Abstract
Background –
Blunted neural reward responsiveness (RR) is observed in youth depression. However, it is unclear whether symptoms of depression experienced early in development relate to adolescent RR beyond current symptoms and further, whether such relationships with RR differ during two key components of reward processing: anticipation and outcome.
Methods –
Within a prospective longitudinal study oversampled for early depression, children and caregivers completed semi-annual diagnostic assessments beginning in preschool. In later adolescence, mean age = 16.49 years (SD = 0.94), youths’ (N=100) neurophysiological responses to cues signaling likely win and loss and these outcomes were assessed. Longitudinally assessed dimensional depression and externalizing symptoms (often co-morbid with depression and also associated with RR) experienced at different developmental periods (preschool 3–5.11 years, school-age 6–9.11 years, early-adolescent 10–14.11 years, current) were used as simultaneous predictors of event-related potentials indexing anticipatory cue processing (cue-P3) and outcome processing (Reward Positivity-RewP/Feedback Negativity-FN, feedback-P3).
Results –
Blunted motivated attention to cues signaling likely win (cue-P3) was specifically predicted by early-adolescent depression symptoms. Blunted initial response to win (RewP) and loss (FN) outcomes was specifically predicted by preschool depression symptoms. Blunted motivational salience of win and loss outcomes (feedback-P3) was predicted by cumulative depression, not specific to any developmental stage.
Conclusions –
Although blunted anticipation and outcome RR is a common finding in depression, specific deficits related to motivated attention to cues and initial outcome processing may map onto the developmental course of these symptoms.
Keywords: Depression, Development, Preschool, Adolescent, EEG/Evoked Potentials, Reward
1. Introduction
Appropriate response to reward is critical for motivated behavior and learning. Although reward responsiveness (RR) increases normatively across adolescence (1), blunted RR is consistently implicated in adolescent depression and may play a role in its pathogenesis (2, 3). For example, blunted RR is observed in remitted depression (4) non-depressed but high-risk groups including first-degree relatives of depressed persons (5), offspring of depressed parents (6, 7), adolescents known to develop later depression (8). Because of this, blunted RR has been conceptualized as a trait vulnerability and potential biomarker of depression risk. In a separate line of inquiry, early experiences, particularly of trauma or deprivation, but also depression, have been linked to blunted RR later in life (9–13) as well as concurrently, during preschool (14). These studies suggest that early life may be a sensitive period, wherein negative experiences known to blunt RR alter the hedonic ‘set-point’ (10). Conversely, negative experiences, such as experiencing depression, at later points in development may relate to other types of reward dysfunction – for example there is particularly strong evidence for blunted responding to cues signaling potential reward in adolescent depression (12, 15, 16). (10) Another related view is that blunted RR reflects a ‘scar’ of earlier experience of depression symptoms and plays a role in heightened risk for reoccurrence, supported by findings of blunted RR in remitted depression (4). However, there has been no prospective investigation of whether early experience of depression symptoms impacts neural RR later in adolescence, the developmental period most commonly examined in studies of reward processing and depression risk in youth (3). Understanding how past early depression versus recent depression in adolescence might impact RR is critical for understanding not only the pathophysiology of depression and its maintenance over development, but also how blunted RR evolves over development and may function as a depression biomarker in the high-risk period of adolescence.
RR is a multifaceted construct and in the fMRI literature it is common to index both reward anticipation (i.e., response to cues signaling that either a win or loss is likely to occur), and response to outcomes (i.e., receipt of win or loss outcomes). Using EEG, which has much higher temporal resolution than fMRI, anticipation and outcome processing can be further broken down into subprocess indexed by specific event-related potentials (ERPs) (17). For example, motivated attention to anticipatory cues is indexed by the cue-P3, a centro-parietal positivity increased following cues indicating likely reward (18). Initial responsiveness to win outcomes is indexed by the reward positivity (RewP), a fronto-central positivity associated with ‘good’ outcomes. The loss-related equivalent of this component, the feedback negativity (FN), is a negativity for ‘bad’ outcomes. The difference between win and loss, the ΔRewP, is frequently blunted in depression and depression risk (19). Motivational salience of win and loss outcomes is indexed by the feedback-P3 (fb-P3), a centro-parietal positivity greater for good or more salient feedback (20, 21).
To date, the literature on depression and reward using ERPs has most often employed the ‘Doors Task’ (22) which, although ideally suited for evaluating responsiveness to win versus loss feedback via the ΔRewP, does not assess anticipation-related components of RR. Recently, there has been a growing call to examine other reward-related components in addition to the RewP/FN (17). Here, for the first time, we have modified an fMRI reward task, the ‘Cards Task’, commonly used in the adolescent depression literature (15) for use with EEG. This task isolates responses not only to win and loss outcomes, but also cues predictive of those outcomes. Only a handful of EEG studies have investigated both anticipation and outcome reward-related ERPs in relation to depression (23–25). Although these studies implicate both phases of RR in depression, the exact ERPs implicated often differ across studies. The common depression-related findings are blunted amplitude or latency for the fb-P3 as well as null effects of current depression on the RewP and cue-P3.
Another concern is that depression is often co-morbid with other forms of psychopathology that may also show disruptions in RR, such as externalizing disorders (26). Further, recent work in adults highlights the importance of considering both depression and externalizing dimensions when investigating RR (23). Thus, including externalizing symptoms as a covariate will be critical for evaluating the specificity of relationships between depression and RR in adolescence.
The current study capitalizes on rich longitudinally-assessed diagnostic information spanning ~13 years, beginning when children were 3 to 5 years of age—combined with EEG assessed later in adolescence—to examine relationships between ERP responses to both anticipation and receipt of wins and losses and depression/externalizing symptom severity at different phases of development (preschool, school-age, early-adolescence, and current). We addressed three main goals with this approach. First, we examined relationships between anticipation and outcome ERPs during adolescence using a modified version of the cards task, originally designed for and commonly used with fMRI, to probe reward anticipation/outcome responses in depression. Second, we examined effects of depression on RR above and beyond externalizing symptoms. We hypothesized that depression symptoms, specifically, would be related to blunted ERP responses to both winning and losing. Third, we examined how symptoms during distinct developmental stages might differentially impact reward responsiveness later in adolescence. We hypothesized that preschool and current depression symptoms would show unique relationships with RR.
2. Methods and Materials
2.1. Participants.
Adolescents from the Preschool Depression Study (PDS; 27) who completed the final follow-up session participated. The PDS is a longitudinal study at Washington University School of Medicine (WUSM) in St. Louis that initially recruited children aged 3.0–5.11 years old from primary caregivers, daycares, and preschools oversampling for depression using the Preschool Feelings Checklist (PFC; 28). The PFC is sensitive for preschool depressive symptoms but also identifies children with other disorders, including disruptive disorders (29). Children with elevated PFC scores (≥3) and scores of 0 (presumed healthy) were contacted for participation. Exclusion criteria included Autism Spectrum disorder (no other psychiatric disorders), chronic illness, speech, language or cognitive delays or neurological disorders. Children then underwent approximately annual diagnostic assessments over 12–15 years that were used to create dimensional symptom scores. Caregivers completed informed consent and child verbal/written assent was obtained before study participation. WUSM institutional review board approved all procedures. The current protocol was completed at the ninth follow-up assessment and of the 170 participants who completed the follow-up session, 158 consented to participate in the EEG, 152 of which completed the EEG (2 no longer interested, 1 due to hairstyle, 3 due to scheduling). After data processing and cleaning to ensure enough usable segments (discussed below), 6 participants were excluded and 3 had technical malfunctions. Of the 144 participants with usable data, 38 were enrolled in the study at later waves and thus symptom counts could not be calculated for preschool age and 6 had missing demographic covariate information from early sessions. This resulted in a sample of 100 participants (see Table 1 for demographic and clinical information and Table S6 for demographic and symptom comparisons between participants in the EEG assessment and the remaining study participants).
Table 1.
Demographic and Clinical Characteristics of the Study Participants (N = 100)
| Characteristic | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Age at EEG Assessment, Years | 16.49 | 0.94 | 14.35 | 18.32 |
| Sex, Female, n (%) | 48 (48%) | |||
| Race, White, n (%) | 55 (55%) | |||
| Baseline Income-to-Needs | 2.09 | 1.16 | 0 | 3.93 |
| Historical Depression Symptoms, Average of PAPA/CAPA Depression Core Symptoms | ||||
| Preschool (3.0–5.11 years) | 2.38 | 1.61 | 0 | 7 |
| School age (6.0–9.11 years) | 2.19 | 1.44 | 0 | 6 |
| Early adolescence (10.0–14.11 years) | 2.23 | 1.53 | 0 | 7 |
| Historical Externalizing Symptoms—Average of PAPA/CAPA Externalizing Dimensional Scores | ||||
| Preschool (3.0–5.11 years) | 6.60 | 6.10 | 0 | 31 |
| School age (6.0–9.11 years) | 5.67 | 5.65 | 0 | 24 |
| Early adolescence (10.0–14.11 years) | 4.31 | 5.55 | 0 | 25 |
| Current Symptoms, Sum of K-SADS Items | ||||
| Depression (core symptoms) | 1.73 | 2.45 | 0 | 9 |
| Externalizing (ADHD/ODD/CD screen items) | 0.23 | 0.53 | 0 | 3 |
| Depression Diagnosis, n | Yes | No | ||
| Preschool | 35 | 65 | ||
| School age/early adolescence | 45 | 55 | ||
| Current | 15 | 85 | ||
| Ever | 57 | 43 | ||
ADHD, attention-deficit/hyperactivity disorder; CAPA, Childhood and Adolescent Psychiatric Assessment; CD, conduct disorder; EEG, electroencephalography; K-SADS, Kiddie Schedule for Affective Disorders and Schizophrenia; ODD, oppositional defiant disorder; PAPA, Preschool-Age Psychiatric Assessment.
2.2. Measures.
2.2.1. Depression and Externalizing Severity Scores.
Dimensional depression and externalizing severity scores were created using diagnostic interviews of caregiver-administered Preschool-Age Psychiatric Assessment (PAPA; 30) through age 7, caregiver and child administered Childhood and Adolescent Psychiatric Assessment (CAPA; 31) from ages 8 and older, and caregiver and child administered Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; 32) at the final assessment. As is standard for the PAPA and CAPA, raters were first trained to reliability and all diagnostic assessments were audiotaped and 20% of tapes were reviewed by a master coder for reliability and discrepancies resolved in consultation with a senior child psychiatrist (J.L.L.). For the KSADS, interrater reliability for depression diagnoses was kappa=0.81, % agreement=93.2.
Depression severity scores were calculated by summing the number of nine core depression symptoms endorsed by the caregiver and/or child at each assessment. Externalizing severity scores were the combined sum scores of symptoms from the ADHD, ODD, and CD modules. Symptoms were considered present if either the parent or child endorsed them. For PAPA/CAPA, this included subthreshold, but for KSADS, symptoms were counted when endorsed at threshold To create mutually exclusive symptom scores for each symptom domain from each developmental period, means of symptoms were calculated for each participant from diagnostic interviews conducted when participants were within each of the following age ranges: preschool age = <6.0 years, school age = 6.0– 9 yrs, 11 months, early adolescence = 10–14 years, 11 months. ‘Early adolescence’ symptoms did not include symptoms from the current EEG assessment wave. Mean of symptoms from preschool up to, but not including, the current wave were used for past cumulative symptoms. Internal consistency scores for depression and externalizing severity scores during the preschool period were alpha= 0.62 and 0.92, respectively. Internal consistency scores for depression and externalizing severity scores across longitudinal assessments were alpha’s = 0.63 and 0.90, respectively. See Figure S2 for histograms depicting the distribution of all symptom measures and Table S5 for bivariate relationships between symptom measures.
2.2.2. Income to Needs Ratio.
At baseline caregivers reported on family income and the income-to-needs ratio was calculated as total family income divided by federal poverty level, based on family size at time of collection (33).
2.2.3. Cards Anticipation and Outcome ERP Reward Task.
Participants completed an EEG card-guessing task (Figure 1) that assessed responses during anticipation and receipt of reward feedback, modeled from a neuroimaging paradigm (34). Participants completed 72 trials across 2 blocks, with 24 likely win trials, 24 likely loss trials, 12 either win or lose trials, and 12 neither win nor lose trials. In total there were 22 possible instances of win feedback, 22 loss feedback, and 28 neutral feedback. Participants were told that their performance would determine a monetary reward after the scan, however, feedback outcomes were presented in a fixed order and were unrelated to the participants high/low value choice. Participants were told that they had “won” $17 following the task but specific values were not assigned to win and loss events. Participants with 10 or fewer usable trials for any of the cue/outcome types of interest were excluded from analyses (n=6) (35, 36). Descriptive statistics for usable segments and Cronbach’s alphas (internal consistency) for all ERPs are presented in Table S1.
Figure 1.
Trial structure for the Card Guessing Task. On each trial, a mystery card was presented during which the participant guessed whether the card’s value was high or low via button press (high or low guess was displayed following choice totaling 4000ms). Next one of four possible cue types were displayed for 2000ms. Cues indicated the type and probability of possible outcomes: likely win (followed by win 67% or neutral 33% of the time), likely loss (followed by loss 67% or neutral 33% of the time), either win or loss (each outcome 50%), or neutral (100% neutral). Cues were followed by 500ms of fixation. Next win, loss, or neutral outcomes were presented for 2000ms. Finally, participants were instructed to press a button to initiate the next trial. If participants did not make a high/low choice, they were told that they didn’t respond and saw fixation and dashes for the remainder of the trial. Following completion of the task, participants were told they “won” $17.
2.3. EEG Data Collection and Processing
Continuous EEG was recorded using the BrainVision ActiChamp, 32 channel active channel amplifier system (BrainVision LLC, Morrisville, NC, USA). Electrodes were mounted using a subset of the International 10/20 System using 32 sites with a ground electrode located at FPz. The electrooculogram (EOG) generated from blinks and eye movements were recorded from five facial electrodes placed around the eyes. Brain Vision Analyzer 2 software (Brain Products, Gilching, Germany) was used for off-line analysis. All data were re-referenced to the average of TP9 and TP10 and high-pass filtered using a 0.1 Hz half-power cutoff. The EEG was then segmented for each trial, from 200 milliseconds before anticipation cue and continuing for 1000 milliseconds. Segmentation for outcome events began 200 milliseconds before feedback and continued for 1000 milliseconds. The EEG was corrected for EOG artifacts (Gratton et al., 1983) and physiological artifacts removed using an automatic procedure with a maximum allowed voltage step of 50 μv within a 400 ms interval length, maximum absolute different between any two points of 175 μv, and a minimum allowed activity of 0.50 μv within a 100 ms interval length. Trials were then averaged and baseline corrected (−200 to 0 ms) and low-pass filtered from 30 Hz.
Anticipation ERPs were quantified separately for likely win and likely loss cues; outcome ERPs were quantified separately for win and loss feedback. Feedback ERPs include all win and loss outcomes irrespective of cue type as feedback valence effects (RewP/FN) did not significantly differ based on cue type (F(1,99) = 0.64, p = .43). Time windows for ERPs were based upon previous literature (35) and a visual inspection of grand average waveforms. The cue-P3 was quantified as mean activity at the Pz electrode site from 350–450 ms after onset of anticipation cues. The RewP/FN was quantified as mean activity at Fz from 250–350 ms following feedback. The feedback-P3 (fb-P3) was quantified as mean activity at Pz from 350–450 ms after feedback. See Table S2 for descriptive statistics for each ERP.
2.4. Statistical Analysis
All analyses were conducted using SPSS 26.0.0.0 (IBM Statistics). Paired samples t-tests tested for effects of anticipatory cue (likely win versus likely lose) and feedback type (win versus loss) for the cue-P3 and RewP/FN/fb-P3, respectively. Bivariate correlations evaluated relationships between components for win or loss.
For each component, a repeated measures ANOVA was conducted with mean amplitude as the dependent measure and cue/outcome valence (win or loss) as the within-subjects factor. Symptoms at four mutually exclusive periods in development (preschool, school-age, early-adolescence, and current) for both depression and externalizing dimensions were included simultaneously. ANOVAs also controlled for sex (1=male, 2=female), baseline income to needs ratio, and age at EEG assessment. Both main effects of symptoms and interactions between symptoms and cue/outcome valence are of interest and reported. This repeated measures ANOVA approach was chosen as it is the most conservative and parsimonious way to test whether specific symptom domains at specific developmental timepoints relate to ERPs in similar or different ways depending on cue/outcome valence, our main question of interest.
Significant interactions between cue/outcome valence and symptoms indicate that the relationship between symptoms and the ERP differ significantly depending on cue/outcome valence. Post hoc regressions (detailed below) are then necessary to identify direction of symptom relationships for each valence (win or loss) controlling for the alternative cue/outcome type. Interactions are particularly expected for the RewP/FN where the sign of this ERP differs for win and loss feedback (i.e. a ‘greater’ response to win is reflected by a more positive RewP whereas a ‘greater’ response to loss is reflected by a more negative FN). Main effects of symptoms suggest that symptoms relate significantly to the ERP in question irrespective of the cue/outcome valence – this would indicate greater responsiveness to feedback or cues generally. Post hoc regressions predicting the mean of win and loss are needed to identify the direction of this effect.
We conducted post hoc regressions for all significant symptom effects in the rmANOVAs. All covariates and symptom measures were regressed on 1) win and loss separately (controlling for the other type of cue/outcome) to examine significant interactions with valence from the rmANOVAs or 2) on the mean of win and loss to examine significant main effects of symptoms. Supplemental bivariate correlations between symptom measures and ERPs were conducted for descriptive purposes, with several correlated symptom measures in the ANOVA/regression analyses, it was possible that individual symptom effects that might be masked or inflated.
False discovery rate (FDR) correction (Benjamini-Hochberg procedure) was applied across the three ANOVAs to determine whether nominally significant main effects of symptoms or interactions between symptoms and cue/outcome valence remained significant after correcting for multiple comparisons.
3. Results
3.1. Group Average Anticipatory ERPs.
The cue-P3 was maximal at Pz with a significantly greater positivity observed for likely win than likely lose cues, t(99)=6.55, p<.001 (Figure 2A).
Figure 2.
Anticipation (A) and outcome (B/C) ERPs and scalp topographies. ERP waveforms are at electrodes Fz (B) or Pz (A/C). Cue or outcome onset was at 0 ms. Shaded regions indicate the time windows during which each ERP was scored.
3.2. Group Average Consummatory ERPs.
The RewP/FN was maximal at frontal midline sites with a significantly greater positivity to win than loss feedback, t(99)=4.76, p<.001 (Figure 2B). The fb-P3 was maximal at Pz with a significantly greater positivity observed for win than loss feedback, t(99) = 3.96, p < .001 (Figure 2C).
The RewP/FN and fbP3 were significantly correlated for residual response to both win feedback (r = .34, p < .001) and loss feedback (r = .31, p = .002). All other relationships were non-significant (Table S3).
3.3. Depression Symptom Effects.
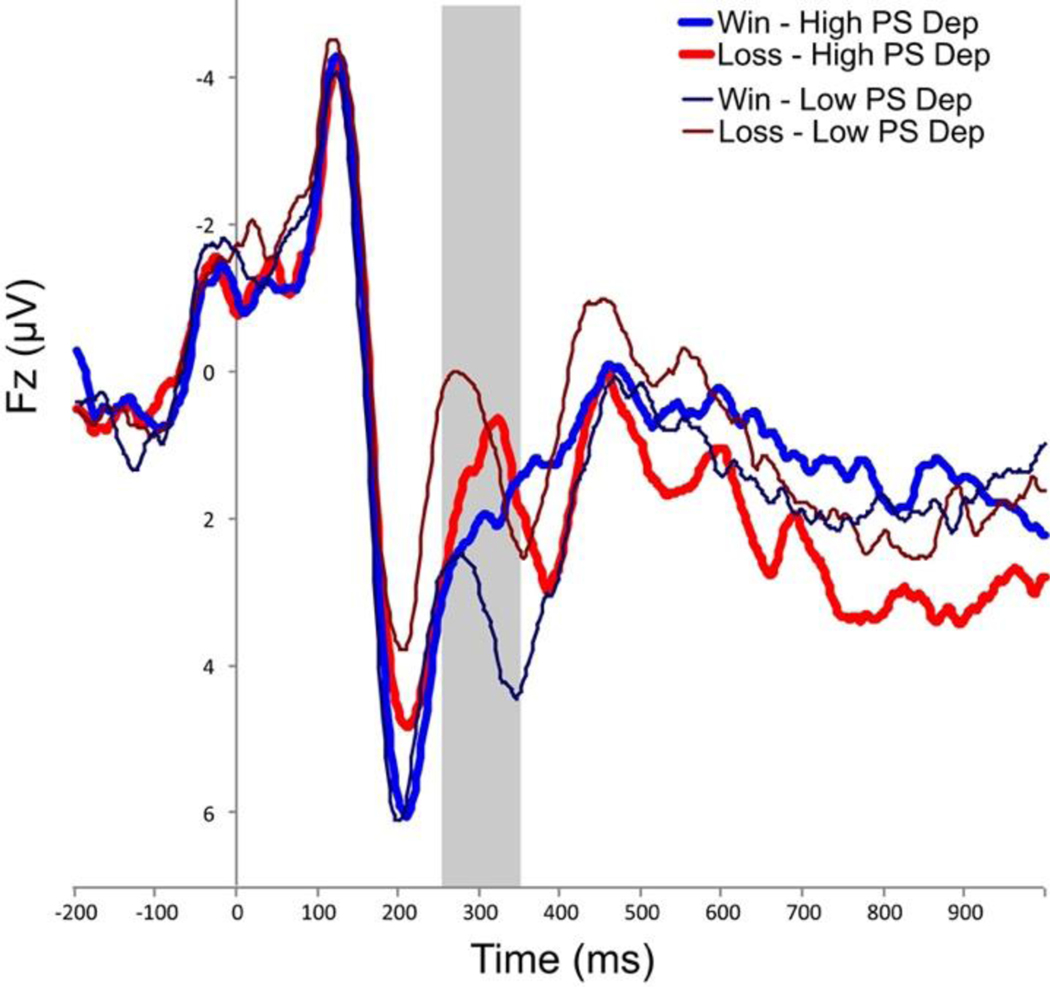
The RewP/FN showed a significant interaction between depression symptoms and feedback valence. Preschool depression symptoms, specifically, significantly interacted with feedback valence (win versus loss) for the RewP/FN (Table 2, Figure 3); this relationship was also observed in bivariate correlation (Table S4). Post hoc regressions to follow up on the significant interaction showed that preschool depression significantly predicted a less positive response to win feedback, indicating blunted response to win, and a less negative response to loss feedback, also indicating a blunted response to loss (Table 3; Figure S1).
Table 2.
Repeated-Measures Analysis of Variance Outputs
| Effect | Cue-P3 | RewP/FN | fb-P3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p (pcorr) | F | p (pcorr) | F | p (pcorr) | ||||
| Valence | 0.75 | .387 | .01 | 1.08 | .302 | .01 | 0.12 | .727 | <.01 |
| Valence×PS DEP | 0.23 | .631 (.631) | <.01 | 6.72 | .011 (.033)a | .07 | 1.35 | .249 (.373) | .02 |
| Valence×SA DEP | 0.15 | .696 | <.01 | 0.12 | .732 | <.01 | 0.50 | .484 | .01 |
| Valence×AD DEP | 4.68 | .033 (.099) | .05 | 0.38 | .540 (.540) | <.01 | 1.31 | .256 (.384) | .02 |
| Valence×Current DEP | 0.31 | .578 | <.01 | 0.14 | .705 | <.01 | 0.11 | .746 | <.01 |
| Valence×PS EXTL | 0.37 | .544 | <.01 | 0.01 | .915 | <.01 | <0.01 | .961 | <.01 |
| Valence×SA EXTL | 0.82 | .369 | .01 | 0.92 | .341 | .01 | 0.05 | .831 | <.01 |
| Valence×AD EXTL | 3.76 | .056 | .04 | 0.98 | .325 | .01 | 1.88 | .174 | .02 |
| Valence×Current EXTL | 1.30 | .257 | .02 | 0.26 | .609 | <.01 | 0.54 | .463 | .01 |
| Valence×Sex | 0.14 | .711 | <.01 | 0.07 | .796 | <.01 | 2.75 | .101 | .03 |
| Valence×Age | 1.78 | .186 | .02 | 2.88 | .093 | .03 | 0.45 | .506 | .01 |
| Valence×Income/Need | 0.26 | .613 | <.01 | 2.10 | .151 | .02 | 1.90 | .172 | .02 |
| PS DEP | 0.40 | .529 | .01 | 0.42 | .518 | .01 | 0.18 | .670 | .00 |
| SA DEP | 1.51 | .223 | .02 | 0.03 | .870 | <.01 | 1.45 | .232 | .02 |
| AD DEP | 0.29 | .592 | <.01 | 1.52 | .221 | .02 | 1.31 | .255 | .02 |
| Current DEP | <0.01 | .956 | <.01 | 0.08 | .780 | <.01 | 0.01 | .946 | <.01 |
| PS EXTL | 0.63 | .428 | .01 | 3.39 | .069 | .04 | 1.83 | .179 | .02 |
| SA EXTL | 2.74 | .102 | .03 | 1.09 | .300 | .01 | 3.59 | .062 | .04 |
| AD EXTL | 0.79 | .378 | .01 | 0.17 | .678 | <.01 | 0.31 | .581 | <.01 |
| Current EXTL | 0.84 | .362 (.362) | .01 | 7.79 | .006 (.018)a | .08 | 3.73 | .057 (.085) | .04 |
| Sex | 0.62 | .435 | .01 | 0.51 | .475 | .01 | 0.06 | .801 | <.01 |
| Age at EEG | 0.58 | .449 | .01 | 0.56 | .456 | .01 | 1.08 | .303 | .01 |
| Income/Need | 0.22 | .640 | <.01 | 0.03 | .854 | <.01 | 0.04 | .851 | <.01 |
AD, early adolescence; DEP, depression; EEG, electroencephalogram; EXTL, externalizing; fb, feedback; pcorr, false discovery rate-corrected p value; PS, preschool; RewP/FN, reward positivity/feedback negativity; SA, school age.
p < .05.
Figure 3.
Outcome locked event-related potential waveforms at Fz for individuals with upper tertile (bold lines) versus lower tertile (thin lines) preschool (PS) depression (Dep) symptoms. Time window for the RewP/FN (250–350ms) is highlighted in gray.
Table 3.
Post Hoc Regressions Investigating Significant Effects From Repeated-Measure Analyses of Variance
| Dependent Variable | Predictor | Cue-P3 | RewP/FN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | Std. β | t | P | B | SE | Std. β | t | p | ||
| Win | (Constant) | −5.80 | 7.56 | −0.77 | .445 | −7.32 | 6.99 | −1.05 | .298 | ||
| PS DEP | −0.17 | 0.39 | −.04 | −0.43 | .671 | −0.81 | 0.38 | −.25 | −2.14 | .035a | |
| SA DEP | 0.15 | 0.52 | .03 | 0.30 | .767 | 0.13 | 0.48 | .04 | 0.27 | .791 | |
| AD DEP | −1.00 | 0.47 | −.22 | −2.15 | .035a | 0.09 | 0.46 | .03 | 0.20 | .839 | |
| Current DEP | 0.11 | 0.20 | .04 | 0.55 | .585 | −0.08 | 0.19 | −.04 | −0.43 | .671 | |
| PS EXTL | 0.06 | 0.12 | .06 | 0.54 | .592 | −0.07 | 0.11 | −.08 | −0.62 | .539 | |
| SA EXTL | −0.13 | 0.17 | −.11 | −0.75 | .454 | −0.09 | 0.16 | −.10 | −0.57 | .573 | |
| AD EXTL | 0.30 | 0.17 | .24 | 1.81 | .074a | 0.12 | 0.16 | .13 | 0.78 | .439 | |
| Current EXTL | −1.03 | 0.99 | −.08 | −1.04 | .301 | 1.17 | 0.95 | .12 | 1.23 | .223 | |
| Age | 0.56 | 0.45 | .08 | 1.24 | .219 | 0.75 | 0.43 | .14 | 1.76 | .082 | |
| Income/Need | 0.21 | 0.40 | .04 | 0.53 | .6 | −0.53 | 0.39 | −.12 | −1.37 | .173 | |
| Sex | 0.27 | 0.87 | .02 | 0.31 | .758 | 0.03 | 0.83 | .00 | 0.03 | .976 | |
| Loss ERP | 0.96 | 0.07 | .84 | 13.66 | <.001 | 0.76 | 0.09 | .69 | 8.57 | <.001 | |
| Model Statistics | R2 = .71, adj. R2 = .67, F = 17.26, p < .001 | R2 = .53, adj. R2 = .47, F = 8.19, p < .001 | |||||||||
| Loss | (Constant) | 11.70 | 6.42 | 1.82 | .072 | 5.52 | 6.29 | 0.88 | .383 | ||
| PS DEP | 0.27 | 0.33 | .07 | 0.81 | .422 | 0.92 | 0.34 | .32 | 2.74 | .007a | |
| SA DEP | −0.46 | 0.44 | −.11 | −1.03 | .308 | −0.18 | 0.43 | −.05 | −0.40 | .687 | |
| AD DEP | 0.75 | 0.41 | .19 | 1.85 | .068 | −0.49 | 0.41 | −.16 | −1.20 | .234 | |
| Current DEP | −0.09 | 0.17 | −.04 | −0.52 | .602 | 0.04 | 0.17 | .02 | 0.21 | .835 | |
| PS EXTL | −0.11 | 0.10 | −.11 | −1.02 | .312 | −0.09 | 0.10 | −.11 | −0.83 | .409 | |
| SA EXTL | 0.26 | 0.15 | .24 | 1.74 | .085 | 0.20 | 0.14 | .25 | 1.42 | .159 | |
| AD EXTL | −0.34 | 0.14 | −.31 | −2.38 | .019a | −0.16 | 0.14 | −.19 | −1.13 | .26 | |
| Current EXTL | 1.36 | 0.84 | .12 | 1.61 | .112 | 0.79 | 0.86 | .09 | 0.92 | .363 | |
| Age | −0.67 | 0.39 | −.11 | −1.72 | .09 | −0.46 | 0.39 | −.09 | −1.18 | .24 | |
| Income/Need | −0.09 | 0.35 | −.02 | −0.26 | .798 | 0.44 | 0.35 | .11 | 1.25 | .215 | |
| Sex | −0.58 | 0.75 | −.05 | −0.78 | .439 | −0.45 | 0.74 | −.05 | −0.61 | .545 | |
| Win ERP | 0.71 | 0.05 | .81 | 13.66 | <.001 | 0.61 | 0.07 | .67 | 8.57 | <.001 | |
| Model Statistics | R2 = .71, adj. R2 = .67, F = 18.05; p < .001 | R2 = .54, adj. R2 = .48, F = 8.49; p < .001 | |||||||||
| Mean Win and Loss | (Constant) | −1.94 | 8.23 | −0.24 | .815 | ||||||
| PS DEP | 0.29 | 0.44 | .10 | 0.65 | .518 | ||||||
| SA DEP | −0.09 | 0.57 | −.03 | −0.16 | .87 | ||||||
| AD DEP | −0.65 | 0.53 | −.22 | −1.23 | .221 | ||||||
| Current DEP | −0.06 | 0.22 | −.03 | −0.28 | .78 | ||||||
| PS EXTL | −0.24 | 0.13 | −.33 | −1.84 | .069 | ||||||
| SA EXTL | 0.20 | 0.19 | .25 | 1.04 | .3 | ||||||
| AD EXTL | −0.08 | 0.18 | −.09 | −0.42 | .678 | ||||||
| Current EXTL | 3.03 | 1.09 | .36 | 2.79 | .006 | ||||||
| Age | 0.38 | 0.50 | .08 | 0.75 | .456 | ||||||
| Income/Need | −0.08 | 0.46 | −.02 | −0.19 | .854 | ||||||
| Sex | −0.70 | 0.97 | −.08 | −0.72 | .475 | ||||||
| Model Statistics | R2 = .14, adj. R2 = .04, F = 1.32, p = .228 | ||||||||||
AD, early adolescence; adj., adjusted; DEP, depression; ERP, event-related potential; EXTL, externalizing; PS, preschool; RewP/FN, reward positivity/feedback negativity; SA, school age; Std., standardized.
p < .05.
Early-adolescent depression symptoms, specifically, interacted with cue valence (likely win versus likely loss) for the cue-P3 (Table 2) – this relationship only approach significance after correcting for multiple corrections. In post hoc regressions early-adolescent depression predicted blunted response to likely win cues, but not likely loss cues (Table 3).
No significant effects of symptoms at specific ages were observed for the fb-P3 in ANOVA/regression analyses with depression symptoms different developmental timepoints as simultaneous predictors (Tables 2–3). However, in bivariate correlations increased depression related to blunted fb-P3 with a similar effect size across developmental timepoints (Table S4). Further, exploratory bivariate correlations showed that cumulative past depression predicted a blunted fb-P3 (Table S4). A further exploratory regression showed that this effect remained when controlling for past cumulative externalizing symptoms and demographic covariates (β = −0.35, p = .023; Table S7).
3.4. Externalizing Symptom Effects.
The cue-P3 and RewP/FN also showed significant effects of externalizing symptoms; no significant effects were observed for the fb-P3. Early-adolescent externalizing symptoms, specifically, significantly interacted with cue valence for the cue-P3 (Table 2). Post hoc regressions showed that early-adolescent externalizing symptoms significantly predicted blunted response to likely lose cues but not response to likely win cues (Table 3). These effects were not observed in the bivariate correlations (Table S4, Figure 3B).
A significant main effect of current externalizing symptoms was observed for the RewP/FN (Table 2). Post hoc regressions showed that current externalizing symptoms positively predicted the RewP/FN across feedback types indicating potentiated response to win and blunted response to loss outcomes (Table 3).
4. Discussion
We modified a well-known reward processing paradigm, the cards task, which has been used extensively with fMRI in the adolescent depression literature (12, 34) for use with EEG. Similar to work in adults, we identified a cue-P3 that was larger for anticipatory cues indicating likely wins versus likely losses, as well as the RewP/FN and fb-P3 as ERPs sensitive to feedback about wins versus losses (17, 23, 35).
Next, drawing on the strength of prospective longitudinal clinician ratings of depression and externalizing symptoms, we investigated how these ERPs, assessed in later adolescence, related to depression and externalizing symptoms experienced at specific periods earlier in development. We found that depression symptoms predicted blunted response to wins during both anticipation (at trend level) and outcome processing as well as blunted response to loss outcomes -- this general pattern is consistent with the extant fMRI and ERP literatures (37, 38). However, there were both general and age-specific effects of depression on RR which depended on the ERP in question.
Depression symptoms experienced during the preschool period were associated with blunted responsiveness during initial responding to both loss and win outcomes (FN and RewP). This relates to fMRI findings from our lab using the same sample where preschool depression specifically predicted adolescent blunted response to reward cues within a distributed network of reward-sensitive regions including the striatum and anterior cingulate cortex (12). These regions are both likely neural generators for the outcome-related RewP/FN (39, 40). Blunted RewP/FN is one of the most frequently reported findings in the depression literature, however it has been suggested that this component may function more as an underlying depression risk-factor than a reflection of current symptoms (2). Blunted RewP/FN may also be more evident during simple gambling tasks rather than anticipation/outcome paradigms (41). Further, studies using similar anticipation/outcome tasks have reported null direct effects of current depression on the RewP/FN (23–25). However, our results do add to a growing literature suggesting that early experiences linked to depression liability also impact RR observed later in life (9, 12, 13, 42). Collectively, findings across studies suggest that early life, including the preschool period, may be a particular window of vulnerability where depression may negatively impact reward system function and development. This interpretation, as well as others – for example, depression symptoms in preschool may reflect a more heritable form of depression with stronger links to adolescent anhedonia, with this underlying genetic risk driving both preschool depression and blunted adolescent RewP/FN - should be investigated in future work assessing both symptoms and RR in concert across development.
Early-adolescent depression, above and beyond symptoms at other ages, predicted blunted cue-P3, possibly reflecting reduced motivated attention specifically to cues signaling likely reward. The few studies investigating the cue-P3 in depression have reported null results for amplitude (23, 24); there is evidence for prolonged latency in adolescent depression (25). However, a blunted P3 to salient stimuli in other task contexts, particularly oddball tasks, is a common finding with a number of types of psychopathology, including depression and externalizing disorders (43, 44). Here we show some interesting specificity to depression versus externalizing symptoms, the latter predicted blunted P3 response to cues signaling a likely loss. Given that depression and externalizing are often comorbid, considering both types of symptoms simultaneously may be particularly important for future studies.
Blunted fb-P3, thought to index motivated attention to win/loss outcomes, was also related to depression, but not at a specific developmental stage. Instead we observed a general effect where depression at each developmental period, as well as cumulative past depression, predicted a blunted fb-P3 for both win and loss feedback. Previous fMRI work from our lab demonstrates that cumulative depression, versus current depression, predicted blunted response to reward in a distributed network of reward sensitive regions (12). This is particularly interesting given that the fb-P3 likely reflects activation within a large and distributed set of neural generators, including the lateral frontal cortex, parietal cortex, and medial temporal lobe (45).
Although the current study has many strengths, it also has limitations. Namely, neural RR was assessed only once in late adolescence precluding analysis of symptom effects on the trajectory of RR over development. Further, diagnostic assessments shifted to the K-SADS at the timepoint when ERPs were assessed, and depression symptoms were more skewed at this timepoint than at earlier ages, which could have influenced historical versus current symptom findings. It is also important to consider that our analytical approach focuses on unique effects of symptoms experienced at different ages on RR – this residualizing of effects is useful analytically and conceptually but may not map onto lived experience of these disorders which typically covary across domains and continue across development. Finally, there are not increases in depression symptoms from childhood to adolescence, as is typically observed in community samples – this is likely because the sample was enriched for depressive symptoms during preschool, and as such, experienced some regression to the mean of symptoms as the children developed. However, this atypical distribution of symptoms across development should be noted and further underscores the need for replication of these findings in other community-based samples.
Blunted RR during both anticipation and outcome phases of reward processing have been implicated in depression and depression risk (19, 37). However, this is the first ERP evidence that depression symptoms experienced during different developmental periods show unique relationships with blunted RR during different phases of reward processing. More work is needed to investigate how these ERPs may act, either alone or in concert, when predicting trajectories of future depression and externalizing symptoms across the high-risk adolescent years.
Supplementary Material
Acknowledgments:
This study was supported by grants 2R01 MH064769–06 and R01 MH098454 (JLL and DMB).
Footnotes
Data Availability Statement; Data supporting these findings are available from the corresponding author by request.
Conflict of Interest Statement: Dr. Luby receives royalties from Guilford Press. The other authors report no financial relationships with commercial interests.
References
- 1.Burani K, Mulligan EM, Klawohn J, Luking KR, Nelson BD, Hajcak G (2019): Longitudinal increases in reward-related neural activity in early adolescence: Evidence from event-related potentials (ERPs). Developmental cognitive neuroscience. 36:100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kujawa A, Burkhouse KL (2017): Vulnerability to Depression in Youth: Advances from Affective Neuroscience. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luking KR, Pagliaccio D, Luby JL, Barch DM (2016): Reward Processing and Risk for Depression Across Development. Trends in cognitive sciences. 20:456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitton AE, Kakani P, Foti D, Van’t Veer A, Haile A, Crowley DJ, et al. (2016): Blunted neural responses to reward in remitted major depression: A high-density event-related potential study. Biol Psychiatry Cogn Neurosci Neuroimaging. 1:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg A, Huiting L, Hajcak G, Shankman SA (2015): Blunted Neural Response to Rewards as a Vulnerability Factor for Depression: Results From a Family Study. Journal of abnormal psychology. 124:878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kujawa A, Proudfit GH, Klein DN (2014): Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of abnormal psychology. 123:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foti D, Kotov R, Klein DN, Hajcak G (2011): Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. Journal of abnormal child psychology. 39:913–924. [DOI] [PubMed] [Google Scholar]
- 8.Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G (2016): Blunted Neural Response to Rewards Prospectively Predicts the Development of Depression in Adolescent Girls. American Journal of Psychiatry. [Google Scholar]
- 9.Birn RM, Roeber BJ, Pollak SD (2017): Early childhood stress exposure, reward pathways, and adult decision making. Proceedings of the National Academy of Sciences of the United States of America. 114:13549–13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novick AM, Levandowski ML, Laumann LE, Philip NS, Price LH, Tyrka AR (2018): The effects of early life stress on reward processing. Journal of psychiatric research. 101:80–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luby JL, Baram TZ, Rogers CE, Barch DM (2020): Neurodevelopmental Optimization after Early-Life Adversity: Cross-Species Studies to Elucidate Sensitive Periods and Brain Mechanisms to Inform Early Intervention. Trends in neurosciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rappaport BI, Kandala S, Luby JL, Barch DM (2020): Brain Reward System Dysfunction in Adolescence: Current, Cumulative, and Developmental Periods of Depression. The American journal of psychiatry.appiajp201919030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, et al. (2013): Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 249:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, et al. (2016): Neural Correlates of Reward Processing in Depressed and Healthy Preschool-Age Children. Journal of the American Academy of Child and Adolescent Psychiatry. 55:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbes EE (2011): fMRI studies of reward processing in adolescent depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 36:372–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes EE, Dahl RE (2012): Research Review: altered reward function in adolescent depression: what, when and how? Journal of child psychology and psychiatry, and allied disciplines. 53:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glazer JE, Kelley NJ, Pornpattananangkul N, Mittal VA, Nusslock R (2018): Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 132:184–202. [DOI] [PubMed] [Google Scholar]
- 18.Pfabigan DM, Seidel EM, Sladky R, Hahn A, Paul K, Grahl A, et al. (2014): P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: an EEG and fMRI experiment. NeuroImage. 96:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proudfit GH (2015): The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology. 52:449–459. [DOI] [PubMed] [Google Scholar]
- 20.Polich J (2007): Updating P300: an integrative theory of P3a and P3b. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 118:2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.San Martin R, Appelbaum LG, Pearson JM, Huettel SA, Woldorff MG (2013): Rapid brain responses independently predict gain maximization and loss minimization during economic decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 33:7011–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajcak G, Moser JS, Holroyd CB, Simons RF (2007): It’s worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 44:905–912. [DOI] [PubMed] [Google Scholar]
- 23.Novak BK, Novak KD, Lynam DR, Foti D (2016): Individual differences in the time course of reward processing: Stage-specific links with depression and impulsivity. Biological psychology. 119:79–90. [DOI] [PubMed] [Google Scholar]
- 24.Ait Oumeziane B, Jones O, Foti D (2019): Neural Sensitivity to Social and Monetary Reward in Depression: Clarifying General and Domain-Specific Deficits. Frontiers in behavioral neuroscience. 13:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landes I, Bakos S, Kohls G, Bartling J, Schulte-Korne G, Greimel E (2018): Altered neural processing of reward and punishment in adolescents with Major Depressive Disorder. Journal of affective disorders. 232:23–33. [DOI] [PubMed] [Google Scholar]
- 26.Baskin-Sommers AR, Foti D (2015): Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 98:227–239. [DOI] [PubMed] [Google Scholar]
- 27.. Luby JL, Si X, Belden AC, Tandon M, Spitznagel E (2009): Preschool depression: homotypic continuity and course over 24 months. Archives of general psychiatry. 66:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.. Luby JL, Heffelfinger A, Koenig-McNaught AL, Brown K, Spitznagel E (2004): ThePreschool Feelings Checklist: a brief and sensitive screening measure for depression in young children. Journal of the American Academy of Child and Adolescent Psychiatry. 43:708–717. [DOI] [PubMed] [Google Scholar]
- 29.Belden AC, Thomson NR, Luby JL (2008): Temper tantrums in healthy versus depressed and disruptive preschoolers: defining tantrum behaviors associated with clinical problems. The Journal of pediatrics. 152:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.. Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A (2006): Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA). Journal of the American Academy of Child and Adolescent Psychiatry. 45:538–549. [DOI] [PubMed] [Google Scholar]
- 31.. Angold A, Costello EJ (2000): The Child and Adolescent Psychiatric Assessment (CAPA). Journal of the American Academy of Child and Adolescent Psychiatry. 39:39–48. [DOI] [PubMed] [Google Scholar]
- 32.. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- 33.McLoyd VC (1998): Socioeconomic disadvantage and child development. Am Psychol. 53:185–204. [DOI] [PubMed] [Google Scholar]
- 34.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. (2009): Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American journal of psychiatry. 166:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novak KD, Foti D (2015): Teasing apart the anticipatory and consummatory processing of monetary incentives: An event-related potential study of reward dynamics. Psychophysiology. 52:1470–1482. [DOI] [PubMed] [Google Scholar]
- 36.Levinson AR, Speed BC, Infantolino ZP, Hajcak G (2017): Reliability of the electrocortical response to gains and losses in the doors task. Psychophysiology. 54:601–607. [DOI] [PubMed] [Google Scholar]
- 37.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J (2013): The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. Journal of affective disorders. 151:531–539. [DOI] [PubMed] [Google Scholar]
- 38.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. (2018): Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. The American journal of psychiatry. 175:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foti D, Weinberg A, Bernat EM, Proudfit GH (2014): Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G (2011): Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. NeuroImage. 57:1608–1616. [DOI] [PubMed] [Google Scholar]
- 41.Moran TP, Schroder HS, Kneip C, Moser JS (2017): Meta-analysis and psychophysiology: A tutorial using depression and action-monitoring event-related potentials. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 111:17–32. [DOI] [PubMed] [Google Scholar]
- 42.Pechtel P, Pizzagalli DA (2011): Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 214:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajcak G, Foti D (2020): Significance?... Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology.e13570. [DOI] [PubMed] [Google Scholar]
- 44.Iacono WG, Malone SM, McGue M (2003): Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 48:147–178. [DOI] [PubMed] [Google Scholar]
- 45.Soltani M, Knight RT (2000): Neural origins of the P300. Crit Rev Neurobiol. 14:199–224. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.