Abstract
Background
Conventional risk score for predicting short and long-term mortality following an ST-segment elevation myocardial infarction (STEMI) is often not population specific.
Objective
Apply machine learning for the prediction and identification of factors associated with short and long-term mortality in Asian STEMI patients and compare with a conventional risk score.
Methods
The National Cardiovascular Disease Database for Malaysia registry, of a multi-ethnic, heterogeneous Asian population was used for in-hospital (6299 patients), 30-days (3130 patients), and 1-year (2939 patients) model development. 50 variables were considered. Mortality prediction was analysed using feature selection methods with machine learning algorithms and compared to Thrombolysis in Myocardial Infarction (TIMI) score. Invasive management of varying degrees was selected as important variables that improved mortality prediction.
Results
Model performance using a complete and reduced variable produced an area under the receiver operating characteristic curve (AUC) from 0.73 to 0.90. The best machine learning model for in-hospital, 30 days, and 1-year outperformed TIMI risk score (AUC = 0.88, 95% CI: 0.846–0.910; vs AUC = 0.81, 95% CI:0.772–0.845, AUC = 0.90, 95% CI: 0.870–0.935; vs AUC = 0.80, 95% CI: 0.746–0.838, AUC = 0.84, 95% CI: 0.798–0.872; vs AUC = 0.76, 95% CI: 0.715–0.802, p < 0.0001 for all). TIMI score underestimates patients’ risk of mortality. 90% of non-survival patients are classified as high risk (>50%) by machine learning algorithm compared to 10–30% non-survival patients by TIMI. Common predictors identified for short- and long-term mortality were age, heart rate, Killip class, fasting blood glucose, prior primary PCI or pharmaco-invasive therapy and diuretics. The final algorithm was converted into an online tool with a database for continuous data archiving for algorithm validation.
Conclusions
In a multi-ethnic population, patients with STEMI were better classified using the machine learning method compared to TIMI scoring. Machine learning allows for the identification of distinct factors in individual Asian populations for better mortality prediction. Ongoing continuous testing and validation will allow for better risk stratification and potentially alter management and outcomes in the future.
Introduction
Half of the global burden related to ischemic heart disease occurs within the Asia-Pacific region [1]. Prediction of mortality risks associated with the acute coronary syndrome (ACS) is often evaluated using risk scores such as the Thrombolysis in Myocardial Infarction (TIMI) or Global Registry of Acute Cardiac Events (GRACE) scores. These scores are extrapolated from studies with predominantly Caucasian patients with limited participation from Asia [2]. Asian countries tend to have younger patients with myocardial infarction, a higher burden of diabetes melitus, hypertension and renal failure as well as higher rates of delayed presentation for medical care [3, 4]. South-East Asia in particular is unique because of its heterogeneity due to inherent genetic variations in an already diverse group of multi-ethnic communities. Conventional risk scores may not be able to account for nuances related to the individual region in terms of disease burden, healthcare resources and available interventions.
The TIMI risk score is widely used due to its simplicity in calculation and accuracy in STEMI patients. TIMI scoring, unlike the GRACE score, was derived from patients with ST-segment elevation myocardial infarction (STEMI) only [5]. Studies using TIMI scores amongst Asians revealed a higher incidence of STEMI when compared to their Caucasian counterpart with somewhat similar mortality risk. This discrepancy is difficult to explain especially in the context of a higher disease burden amongst Asian patients.
Conventional cardiovascular disease (CVD) risk assessment models assume that risk factors have a linear relationship to clinical outcomes, leading to the oversimplification of a truly complex correlation. There is a need to develop models which consider these multiple risk factors and outcomes, including the use of machine learning (ML) algorithms [2, 6–8].
Current evidence supporting the use of ML over statistically-based models in mortality predictions include Logistic Regression (LR), Support Vector Machine (SVM) and Random Forest (RF). ML has been shown to outperform the conventional risk scoring model in population-specific mortality studies, post-STEMI, in countries like China, Israel and Korea [2, 7, 9].
To our knowledge, the development, and application of ML algorithms to predict short- and long-term mortality post-STEMI in a heterogeneous Asian population has yet to be reported. The study aims to identify factors and develop an ML model risk calculator that predicts short and long-term mortality in a heterogeneous South-East Asian population.
Methods
Study data
We used retrospective data from the Malaysian National Cardiovascular Database (NCVD-ACS) registry collected between 2006 until 2016. The NCVD registry was approved by the Medical Review & Ethics Committee (MREC), Ministry of Health (MOH) Malaysia in 2007 (Approval Code: NMRR-07-20-250). MREC waived informed patient consent for NCVD. The registry collects data on a standardised set of clinical, demographic, and procedural variables, along with outcomes, for consecutive patients treated at participating institutions [10, 11]. The study was also approved by the UITM ethic committee (Reference number: 600-TNCPI (5/1/6)) and the National Heart Association of Malaysia (NHAM) for data acquisition.
All patients from the ACS registry without exclusion were used including patients who received reperfusion (fibrinolysis, primary PCI (PPCI), angiography demonstrating spontaneous reperfusion, or urgent coronary artery bypass grafting (CABG)) for STEMI. In this context, STEMI was defined as persistent ST-segment elevation ≥ 1 mm in two contiguous electrocardiographic leads, or the presence of a new left bundle branch block in the setting of positive cardiac markers. 50 variables from a complete set of data were used in this study based on clinical recommendation. Categories of variables used were sociodemographic characteristics, CVD diagnosis and severity, CVD risk factors, CVD comorbidities, non-CVD comorbidities, biomarkers and medication used. The mortality time frame was calculated from first hospital admission for in-hospital, 30 days and 1-year. Confirmation of deaths was done yearly via record linkages with the Malaysian National Registration Department. The data collected by the registry does not include data on short term complication such as heart failure. The follow-up data points are meant to collect these variables but unfortunately are excessive in terms of missing values and hence we omitted this from the study. We focused our algorithm to policy changing endpoints for example hard endpoints such as death to increase the impact of the study. This is similarly done in other publications [2, 7, 9].
Classification and sample pre-processing
We developed the ML using a complete set of data to ensure the validity of the findings. A total of 27,592 STEMI cases from the registry were collected 12,368 were identified as complete cases (with no missing values on predictors). Out of the 12 368 datasets, a total of 6299, 3130 and 2939 complete cases were used for in-hospital, 30-days and 1-year respectively for the model development. This rendered almost 50% complete cases of patients with a full predictor set of 50 variables for each time frame (9 continuous, 41 categorical) for the study (Table 1). Stratified random sampling of data was used [12]. Data were split for model development (70%) and validation (30%). We accessed the performance of ML and TIMI using a validation set that accounts for 30% of data for each time frame that is not used for model development.
Table 1. Patients characteristics for the in-hospital, 30-days and 1-year dataset.
| Variables | Description | In-hospital | 30 days | 1-year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Survival | Non-survival | p-value | Total | Survival | Non-survival | p-value | Total | Survival | Non-survival | p-value | ||
| N | 6299 | 5961 (94.6) | 338 (5.4) | 3130 | 2878 (91.9) | 252 (8.1) | 2939 | 2516 (85.6) | 423 (14.4) | ||||
| Age | 55.8 ± 11.5 | 55.4 ± 11.3 | 63.8 ± 12.0 | 0.81 | 56.6 ± 11.7 | 56.0 ±11.4 | 64.2 ±12.5 | 0.054 | 56.6 ± 11.6 | 55.5 ± 11.2 | 62.8 ± 12.0 | 0.028 | |
| Race | Malay | 3574 (56.7) | 3365 (56.5) | 209 (61.8) | 0.050 | 1763 (56.3) | 1608 (55.9) | 155 (61.5) | 0.003 | 1625 (55.3) | 1370 (54.5) | 255 (60.3) | 0.004 |
| Chinese | 1194 (19.0) | 1126 (18.9) | 68 (20.1) | 552 (13.6) | 498 (17.3) | 54 (21.4) | 531 (18.1) | 453 (18.0) | 78 (18.4) | ||||
| Indian | 1217 (19.3) | 1170 (19.6) | 47 (13.9) | 640 (20.5) | 602 (20.9) | 38 (15.1) | 610 (20.8) | 530 (21.1) | 80 (18.9) | ||||
| Others | 314 (5.0) | 300 (5.0) | 14 (4.1) | 175 (5.6) | 170 (5.9) | 5 (2.0) | 173 (5.9) | 163 (6.5) | 10 (2.4) | ||||
| Gender | Male | 5417 (86.0) | 5152 (86.4) | 265 (78.4) | <0.0001 | 2681 (85.7) | 2486 (86.4) | 195 (77.4) | <0.0001 | 2533 (86.2) | 2214 (88.0) | 319 (75.4) | <0.0001 |
| Female | 882 (14.0) | 809 (13.6) | 73 (21.6) | 448 (14.4) | 392 (13.6) | 57 (22.6) | 406 (13.8) | 302 (12.0) | 104 (24.6) | ||||
| Smoking status | Never | 2003 (31.8) | 1866 (31.3) | 137 (40.5) | <0.0001 | 1053 (33.6) | 941 (32.7) | 112 (44.4) | <0.0001 | 977 (33.2) | 786 (31.2) | 191 (45.2) | <0.0001 |
| Former (quit tobacco > 30days) | 1019 (16.2) | 952 (16.0) | 67 (19.8) | 472 (15.1) | 424 (14.7) | 48 (19.0) | 440 (15.0) | 371 (14.7) | 69 (16.3) | ||||
| Current (tobacco < 30days) | 3277 (52.0) | 3143 (52.7) | 134 (39.6) | 1605 (51.3) | 1513 (52.6) | 92 (36.5) | 1522 (51.8) | 1359 (54.0) | 163 (38.5) | ||||
| History of hypertension | 3344 (53.1) | 3112 (52.2) | 232 (68.6) | <0.0001 | 1697 (54.2) | 1538 (53.4) | 159 (63.1) | 0.003 | 1587 (54.0) | 1316 (52.3) | 271 (64.1) | <0.0001 | |
| History of diabetes | 2482 (39.4) | 2291 (38.4) | 191 (56.5) | <0.0001 | 1271 (40.6) | 1129 (39.2) | 142 (56.3) | <0.0001 | 1187 (40.4) | 945 (37.6) | 242 (57.2) | <0.0001 | |
| Family history of premature cardiovascular disease | 892 (14.2) | 869 (14.6) | 23 (6.8) | <0.0001 | 435 (13.9) | 419 (14.6) | 16 (6.3) | <0.0001 | 410 (14.0) | 372 (14.8) | 38 (9.0) | 0.0001 | |
| History of myocardial infarction | 625 (9.9) | 580 (9.7) | 45 (13.3) | 0.032 | 299 (9.6) | 271 (9.4) | 28 (11.1) | 0.380 | 278 (9.5) | 231 (9.2) | 47 (11.1) | 0.210 | |
| Documented CAD | 583 (9.3) | 552 (9.3) | 31 (9.2) | 0.956 | 358 (11.4) | 323 (11.2) | 35 (13.9) | 0.202 | 341 (11.6) | 273 (10.9) | 68 (16.1) | 0.002 | |
| History of heart failure | 124 (2.0) | 109 (1.8) | 15 (4.4) | 0.001 | 56 (1.8) | 49 (1.7) | 7 (2.8) | 0.217 | 49 (1.7) | 32 (1.3) | 17 (4.0) | <0.0001 | |
| Chronic lung disease | 114 (1.8) | 101 (1.7) | 13 (3.8) | 0.004 | 61 (1.9) | 54 (1.9) | 7 (2.8) | 0.321 | 60 (2.0) | 44 (1.7) | 16 (3.8) | 0.006 | |
| Chronic renal disease | 191 (3.0) | 158 (2.7) | 33 (9.8) | <0.0001 | 104 (3.3) | 77 (2.7) | 27 (10.7) | <0.0001 | 98 (3.3) | 52 (2.1) | 46 (10.9) | <0.0001 | |
| Cerebrovascular disease | 171 (2.7) | 156 (2.6) | 15 (3.3) | 0.045 | 88 (2.8) | 80 (2.8) | 8 (3.2) | 0.716 | 84 (2.9) | 63 (2.5) | 21 (5.0) | 0.005 | |
| Heart rate | 82.4 ± 21.1 | 81.7 ± 20.6 | 93.9 ± 26.6 | <0.0001 | 82.9 ± 20.9 | 81.9 ± 20.0 | 94.5 ±27.0 | <0.0001 | 82.6 ±20.6 | 81.1 ± 19.6 | 91.7 ± 24.2 | <0.0001 | |
| Systolic blood pressure | 132.8 ± 27.8 | 135.6 ± 27.4 | 120.4 ± 30.2 | 0.011 | 134.9 ± 28.2 | 135.6 ±28.0 | 126.4 ± 29.4 | <0.0001 | 153.1 ± 28.0 | 135.9 ± 27.3 | 130.1 ± 3.1 | 0.010 | |
| Diastolic blood pressure | 82.8 ± 94.1 | 83.4 ± 96.5 | 73.6 ± 20.2 | 0.965 | 81.3 ± 18.5 | 81.8 ±18.3 | 76.2 ±19.8 | <0.0001 | 81.5 ± 18.4 | 82.1 ±18.1 | 78.4 ±20.0 | 0.066 | |
| Killip class | I | 4300 (68.3) | 4210 (70.6) | 90 (26.6) | <0.0001 | 2072 (66.2) | 1998 (69.4) | 74 (29.4) | <0.0001 | 1980 (67.4) | 1809 (71.9) | 141 (40.4) | <0.0001 |
| II | 1190 (18.9) | 1132 (19.0) | 58 (17.2) | 558 (17.8) | 506 (17.6) | 52 (20.6) | 512 (17.4) | 413 (16.4) | 99 (23.4) | ||||
| III | 237 (3.8) | 200 (3.4) | 37 (10.9) | 128 (4.1) | 98 (3.4) | 30 (11.9) | 110 (3.7) | 71 (2.8) | 39 (9.2) | ||||
| IV | 572 (9.1) | 419 (7.0) | 153 (45.3) | 372 (11.9) | 276 (9.6) | 96 (38.1) | 337 (11.5) | 223 (8.9) | 114 (27.0) | ||||
| Total cholesterol | 5.4 ± 1.6 | 5.4 ± 1.6 | 4.8 ± 1.7 | 0.10 | 5.2 ± 1.4 | 5.3 ± 1.3 | 4.9 ± 1.6 | <0.0001 | 5.2 ± 1.4 | 5.3 ± 1.3 | 4.9 ± 1.6 | 0.005 | |
| HDL | 1.1±1.2 | 1.1 ± 1.2 | 1.0 ±0.3 | 0.952 | ± 0.4 | ±0.4 | 1.1 ± 0.3 | 0.140 | 1.1 ± 0.3 | 1.1 ±0.3 | 1.1 ±0.4 | 0.130 | |
| LDL | 3.8 ± 10.7 | 3.8 ± 11.0 | 3.0 ± 1.4 | 0.706 | 3.5 ± 5.4 | 3.5 ± 5.6 | 3.1 ± 1.4 | 0.295 | 3.1 ± 1.2 | 3.4 ± 1.2 | 3.1 ±1.4 | 0.026 | |
| Triglycerides | 1.8 ± 1.7 | 1.8 ± 1.7 | 1.7 ± 1.0 | 0.529 | 1.7 ± 0.9 | 1.7 ± 0.9 | 1.7 ±0.9 | 0.762 | 1.7 ± 0.9 | 1.7 ±0.9 | 1.6 ±0.8 | 0.206 | |
| Fasting blood glucose | 8.7 ± 4.4 | 8.5 ± 4.1 | 12.3 ± 6.7 | <0.0001 | 8.8 ± 4.4 | 8.6 ± 4.1 | 11.7 ±6.5 | <0.0001 | 8.8 ± 4.2 | 8.4 ± 3.8 | 10.8 ±5.8 | <0.0001 | |
| ECG abnormalities type | ST segment elevation ≥1mm in ≥2 contiguous limb leads | 2804 (44.5) | 2565 (44.6) | 148 (43.8) | 0.782 | 1518 (48.5) | 1403 (48.7) | 115 (45.6) | 0.343 | 1437 (48.9) | 1241 (49.3) | 196 (46.3) | 0.255 |
| ST segment elevation ≥2mm in ≥2 contiguous frontal leads or chest leads | 3373 (59.9) | 3563 (59.8) | 210 (62.1) | 0.389 | 1828 (58.4) | 1664 (57.8) | 164 (65.1) | 0.025 | 1710 (58.2) | 1447 (57.5) | 263 (62.2) | 0.072 | |
| ST segment depression ≥0.5mm in ≥2 contiguous leads | 627 (10.0) | 589 (9.9) | 38 (11.2) | 0.416 | 280 (8.9) | 254 (8.8) | 26 (10.3) | 0.426 | 267 (9.1) | 219 (8.7) | 48 (11.3) | 0.080 | |
| T-wave inversion ≥1mm | 394 (6.3) | 378 (6.3) | 16 (4.7) | 0.235 | 197 (6.3) | 184 (6.4) | 13 (5.2) | 0.439 | 189 (6.4) | 152 (6.0) | 37 (8.7) | 0.036 | |
| Bundle branch block | 138 (2.2) | 111 (1.9) | 27 (8.0) | <0.0001 | 72 (2.4) | 56 (1.9) | 18 (7.1) | <0.0001 | 59 (2.0) | 38 (1.5) | 21 (5.0) | <0.0001 | |
| ECG abnormalities location | Inferior leads: II, III, aVF | 2998 (47.6) | 2859 (48.0) | 139 (41.1) | 0.014 | 1520 (48.6) | 1415 (49.2) | 105 (41.7) | 0.022 | 1433 (48.8) | 1251 (49.7) | 182 (43.0) | 0.011 |
| Anterior leads: V1 to V4 | 3435 (54.5) | 3233 (54.2) | 202 (59.8) | 0.047 | 1655 (52.9) | 1498 (52.1) | 157 (62.3) | 0.022 | 1545 (52.6) | 1287 (51.2) | 258 (61.0) | <0.0001 | |
| Lateral leads: I, aVL, V5 to V6 | 1396 (22.2) | 1295 (21.7) | 101 (29.9) | <0.0001 | 744 (23.8) | 659 (22.9) | 85 (33.7) | <0.0001 | 705 (24.0) | 567 (22.5) | 138 (32.6) | <0.0001 | |
| True posterior: V1, V2 | 515 (8.2) | 484 (8.1) | 321 (9.2) | 0.492 | 258 (8.2) | 235 (8.2) | 23 (9.1) | 0.595 | 243 (8.3) | 213 (8.5) | 30 (7.1) | 0.343 | |
| Right ventricle: ST elevation in lead V4R | 524 (8.3) | 494 (8.3) | 30 (8.9) | 0.703 | 286 (9.1) | 262 (9.1) | 24 (9.5) | 0.824 | 269 (9.2) | 231 (9.2) | 38 (9.0) | 0.896 | |
| FB status | 4530 (71.9) | 4288 (71.9) | 242 (71.6) | 0.893 | 2144 (68.5) | 1973 (68.6) | 171 (67.9) | 0.819 | 1989 (67.7) | 1717 (68.2) | 272 (64.3) | 0.109 | |
| Cardiac catheterization | 2950 (46.8) | 2812 (47.2) | 138 (40.8) | 0.045 | 1727 (55.2) | 1619 (56.3) | 108 (42.9) | <0.0001 | 1629 (55.4) | 1455 (57.8) | 174 (41.1) | <0.0001 | |
| PCI | 2414 (38.3) | 2298 (38.6) | 116 (34.3) | 0.120 | 1396 (44.6) | 1304 (45.3) | 92 (36.5) | 0.007 | 1323 (45.0) | 1188 (47.2) | 135 (31.9) | <0.0001 | |
| CABG | 33 (0.5) | 30 (0.5) | 3 (0.9) | 0.341 | 33 (1.1) | 27 (0.9) | 6 (2.4) | 0.032 | 22 (0.7) | 18 (0.7) | 4 (0.9) | 0.611 | |
| ASA | 6180 (98.1) | 5862 (98.3) | 318 (94.1) | <0.0001 | 3070 (98.1) | 2829 (98.3) | 241 (95.6) | 0.003 | 2883 (98.1) | 2473 (98.3) | 410 (96.9) | 0.058 | |
| GP receptor inhibitor | 173 (2.7) | 162 (2.7) | 11 (3.3) | 0.557 | 62 (2.0) | 56 (1.9) | 6 (2.4) | 0.635 | 58 (2.0) | 51 (2.0) | 7 (1.7) | 0.611 | |
| Heparin | 962 (15.3) | 900 (15.1) | 62 (18.3) | 0.107 | 549 (17.5) | 501 (17.4) | 48 (19.0) | 0.512 | 523 (17.9) | 459 (18.2) | 64 (15.1) | 0.121 | |
| LMWH | 1546 (24.5) | 1450 (24.3) | 96 (28.4) | 0.09 | 479 (15.3) | 414 (14.4) | 65 (25.8) | <0.0001 | 406 (13.8) | 313 (12.4) | 93 (22.0) | <0.0001 | |
| Beta blockers | 4066 (64.5) | 3978 (66.7) | 88 (26.0) | <0.0001 | 1896 (60.6) | 1800 (62.5) | 96 (38.1) | <0.0001 | 1754 (59.7) | 1558 (61.9) | 196 (46.3) | <0.0001 | |
| ACE inhibitor | 3320 (52.7) | 3251 (54.5) | 69 (20.4) | <0.0001 | 1509 (48.2) | 1452 (50.5) | 57 (22.6) | <0.0001 | 1388 (47.2) | 1268 (50.4) | 120 (28.4) | <0.0001 | |
| Angiotensin II receptor blocker | 181 (2.9) | 176 (3.0) | 5 (1.5) | 0.115 | 61 (1.9) | 55 (1.9) | 6 (2.4) | 0.605 | 52 (1.8) | 43 (1.7) | 9 (2.1) | 0.564 | |
| Statin | 6013 (95.5) | 5713 (95.8) | 300 (88.8) | <0.0001 | 3003 (95.9) | 2774 (96.4) | 229 (90.9) | <0.0001 | 2820 (96.0) | 2433 (96.7) | 387 (91.5) | <0.0001 | |
| Other lipid lowering agent | 127 (2.0) | 124 (2.1) | 3 (0.9) | 0.129 | 51 (1.6) | 48 (1.7) | 3 (1.2) | 0.566 | 47 (1.6) | 38 (1.5) | 9 (2.1) | 0.349 | |
| Diuretics | 1349 (21.4) | 1201 (20.1) | 148 (43.8) | <0.0001 | 720 (23.0) | 610 (21.2) | 110 (43.7) | <0.0001 | 651 (22.2) | 473 (18.8) | 178 (42.1) | <0.0001 | |
| Calcium antagonist | 367 (5.8) | 352 (5.9) | 15 (4.4) | 0.263 | 183 (5.8) | 176 (6.1) | 7 (2.8) | 0.030 | 161 (5.5) | 139 (5.5) | 22 (5.2) | 0.787 | |
| Oral hypoglycaemic agent | 1345 (21.4) | 1312 (22.0) | 33 (9.8) | <0.0001 | 597 (19.1) | 567 (19.7) | 30 (11.9) | 0.003 | 546 (18.6) | 478 (19.0) | 68 (16.1) | 0.153 | |
| Insulin | 1658 (26.3) | 1516 (25.4) | 142 (42.0) | <0.0001 | 869 (27.8) | 757 (26.3) | 112 (44.4) | <0.0001 | 804 (27.3) | 624 (24.8) | 180 (42.6) | <0.0001 | |
| Anti-arrhythmic agent | 313 (5.0) | 276 (4.6) | 37 (0.9) | <0.0001 | 178 (5.7) | 144 (5.0) | 34 (13.5) | <0.0001 | 151 (5.1) | 114 (4.5) | 37 (8.7) | <0.0001 | |
Abbreviations: CAD = coronary artery disease, HDL = high-density lipoprotein, LDL = low-density lipoprotein, ECG = electrocardiogram, FB = fibrinolytic therapy, PCI = percutaneous coronary intervention, CABG = coronary artery bypass graft, ASA = acetylsalicylic acid (aspirin), GP = glycoprotein, LMWH = low-molecular-weight heparin, ACE = Angiotensin-converting enzyme.
Data are shown as n (%) for categorical variables and mean ± SD for continuous variables.
p value is statistically highly significant as p < 0.001.
Model development and calibration
Prediction models post-STEMI were developed using three selected ML algorithms. Next, feature selection (see below) was carried out on the ranked variables in an ascending order iteratively [13]. 10-fold cross-validation was used to avoid overfitting for model development on the training set [14]. The prediction models were trained and tested for each iteration, and the models with the highest performance consisting of the least number of variables were selected. Predictive performances of the models were calculated using the validation dataset.
Secondary analyses were carried out after adding 15224 missing cases imputed using multivariable imputation using chained equations and predictive mean matching that yields a total of 27 592 cases [15]. This method imputes missing values based on real values from other cases where predicted values are closest. Our reference for incomplete dataset refers to missing sets of variables up to 50%. The missing dataset mentioned refers to patient characteristics and not outcome data. As our dataset is a prospective dataset, with retrospective data management, the level of missingness in values across all variables was completely random and beyond our control. The probability of missingness in our dataset depends neither on the observed values in any variable of the dataset nor on the unobserved part of the dataset.
Hence the dataset is classified as missing completely at random (MCAR) which is the highest level of randomness and it implies that the pattern of missing value is random and does not depend on any variable which may or may not be included in the analysis. We had complete data for all our outcomes. The models were tested with a similar validation dataset for ML models trained with a complete cases dataset.
Machine learning algorithms and calibration
Supervised classification ML algorithms RF [16], SVM [17] and LR [18] were selected in this study. They are the classifiers that have resulted in high predictive performance compared to conventional methods in mortality studies [7, 19]. RF and SVM are black-box models (models without interpretability) meanwhile LR is a white-box model (model with good interpretability) [16, 18, 20]. The ML algorithms’ parameters were set to the optimized value to obtain higher predictive performance (S1 Table). Tuned hyperparameters improve ML model performance over the default setting provided [21]. The area under the receiver operating curve (AUC) was used as a predictive performance metric [22]. Additional performance metrics were accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for model calibration [23]. Paired resampled t-test was used to compare ML models predictive performances [12, 24].
Feature selection
Feature selection is the process of ranking variables using classifier specific variable evaluator. RF, SVM and LR variable importance method were used to rank variables importance associated with outcome (survival / non-survival at in-hospital, 30 days, and 1-year).
Feature reduction involves the elimination of existing variables to a minimal set, which reduces training time, produces better results, and increases the accuracy of results. Next, sequential backward elimination (SBE) [13] was used in this study for feature reduction on the ranked variables in ascending order of importance iteratively from the ML variables importance methods. The ML prediction model is retrained and tested each time a variable is eliminated. The variable that causes a significant decrease in the AUC of the prediction model upon elimination based on the ranked variable list using the feature selection method is deemed as important. We selected the important variables and ranked them again and the elimination process is repeated until a model with a reduced number of variables and the highest AUC value is achieved.
RFE combined with ML classifiers have been used in various clinical dataset successfully [25–28]. We also used Recursive feature elimination (RFE) to find a minimal and best set of variables by removing the least important features and compare them with feature selection by ML methods (RF, SVM and LR) [29, 30].
Comparison with TIMI score
Calculated TIMI scores were used from the NCVD registry for the validation data performance. TIMI score performance (AUC) was compared with the developed ML-based models using the validation set. We derived a graph to compare performance between ML and TIMI score based on cutoff points applicable in clinical practice and literature [31]. A high risk of death was defined as a probability risk of death of more than 8% similar to reported in Correia et al. [31].
Net reclassification improvement (NRI) was used to determine the changes in discrimination between the TIMI risk score for STEMI and ML algorithm. The NRI uses reclassification tables to examine whether there is an additive benefit gained from reclassifying patients using a different approach in mortality assessment. By calculating the NRI, we were able to quantify the degree to which the different mortality risk assessment approaches driving correct movement between categories. An NRI can be interpreted as the percentage by which the net classification has improved by using a new different approach. The NRI was used to evaluate the improvement in classification obtained by comparing the TIMI risk score for STEMI with ML for STEMI [32].
Additional statistics
The results are expressed as mean and SD for continuous variable and as frequencies for categorical variables. Correlation analysis was carried out to identify a significant relationship between variables. Univariate analysis was performed using a Chi-Square test to identify significant variables and a two-sided independent student t-test (p < 0.05). The ML performance was compared using a pair-wise corrected resampled t-test [33, 34]. Statistical significance was considered if the p-value was less than 0.05.
Software
R package (Version 3.5.2) was used in ML algorithm development. Statistical analysis was conducted using Statistical Package for Social Sciences (SPSS) program version 16.0 [35].
Results
Patient characteristics
A total of 27,592 STEMI patients were identified. Incomplete data made up 55.2% of patients enrolled. Table 1 illustrates patients’ characteristic used in this study on the complete dataset. The mean age was 56.6 (SD = 11.7). The majority of patients (~87%) were males. The overall mortality reported for in-hospital, 30 days and 1-year was 5.4%, 8.1% and 14.4%. There was a significant difference between survivors to non-survivors for in-hospital, 30-days and 1-year mortality in terms of gender, smoking status, diabetes, renal disease, heart rate, Killip class, fasting blood glucose, ECG abnormalities, beta-blocker, ACE inhibitor, statin, diuretics, insulin and anti-arrhythmic agent use (p < 0.0001 for all). S2 Table illustrates patient’s characteristics for secondary analysis using an imputed dataset. Both statistical analyses on the complete and imputed dataset are almost similar.
ML prediction
Maximal predictive performances on the validation dataset were observed for ML models constructed using reduced and complete sets of variables compared to TIMI risk score using untouched 30% validation dataset (Table 2). TIMI only outperformed the RFE-LR model for 30 days and 1-year mortality. The best-selected ML model (SVMvarImp–SBE–SVM) performed better against TIMI based on the AUC value using the untouched 30% validation dataset (p < 0.0001 for all models). Detailed performance evaluation of the best ML model against TIMI risk score is presented in Table 3.
Table 2. The AUC of TIMI risk score and ML models with and without feature selection based on a 30% validation dataset.
| Classifiers | The area under the ROC Curve (95% CI) | ||
|---|---|---|---|
| In-hospital | 30 days | 1-year | |
| RF | 0.86 (0.820–0.88) | 0.83 (0.786–0.879) | 0.78 (0.741–0.827) |
| RFvarImp-SBE-RF | 0.87 (0.832–0.907) | 0.85 (0.10–0.890) | 0.80 (0.750–0.834) |
| RFE-RF | 0.86 (0.821–0.893) | 0.82 (0.772–0.872) | 0.79 (0.748–0.833) |
| SVM | 0.86 (0.824–0.895) | 0.87 (0.831–0.912) | 0.84 (0.801–0.877) |
| SVMvarImp-SBE-SVM | 0.88 (0.846–0.910) | 0.90 (0.870–0.935) | 0.84 (0.798–0.872) |
| RFE-SVM | 0.85 (0.811–0.887) | 0.88 (0.837–0.920) | 0.84 (0.806–0.880) |
| LR | 0.88 (0.846–0.911) | 0.85 (0.803–0.897) | 0.76 (0.710–0.807) |
| LRstepwise—SBE-LR | 0.89 (0.861–0.920) | 0.85 (0.812–0.906) | 0.80 (0.767–0.848) |
| RFE- LR | 0.87 (0.842–0.897) | 0.83 (0.783–0.882) | 0.78 (0.737–0.826) |
| TIMI | 0.81 (0.772–0.802) | 0.80 (0.746–0.838) | 0.76 (0.715–0.802) |
Abbreviations:
RF = Random Forest.
RFvarImp-SBE-RF = RF variable importance with sequential backward elimination and RF classifier.
RFE-RF = Recursive feature elimination with RFclassifier.
SVM = Support Vector Machine.
SVMvarImp-SBE-SVM = SVM variable importance with sequential backward elimination and SVM classifier.
RFE-SVM = Recursive feature elimination with SVM classifier.
LR = Logistic Regression.
LRstepwise—SBE-LR = LR stepwise feature elimination and LR classifier.
RFE- LR = Recursive feature elimination with LR classifier.
TIMI = Thrombolysis in Myocardial Infarction.
Table 3. Additional performance metrics based on a 30% validation dataset for TIMI risk score and ML models with and without feature selection.
| PPV | NPV | Sensitivity | Specificity | Accuracy (Cl 95%) | Mcnemar’s test (p-value) | |
|---|---|---|---|---|---|---|
| In-hospital | ||||||
| Classifier | ||||||
| RF | 0.380 | 0.963 | 0.347 | 0.968 | 0.935 (0.923,0.946) | <0.0001 |
| RFvarImp-SBE-RF | 0.447 | 0.963 | 0.337 | 0.977 | 0.942 (0.931,0.952) | <0.0001 |
| RFE-RF | 0.350 | 0.963 | 0.347 | 0.964 | 0.931 (0.918,0.942) | <0.0001 |
| SVM | 0.242 | 0.976 | 0.614 | 0.892 | 0.877 (0.861, 0.891) | <0.0001 |
| SVMvarImp-SBE-SVM | 0.219 | 0.980 | 0.693 | 0.861 | 0.852 (0.835,0.868) | <0.0001 |
| RFE-SVM | 0.202 | 0.982 | 0.723 | 0.838 | 0.832 (0.815, 0.849) | <0.0001 |
| LR | 0.211 | 0.981 | 0.713 | 0.850 | 0.842 (0.825,0.858) | <0.0001 |
| LRstepwise—SBE-LR | 0.211 | 0.984 | 0.752 | 0.841 | 0.836 (0.814,0.852) | <0.0001 |
| RFE- LR | 0.185 | 0.978 | 0.663 | 0.834 | 0.825 (0.807,0.842) | <0.0001 |
| TIMI | 0.180 | 0.976 | 0.644 | 0.834 | 0.824 (0.806, 0.841) | <0.0001 |
| 30 days | ||||||
| Classifier | ||||||
| RF | 0.389 | 0.946 | 0.373 | 0.949 | 0.903 (0.882,0.921) | <0.0001 |
| RFvarImp-SBE-RF | 0.341 | 0.948 | 0.413 | 0.930 | 0.889 (0.867,0.909) | <0.0001 |
| RFE-RF | 0.414 | 0.947 | 0.387 | 0.952 | 0.907 (0.887,0.925) | <0.0001 |
| SVM | 0.258 | 0.972 | 0.733 | 0.817 | 0.810 (0.784,0.835) | <0.0001 |
| SVMvarImp-SBE-SVM | 0.258 | 0.983 | 0.840 | 0.790 | 0.794 (0.767,0.820) | <0.0001 |
| RFE-SVM | 0.261 | 0.980 | 0.813 | 0.800 | 0.801 (0.774,0.829) | <0.0001 |
| LR | 0.248 | 0.973 | 0.747 | 0.803 | 0.799 (0.771,0.824) | <0.0001 |
| LRstepwise—SBE-LR | 0.281 | 0.974 | 0.747 | 0.834 | 0.827 (0.802,0.851) | <0.0001 |
| RFE- LR | 0.248 | 0.971 | 0.720 | 0.810 | 0.803 (0.776,0.828) | <0.0001 |
| TIMI | 0.245 | 0.962 | 0.627 | 0.832 | 0.816 (0.789, 0.840) | <0.0001 |
| 1-year | ||||||
| Classifier | ||||||
| RF | 0.410 | 0.909 | 0.373 | 0.949 | 0.827 (0.801,0.852) | <0.0001 |
| RFvarImp-SBE-RF | 0.436 | 0.909 | 0.460 | 0.901 | 0.838 (0.811,0.861) | <0.0001 |
| RFE-RF | 0.425 | 0.913 | 0.492 | 0.889 | 0.8318 (0.805,0.856) | <0.0001 |
| SVM | 0.382 | 0.950 | 0.746 | 0.798 | 0.7909 (0.763,0.817) | <0.0001 |
| SVMvarImp-SBE-SVM | 0.357 | 0.950 | 0.754 | 0.773 | 0.771 (0.741,0.798) | <0.0001 |
| RFE-SVM | 0.387 | 0.953 | 0.762 | 0.798 | 0.793 (0.765,0.820) | <0.0001 |
| LR | 0.329 | 0.924 | 0.611 | 0.792 | 0.766 (0.737,0.794) | <0.0001 |
| LRstepwise—SBE-LR | 0.372 | 0.935 | 0.659 | 0.814 | 0.792 (0.764,0.818) | <0.0001 |
| RFE- LR | 0.344 | 0.926 | 0.619 | 0.802 | 0.776 (0.747,0.803) | <0.0001 |
| TIMI | 0.332 | 0.907 | 0.484 | 0.837 | 0.786 (0.758, 0.813) | <0.0001 |
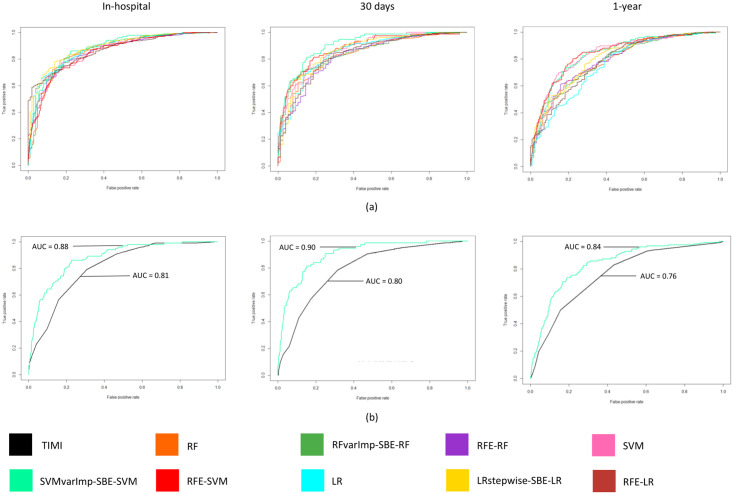
Fig 1(a) illustrates ML model performances and Fig 1(b) the best selected ML model against TIMI based on the AUC value using the untouched 30% validation dataset. Combination of SVMvarImp-SBE-SVM algorithm demonstrated the highest predictive performance with the least number of predictors for in-hospital, 30 days and 1-year model. There was no significant difference for in-hospital model for LRstepwise—SBE-LR (AUC = 0.89, 95% CI:0.861–0.920) with 24 predictors and SVMvarImp-SBE-SVM (AUC = 0.88, 95% CI: 0.846–0.910) with 15 predictors (p = 0.143; 95% CI, -0.026 to 0.004). 30-days ML mortality prediction for SVMvarImp–SBE–SVM (AUC = 0.90, 95% CI: 0.867–0.935) model reported no significant difference to RFE-SVM (AUC = 0.88,95% CI:0.837–0.920) (p = 0.115; 95% CI, -0.013 to 0.001). Model performances were observed to be similar (AUC = 0.84) and significantly better (p <0.0001) between the following 1-year mortality models (SVMvarImp vs SVM; 95% CI, 0.035 to 0.052, RFE-SVM vs SVM, 95% CI, 0.005 to 0.011, SVMvarImp-SBE-SVM vs RFE-SVM; 95% CI, 0.027 to 0.044). However, SVMvarImp-SBE-SVM model consisted of the least number of predictors (14 predictors) compared to SVM without feature selection (50 predictors) and RFE-SVM (44 predictors). Similar performance (p = 0.828; 95% CI, -0.007 to 0.009) were reported for LR with a reduced set of predictors (AUC = 0.85, 95% CI: 0.812–0.907) and a complete set of predictors for 30 days (AUC = 0.85, 95% CI: 0.803–0.897).
Fig 1. The receiver operating characteristics (ROC) curves of ML models and TIMI score based on a 30% validation dataset.
ROC curves show the performance for In-hospital, 30 days and 1-year ML mortality prediction models (a). The ROC values for TIMI against the best ML model (SVMvarImp-SBS-SVM). Abbreviations: varImp = variable importance.
Secondary analysis on best ML models (SVMvarImp–SBE–SVM) performance trained with imputed data reported for in-hospital (AUC = 0.87, 95% CI: 0.845–0.912), 30day (AUC = 0.90, 95% CI: 0.857–0.923), and 1-year (AUC = 0.83, 95% CI: 0.796–0.871). Complete case ML model dataset resulted in an almost similar AUC result for in-hospital (AUC = 0.88, 95% CI: 0.846–0.910), 30 days (AUC = 0.90, 95% CI: 0.870–0.935), and 1-year mortality (AUC = 0.84, 95% CI: 0.798–0.872). For in-hospital and 30 days, the imputed and the complete case model is significant (p<0.0001; 95% CI, 0.011 to 0.018, p = 0.001; 95% CI, 0.004 to 0.016 respectively). As for 1-year model, it is not statistically significant between imputed and complete case model (p = 0.931; 95% CI, -0.006 to 0.005).
Feature selection
RFE and SBE feature selection methods were combined with ML algorithms to construct predictive models with optimal performance (refer to methods). Initial ranking using all 50 variables for best model (SVMvarimp-SBE-SVM) using SVM variable importance is shown in S1–S3 Figs. SBE was then used to identify features that result in model optimal performances.
Common predictors observed for in-hospital, 30 days and 1-year mortality across all ML models in this study are (age, heart rate, Killip class, and fasting blood glucose). Diuretics were an additional common predictor for the best model (SVMvarImp-SBE-SVM). Age, heart rate and Killip class are identified as common predictors for the best ML model (SVMvarImp-SBE-SVM) in-hospital, 30 days and 1-year against TIMI (Table 4).
Table 4. Selected variables that resulted in optimum AUC for the best ML models (SVMvarImp-SBE-SVM) in in-hospital, 30-days, and 1-year against TIMI risk score variables.
| Variables | Machine learning best model | TIMI Score | ||
|---|---|---|---|---|
| In-hospital | 30days | 1-year | ||
| Age | • | • | • | • |
| Race | • | |||
| Smoking status | • | |||
| Hypertension | • | • | ||
| Diabetes | • | • | ||
| Family history of premature CVD | • | • | ||
| Chronic renal disease | • | |||
| Heart rate | • | • | • | • |
| Systolic bp | • | • | • | |
| Diastolic bp | • | |||
| Killip class | • | • | • | • |
| HDL | • | |||
| Fasting blood glucose | • | • | • | |
| Weight | • | |||
| ECG-type bundle branch block | • | • | ||
| ECG- location lateral lead | • | |||
| Time to treatment | • | |||
| Cardiac catheterization | • | • | ||
| PCI | • | • | ||
| ASA | • | |||
| Beta blockers | • | • | ||
| ACE inhibitor | • | |||
| Statin | • | |||
| Diuretics | • | • | • | |
| Oral hypoglycaemic agent | • | • | ||
| Insulin | • | |||
The best ML model (SVMvarImp–SBE–SVM) was converted into a short- and long-term online mortality calculator available at http://myheartstemi.uitm.edu.my/home.php.
Comparison of ML to TIMI risk score when applied to validation dataset
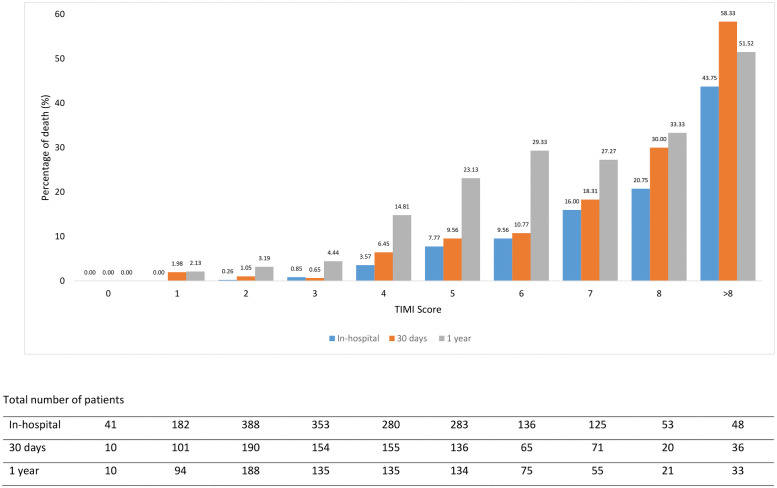
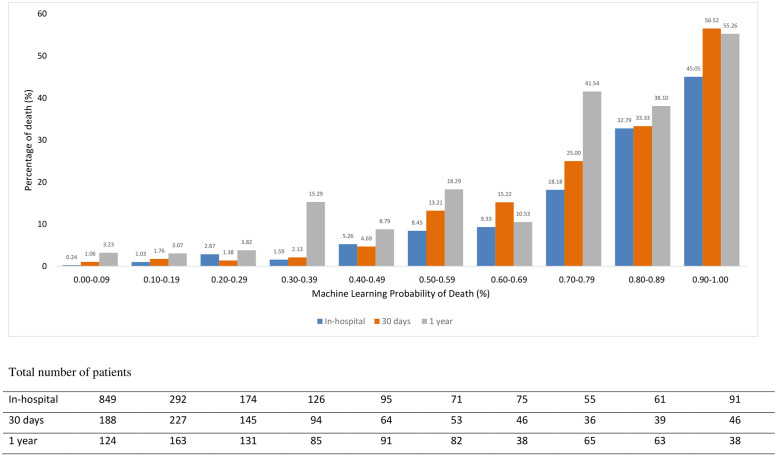
Figs 2 and 3 illustrate the comparison of the best ML model mortality rate against the TIMI score. ML score categorized patients as low risk with the probability <50% and high-risk stratum as ≥50%. This is equivalent to TIMI low risk of score ≤5 and a high-risk score of > 5 [5].
Fig 2. The rate of death across the risk score of TIMI.
Fig 3. The rate of death across ML probability of death.
In the high-risk group, ML better-predicted mortality in comparison to TIMI for in-hospital death (21.94% vs 16.15%) but similar for prediction for 30 days and 1-year deaths. (25.61% vs 23.15% and 35.71% vs 34.48%).
ML predicted a better mortality rate when compared to TIMI in the lower risk group for in-hospital (1.97% vs 2.59%), 30 days (1.73% vs 3.46%) and 1-year (5.05% vs 9.35%).
Regarding the NRI for the in-hospital model, the net reclassification of patients improved using the ML produced a net reclassification improvement of 0.20 with p<0.0001 over the original TIMI risk score, that is, a 20% improved classification. NRI for 30 days reported the net reclassification of patients improved using the ML produced a net reclassification improvement of 0.19 with p<0.0001 over the original TIMI risk score, that is, a 19% improved classification. In the 1-year model, the net reclassification of patients improved using the ML produced a net reclassification improvement of 0.14 with p<0.0001 over the original TIMI risk score, that is, a 14% improved classification (Table 5).
Table 5. Predicted risks and reclassification of STEMI patient’s mortality between ML best model (SVMvarImp-SBE-SVM) and TIMI risk score for in-hospital, 30-days, and 1-year on 30% validation dataset.
| In-hospital | ||||||
| Individuals with events (n = 101) | ||||||
| Number of individuals | Reclassified | Net correctly reclassified (%) | ||||
| Low risk | High risk | Increased risk | Decreased risk | 18.81 | ||
| Machine Learning | 23 | 4 | ||||
| TIMI Score | ||||||
| Low risk | 13 | 23 | ||||
| High risk | 4 | 61 | ||||
| Individuals without events (n = 1788) | ||||||
| Machine Learning | 116 | 144 | 1.57 | |||
| TIMI Score | ||||||
| Low risk | 1375 | 116 | ||||
| High risk | 144 | 153 | ||||
| Net reclassification index (NRI) | 20.38 | |||||
| p-value | <0.0001 | |||||
| 30 days | ||||||
| Individuals with events (n = 75) | ||||||
| Number of individuals | Reclassified | Net correctly reclassified (%) | ||||
| Low risk | High risk | Increased risk | Decreased risk | 20.00 | ||
| Machine Learning | 17 | 2 | ||||
| TIMI Score | ||||||
| Low risk | 11 | 17 | ||||
| High risk | 2 | 45 | ||||
| Individuals without events (n = 863) | ||||||
| Machine Learning | 66 | 53 | -1.51 | |||
| TIMI Score | ||||||
| Low risk | 652 | 66 | ||||
| High risk | 53 | 92 | ||||
| Net reclassification index (NRI) | 18.49 | |||||
| p-value | <0.0001 | |||||
| 1-year | ||||||
| Individuals with events (n = 126) | ||||||
| Number of individuals | Reclassified | Net correctly reclassified (%) | ||||
| Low risk | High risk | Increased risk | Decreased risk | 23.81 | ||
| Machine Learning | 44 | 14 | ||||
| TIMI Score | ||||||
| Low risk | 21 | 44 | ||||
| High risk | 14 | 47 | ||||
| Individuals without events (n = 754) | ||||||
| Machine Learning | 108 | 36 | -9.55 | |||
| TIMI Score | ||||||
| Low risk | 523 | 108 | ||||
| High risk | 36 | 87 | ||||
| Net reclassification index (NRI) | 14.26 | |||||
| p-value | <0.0001 | |||||
Discussion
Our study is the first to show better short- and long-term mortality prediction using the ML method in a multi-ethnic Asian patient with STEMI. We demonstrated high performance on validation dataset for ML models with a combination of feature selection and classifier algorithms. Overall ML model performed better than TIMI for in-hospital, 30days and 1-year AUC of (0.88vs 0.81, 0.90 vs 0.80, 0.84 vs 0.76). SVMvarImp-SBE-SVM for in-hospital, 30 days and 1-year mortality prediction had better performance compared to RF, LR and TIMI scoring as well.
The TIMI risk score was originally developed to estimate 30 days mortality risk. In the absence of a more convenient risk score system, it has since been exploited to predict in-hospital, 30 days and 1-year mortality post-STEMI in other Asian countries as well as Mexico [2, 36–38]. This is despite its moderate accuracy for risk prediction in Asians with an AUC of 0.78 [3]. In this validation study, the Asian cohort was found to be carrying an overall higher disease burden and risk compared to the TIMI cohort. The mortality rate, however, was no different suggesting an inherent inaccuracy within the algorithm. Not only that, TIMI is known to underestimate mortality risk in the lower risk group. This may delay treatment incurring excess avoidable deaths.
TIMI risk score for STEMI consists of the following components: age; systolic blood pressure; heart rate; Killip classification; infarct location or left bundle branch block; a history of diabetes, hypertension, angina pectoris, weight, and time to reperfusion (thrombolysis or pPCI). Previous studies have modified ‘time to reperfusion’ to be ‘door-to-needle’ or ‘door-to-balloon’ time instead of ‘symptom onset to-reperfusion’ time because of inconsistencies in the reporting of symptom onset time [39]. Our study excluded some variables such as angina pectoris, weight and time to reperfusion in the model development as over 50% of data was missing. Additional parameters (Table 1) were included including ethnicity, smoking status, invasive and non-invasive treatments, lipid profile and features from the complete blood chemistry at admission.
Feature selection algorithms are essential in mortality prediction. A combination of feature selection methods with classification algorithms resulted in higher performance versus using standalone classifiers [29]. Applications of feature selection algorithms improved ML model performance using a reasonable number of predictors by reducing the predictor’s dimensionality [40]. The model performance in this study increased with the reduction in the number of predictors. Our results indicate that ML model predictive performance requires 15 predictors for in-hospital, 13 for 30 days and 12 for 1-year mortality prediction that performs better than models developed using a conventional statistical approach.
We used univariate analysis to support the relationship between variables selected from ML algorithms and outcomes (Table 1). Age, heart rate, Killip class and fasting blood glucose were ranked and selected by all short- and long-term mortality prediction ML models. Older age and higher Killip class were significant predictors of mortality [41, 42]. Age, Killip class and fasting blood glucose were also selected as a factor that affects mortality post-STEMI by ML models in previous studies [7, 19]. Glucose levels were ranked by all ML models, supporting the relationship between hyperglycemia and increased risk in mortality for patients with STEMI in the Asian population [43]. STEMI patients with higher heart rates were associated with an increased risk of mortality, even after primary PCI [44]. This may be a reflection of worse presentation (higher Killip class) or even higher pain intensity from a larger infarct.
Incorporating variables like having invasive or non-invasive management into the SVMvarImp-SBE-SVM model to predict in-hospital, 30 days and 1-year mortality yield interesting results. Invasive treatment such as PCI received by STEMI patients showed a trend towards better outcomes for in-hospital and 30 days after discharge. Mortality risk at 1-year was reduced by 40% for patients who received PCI compared to those who did not [4, 39, 45].
TIMI and GRACE scores were calculated based on data during an era where early reperfusion therapy and routine use of drug-eluting stents were not common. Non-invasive treatment predictors such as pharmacological therapy (medications including anti-hypertensive (ACE inhibitor, beta-blockers, diuretics), anti-diabetic agents (oral hypoglycaemic agents, insulin and antiplatelet) were selected for in-hospital, 30 days and 1-year mortality prediction in our study. These drugs are often prescribed in the acute setting to augment neurohumoral modulation associated with left ventricular negative remodelling. Being on these medications could signal a sicker ventricle hence the strong association with death.
Systolic and diastolic blood pressure were ranked as predictors for in-hospital, 1-year and 30 days models. Cardiogenic shock at presentation increases the risk of death. STEMI patients with cardiogenic shock who survived in-hospital death are at an increased risk of long-term death, probably as a reflection of the severity during initial admission [46].
Other CVD risk such as hypertension, diabetes, smoking and chronic renal disease, were associated with a poorer 1-year outcome. Poorly controlled CVD risk leads to an adverse systemic remodeling, leading to a plethora of cardiovascular conditions including heart failure, stroke, renal failure, and peripheral vascular disease [47].
By having continuous data collection through an electronic health records system, we were able to allow for the adaptation of ML predictive algorithm tailored to patient’s risk grouping. ML methods discussed in this study are needed to rank and select significant risk factors associated with short- and long-term STEMI mortality. Feature selection allows better interpretation of the models by restricting the scope of predictors used, selecting only those clinically relevant.
ML models in this study have demonstrated higher performance compared to TIMI scoring that was extrapolated from a Caucasian cohort. Asian patients present at a younger age with acute coronary syndromes. The average age in the GRACE registry was 61, whereas it is 58 in Malaysia and 51 in the Middle East [48]. Numerous factors are associated with differences in presentation. Hence risk scoring tools should be adapted to a specific population to better reflect the differences with greater accuracy.
Data imputation was performed to ensure the validity of the findings. We used multivariable imputation using chained equations and predictive mean matching method for data imputation instead of using machine learning-based method such as missForest in this study. The data imputation method used in this study was selected as recommended in a similar study conducted on the Swedish heart registry dataset that resulted in high model performance [19]. Moreover, Solaro et al. demonstrated that the relative performance of missForest varied with the MCAR data patterns and did not show a clear advantage. Overall, the imputation accuracy and applicability of missForest is still unclear [49]. We initially did not include patients with more than 50% missing data as it will require data imputation, which may affect our result. We do not feel it is a limitation for the population as it is still a large dataset. As the dataset had completed dataset for all follow-up time points, generation of risk calculator was possible for both ML and TIMI calculator. However, identifying factors associated with short- and long-term mortality prediction usage of complete cases would lead to more reliable findings. We went back to use an incomplete dataset and imputed data and showed similar results.
The cross-validation approach used in this study increases the efficacy of the models during model construction as it reduces the risk of model over-fitting. Also, the classification performance is highly influenced by data pre-processing and tuning of algorithms [50]. A pair-wise corrected resampled t-test was used to evaluate the differences between ML models predictive performances. The resampled t-test is a validated tool for the comparison of outcome between two classifiers [33, 34]. ML algorithms SVM and RF have demonstrated high predictive performance when combined with feature selection in mortality related studies [7, 19]. Both RF and SVM models were used to determine the list of variable importance that is an essential part of contributing to good model performance. RF, SVM and LR with SBE a feature reduction algorithm reported higher performance compared to RFE. SBE algorithm depends only on importance as an adequate term to eliminate unimportant variables one-by-one from a model [51]. Meanwhile, RFE is reported to have poor generalisation ability.
ML models in this study were validated with untouched validation data that was not used for model development, to confirm the reliability of the current study. We also demonstrated the ML model using complete sets of variables collected, without a variable selection process that resulted in a similar performance to models with feature selection. This shows that feature selection does not lead to the loss of important prognostic information.
Despite a large proportion of missing values in the original dataset, we were still able to apply both TIMI and ML algorithm and compare outcomes. This is likely because we used a hard endpoint of death that is not affected by missing values. Another possibility is that the variables extracted (15 for in-hospital, 13 for 30 days and 12 for 1-year) was sufficient to increase the model’s precision to predict death reliably.
Future study will focus on validation of the ML algorithm in real-time involving several local hospitals for continuous assessment of its reliability. Application of ML models that are population-specific together with conventional risk scoring method allows better outcome in mortality prediction, communication and increases awareness of patients that enables behavioural modifications and better management of limited resources by clinicians.
Study limitations
This study compared the performance of an ML-based model for in-hospital, 30 days and 1-year with a clinical prognostic model that was designed for 30 days’ mortality. Its robustness would be increased had we included variables and compared them to other scoring systems such as GRACE and the Heart Score. The lack of certain variables precluded this attempt. We recognised that missing variable may result in a bias finding. We attempted to reduce this effect by applying TIMI score and ML-based score to the same population. Selection bias that exists within registries is difficult to control. We hope that future real-world study would validate our findings.
Conclusions
In conclusion, our study illustrates the capability of ML algorithms application for feature selection and prediction of in-hospital, 30 days and 1-year population-specific mortality in STEMI patients. ML models that are population-specific demonstrate better performance compared to TIMI score. A combination of feature selection techniques with classification algorithm allows for reliable selection of significant variables and improvement in model predictive performance, with subsequent benefits by allowing effective resource allocation in the management of STEMI patients.
Supporting information
Abbreviations = ptagenotification = Age, ptrace = Race, ptsex = Gender, smokingstatus = Smoking status, chpt = History of hypertension, cdm = History of diabetes, cpremcvd = Family history of premature cardiovascular disease, cmi = History of myocardial infarction, ccap = Documented CAD, cheartfail = History of heart failure, clung = Chronic lung disease, crenal = Chronic renal disease, ccerebrovascular = Cerebrovascular disease, heartrate = Heart rate, bpsys = Systolic blood pressure, bpdias = Diastolic blood pressure, killipclass = Killip Class, tc = Total cholesterol, hdlc = High-Density Lipoprotein, ldlc = Low-Density Lipoprotein, tg = Triglycerides, fbg = Fasting blood glucose, ecgabnormstylestelev1 = ST segment elevation ≥1mm in ≥2 contiguous limb leads, ecgabnormstylestelev2 = ST segment elevation ≥2mm in ≥2 contiguous frontal leads or chest leads, ecgabnormstylestdep = ST segment depression ≥0.5mm in ≥2 contiguous leads, ecgabnormtypetwave = T-wave inversion ≥1mm, ecgabnormtypebbb = Bundle branch block, ecgabnormlocationil = Inferior leads: II, III, aVF, ecgabnormlocational = Anterior leads: V1 to V4, ecgabnormlocationll = Lateral leads: I, aVL, V5 to V6, ecgabnormlocationtp = True posterior: V1, V2, ecgabnormlocationrv = Right ventricle: ST elevation in lead V4R, fbstatus = Fibrinolytic status, pci = Percutaneous Coronary Intervention, cabg = Coronary artery bypass graft, asa = Aspirin, gpri = GP receptor inhibitor, heparin = Unfractionated heparin, lmwh = Low-molecular-weight Heparin, bb = Beta blockers, acei = ACE inhibitor, arb = Angiotensin II receptor blocker, statin = Statin, lipidla = Other lipid lowering agent, diuretic = Diuretics, calcantagonist = Calcium antagonist, oralhypogly = Oral hypoglycaemic agent, insulin = Insulin, antiarr = Anti-arrhythmic agent.
(TIF)
Abbreviations = ptagenotification = Age, ptrace = Race, ptsex = Gender, smokingstatus = Smoking status, chpt = History of hypertension, cdm = History of diabetes, cpremcvd = Family history of premature cardiovascular disease, cmi = History of myocardial infarction, ccap = Documented CAD, cheartfail = History of heart failure, clung = Chronic lung disease, crenal = Chronic renal disease, ccerebrovascular = Cerebrovascular disease, heartrate = Heart rate, bpsys = Systolic blood pressure, bpdias = Diastolic blood pressure, killipclass = Killip Class, tc = Total cholesterol, hdlc = High-Density Lipoprotein, ldlc = Low-Density Lipoprotein, tg = Triglycerides, fbg = Fasting blood glucose, ecgabnormstylestelev1 = ST segment elevation ≥1mm in ≥2 contiguous limb leads, ecgabnormstylestelev2 = ST segment elevation ≥2mm in ≥2 contiguous frontal leads or chest leads, ecgabnormstylestdep = ST segment depression ≥0.5mm in ≥2 contiguous leads, ecgabnormtypetwave = T-wave inversion ≥1mm, ecgabnormtypebbb = Bundle branch block, ecgabnormlocationil = Inferior leads: II, III, aVF, ecgabnormlocational = Anterior leads: V1 to V4, ecgabnormlocationll = Lateral leads: I, aVL, V5 to V6, ecgabnormlocationtp = True posterior: V1, V2, ecgabnormlocationrv = Right ventricle: ST elevation in lead V4R, fbstatus = Fibrinolytic status, pci = Percutaneous Coronary Intervention, cabg = Coronary artery bypass graft, asa = Aspirin, gpri = GP receptor inhibitor, heparin = Unfractionated heparin, lmwh = Low-molecular-weight Heparin, bb = Beta blockers, acei = ACE inhibitor, arb = Angiotensin II receptor blocker, statin = Statin, lipidla = Other lipid lowering agent, diuretic = Diuretics, calcantagonist = Calcium antagonist, oralhypogly = Oral hypoglycaemic agent, insulin = Insulin, antiarr = Anti-arrhythmic agent.
(TIF)
Abbreviations = ptagenotification = Age, ptrace = Race, ptsex = Gender, smokingstatus = Smoking status, chpt = History of hypertension, cdm = History of diabetes, cpremcvd = Family history of premature cardiovascular disease, cmi = History of myocardial infarction, ccap = Documented CAD, cheartfail = History of heart failure, clung = Chronic lung disease, crenal = Chronic renal disease, ccerebrovascular = Cerebrovascular disease, heartrate = Heart rate, bpsys = Systolic blood pressure, bpdias = Diastolic blood pressure, killipclass = Killip Class, tc = Total cholesterol, hdlc = High-Density Lipoprotein, ldlc = Low-Density Lipoprotein, tg = Triglycerides, fbg = Fasting blood glucose, ecgabnormstylestelev1 = ST segment elevation ≥1mm in ≥2 contiguous limb leads, ecgabnormstylestelev2 = ST segment elevation ≥2mm in ≥2 contiguous frontal leads or chest leads, ecgabnormstylestdep = ST segment depression ≥0.5mm in ≥2 contiguous leads, ecgabnormtypetwave = T-wave inversion ≥1mm, ecgabnormtypebbb = Bundle branch block, ecgabnormlocationil = Inferior leads: II, III, aVF, ecgabnormlocational = Anterior leads: V1 to V4, ecgabnormlocationll = Lateral leads: I, aVL, V5 to V6, ecgabnormlocationtp = True posterior: V1, V2, ecgabnormlocationrv = Right ventricle: ST elevation in lead V4R, fbstatus = Fibrinolytic status, pci = Percutaneous Coronary Intervention, cabg = Coronary artery bypass graft, asa = Aspirin, gpri = GP receptor inhibitor, heparin = Unfractionated heparin, lmwh = Low-molecular-weight Heparin, bb = Beta blockers, acei = ACE inhibitor, arb = Angiotensin II receptor blocker, statin = Statin, lipidla = Other lipid lowering agent, diuretic = Diuretics, calcantagonist = Calcium antagonist, oralhypogly = Oral hypoglycaemic agent, insulin = Insulin, antiarr = Anti-arrhythmic agent.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank National Heart Association Malaysia for providing us with the data for this study.
Data Availability
The data that support the findings of this study are available from the National Heart Association of Malaysia (NHAM) but restrictions apply to the availability of these data, and so are not publicly available. The data belongs to the individual ministry of health universities hospitals and private hospitals that require multiple institutional agreements for data release to third parties hence ethical approval is needed for analysis. Data are however available from NHAM upon request using https://www.malaysianheart.org/?p=contact or email them at secretariat@malaysianheart.org. Any findings from the data need to be reported and permission needs to be obtained from the NHAM committee before publication.
Funding Statement
This work was supported by the University of Malaya Internal grant (Project No: GPF013B- 2018). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Health at a Glance: Asia/Pacific 2018 Measuring Progress towards Universal Health Coverage. OECD Publishing; 2018. Dec 3. [Google Scholar]
- 2.Peng Y, Du X, Rogers KD, Wu Y, Gao R, Patel A. Predicting In-Hospital Mortality in Patients With Acute Coronary Syndrome in China. The American Journal of Cardiology. 2017. Oct 1;120(7):1077–1083. doi: 10.1016/j.amjcard.2017.06.044 [DOI] [PubMed] [Google Scholar]
- 3.Selvarajah S, Fong AY, Selvaraj G, Haniff J, Uiterwaal CS, Bots ML. An Asian validation of the TIMI risk score for ST-segment elevation myocardial infarction. PLoS One. 2012. Jul 16;7(7):e40249. doi: 10.1371/journal.pone.0040249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuhdi AS, Ahmad WA, Zaki RA, Mariapun J, Ali RM, Sari NM, et al. Acute coronary syndrome in the elderly: the Malaysian National Cardiovascular Disease Database-Acute Coronary Syndrome registry. Singapore medical journal. 2016. Apr;57(4):191. doi: 10.11622/smedj.2015145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000. Oct 24;102(17):2031–2037. doi: 10.1161/01.cir.102.17.2031 [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Groeneveld PW. Big data, health informatics, and the future of cardiovascular medicine. 2017:899–902. [DOI] [PubMed] [Google Scholar]
- 7.Shouval R, Hadanny A, Shlomo N, Iakobishvili Z, Unger R, Zahger D, et al. Machine learning for prediction of 30-day mortality after ST elevation myocardial infraction: An Acute Coronary Syndrome Israeli Survey data mining study. International Journal of Cardiology. 2017. Nov 1;246:7–13. doi: 10.1016/j.ijcard.2017.05.067 [DOI] [PubMed] [Google Scholar]
- 8.Obermeyer Z, Emanuel EJ. Predicting the future—big data, machine learning, and clinical medicine. The New England journal of medicine. 2016. Sep 29;375(13):1216. doi: 10.1056/NEJMp1606181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon JM, Jeon KH, Kim HM, Kim MJ, Lim S, Kim KH, et al. Deep-learning-based out-of-hospital cardiac arrest prognostic system to predict clinical outcomes. Resuscitation. 2019. Jun 1;139:84–91. doi: 10.1016/j.resuscitation.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 10.Ahmad WA, Ali RM, Khanom M, Han CK, Bang LH, Yip AF, et al. The journey of Malaysian NCVD—PCI (National Cardiovascular Disease Database—Percutaneous Coronary Intervention) Registry: A summary of three years report. International journal of cardiology. 2013. Apr 30;165(1):161–164. doi: 10.1016/j.ijcard.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 11.Ahmad WA, Zambahari R, Ismail O, Sinnadurai J, Rosman A, Piaw CS, et al. Malaysian national cardiovascular disease database (NCVD)–acute coronary syndrome (ACS) registry: how are we different. CVD Prevention and Control. 2011. Sep 1;6(3):81–89. [Google Scholar]
- 12.Kuhn M, Johnson K. Applied predictive modeling. New York: Springer; 2013. Sep. [Google Scholar]
- 13.Genuer R, Poggi JM, Tuleau-Malot C. Variable selection using random forests Pattern Recognition Letters, 31, 2225 10.1016. J. PATREC. 2010;14. [Google Scholar]
- 14.Geisser S. Predictive inference. CRC press; 1993. Jun 1. [Google Scholar]
- 15.Buuren SV, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. Journal of statistical software. 2010:1–67. [Google Scholar]
- 16.Breiman L. Random forests. Machine learning. 2001. Oct 1;45(1):5–32. [Google Scholar]
- 17.Vapnik V. The support vector method of function estimation. InNonlinear Modeling 1998. (pp. 55–85). Springer, Boston, MA. doi: 10.1162/089976698300017269 [DOI] [Google Scholar]
- 18.Menard S. Applied logistic regression analysis. Sage; 2002. [Google Scholar]
- 19.Wallert J, Tomasoni M, Madison G, Held C. Predicting two-year survival versus non-survival after first myocardial infarction using machine learning and Swedish national register data. BMC medical informatics and decision making. 2017. Dec 1;17(1):99. doi: 10.1186/s12911-017-0500-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Pei J, Kamber M. Data mining: concepts and techniques. Elsevier; 2011. Jun 9. [Google Scholar]
- 21.Feurer M, Hutter F. Hyperparameter optimization. InAutomated Machine Learning 2019. (pp. 3–33). Springer, Cham. [Google Scholar]
- 22.Fawcett T. Roc analysis in pattern recognition. Pattern Recognition Letters. 2005;8:861–874. [Google Scholar]
- 23.Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Frontiers in public health. 2017. Nov 20; 5:307. doi: 10.3389/fpubh.2017.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bengio Y, Nadeau C. Inference for the Generalization Error. CIRANO; 1999. Jul 1. [Google Scholar]
- 25.Lin X, Li C, Zhang Y, Su B, Fan M, Wei H. Selecting feature subsets based on SVM-RFE and the overlapping ratio with applications in bioinformatics. Molecules. 2018. Jan;23(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra A, Dimri A, Pradhan T. Prediction of factors affecting amlodipine induced pedal edema and its classification. In2017 International Conference on Advances in Computing, Communications and Informatics (ICACCI) 2017 Sep 13 (pp. 1684–1689). IEEE.
- 27.Yang X. Identification of risk genes associated with myocardial infarction based on the recursive feature elimination algorithm and support vector machine classifier. Molecular medicine reports. 2018. Jan 1;17(1):1555–1560. doi: 10.3892/mmr.2017.8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Riverol Y, Kuhn M, Vizcaíno JA, Hitz MP, Audain E. Accurate and fast feature selection workflow for high-dimensional omics data. PloS one. 2017. Dec 20;12(12): e0189875. doi: 10.1371/journal.pone.0189875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jafarian A, Ngom A, Rueda L. A Novel Recursive Feature Subset Selection Algorithm. In2011 IEEE 11th International Conference on Bioinformatics and Bioengineering 2011 Oct 24 (pp. 78–83). IEEE.
- 30.Díaz-Uriarte R, De Andres SA. Gene selection and classification of microarray data using random forest. BMC bioinformatics. 2006. Dec 1;7(1):3. doi: 10.1186/1471-2105-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correia LC, Garcia G, Kalil F, Ferreira F, Carvalhal M, Oliveira R, et al. Prognostic value of TIMI score versus GRACE score in ST-segment elevation myocardial infarction. Arquivos brasileiros de cardiologia. 2014. Aug;103(2):98–106. doi: 10.5935/abc.20140095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008. Jan 30;27(2):157–172. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 33.Dietterich TG. Approximate statistical tests for comparing supervised classification learning algorithms. Neural computation. 1998. Oct 1;10(7):1895–1923. doi: 10.1162/089976698300017197 [DOI] [PubMed] [Google Scholar]
- 34.Raschka S. Model evaluation, model selection, and algorithm selection in machine learning. arXiv preprint arXiv:1811.12808. 2018. Nov 13. [Google Scholar]
- 35.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. 2011. https://www.R-project.org.
- 36.Chimparlee N, Chaipromprasit J, Athisakul S, Lertsuwunseri V, Buddhari W, Udayachalerm W, et al. COMPARISON BETWEEN TIMI AND GRACE SCORES AS A PREDICTOR FOR SHORT-AND LONG-TERM OUTCOME IN PATIENTS WITH ACUTE ST-ELEVATION MYOCARDIAL INFARCTION. Journal of the American College of Cardiology. 2018. Mar 10;71(11S): A246-. [Google Scholar]
- 37.Timbol AB, Villaluna RA, Punzalan FE. 106: TIMI RISK SCORE FOR STEMI A VALIDATION STUDY AMONG FILIPINOS FOR PREDICTING IN-HOSPITAL MORTALITY. Critical care medicine. 2015. Dec 1;43(12):28. [Google Scholar]
- 38.González-Pacheco H, Arias-Mendoza A, Álvarez-Sangabriel A, Juárez-Herrera Ú, Damas F, Eid-Lidt G, et al. The TIMI risk score for STEMI predicts in-hospital mortality and adverse events in patients without cardiogenic shock undergoing primary angioplasty. Archivos de cardiología de México. 2012. Mar;82(1):7–13. [PubMed] [Google Scholar]
- 39.Selvarajah S, Fong AY, Selvaraj G, Haniff J, Hairi NN, Bulgiba A, et al. Impact of cardiac care variation on ST-elevation myocardial infarction outcomes in Malaysia. The American journal of cardiology. 2013. May 1;111(9):1270–1276. doi: 10.1016/j.amjcard.2013.01.271 [DOI] [PubMed] [Google Scholar]
- 40.Vomlel J, Kruzık H, Tuma P, Precek J, Hutyra M. Machine learning methods for mortality prediction in patients with st elevation myocardial infarction. Proceedings of WUPES. 2012; 2012:204–213. [Google Scholar]
- 41.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Archives of internal medicine. 2003. Oct 27;163(19):2345–2353. doi: 10.1001/archinte.163.19.2345 [DOI] [PubMed] [Google Scholar]
- 42.Cheng JM, Helming AM, van Vark LC, Kardys I, Den Uil CA, Jewbali LS, et al. A simple risk chart for initial risk assessment of 30-day mortality in patients with cardiogenic shock from ST-elevation myocardial infarction. European Heart Journal: Acute Cardiovascular Care. 2016. Apr;5(2):101–107. doi: 10.1177/2048872615568966 [DOI] [PubMed] [Google Scholar]
- 43.Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovascular Disorders. 2017. Dec;17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng YY, Wu TT, Chen Y, Hou XG, Yang Y, Ma X, et al. Resting heart rate and long-term outcomes in patients with percutaneous coronary intervention: results from a 10-year follow-up of the CORFCHD-PCI study. Cardiology research and practice. 2019. Apr 1;2019. doi: 10.1155/2019/5432076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatason P, Zubairi YZ, Ahmad WA, Hafidz MI, Ismail MD, Hadi MF, et al. In-hospital mortality of cardiogenic shock complicating ST-elevation myocardial infarction in Malaysia: a retrospective analysis of the Malaysian National Cardiovascular Database (NCVD) registry. BMJ open. 2019. May 1;9(5): e025734. doi: 10.1136/bmjopen-2018-025734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laufer-Perl M, Shacham Y, Letourneau-Shesaf S, Priesler O, Keren G, Roth A, et al. Gender-Related Mortality and In-Hospital Complications Following ST-Segment Elevation Myocardial Infarction: Data From a Primary Percutaneous Coronary Intervention Cohort. Clinical cardiology. 2015. Mar;38(3):145–149. doi: 10.1002/clc.22363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajadurai J., Zambahar R., Abdul Rahman A. R., Suhaimi et al. Clinical Practices and Guidelines on Primary & Secondary Prevention of Cardiovascular Disease 2017. Putrajaya, WP Putrajaya: CPG Secretariat; 2017. https://www.malaysianheart.org/files/597736485dd17.pdf [Google Scholar]
- 48.Thalib L, Furuya-Kanamori L, AlHabib KF, Alfaleh HF, AlShamiri MQ, Amin H, et al. Validation of the 6-month GRACE score in predicting 1-year mortality of patients with acute coronary syndrome admitted to the Arabian Gulf hospitals. Angiology. 2017. Mar;68(3):251–256. doi: 10.1177/0003319716659179 [DOI] [PubMed] [Google Scholar]
- 49.Solaro N, Barbiero A, Manzi G, Ferrari PA. A simulation comparison of imputation methods for quantitative data in the presence of multiple data patterns. Journal of Statistical Computation and Simulation. 2018. Dec 12;88(18):3588–35619. [Google Scholar]
- 50.Kesavaraj G, Sukumaran S. A study on classification techniques in data mining. In2013 Fourth International Conference on Computing, Communications and Networking Technologies (ICCCNT) 2013 Jul 4 (pp. 1–7). IEEE.
- 51.Mao KZ. Orthogonal forward selection and backward elimination algorithms for feature subset selection. IEEE Transactions on Systems, Man, and Cybernetics, Part B (Cybernetics). 2004. Jan 30;34(1):629–634. doi: 10.1109/tsmcb.2002.804363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations = ptagenotification = Age, ptrace = Race, ptsex = Gender, smokingstatus = Smoking status, chpt = History of hypertension, cdm = History of diabetes, cpremcvd = Family history of premature cardiovascular disease, cmi = History of myocardial infarction, ccap = Documented CAD, cheartfail = History of heart failure, clung = Chronic lung disease, crenal = Chronic renal disease, ccerebrovascular = Cerebrovascular disease, heartrate = Heart rate, bpsys = Systolic blood pressure, bpdias = Diastolic blood pressure, killipclass = Killip Class, tc = Total cholesterol, hdlc = High-Density Lipoprotein, ldlc = Low-Density Lipoprotein, tg = Triglycerides, fbg = Fasting blood glucose, ecgabnormstylestelev1 = ST segment elevation ≥1mm in ≥2 contiguous limb leads, ecgabnormstylestelev2 = ST segment elevation ≥2mm in ≥2 contiguous frontal leads or chest leads, ecgabnormstylestdep = ST segment depression ≥0.5mm in ≥2 contiguous leads, ecgabnormtypetwave = T-wave inversion ≥1mm, ecgabnormtypebbb = Bundle branch block, ecgabnormlocationil = Inferior leads: II, III, aVF, ecgabnormlocational = Anterior leads: V1 to V4, ecgabnormlocationll = Lateral leads: I, aVL, V5 to V6, ecgabnormlocationtp = True posterior: V1, V2, ecgabnormlocationrv = Right ventricle: ST elevation in lead V4R, fbstatus = Fibrinolytic status, pci = Percutaneous Coronary Intervention, cabg = Coronary artery bypass graft, asa = Aspirin, gpri = GP receptor inhibitor, heparin = Unfractionated heparin, lmwh = Low-molecular-weight Heparin, bb = Beta blockers, acei = ACE inhibitor, arb = Angiotensin II receptor blocker, statin = Statin, lipidla = Other lipid lowering agent, diuretic = Diuretics, calcantagonist = Calcium antagonist, oralhypogly = Oral hypoglycaemic agent, insulin = Insulin, antiarr = Anti-arrhythmic agent.
(TIF)
Abbreviations = ptagenotification = Age, ptrace = Race, ptsex = Gender, smokingstatus = Smoking status, chpt = History of hypertension, cdm = History of diabetes, cpremcvd = Family history of premature cardiovascular disease, cmi = History of myocardial infarction, ccap = Documented CAD, cheartfail = History of heart failure, clung = Chronic lung disease, crenal = Chronic renal disease, ccerebrovascular = Cerebrovascular disease, heartrate = Heart rate, bpsys = Systolic blood pressure, bpdias = Diastolic blood pressure, killipclass = Killip Class, tc = Total cholesterol, hdlc = High-Density Lipoprotein, ldlc = Low-Density Lipoprotein, tg = Triglycerides, fbg = Fasting blood glucose, ecgabnormstylestelev1 = ST segment elevation ≥1mm in ≥2 contiguous limb leads, ecgabnormstylestelev2 = ST segment elevation ≥2mm in ≥2 contiguous frontal leads or chest leads, ecgabnormstylestdep = ST segment depression ≥0.5mm in ≥2 contiguous leads, ecgabnormtypetwave = T-wave inversion ≥1mm, ecgabnormtypebbb = Bundle branch block, ecgabnormlocationil = Inferior leads: II, III, aVF, ecgabnormlocational = Anterior leads: V1 to V4, ecgabnormlocationll = Lateral leads: I, aVL, V5 to V6, ecgabnormlocationtp = True posterior: V1, V2, ecgabnormlocationrv = Right ventricle: ST elevation in lead V4R, fbstatus = Fibrinolytic status, pci = Percutaneous Coronary Intervention, cabg = Coronary artery bypass graft, asa = Aspirin, gpri = GP receptor inhibitor, heparin = Unfractionated heparin, lmwh = Low-molecular-weight Heparin, bb = Beta blockers, acei = ACE inhibitor, arb = Angiotensin II receptor blocker, statin = Statin, lipidla = Other lipid lowering agent, diuretic = Diuretics, calcantagonist = Calcium antagonist, oralhypogly = Oral hypoglycaemic agent, insulin = Insulin, antiarr = Anti-arrhythmic agent.
(TIF)
Abbreviations = ptagenotification = Age, ptrace = Race, ptsex = Gender, smokingstatus = Smoking status, chpt = History of hypertension, cdm = History of diabetes, cpremcvd = Family history of premature cardiovascular disease, cmi = History of myocardial infarction, ccap = Documented CAD, cheartfail = History of heart failure, clung = Chronic lung disease, crenal = Chronic renal disease, ccerebrovascular = Cerebrovascular disease, heartrate = Heart rate, bpsys = Systolic blood pressure, bpdias = Diastolic blood pressure, killipclass = Killip Class, tc = Total cholesterol, hdlc = High-Density Lipoprotein, ldlc = Low-Density Lipoprotein, tg = Triglycerides, fbg = Fasting blood glucose, ecgabnormstylestelev1 = ST segment elevation ≥1mm in ≥2 contiguous limb leads, ecgabnormstylestelev2 = ST segment elevation ≥2mm in ≥2 contiguous frontal leads or chest leads, ecgabnormstylestdep = ST segment depression ≥0.5mm in ≥2 contiguous leads, ecgabnormtypetwave = T-wave inversion ≥1mm, ecgabnormtypebbb = Bundle branch block, ecgabnormlocationil = Inferior leads: II, III, aVF, ecgabnormlocational = Anterior leads: V1 to V4, ecgabnormlocationll = Lateral leads: I, aVL, V5 to V6, ecgabnormlocationtp = True posterior: V1, V2, ecgabnormlocationrv = Right ventricle: ST elevation in lead V4R, fbstatus = Fibrinolytic status, pci = Percutaneous Coronary Intervention, cabg = Coronary artery bypass graft, asa = Aspirin, gpri = GP receptor inhibitor, heparin = Unfractionated heparin, lmwh = Low-molecular-weight Heparin, bb = Beta blockers, acei = ACE inhibitor, arb = Angiotensin II receptor blocker, statin = Statin, lipidla = Other lipid lowering agent, diuretic = Diuretics, calcantagonist = Calcium antagonist, oralhypogly = Oral hypoglycaemic agent, insulin = Insulin, antiarr = Anti-arrhythmic agent.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
The data that support the findings of this study are available from the National Heart Association of Malaysia (NHAM) but restrictions apply to the availability of these data, and so are not publicly available. The data belongs to the individual ministry of health universities hospitals and private hospitals that require multiple institutional agreements for data release to third parties hence ethical approval is needed for analysis. Data are however available from NHAM upon request using https://www.malaysianheart.org/?p=contact or email them at secretariat@malaysianheart.org. Any findings from the data need to be reported and permission needs to be obtained from the NHAM committee before publication.