Abstract
Background and Aims
Inflammatory bowel diseases [IBD], comprising Crohn’s disease [CD] and ulcerative colitis [UC], are chronic conditions characterized by severe dysregulation of innate and adaptive immunity resulting in the destruction of the intestinal mucosa. Natural killer [NK] cells play a pivotal role in the dynamic interaction between the innate and adaptive immune response. There is an increasing appreciation for the key role immunometabolism plays in the regulation of NK cell function, yet little remains known about the metabolic profile, cytokine secretion, and killing capacity of human NK cells during active IBD.
Methods
Peripheral blood mononuclear cells were isolated from peripheral blood of patients with moderate to severely active IBD and healthy controls. NK cells were stained with a combination of cell surface receptors, intracellular cytokines, and proteins and analyzed by flow cytometry. For measurements of NK cell cytotoxicity, the calcein-AM release assay was performed. The metabolic profile was analyzed by an extracellular flux analyzer.
Results
NK cells from IBD patients produce large quantities of pro-inflammatory cytokines, IL-17A and TNF-α ex vivo, but have limited killing capability. Furthermore, patient NK cells have reduced mitochondrial mass and oxidative phosphorylation. mTORC1, an important cell and metabolic regulator, demonstrated limited activity in both freshly isolated cells and cytokine-stimulated cells.
Conclusions
Our results demonstrate that circulating NK cells of IBD patients have an unbalanced metabolic profile, with faulty mitochondria and reduced capacity to kill. These aberrations in NK cell metabolism may contribute to defective killing and thus the secondary infections and increased risk of cancer observed in IBD patients.
Keywords: NK cell, mTORC, mitochondria, immune metabolism
1. Introduction
Inflammatory bowel diseases [IBD], comprising Crohn’s disease [CD] and ulcerative colitis [UC], are chronic inflammatory disorders of the gastrointestinal tract. The incidence and prevalence of IBD across the world have shown consistent increases in recent decades.1 Symptoms of IBD include pain, cramps, bloating, recurrent or bloody diarrhea, weight loss, and extreme tiredness.2 There is currently no cure for CD or UC.2 Treatment aims to relieve and prevent symptoms from returning, and includes specific diets, lifestyle changes, medication, and surgery.2 Yet, it is not clear what drives the immune dysregulation underpinning IBD pathogenesis. While generally thought to involve the combination of intestinal microbiota and genetic susceptibility, we still do not fully understand the mechanisms behind the dysregulation of pro- and anti-inflammatory cytokines.3
Natural killer [NK] cells are an essential component of anti-cancer and anti-viral immunity.4,5 More recently, a role for NK cells in the fight against bacterial and fungal infection has also been demonstrated.6–8 NK cells have immunomodulatory properties in vivo during chronic inflammation and autoimmunity.4,9,10 Their capacity for secreting pro-inflammatory cytokines such as TNF-α and IFN-γ mean NK cells are important drivers for the polarization and activation of other immune cells during inflammation.9 Peripheral blood NK cells obtained from IBD patients have impaired killing capability and differential expression of killer cell immunoglobulin-like receptors [KIRs].11–13 Interestingly, the NK cell phenotype differs with location: gut-resident NK cells produce IFN-γ but are less cytotoxic and express lower levels of CD16 compared to cells in the peripheral blood.9,14 Understanding how NK cells behave in the peripheral blood of IBD patients is important not only in terms of understanding cell defects in the course of disease, but may also inform new therapeutic approaches for these patients.15
Human peripheral blood NK cells can be functionally divided into two subsets based on the level of surface expression of CD56 [CD56dim and CD56bright cells].16 Approximately 90% of human NK cells are accounted for by CD56dim cells.16 CD56dim cells are strongly cytotoxic and express high levels of CD16. CD56bright cells account for the remaining 10% of human NK cells in peripheral blood; these cells weakly express CD16, but express many cytokine receptors [e.g. IL2-R, IL-15-R, IL-12-R, IL-18-R, IL-21-R], and produce large quantities of cytokines and chemokines upon stimulation.16 Recently, it has been shown that these two NK cell populations can be distinguished by their metabolic profile.15,17 Cell metabolism is not only important for ATP production, an essential energy molecule for cell function, but has also been implicated as a fine-tune regulator of NK cell effector functions.17 After cytokine stimulation [e.g. IL-12 plus IL-15], CD56dim NK cells exhibit minimal metabolic activity as they are poised to kill target cells with high basal levels of cytotoxic granules, whereas cytokine stimulation promotes glucose uptake by CD56bright NK cells and increased mammalian target of rapamycin 1 [mTORC1] activity, an important protein kinase that upregulates NK cell metabolism.17 This metabolic adaptation is necessary for CD56bright cells to produce large quantities of IFN-γ.17 In other inflammatory diseases, such as rheumatoid arthritis and obesity, alterations in immunometabolism correlate with changes in immune cell phenotype and function.18 The metabolic profile of NK cells isolated from IBD patients during a flare is currently unknown, but it may be possible to manipulate this, offering a new avenue for therapeutic intervention.
In the present study, we investigated the phenotype, function, and metabolic status of NK cells isolated from peripheral blood of patients with active IBD and compared this with those of healthy controls. Our data show that patient NK cells have impaired cytotoxicity response, increased expression of IL-17 and TNF-α, and diminished cell metabolism compared to those obtained from healthy controls. Cytokine stimulation of NK cells of IBD patients results in reduced activation of mTORC1 and impaired IFN-γ production. We demonstrate that these NK cells have defective mitochondrial fitness, suggesting a link between metabolism and NK cell defects in IBD patients.
2. Methods
2.1. Patients
Patients with biopsy-proven CD or UC and healthy controls were recruited from a single tertiary referral center at the Centre for Colorectal Disease [CCD], St Vincent’s University Hospital [SVUH]. Patients attending for routine clinical review with evidence of clinical disease activity were invited to participate [n = 53]. In addition, control samples were obtained from patients and volunteers without IBD (n = 47, mean age 37 years [range 25–67], 55% female and 45% male). Informed consent was obtained from all participants and the protocol was approved by the local research and ethics committee at SVUH. Active disease was defined using a validated clinical scoring system (Partial Mayo Score [PMS] > 3] [in UC]; or Harvey Bradshaw Index [HBI] > 5] [in CD]) or evidence of disease activity on recent stool [fecal calprotectin] or endoscopic evaluation. Disease distribution and clinical characteristics are outlined in Table 1. The majority of CD patients had an ileal or colonic disease location as defined by the Montreal classification. The majority of UC patients had left-sided or total colon [pancolitis] involvement of the disease. Mean HBI and PMS was 5 in both groups, indicating patients with active clinical disease. Fecal calprotectin was significantly elevated in both CD and UC groups with median levels of 605 and 1000 µg/g, respectively [normal < 15 µg/g].
Table 1.
Characteristics of patients included in the study
| Characteristics | CD | UC/IBD-U |
|---|---|---|
| Number of patients | n = 27 | n = 26 [IBD-U = 2] |
| Age at assessment, years [median, range] | 39 [18–63] | 30.5 [18–81] |
| Sex [M/F] | 15/12 | 15/11 |
| Disease extenta | ||
| L1 | 12 | |
| L2 | 4 | |
| L3 | 10 | |
| L4 | 4 | |
| Perianal | 5 | |
| Proctitis | 2 | |
| Left sided | 11 | |
| Pancolitis | 13 | |
| HBI score, mean ± SD | 4.74 [±2.4] | |
| PMS score, mean ± SD | 4.8 [±1.85] | |
| Therapy [n] | ||
| 5-ASA | 2 | 13 |
| Steroids | 3 | 8 |
| Biologics | 16 | 13 |
| Immunomodulators | 3 | 6 |
| Prior IBD surgery | 10 | 1 |
| Smoker | 6 | 1 [+3 EX] |
| Fecal calprotectin [FCP], µg/g, median [IQR] | 605 [38–1000] | 1000 [18–1118] |
HBI, Harvey Bradshaw Index; PMS, Partial Mayo Score; 5-ASA, 5-aminosalicylic acid; IQR, interquartile range.
aMontreal classification of CD [L1: ileal, L2: colonic, L3: ileocolonic, L4: upper gastrointestinal]
The demographic and clinical characteristics of the analyzed patients are described in Table 1.
2.2. Cell isolation and cell culture
In total, 30 mL of blood was drawn into lithium-heparin tubes [BD Bioscience] and peripheral blood mononuclear cells [PBMCs] were isolated by density gradient centrifugation using Lymphoprep [Axis-Shield]. For Seahorse extracellular flux experiments, NK cells were purified using the EasySep Human NK Cell Isolation Kit [STEMCELL Technologies] as per the manufacturer’s instructions. Cell viability was assessed by trypan blue exclusion. For overnight cell stimulation experiments, 2 × 106 cells/mL PBMCs were incubated at 37°C for 18 h in RPMI 1640 Glutamax medium [Gibco, Invitrogen] supplemented with 10% fetal calf serum and 1% penicillin/streptomycin [Invitrogen] and stimulated with IL-12 [30 ng/mL] and IL-15 [100 ng/mL] [Miltenyi Biotec].
2.3. Cytotoxicity assay
For measurements of NK cell cytotoxicity, the calcein-AM release assay was performed as previously described with minor changes.19 K562 target cells were stained with Calcein-AM [10 μM; BD Pharmingen] at 37°C in 5% CO2 for 30 min. Calcein-AM-stained cells were thoroughly washed to remove excess dye. PBMCs and stained K562 cells were co-cultured in triplicate at an effector-to-target ratio [E:T] of 10:1 and 5:1. For maximum release [positive control], stained target cells were lysed with 10% Triton-X solution. Cells were incubated for 2 h at 37°C in 5% CO2. After the incubation period, the cell plate was gently centrifuged, and supernatant was carefully aspirated and placed into a black 96-well plate, and calcein-AM fluorescence was measured by a FLUOstar OPTIMA Microplate Reader [BMG LABTECH]. When NK cells lyse target cells, the fluorescent dye is released into the supernatant. Therefore, fluorescence is proportional to the target cell killing calculated as follows:
2.4. Flow cytometry
For surface staining, cells were washed and stained for 20 min at 4°C with saturating concentrations of the following antibodies: CD56 [REA196, NCAM 16.2], CD3 [SK7], CD71 [M-A172]; TRAIL [REA1113]; and NKG2D [1D11], 2B4 [C1.7], CD94 [REA113], NKp46 [9E2], NKp30 [P30-15], NKp44 [REA1163] [Miltenyi Biotech, BD Pharmingen or Biolegend]. For intracellular staining, stimulated cells were incubated for 3 h with the protein-transport inhibitor Brefeldin A [Biolegend] prior to staining. For fixation and permeabilization of the cells, the Cytofix/Cytoperm kit from BD Pharmingen was used according to the manufacturer’s instructions. After fixation, cells were washed and stained for 30 min with IFN-γ [B27], Granzyme B [REA226], IL-17 [BL168], or TNF-α [Mab11] antibody. For the phospho-S6 ribosomal protein staining, freshly isolated PBMCs were washed in FACS buffer and stained for 20 min for surface staining [CD56 and CD3]. After being washed again, cells were fixed and permeabilized. Cells were incubated with anti-phospho-S6 ribosomal protein Ser 235/236 [Cell Signaling Technologies] for 30 min at 4°C. Live cells were gated according to their forward scatter area [FSC-A] and side scatter area [SSC-A]; single cells were gated according to their forward scatter width [FSC-W] and FSC-A; and NK cells were identified as CD56+ CD3− cells. Samples were acquired via a FACS Canto II [Becton Dickinson] and analyzed by FlowJo software [TreeStar].
2.5. Metabolism analysis
Measurement of cellular bioenergetics [respiratory activity] was performed using the Seahorse extracellular flux analyzer [Agilent]. The metabolic profiles of freshly isolated NK cells from IBD patients and controls were assessed using the XFp Seahorse analyzer. In brief, 3 × 105 purified NK cells were allowed to adhere to an eight-well XF cell culture microplate [Seahorse Biosciences] coated with Cell-Tak [Corning]. Sequential measurements of oxygen consumption rate [OCR, representative of OXPHOS] and extracellular acidification rate [ECAR, representative of glycolysis] were made after the addition of the inhibitors oligomycin [2 µM, ATP synthase blocker, used to measure ATP turnover and to determine proton leak], carbonyl cyanide-4-[trifluoromethoxy] phenylhydrazone [FCCP, 0.5 µM, a mitochondria uncoupler, which allows calculation of the maximum respiratory capacity], rotenone [100 nM, inhibitor of mitochondrial complex I] plus antimycin A [4 µM, inhibitor of mitochondrial complex III] were used to measure non-mitochondrial respiration and 2-deoxyglucose [2DG, 30 mM, a glycolysis inhibitor, which provides a baseline ECAR measurement]. This allowed calculation of basal mitochondrial respiration, maximal mitochondrial respiration, ATP synthesis, basal glycolysis, glycolytic capacity, and OCR/ECAR ratio.
2.6. Mitochondria mass and mitochondria membrane potential
Mitochondrial mass was measured via staining of cells for 30 min with MitoTracker Green [100 nM; ThermoFisher] and mitochondria membrane potential analysis was performed by staining cells with TMRM [tetramethylrhodamine, methyl ester, 0.1 µM; ThermoFisher] for 30 min. After washing, cells were stained with surface markers [CD56 and CD3] and live-dead aqua dye was used to exclude dead cells [ThermoFisher].
2.7. Statistical analysis
GraphPad Prism 8.00 [GraphPad Software] was used for statistical analysis. Unpaired, non-parametric data were compared using the Mann–Whitney test. Two-way ANOVA with multiple comparisons was performed when more than two data sets were being compared.
3. Results
3.1. NK cells from IBD patients have impaired killing capability and abnormal phenotype
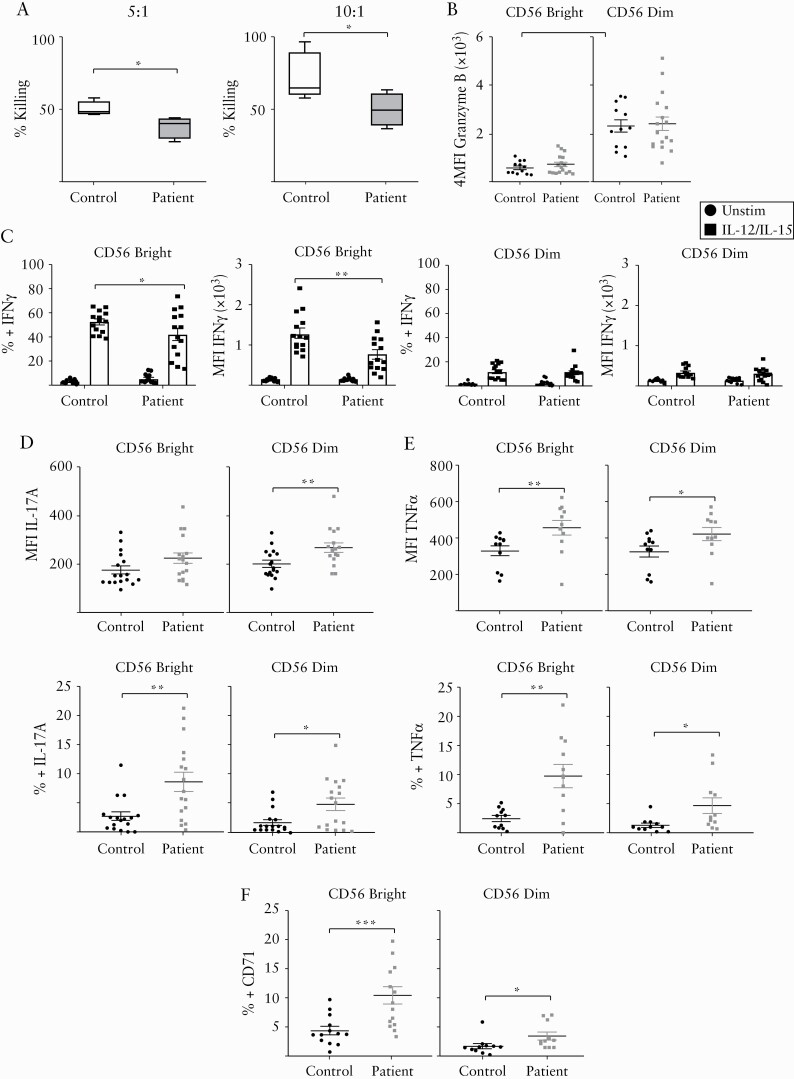
We analyzed the percentage of CD56bright and CD56dim NK cells in the peripheral blood of IBD patients and healthy controls. We did not observe a significant difference in CD56bright cells [mean 7.4% control vs 6.02% patient, p = 0.2] or CD56dim cells [mean 88.98% control vs 90.57% patient, p = 0.3] [Supplementary Figure 1A]. To determine NK cell killing efficiency, we challenged NK cells from patients and healthy controls against the K562 cell line. We found that NK cells obtained from IBD patients have defects in cellular cytotoxicity, as they were unable to kill K562 cells at two different effector to target cell ratios [Figure 1A]. This is in line with previous reports by others.11,20 However, we found no significant difference in levels of granzyme B expressed by NK cells when comparing IBD patients to controls [Figure 1B].
Figure 1.
NK cells from IBD patients have cytotoxic defects and altered phenotype. [A] Different effector to target [E:T] ratios of freshly isolated PBMCs from IBD patients and controls were incubated with K562 cells stained with Calcein-AM. Cytotoxicity was detected by quantification of fluorescence released in the media. Left: E:T ratio of 5:1; right: E:T ratio of 10:1 [n = 4–6]. [B] Flow-cytometry analysis of the production of granzyme B (mean fluorescence intensity [MFI]) of freshly isolated CD56bright cells and CD56dim cells from patients and controls [n = 12–16]. [C] Frequency and expression [MFI] of IFN-γ on CD56bright cells [left panel] or CD56dim cells [right panel] from patients and controls left unstimulated or stimulated for 18 h with IL-12 plus IL-15 cytokine combination [n = 14]. [D] Expression [MFI] of IL-17A [upper panel] and frequency [lower panel] of CD56bright and CD56dim cells of patients and controls [n = 17]. [E] Expression [MFI] of TNF-α [upper panel] and frequency [lower panel] of CD56bright and CD56dim cells of patients and controls [n = 11]. [F] Frequency of CD71 expression on CD56bright cells and CD56dim cells of patients and controls [n = 13]. Bars show the average value ± SEM. Samples were compared using a Student t test or two-way ANOVA as appropriate. *p < 0.05, **p < 0.01, ***p < 0.001.
NK cells are an important source of IFN-γ.4,5,21,22 We observed no difference in basal levels of IFN-γ production [data not shown] when we compared NK cells isolated from IBD patients and controls. To determine if NK cells from IBD patients have defects in the production of IFN-γ in response to stimulation, cells obtained from patients and controls were incubated overnight in the presence of IL-12 and IL-15. This cytokine stimulus induced IFN-γ expression by NK cells isolated from both patients and controls. However, we noted that CD56bright NK cells of IBD patients produced significantly lower levels of IFN-γ compared to those of healthy controls [Figure 1C; Supplementary Figure 1B].
Next, we investigated if NK cell expression of the pro-inflammatory cytokines IL-17A and TNF-α differs between patients and controls as both have been implicated in the pathogenesis of IBD.23 IBD patient NK cells [both CD56bright and CD56dim] had significantly increased expression of both IL-17A and TNF-α [Figure 1D, E].
We further evaluated the phenotype of NK cells freshly isolated from peripheral blood of IBD patients. NK cells possess various surface receptors that activate or inhibit NK cell function and cytotoxicity. A balance between these receptors is required to maintain NK cell tolerance toward normal cells. We found that the frequency and/or expression of many surface receptors related to activation of NK cell function such as TRAIL, NKG2D, 2B4 [CD244], NKp46, NKp30, NKp44, or inhibition-like CD94 were similar when we compared the patients and controls in our cohort [Supplementary Figure 2A–G].
Anemia is a common extraintestinal manifestation of IBD patients and lymphocytes require iron uptake for their immune cell functions.24,25 We therefore analyzed the expression of CD71, the transferrin receptor which is highly expressed on activated NK cells.17,26 Interestingly, we found that both CD56bright and CD56dim cells from patients have increased basal expression of CD71 compared to controls [Figure 1F].
Collectively, these findings show that IBD patient NK cells have reduced cytotoxicity and a dysregulated pattern of cytokine secretion, reduced IFN-γ production, but elevated TNF-α and IL-17A. CD71 in NK cells is regulated by mTORC1,17 so we hypothesized that NK cells from IBD patients may have disrupted metabolic signaling.
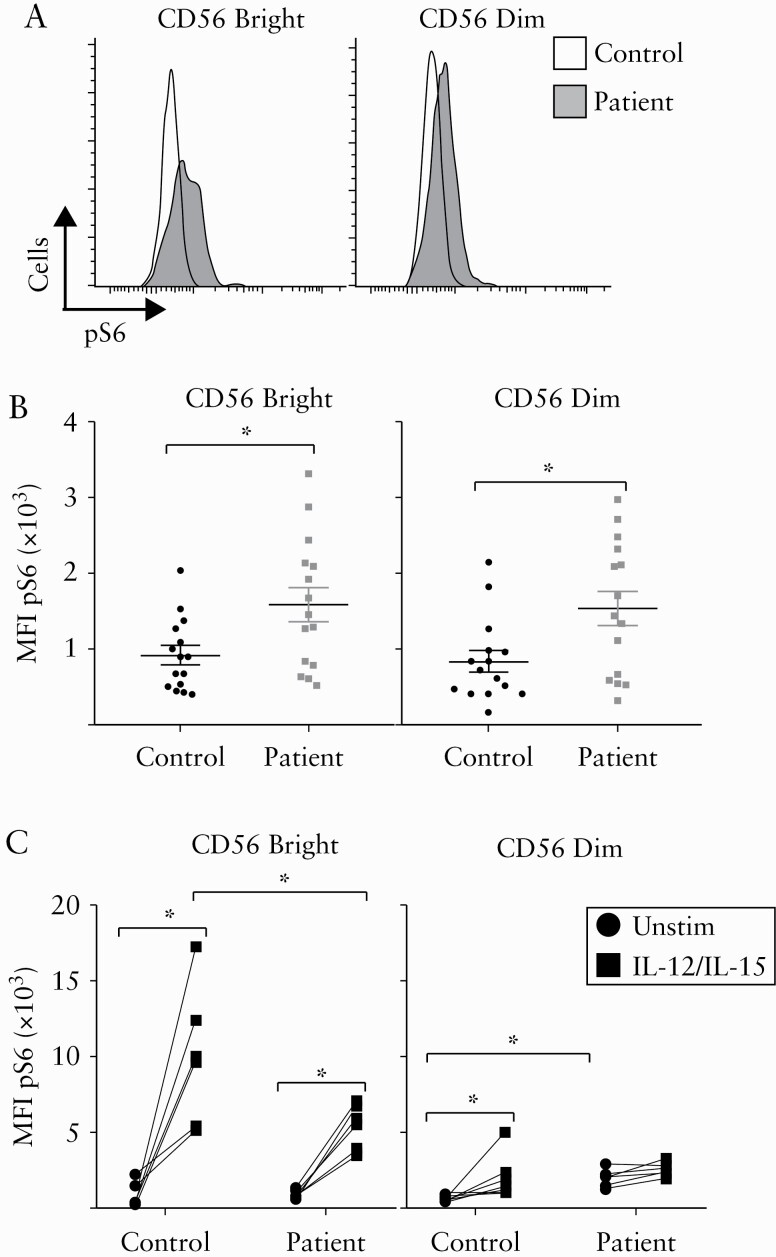
3.2. NK cells from IBD patients have dysregulated mTORC1 activity
The protein kinase mTORC1 is required for optimal NK cell cytotoxicity and cytokine production.17,22,27 Given the defects we observed in patient NK cells [Figure 1], we investigated mTORC1 activity in these cells by measuring phosphorylation of S6 protein [pS6], a downstream target of mTORC1 signaling. Figure 2 shows that freshly isolated NK cells from IBD patients have significantly elevated basal levels of mTORC1 activity compared to those of healthy controls. pS6 levels were significantly elevated in both CD56dim and CD56bright cells of IBD patients [Figure 2A, B]. Following overnight culture in the presence of IL-12 and IL-15, healthy control CD56bright NK cells had significantly upregulated levels of pS6 compared to patient cells [Figure 2C left panel]. Though IBD CD56dim NK cells have elevated basal pS6 levels, this was not further augmented following in vitro cytokine stimulation [Figure 2C right panel]. Our data showing differences in IBD patients and controls in NK cell expression of pS6 suggest that NK cells from IBD patients have aberrant mTORC1 activity and altered responses to cytokine stimulation.
Figure 2.
Impaired mTORC1 activity in NK cells from IBD patients. Freshly isolated PBMCs were analysed for pS6 levels by intracellular flow cytometry staining. [A,B] Representative histogram [A] and collective data [B] are shown for NK cells stratified into CD56bright and CD56dim cells [n = 15]. [C] PBMCs were stimulated for 18 h with IL-12 plus IL-15 or left unstimulated. Cells were then analyzed for pS6 levels by intracellular flow cytometry staining. NK cells were stratified into CD56bright [right panel] and CD56dim cells [left panel]. Bars show the average value ± SEM. Samples were compared using a Student t test or two-way ANOVA as appropriate. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. NK cells from IBD patients have defective metabolism
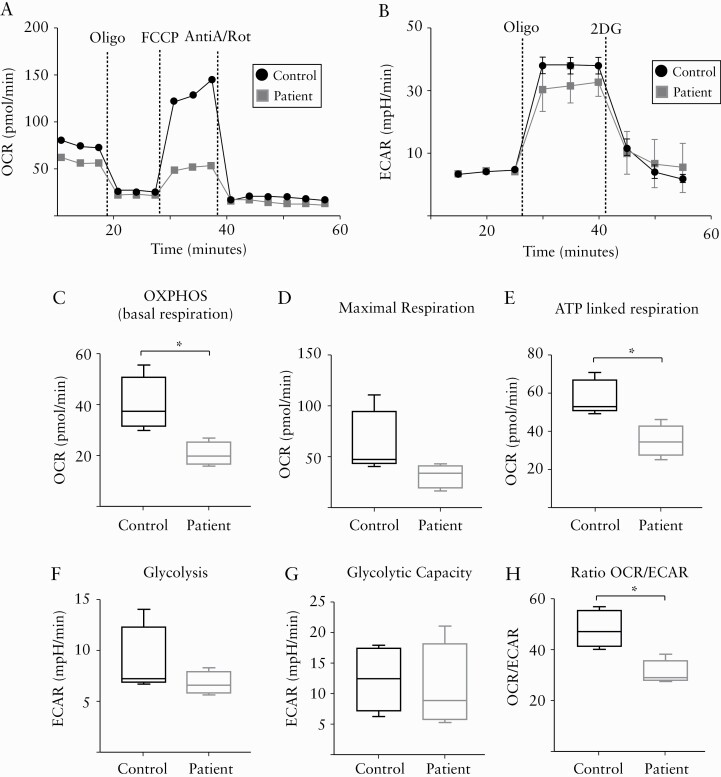
We have demonstrated that NK cells isolated from IBD patients have impaired killing, dysregulated cytokine production, and altered mTORC1 activity. Therefore, we investigated whether peripheral blood NK cells from IBD patients have a different metabolic profile compared to healthy controls. To investigate metabolic changes, NK cells were freshly purified from PBMCs and glucose metabolism was assessed before or after injections of oligomycin, FCCP, antimycin A, and 2-DG, which are specific inhibitors of mitochondria function or glycolysis. Our data show that NK cells from IBD patients have a significant decrease in OXPHOS [basal respiration] and no significant changes in maximal respiration when compared to controls [Figure 3A, C, D]. Limited respiration correlated with diminished ATP production under basal conditions [Figure 3E]. No significant differences in basal glycolysis and glycolytic capacity were observed [Figure 3B, F, G]. In addition to the defects observed in OXPHOS, our data indicate that IBD NK cells have an altered balance between OXPHOS and the glycolysis pathways [Figure 3H]. Together, these metabolic analyses suggest that NK cells from IBD patients rely mostly on glycolysis rather than mitochondrial respiration, indicating a distinct NK cell bioenergetic program in IBD.
Figure 3.
NK cells from IBD patients have an abnormal metabolic profile. PBMCs were freshly isolated from IBD patients [two patients with CD, one patient with UC and one patient with IBD-unclassified] or controls, and NK cells were purified. Detailed metabolic analysis was performed using the Seahorse extracellular flux analyzer. [A,B] Representative traces for the oxygen consumption rate [OCR] and extracellular acidification rate [ECAR]. [C–H] Pooled data for OXPHOS [C], maximal respiration [D], ATP production [E], glycolysis [F], glycolytic capacity [G], and OCR and ECAR ratio [H] [n = 4]. Bars show the average value ± SEM. Samples were compared using a Student t test. *p < 0.05.
3.4. Defective mitochondria activity in NK cells from patients with IBD
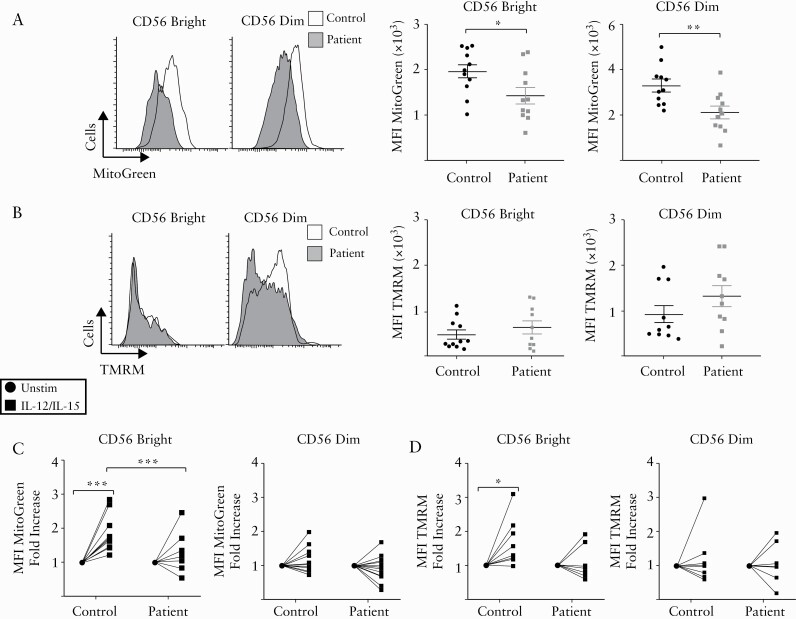
As NK cells from IBD patients displayed significant defects in OXPHOS, we further investigated the properties of mitochondria in CD56bright and CD56dim NK cells from these patients. In line with the defects in OXPHOS, freshly isolated NK cells from IBD patients show decreased mitochondrial mass [as indicated by MitoTracker Green staining; Figure 4A], while no significant differences were observed in mitochondrial membrane potential [as indicated by TMRM staining; Figure 4B]. Overnight culture of NK cells with IL-12 and IL-15 revealed important differences in mitochondrial mass and membrane potential between IBD patients and controls. CD56bright NK cells significantly upregulate their mitochondria mass and polarization compared to CD56dim NK cells [Figure 4C, D]. When we compared the changes in the properties of mitochondria between NK cells from patients and controls, we observed that CD56bright NK cells from IBD patients have impaired upregulation of mitochondria mass and membrane potential in response to cytokine stimulation [Figure 4C, D]. Together, these data indicate significant defects in the mitochondria of patient-derived NK cells, which have reduced mass and do not alter their membrane potential in response to cytokine signals.
Figure 4.
Mitochondria from NK cells of IBD patients have inadequate mitochondria activity. Freshly isolated PBMCs from patients and healthy donors were stained with MitoTracker Green [100 nM] and TMRM [100 nM] for 30 min and analysed by flow cytometry. [A] Representative histogram [left panel] and pooled data of NK cells stratified into CD56bright and CD56dim cells stained with MitoTracker Green [n = 11]. [B] Representative histogram [left panel] and pooled data of NK cells stratified into CD56bright and CD56dim cells stained with TMRM [n = 10]. [C,D] PBMCs were stimulated for 18 h with IL-12 plus IL-15 or left unstimulated. Cells were then analyzed for mitochondria activity by flow cytometry staining. [C] NK cells were stratified into CD56bright [right panel] and CD56dim cells [left panel] and MitoTracker Green intensity was detected [n = 12]. [D] NK cells were stratified into CD56bright [right panel] and CD56dim cells [left panel] and the expression of TMRM was determined [n = 8]. Bars show the average value ± SEM. Samples were compared using a Student t test or two-way ANOVA as appropriate. *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
NK cells are key immune cells in the fight against infection and cancer. They can directly kill pathogens as well as modulate the innate and adaptive immune response.15 The exact role of NK cells in the pathogenesis of IBD is not clear. In the inflamed gut tissue of IBD patients, NK cells are normally described as innate lymphoid cells [ILCs] and are recognized as inflammation promoters with a distinct phenotype and cell lineage origin compared to the NK cells found in peripheral blood or other solid organs.9,14,28 In the present study, we focus on investigating metabolic and phenotypic alterations of circulating NK cells, also termed conventional NK cells.14
We now understand that immunity and metabolism are inextricably linked. Referred to as immunometabolism, this growing scientific field aims to investigate changes in metabolic pathways associated with specific cell behaviors and how these changes can be targeted to regulate immune cell function and potentially treat a variety of diseases.18 Our study, which clearly demonstrates defects in NK cell immunometabolism in IBD patients, highlights the urgent need to further explore metabolic defects of different cell types in IBD patients to clarify if this may be a future therapeutic avenue. For instance, methotrexate, which is sometimes used to treat CD, limits immune cell function through regulation of cell metabolism signaling and nucleotide synthesis.18 Drugs limiting immune cell metabolism may be beneficial to control a flare, but persistent metabolic inhibition may favor secondary infections or even cancer development.29
In our study, we investigated the two main NK cell subpopulations found in the peripheral blood of humans: CD56bright and CD56dim cells.15 Our aim was to investigate NK cells in the circulation of IBD patients with active disease based on HBI/PMS score values and to detect cytotoxic, phenotypic, and metabolic alterations. Peripheral blood-derived NK cells of IBD patients have defective cytotoxicity effector function.11,20,30 To kill K562 cells, NK cells require direct contact, microtubule-organizing center [MTOC] formation, and proper lysosome polarization.31,32 In line with previous studies on freshly isolated cells, we observed that cells from the peripheral blood of IBD patients were unable to kill K562 cells when compared to controls, despite expressing normal levels of granzyme B.11,20,30 We also observed significant defects in mitochondria function and ATP production in patient-derived NK cells which could be related to defective killing, as mitochondria clustering and microtubule network relocation together with MTOC towards the immune synapse are required for effective target cell killing.31,33–35
We found that peripheral blood NK cells isolated from IBD patients had elevated basal expression of the transferrin receptor CD71 and increased IL-17 and TNF-α production but diminished IFN-γ expression after cytokine stimulation. Reduced IFN-γ production but increased expression of other pro-inflammatory cytokines supports the hypothesis that these cells are receiving signals, probably factors circulating in the serum of patients, supporting NK cell dysregulation.11 We focus on IFN-γ production by NK cells in response to a combination of IL-12/IL-15, cytokines derived from innate immune cells which are normally used to activate human NK cells in vitro. Studies exploring IFN-γ production by NK cells isolated from IBD patients with different cytokines have reported different results. Liu et al. demonstrated that IL-21R-positive NK cells were significantly increased in the peripheral blood of IBD patients and produce high levels of IFN-γ and TNF-α after IL-21 stimulation.36 Although IL-21 and IL-15 share the β chain of the IL-15 receptor, IL-21 phosphorylates different signaling pathways and promotes an increase in NK cell IFN-γ production whereas, in contrast to IL-15, it has no impact on NK cell viability.37 The impact of IL-21 on NK cell metabolism is not clear, and IL-21 also has a complex role in promoting and regulating intestinal inflammation that should be investigated and tackled with care.38,39 It would be necessary to further investigate the impact of different cytokines on metabolism and function of NK cells from IBD patients to support the discovery of novel targets to limit NK cell pro-inflammatory cytokine production in IBD.13,40,41
Efficient NK cell metabolism is necessary to meet the biosynthetic and energetic demands associated with large amounts of IFN-γ production upon activation−.41–43 However, in our hands, patient-derived NK cells have reduced mitochondria mass and limited production of IFN-γ. This finding agrees with previous data indicating that OXPHOS is important for NK cell IFN-γ production.17 Limited production of IFN-γ by circulating NK cells is associated with increased cancer and infection risk, both of which are observed in IBD patients.3,8,44,45
Molecular evidence of metabolic defects in NK cells and other lymphocytes isolated from IBD patients is lacking. Because of the large amounts of NK cells necessary for the metabolic analysis, precluding the stratification of CD56bright and CD56dim NK cells, the present results portray an overall change in NK cell metabolism. Our results demonstrate that NK cells from IBD patients together with their defective function also have abnormal metabolism.46 To complement the metabolic data, analyses of mitochondria mass and mitochondria polarization were performed. We found that NK cells from IBD patients rely on glycolysis while enduring limited mitochondria function. Freshly isolated patient NK cells demonstrated reduced mitochondrial mass. Upon cytokine stimulation, patient NK cells were incapable of increasing mitochondrial mass and polarizing adequately like control NK cells. An important observation in our data regarding the basic biology of NK cells was the difference in mitochondria behavior between CD56bright and CD56dim cells after cytokine stimulation. Mitochondria from CD56bright cells preferentially upregulated mitochondrial mass and polarization in response to cytokines when compared with CD56dim cells. These data are further supported by the knowledge that CD56bright cells are more metabolically active than CD56dim cells.17
Our data resemble, in some ways, the defects observed in NK cells and other lymphocytes during chronic inflammatory disease such as cancer, obesity and rheumatoid arthritis−.21,22,47–49 In these chronic diseases, NK cells are metabolically exhausted, and have aberrant mTORC1 signaling and impaired mitochondrial plasticity. These NK cell defects correlate with susceptibility to infection and reduced anti-tumor activity.50
mTORC1 activity is regulated by growth factors, cellular energy, and stress.51 Cytokine stimulation upregulates human NK cell mTORC1 activity, which is important for effector functions.17 Our data indicate that both CD56bright and CD56dim cells from IBD patients have elevated basal levels of mTORC1 activity, as determined by measuring phosphorylation of S6 protein. To further investigate defects on mTORC1 signaling, patient and control NK cells were stimulated overnight with cytokines. Our data revealed that pS6 levels were reduced in NK cells from IBD patients. Patient CD56bright cells were unable to upregulate pS6 at the same magnitude as control CD56bright cells. Furthermore, after overnight incubation with IL-12/IL-15, patient CD56dim cells still expressed higher levels of mTORC1 activity compared to controls. This aberrant mTORC1 activity is also observed in NK cells isolated from cancer patients and individuals with obesity. The precise reason for this is unknown, but one possible explanation is that accumulation of lipids in NK cells inhibits mTORC1 signaling.21,22 mTORC1 is known to regulate mitochondria function through control of translation and transcription of different metabolic genes such as PGC-1α, an important regulator of mitochondrial biogenesis and OXPHOS.51 PGC-1α is important for optimal NK cell effector functions and its expression may be altered in NK cells of IBD patients.52
Our cohort included patients with active CD or UC who were receiving different medications, enabling an overall view of NK cell phenotype in IBD. Further investigation on the functional role of NK cells in IBD is necessary. NK cells from IBD patients with microbial dysbiosis and increased viral loads should also beinvestigated based on the importance of these cells in the elimination of pathogens.7,53 A larger cohort of CD and UC patients is essential to identify potential differences between NK cells of these two conditions that differ not only genetically but also in their inflammatory responses.3 Additionally, differences in the phenotype of NK cells isolated from treatment-naïve patients vs patients receiving a specific treatment will also give valuable information about the role of NK cells in IBD.12,54
Although we observed no difference in NKG2D expression by NK cells isolated from the peripheral blood of IBD patients [Supplementary Figure 2B], an IBD treatment using anti-human NKG2D antibody is promising. Allez et al. observed significant patient improvement after week 12 of anti-NKG2D antibody treatment without significant adverse events.55 NKG2D is expressed by both T cells and innate lymphoid cells. NKG2D+ lymphocytes located in the inflamed gut correlate with inflammation, but most of these NKG2D-expressing cells seem to be CD8+ T cells.56 Investigations of NK cells in tissue have shown NKG2D expression to be highly variable, although some interesting correlations were found. For instance, increased expression of NKG2D by NK cells correlated to C-reactive protein.56 A deeper understanding of NK cell biology in IBD may help progress the NKG2D antibody and other NK cell-targeted therapies into the clinic.
In conclusion, we have demonstrated phenotypic and metabolic defects of NK cells isolated from the peripheral blood of IBD patients with active disease. Identifying novel ways to stratify ILCs from conventional NK cells in IBD research will support understanding of IBD pathogenesis and potential development of personalized therapies.14 Further understanding of the role of NK cells in IBD may yield new interventions to limit inflammation and problematic consequences in patients receiving long-term immunomodulatory treatments.57
Supplementary Material
Acknowledgments
We thank the subjects for donating the samples used in our study. We also thank Dr David Finlay and Dr Clair Gardiner for use of their facilities and the Extracellular Flux Analyzer at Trinity Biomedical Sciences Institute, Dublin, Ireland. Images from the Servier Medical ART database were used when making the graphical abstract.
Funding
This work was supported by AbbVie Newman Fellowship in Inflammatory Bowel Disease 2019 awarded to V.Z.B.
Conflict of Interest
The authors have no financial conflicts of interest.
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Material.
Author Contributions
V.Z.B.: experimental design, sample preparation, data collection, data acquisition, analysis and interpretation of the data, writing the first draft of the paper; F.J.: patient and control recruitment, article revision; M.T.: sample preparation, data collection; G.A.D.: patient and control recruitment, article revision, interpretation of the data; E.J.R.: article revision, interpretation of the data, study supervision.
References
- 1. GBDIBD Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017;152:313–321.e2. [DOI] [PubMed] [Google Scholar]
- 3. Loddo I, Romano C. Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol 2015;6:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zitti B, Bryceson YT. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev 2018;42:37–46. [DOI] [PubMed] [Google Scholar]
- 5. Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2016;16:7–19. [DOI] [PubMed] [Google Scholar]
- 6. Esin S, Batoni G, Counoupas C, et al. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun 2008;76:1719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol 2017;44:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt S, Tramsen L, Lehrnbecher T. Natural killer cells in antifungal immunity. Front Immunol 2017;8:1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poggi A, Benelli R, Venè R, et al. Human gut-associated natural killer cells in health and disease. Front Immunol 2019;10:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lees JR. Interferon gamma in autoimmunity: a complicated player on a complex stage. Cytokine 2015;74:18–26. [DOI] [PubMed] [Google Scholar]
- 11. Giacomelli R, Passacantando A, Frieri G, et al. Circulating soluble factor-inhibiting natural killer (NK) activity of fresh peripheral blood mononuclear cells (PBMC) from inflammatory bowel disease (IBD) patients. Clin Exp Immunol 1999;115:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samarani S, Sagala P, Jantchou P, et al. Phenotypic and functional changes in peripheral blood natural killer cells in crohn disease patients. Mediators Inflamm 2020;2020:6401969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yadav PK, Chen C, Liu Z. Potential role of NK cells in the pathogenesis of inflammatory bowel disease. J Biomed Biotechnol 2011;2011:348530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldberg R, Prescott N, Lord GM, MacDonald TT, Powell N. The unusual suspects–innate lymphoid cells as novel therapeutic targets in IBD. Nat Rev Gastroenterol Hepatol 2015;12:271–83. [DOI] [PubMed] [Google Scholar]
- 15. O’Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol 2019;19:282–90. [DOI] [PubMed] [Google Scholar]
- 16. Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol 2018;9:1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keating SE, Zaiatz-Bittencourt V, Loftus RM, et al. Metabolic reprogramming supports IFN-γ production by CD56bright NK cells. J Immunol 2016;196:2552–60. [DOI] [PubMed] [Google Scholar]
- 18. Pålsson-McDermott EM, O’Neill LAJ. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res 2020;30:300–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol 2001;8:1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ginsburg CH, Dambrauskas JT, Ault KA, Falchuk ZM. Impaired natural killer cell activity in patients with inflammatory bowel disease: evidence for a qualitative defect. Gastroenterology 1983;85:846–51. [PubMed] [Google Scholar]
- 21. Tobin LM, Mavinkurve M, Carolan E, et al. Nk cells in childhood obesity are activated, metabolically stressed, and functionally deficient. JCI Insight 2017;2:e94939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michelet X, Dyck L, Hogan A, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 2018;19:1330–40. [DOI] [PubMed] [Google Scholar]
- 23. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- 24. Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol 2015;6:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lo B. The requirement of iron transport for lymphocyte function. Nat Genet 2016;48:10–1. [DOI] [PubMed] [Google Scholar]
- 26. Salzberger W, Martrus G, Bachmann K, et al. Tissue-resident NK cells differ in their expression profile of the nutrient transporters Glut1, CD98 and CD71. PLoS One 2018;13:e0201170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaiatz-Bittencourt V, Finlay DK, Gardiner CM. Canonical TGF-β signaling pathway represses human NK cell metabolism. J Immunol 2018;200:3934–41. [DOI] [PubMed] [Google Scholar]
- 28. Takayama T, Kamada N, Chinen H, et al. Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology 2010;139:882–92, 892.e1–3. [DOI] [PubMed] [Google Scholar]
- 29. Patel CH, Leone RD, Horton MR, Powell JD. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat Rev Drug Discov 2019;18:669–88. [DOI] [PubMed] [Google Scholar]
- 30. Hirata I, Hayashi K, Asada S, Miyoshi H, Iwakoshi K, Ohshiba S. Alteration of natural killer cell subsets (two color analysis) and their activity in peripheral blood in inflammatory bowel disease. Bull Osaka Med Coll 1990;36:47–55. [PubMed] [Google Scholar]
- 31. Carpen O, Virtanen I, Saksela E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J Immunol 1982;128:2691–7. [PubMed] [Google Scholar]
- 32. Topham NJ, Hewitt EW. Natural killer cell cytotoxicity: how do they pull the trigger? Immunology 2009;128:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abarca-Rojano E, Muñiz-Hernández S, Moreno-Altamirano MM, Mondragón-Flores R, Enriquez-Rincón F, Sánchez-García FJ. Re-organization of mitochondria at the NK cell immune synapse. Immunol Lett 2009;122:18–25. [DOI] [PubMed] [Google Scholar]
- 34. Liu D, Martina JA, Wu XS, Hammer JA 3rd, Long EO. Two modes of lytic granule fusion during degranulation by natural killer cells. Immunol Cell Biol 2011;89:728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maccari I, Zhao R, Peglow M, et al. Cytoskeleton rotation relocates mitochondria to the immunological synapse and increases calcium signals. Cell Calcium 2016;60:309–21. [DOI] [PubMed] [Google Scholar]
- 36. Liu Z, Yang L, Cui Y, et al. Il-21 enhances NK cell activation and cytolytic activity and induces Th17 cell differentiation in inflammatory bowel disease. Inflamm Bowel Dis 2009;15:1133–44. [DOI] [PubMed] [Google Scholar]
- 37. Kasaian MT, Whitters MJ, Carter LL, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 2002;16:559–69. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Jiang X, Zhu J, et al. IL-21/IL-21R signaling suppresses intestinal inflammation induced by DSS through regulation of Th responses in lamina propria in mice. Sci Rep 2016;6:31881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov 2014;13:379–95. [DOI] [PubMed] [Google Scholar]
- 40. Pallone F, Fina D, Caruso R, Monteleone G. Role of IL-21 in inflammatory bowel disease. Expert Rev Clin Immunol 2010;6:537–41. [DOI] [PubMed] [Google Scholar]
- 41. Mah AY, Cooper MA. Metabolic regulation of natural killer cell IFN-γ production. Crit Rev Immunol 2016;36:131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015;42:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science 2013;342:1242454.24115444 [Google Scholar]
- 44. Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology 2009;128:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barrow AD, Colonna M. Exploiting NK cell surveillance pathways for cancer therapy. Cancers [Basel] 2019;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poznanski SM, Ashkar AA. What defines NK cell functional fate: phenotype or metabolism? Front Immunol 2019;10:1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tschopp J. Mitochondria: sovereign of inflammation? Eur J Immunol 2011;41:1196–202. [DOI] [PubMed] [Google Scholar]
- 48. Fearon U, Hanlon MM, Wade SM, Fletcher JM. Altered metabolic pathways regulate synovial inflammation in rheumatoid arthritis. Clin Exp Immunol 2019;197:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riley JS, Tait SW. Mitochondrial DNA in inflammation and immunity. EMBO Rep 2020;21:e49799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gardiner CM, Finlay DK. What fuels natural killers? Metabolism and NK cell responses. Front Immunol 2017;8:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de la Cruz Lopez KG, Toledo Guzman ME, Sanchez EO, Garcia Carranca A. Mtorc1 as a regulator of mitochondrial functions and a therapeutic target in cancer. Front Oncol 2019;9:1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miranda D, Jara C, Ibañez J, et al. PGC-1α-dependent mitochondrial adaptation is necessary to sustain IL-2-induced activities in human NK cells. Mediators Inflamm 2016;2016:9605253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hall LJ, Murphy CT, Hurley G, et al. Natural killer cells protect against mucosal and systemic infection with the enteric pathogen Citrobacter rodentium. Infect Immun 2013;81:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yusung S, McGovern D, Lin L, Hommes D, Lagishetty V, Braun J. NK cells are biologic and biochemical targets of 6-mercaptopurine in Crohn’s disease patients. Clin Immunol 2017;175:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Allez M, Skolnick BE, Wisniewska-Jarosinska M, Petryka R, Overgaard RV. Anti-NKG2D monoclonal antibody (NNC0142-0002) in active Crohn’s disease: a randomised controlled trial. Gut 2017;66:1918–25. [DOI] [PubMed] [Google Scholar]
- 56. Vadstrup K, Galsgaard ED, Jensen H, et al. NKG2D ligand expression in Crohn’s disease and NKG2D-dependent stimulation of CD8+ T cell migration. Exp Mol Pathol 2017;103:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Irving PM, Gibson PR. Infections and IBD. Nat Clin Pract Gastroenterol Hepatol 2008;5:18–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary Material.