bath salts’ constituents 3,4-methylenedioxypyrovalerone and α-pyrrolidinopentiophenone and their enantiomers. Behav Pharmacol 7:578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE (2016) Stereoselective effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone in mice: Drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther 356:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2015) Comparative behavioral pharmacology of three pyrrolidine-containing synthetic cathinone derivatives. J Pharmacol Exp Ther 354:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R, Hauck AE, McKenney JD (1984) Structure-activity studies on amphetamine analogs using drug discrimination methodology. Pharmacol Biochem Behav 21:895–901. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R (2016) Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain Res Bull 126:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie AJ (1979) Aversive stimulus properties of drugs. Neuropharmacology 18:971–979. [DOI] [PubMed] [Google Scholar]
- Gregg RA, Baumann MH, Partilla JS, Bonano JS, Vouga A, Tallarida CS, Velvadapu V, Smith GR, Peet MM, Reitz AB, Negus SS, Rawls SM (2015) Stereochemistry of mephedrone neuropharmacology: Enantiomer-specific behavioural and neurochemical effects in rats. Brit J Pharmacol 172:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T, Amit Z (1987) Conditioned taste aversion induced by self-administered drugs: Paradox revisited. Neurosci Biobehav R 11:107–130. [DOI] [PubMed] [Google Scholar]
- Hunt T, Switzman L, Amit Z (1985) Involvement of Dopamine in the aversive stimulus properties of cocaine in rats. Pharmacol Biochem Behav 22:945–948. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB (2017) Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology 234:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Baumann MH, Partilla JS, Banks ML, Vekariya R., Glennon RA, Negus SS (2016) Abuse-related neurochemical and behavioral effects of cathinone and 4-methylcathinone stereoisomers in rats. Eur Neuropsychopharm 26:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Vandewater SA, Dickerson TJ, Taffe MA (2017) Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats. Neuropharmacology 134:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalix P (1986) The releasing effect of the isomers of the alkaloid cathinone at central and peripheral catecholamine storage sites. Neuropharmacology 25:499–501. [DOI] [PubMed] [Google Scholar]
- King HE, Riley AL (2017) The affective properties of synthetic cathinones: Role of reward and aversion in their abuse. Curr Topics Behav Neurosci 32:165–182. [DOI] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL (2015) Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacol Biochem Behav 137:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL (2014) 3,4-Methylenedioxypyrovalerone (MDPV)-induced conditioned taste avoidance in the F344/N and LEW rat strains. Pharmacol Biochem Behav 126: 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosetrhalfen S, Klosetrhalfen W (1985) Conditioned taste aversion and traditional learning. Psychol Res-Psych Fo 47:71–94. [DOI] [PubMed] [Google Scholar]
- Kolanos R, Sakloth F, Jain AD, Partilla JS, Baumann MH, Glennon RA (2015) Structural modification of the designer stimulant α-pyrrolidinovalerophenone (α-PVP) influences potency at dopamine transponders. ACS Chem Neurosci 6:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S (2017) Conditioned taste aversions: From poisons to pain to drugs of abuse. Psychon Bull Rev 24:335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH (2014) Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL (2016) Pharmacological effects of methamphetamine and alphα-PVP vapor and injection. Neurotoxicology 55:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung J, Fantegrossi W, Howell LL (2010) Reinstatement of extinguished amphetamine self-administration by 3,4-methylenedioxymethamphetamine (MDMA) and its enantiomers in rhesus monkeys. Psychopharmacology 210:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK (2006) 1-(4-methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogues: A promising class of monoamine uptake inhibitors. J Med Chem 49:1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merluzzi AP, Hurwitz ZE, Briscione MA, Cobuzzi JL, Wetzell B, Rice KC, Riley AL (2014) Age-dependent MDPV-induced taste aversions and thermoregulation in adolescent and adult rats. Dev Psychobiol 56:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2003) Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy, Washington, D.C. [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals. National Academy, Washington, D.C. [Google Scholar]
- Nelson KH, Hempel BJ, Clasen MM, Rice KC, Riley AL (2017) Conditioned taste avoidance, conditioned place preference and hyperthermia induced by the second generation ‘bath salt’ α-pyrrolidinopentiophenone (α-PVP). Pharmacol Biochem Behav 156:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley AL (2011) The paradox of drug taking: The role of the aversive effects of drugs. Physiol Behav 103:69–78. [DOI] [PubMed] [Google Scholar]
- Riley AL, Davis CM, Roma PG (2009) Strain differences in taste aversion learning: Implications for animal models of drug abuse. In: Reilly S and Schachtman T (eds), Conditioned Taste Aversion: Behavioral and Neural Processes. Academic Press, New York, pp 226–261. [Google Scholar]
- Riley AL, Freeman KB (2004) Conditioned flavor aversions: Assessment of drug-induced suppression of food intake. Curr. Protoc. Neurosci 8.6E.1–8.6E.12. [DOI] [PubMed] [Google Scholar]
- Riley AL, Nelson KH, To P, López-Arnau R, Xu P, Wang D, Wang Y, Shen HW, Kuhn DM, Angoa-Perez M, Anneken JH, Muskiewicz D, Hall FS (2018) Abuse potential and toxicity of the synthetic cathinones (i.e., “Bath Salts”). Neurosci Biobehav R; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley AL, Tuck DL (1985) Conditioned Food Aversions: A bibliography. Ann NY Acad Sci 443:381–437. [DOI] [PubMed] [Google Scholar]
- Rozin P, Kalat JW (1971) Specific hungers and poison avoidance as adaptive specializations of learning. Psychol Rev 78:459–486. [DOI] [PubMed] [Google Scholar]
- Schechter MD (1986) Discriminative properties of l-cathinone compared to dl- and d-cathinone. Pharmacol Biochem Behav 24:1161–1165. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Briscione MA, Rice KC, Riley AL (2012) Dopamine mediates cocaine induced conditioned taste aversions as demonstrated with cross-drug preexposure to GBR 12909. Pharmacol Biochem Behav 102:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Briscione MA, Riley AL (2011) The effects of haloperidol on cocaine-induced conditioned taste aversions. Physiol Behav 105:1161–1167. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Riley AL (2009) Possible role of norepinephrine in cocaine-induced conditioned taste aversions. Pharmacol Biochem Behav 92:111–116. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Riley AL (2010) Preexposure to cocaine attenuates aversions induced by both cocaine and fluoxetine: implications for the basis of cocaine-induced conditioned taste aversions. Pharmacol Biochem Behav 95:230–234. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Riley AL (2013) Cocaine-induced conditioned taste aversions: Role of monoamine reuptake inhibition. In: Hall FS (ed) Serotonin: Biosynthesis, Regulation and Health Implications. Nova Science Publishers, Inc., New York, pp 256–291. [Google Scholar]
- Stolerman IP, D’Mello GD (1981) Oral self-administration and the relevance of conditioned taste aversions. In: Thompson T, Dews PB, McKim WA (eds.), Advances in Behavioral Pharmacology. Lawrence Erlbaum, Hillsdale, NJ, pp 169–214. [Google Scholar]
- Verendeev A, Riley AL (2011) Relationship between the rewarding and aversive effects of morphine and amphetamine in individual subjects. Learn Behav 39:399–408. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL (2012) Conditioned taste aversion and drugs of abuse: History and interpretation. Neurosci Biobehav R 36:2193–2205. [DOI] [PubMed] [Google Scholar]
- Vouga A, Gregg RA, Haidery M, Ramnath A, Al-Hassani HK, Tallarida CS, Grizzanti D, Raffa RB, Smith GR, Reitz AB, Rawls SM (2015) Stereochemistry and neuropharmacology of a ‘bath salt’ cathinone: S-enantiomer of mephedrone reduces cocaine-induced reward and withdrawal in invertebrates. Neuropharmacology 91:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL (2007) Estimating the relative reinforcing strength of (±)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: Comparison to (+)-methamphetamine. Psychopharmacology 189:483–488. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF (2014) Effects of α-pyrrolidinopentiophenone and 4-methyl-n-ethylcathinone, two synthetic cathinones commonly found in second-generation “bath salts,” on intracranial self-stimulation thresholds in rats. Int J Neuropsychoph 18:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, DeWit H (1976) Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science 191:1273–1275. [DOI] [PubMed] [Google Scholar]
- Woloshchuk CJ, Nelson KH, Rice KC, Riley AL (2016) Effects of 3,4-methylenedioxypyrovalerone (MDPV) pre-exposure on the aversive effects of MDPV, cocaine and lithium chloride: Implications for abuse. Drug Alcohol Depen 167:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]


