Abstract
SARS-CoV-2 survivors may report persistent symptoms that resemble myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). We explored (a) ME/CFS-like symptom prevalence and (b) whether axonal, inflammatory, and/or lung changes may contribute to ME/CFS-like symptoms in SARS-CoV-2 survivors through clinical, neuropsychiatric, neuropsychological, lung function assessment, and serum neurofilament light chain, an axonal damage biomarker. ME/CFS-like features were found in 27% of our sample. ME/CFS-like group showed worse sleep quality, fatigue, pain, depressive symptoms, subjective cognitive complaints, Borg baseline dyspnea of the 6-min walking test vs. those without ME/CFS-like symptoms. These preliminary findings raise concern on a possible future ME/CFS-like pandemic in SARS-CoV-2 survivors.
Keywords: Cognition, Mood alterations, COVID-19, Fatigue, Functional neurological disorders, Viral infection
Introduction
COVID-19 long-haulers fail to revert to normal routine after SARS-CoV-2 infection and report persistent debilitating symptoms, i.e., fatigue, “brain fog”, pain, disrupted sleep, and mood alterations (Rubin 2020).
Chronic COVID-19 may resemble myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a well-documented post-viral condition (Rubin 2020) affecting 0.5–2.5% of the population (Johnston et al. 2013). ME/CFS is characterized by severe post-exertional fatigue not improving with rest, subjective/objective cognitive difficulties, pain, sleep abnormalities, neurosensory, immune, gastro-intestinal disturbances, and cellular energy metabolism dysfunction (Carruthers et al. 2011). Several ME/CFS inconclusive pathophysiological hypotheses have been proposed, including immunological changes (Carruthers et al. 2011). ME/CFS cognitive complaints have been suggested to share pathogenetic commonalities with functional neurological disorders (Teodoro et al. 2018).
Studies in survivors of other coronavirus infections, namely, Severe Acute Respiratory Syndrome (SARS) and Middle East respiratory syndrome, documented a ME/CFS-like picture (Lam et al. 2009; Lee et al. 2019), supporting a link with viral infection (Ahmed et al. 2020), but data on SARS-CoV-2 are preliminary (Townsend et al. 2020).
Serum levels of neurofilament light chain (NfL), a biomarker of axonal damage, may be increased during COVID-19 acute phase (Aamodt et al. 2021), even in the absence of overt neurological signs, suggesting a possible SARS-CoV-2 neuronal trophism (Mariotto et al. 2020). The possible association between chronic fatigue and serum NfL levels has not been determined in this condition, yet.
Persistent lung impairment has been reported after SARS-CoV-2 infection and might contribute to chronic COVID-19 (Rubin 2020).
Our aims were to explore (a) the extent of ME/CFS-like symptoms and (b) whether baseline inflammatory markers, and axonal, and/or lung abnormalities may contribute to ME/CFS-like symptoms in COVID-19 survivors. To this aim, a group of COVID-19 survivors underwent a multidimensional evaluation, including neuropsychiatric, neuropsychological, lung function assessment, and NfL serum level measurement.
Methods
Subjects
Inpatients and outpatients positive to SARS-CoV-2 PCR testing in February–May 2020 were recruited at the Department of Internal Medicine, Verona University Hospital, Italy, after informed consent.
Inclusion criteria include (a) age 18–65 years; (b) no history of neurological, cerebrovascular, psychiatric disorders, or substance use disorders that might interfere with cognition; (c) > 6-month follow-up after SARS-CoV-2 infection; (d) negative nasopharyngeal swab test; and (e) no history of fatigue before SARS-CoV-2 infection.
Socio-demographic data, baseline comorbidities, and clinical and inflammatory features of SARS-CoV-2 infection features were collected. The study was approved by the local ethic committee (#2785CESC).
ME/CFS-like symptoms
ME/CFS-like symptoms were assessed according to the ME International Consensus Criteria (Carruthers et al. 2011) with a semi-structured clinical interview. Patients were classified as typical ME/CFS-like if they met (a) post-exertional neuroimmune exhaustion criteria, (b) ≥ 1 neurological impairment symptom, (c) ≥ 1 immune/gastro-intestinal/genitourinary impairment symptom, and (d) ≥ 1 energy metabolism/transport impairment symptom. Patients meeting post-exertional neuroimmune exhaustion criteria but 1–2 of the remaining criteria (points b–d) were classified as atypical ME/CFS-like. Typical and atypical ME/CFS-like patients were lumped in the ME/CFS + group, while other patients represented the ME/CFS − group.
Neuropsychiatric symptoms
Fatigue was explored with the Multidimensional Fatigue Inventory (MFI). The Pittsburgh Sleep Quality Index (PSQI) evaluated sleep quality over the previous month. The Hospital Anxiety and Depression Scale (HADS) assessed mood changes. Pain severity was evaluated with a 0–10 Numerical Rating Scale.
Neuropsychological assessment
Subjective cognitive complaints (e.g., attention, concentration, and memory difficulties) were assessed through patients’ reports.
Global cognition was explored with the Montreal Cognitive Assessment (MoCA). A reduced neuropsychological test battery was administered to assess the most frequently affected cognitive domains in ME/CFS. Attention and psychomotor speed were evaluated with the Symbol Digit Test and executive function was explored with the Stroop test.
Neurofilament light chain
Investigators blinded to clinical data measured serum NfL levels in duplicated using SIMOA Nf-light kit in SR-X immunoassay analyser, Simoa (Quanterix Corporation, Billerica, Massachusetts, USA), which runs ultrasensitive paramagnetic bead-based enzyme-linked immunosorbent assays (Mariotto et al. 2019). Age-matched healthy controls and normative NfL values were used as comparison (Hviid et al. 2020).
Lung function
Lung function was performed according to the International Recommendations (Miller et al. 2005). A flow-sensing spirometer (Jaeger MasterScreen PFT System) was used. Forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and total lung capacity (TLC) were recorded. FEV1/FVC ratio was taken as index of airflow obstruction. Carbon monoxide diffusion capacity (DLCO) and carbon monoxide transfer coefficient (KCO) were measured by single breath method. FEV1, FVC, TLC, DLCO, and KCO were expressed as percentage of the predicted values. The arterial blood partial pressure of carbon dioxide and oxygen was measured. Walking capacity was assessed by 6-min walking test (6MWT) according to the reference equation for healthy adults, and the individual’s perceived dyspnea and fatigue at baseline and at end-effort were measured with a 10-point modified Borg scale.
Statistical analysis
Continuous variables were assessed with non-parametric Mann–Whitney U test. Chi square test was applied to categorical variables. Statistical significance was set at p < 0.05.
Results
After screening the initial cohort (N = 131) for eligibility (Fig. 1), we included 37 patients (age 51.9 ± 10.9; 25 men, 12 women), of whom 10 were classified as ME/CFS + (age 50.7 ± 12.3; 5 women) and 27 as ME/CFS- (age 52.3 ± 10.5; 7 women; Table 1).
Fig. 1.
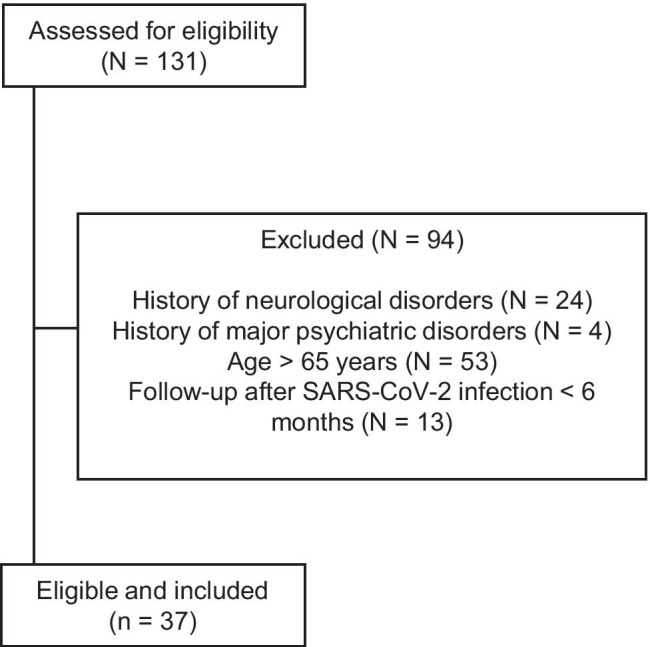
Flow diagram of the study and reasons for patients’ exclusion
Table 1.
Socio-demographic and clinical characteristics
| Characteristics | All (n = 37) | ME/CFS + (n = 10) | ME/CFS − (n = 27) | P valuec |
|---|---|---|---|---|
| Socio-demographic | ||||
| Gender (men/women) | 25/12 | 5/5 | 20/7 | 0.17 |
| Agea | 51.9 ± 10.9, 54 | 50.7 ± 12.3, 55.5 | 52.3 ± 10.5 | 0.90 |
| Education (y)a | 12.9 ± 3.4, 13 | 14.5 ± 3.1, 15 | 12.3 ± 3.3, 13 | 0.07 |
| BMIa | 26.8 ± 4.3, 26.4 | 27.0 ± 4.4, 26.4 | 26.2 ± 4.2, 26.3 | 0.90 |
| Baseline Comorbidities (yes/no) | ||||
| Hypertension | 9/28 | 4/6 | 5/22 | 0.36 |
| Cardiac disease | 2/35 | 1/9 | 1/26 | 1.00 |
| Diabetes | 1/36 | 0/10 | 1/26 | 1.00 |
| Cancer | 1/36 | 0/10 | 1/26 | 1.00 |
| COPD | 2/35 | 1/9 | 1/26 | 1.00 |
| Kidney disease | 1/36 | 0/10 | 1/26 | 1.00 |
| Liver disease | 0/37 | 0/10 | 0/26 | 1.00 |
| Psychiatricb | 4/33 | 0/10 | 4/23 | 0.49 |
| SARS-CoV-2 infection—Clinical | ||||
| Hospitalization (yes/no) | 33/4 | 8/2 | 25/2 | 0.62 |
| Months since infectiona | 6.1 ± 0.3, 6.0 | 6.1 ± 0.4, 6.0 | 6.0 ± 0.1, 6.0 | 0.54 |
| ICU (yes/no) | 8/29 | 3/7 | 5/22 | 0.76 |
| O2 therapy (yes/no) | 22/15 | 4/6 | 18/9 | 0.28 |
| SARS-CoV-2 infection—Inflammatory | ||||
| C-reactive protein (mg/L) | 72.4 ± 52.1, 70 | 72.9 ± 68.2, 83 | 72.3 ± 48.0, 68 | 0.84 |
| Procalcitonin (µg/L) | 0.3 ± 0.4, 0.1 | 0.6 ± 0.6, 0.3 | 0.3 ± 0.4, 0.1 | 0.31 |
| White blood cells (1000/µL) | 7.5 ± 3.6, 7.1 | 8.5 ± 6.1, 6.1 | 7.2 ± 2.7, 7.1 | 0.81 |
| Neutrophil granulocytes (1000/µL) | 6.0 ± 4.0, 5.3 | 7.1 ± 4.2, 5.9 | 5.7 ± 4.0, 4.7 | 0.32 |
| Lymphocytes (1000/µL) | 0.9 ± 0.3, 0.9 | 1.0 ± 0.4, 1.1 | 0.9 ± 0.3, 0.9 | 0.27 |
| I nterleukin 6 (pg/mL) | 32.5 ± 23.2, 24.0 | 34.9 ± 25.1, 24.0 | 31.2 ± 24.5, 25.5 | 0.80 |
| Neuropsychiatric symptoms | ||||
| Sleep disorders (yes/no) | 19/18 | 9/1 | 10/17 | 0.013* |
| PSQI scorea | 5.8 ± 2.8, 6.0 | 7.0 ± 2.2, 6.0 | 5.3 ± 2.9, 5.0 | 0.037* |
| Fatigue | ||||
| MFI scorea | 42.5 ± 20.0, 36.0 | 64.4 ± 17.4, 64.0 | 34.3 ± 13.7, 30.0 | < 0.001* |
| MFI-FG scorea | 9.5 ± 4.8, 8.0 | 13.6 ± 4.6, 14.0 | 7.9 ± 3.9, 6.0 | 0.002* |
| MFI-FF scorea | 8.7 ± 4.7, 8.0 | 13.1 ± 5.0, 12.5 | 7.0 ± 3.4, 6.0 | 0.001* |
| MFI-RA scorea | 8.7 ± 4.8, 7.0 | 13.6 ± 4.7, 14.5 | 6.9 ± 3.4, 6.0 | < 0.001* |
| MFI-RM scorea | 7.5 ± 3.8, 6.0 | 10.9 ± 4.1, 11.0 | 6.3 ± 2.9, 5.0 | 0.001* |
| MFI-FM scorea | 8.0 ± 4.3, 6.0 | 13.2 ± 3.5, 14.0 | 6.0 ± 2.7, 5.0 | < 0.001* |
| Pain (yes/no) | 14/23 | 8/2 | 6/21 | 0.005* |
| NRS scorea | 1.8 ± 2.5, 0.0 | 4.2 ± 2.8, 5.0 | 0.9 ± 1.7, 0.0 | 0.001* |
| Anxiety (yes/no) | 10/27 | 4/6 | 6/21 | 0.51 |
| HADS score (anxiety)a | 4.6 ± 3.4, 5.0 | 5.9 ± 3.5 | 4.1 ± 3.5, 3.0 | 0.11 |
| Depression (yes/no) | 6/31 | 4/6 | 2/25 | 0.06 |
| HADS score (depression)a | 2.9 ± 3.5, 1.0 | 5.6 ± 2.8, 6.0 | 1.9 ± 2.9, 1.0 | 0.002* |
| Neuropsychological assessment | ||||
| Subjective complaints (yes/no) | 14/23 | 10/0 | 4/23 | < 0.001* |
| General (MoCA raw score)a | 26.0 ± 2.0, 26.0 | 27.0 ± 1.6, 27.0 | 25.6 ± 2.0, 26.0 | 0.05 |
| General (MoCA corrected score)a | 25.4 ± 2.4, 24.5 | 26.1 ± 2.2, 26.2 | 25.1 ± 2.4, 24.5 | 0.22 |
| Psychomotor speed (SDT score)a | 46.9 ± 10.4, 46.0 | 51.3 ± 8.7, 48.5 | 45.2 ± 10.7, 44.0 | 0.10 |
| Executive function (Stroop time)a | 18.5 ± 8.3, 17.5 | 16.2 ± 7.5, 16.9 | 19.4 ± 8.6, 17.8 | 0.39 |
| Neurofilament light chain (pg/mL) | 9.5 ± 4.9, 8.5 | 10.4 ± 7.7, 8.2 | 9.2 ± 3.5, 8.5 | 0.89 |
| Spirometry | ||||
| FEV1 (% predicted)a | 115.9 ± 15.6, 116.0 | 113.5 ± 11.2, 111.5 | 116.6 ± 16.9, 119.0 | 0.36 |
| FVC (% predicted)a | 120.8 ± 17.0, 120.0 | 114.8 ± 10.7, 117.0 | 122.7 ± 18.3, 126.0 | 0.17 |
| FEV/FVC (%)a | 100.5 ± 6.7, 101.0 | 103.9 ± 8.6, 107.0 | 99.4 ± 5.7, 100.0 | 0.10 |
| TLC (% predicted)a | 102.3 ± 13.0, 103.0 | 98.3 ± 12.1, 102.5 | 103.6 ± 13.3, 103.0 | 0.42 |
| DLCO (% predicted)a | 89.1 ± 13.3, 87.0 | 84.5 ± 15.0, 81.0 | 90.5 ± 12.7, 87.0 | 0.23 |
| KCO (% predicted)a | 95.4 ± 15.5, 93.0 | 97.6 ± 21.8, 89.5 | 94.7 ± 13.4, 94.0 | 0.85 |
| Arterial blood gas analysis | ||||
| pCO2 (mm Hg)a | 36.5 ± 7.2, 38.0 | 38.8 ± 1.5, 39.0 | 35.8 ± 8.2, 37.0 | 0.15 |
| pO2 (mm Hg)a | 97.0 ± 21.7, 99.0 | 99.3 ± 6.6, 100.0 | 96.2 ± 24.7, 99.0 | 0.95 |
| Six-minute walking test | ||||
| Distance walked (m)a | 589.4 ± 66.9, 600.0 | 602.9 ± 80.2, 608.0 | 584.9 ± 63.2, 576.0 | 0.27 |
| Distance walked (% predicted)a | 103.2 ± 17.8, 100.3 | 106.0 ± 18.1, 106.5 | 102.2 ± 17.9, 98.7 | 0.32 |
| SatO2 (baseline)a | 97.0 ± 1.1, 97.0 | 97.1 ± 0.8, 97.0 | 97.0 ± 1.1, 97.0 | 0.84 |
| SatO2 (end-effort)a | 96.6 ± 1.8, 97.0 | 96.8 ± 1.7, 97.0 | 96.5 ± 2.0, 96.5 | 0.77 |
| Borg dyspnea scale (baseline)a | 0.16 ± 0.45, 0.0 | 0.50 ± 0.76, 0.3 | 0.04 ± 0.20, 0.0 | 0.014* |
| Borg fatigue scale (baseline)a | 0.09 ± 0.30, 0.0 | 0.25 ± 0.46, 0.0 | 0.04 ± 0.20, 0.0 | 0.085 |
| Borg dyspnea scale (end-effort)a | 2.16 ± 1.54, 3.0 | 2.38 ± 0.92, 3.0 | 2.08 ± 1.72, 2.0 | 0.55 |
| Borg fatigue scale (end-effort)a | 1.69 ± 1.38, 1.0 | 2.13 ± 1.73, 2.0 | 1.54 ± 1.25, 1.0 | 0.36 |
BMI body mass index, COPD chronic obstructive pulmonary disease, DLCO diffusing capacity of the lungs for carbon monoxide, FEV1 forced expiratory volume in the first second, FVC forced vital capacity, HADS Hospital Anxiety and Depression Scale, ICU intensive care unit, KCO carbon monoxide transfer coefficient, ME/CFS + patients with myalgic encephalomyelitis/chronic fatigue syndrome-like symptoms, ME/CFS − patients without myalgic encephalomyelitis/chronic fatigue syndrome-like symptom, MFI Multidimensional Fatigue Inventory, MFI-FG general fatigue, MFI-FF physical fatigue, MFI-RA reduced activity, MFI-RM reduced motivation, MFI-FM mental fatigue, MoCA Montreal Cognitive Assessment, NRS numerical rating scale, pCO2 partial pressure of arterial carbon dioxide, pO2 partial pressure of oxygen, PSQI Pittsburgh Sleep Quality Index, SatO2 oxygen saturation, SDT Symbol Digit Test, TLC total lung capacity
aMean ± SD, median
bMild severity
cNon-parametric Mann–Whitney U test for continuous variables; chi square test for categorical variables
*P < 0.05 for ME/CFS + vs ME/CFS - comparison
Socio-demographic data, baseline comorbidities, and clinical and inflammatory features of SARS-CoV-2 infection did not differ between groups.
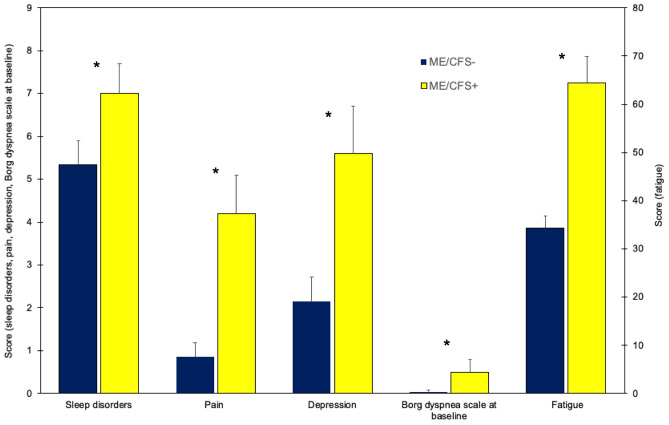
Sleep quality, fatigue, pain, and depressive symptoms were significantly more common and more severe in the ME/CFS + vs. ME/CFS − group (Fig. 2). Anxiety did not differ between groups.
Fig. 2.
Sleep disorders, pain, depression, perceived dyspnea (Borg dyspnea scale at baseline), and fatigue in patients who tested positive (ME/CFS +) and negative (ME/CFS −) to ME/CFS-like symptoms. All the measures were significantly worse in ME/CFS + than ME/CFS − patients (*p < 0.05 for ME/CFS + vs ME/CFS − comparison). Horizontal bars equal 1 S.E.M
Subjective cognitive complaints were significantly worse in the ME/CFS + vs. ME/CFS − group (p < 0.001), but the neuropsychological battery scores did not differ between groups.
Lung function was within normal range and did not differ between groups, except for Borg dyspnea baseline measure of the 6MWT (p = 0.014), which was significantly higher in the ME/CFS + vs. ME/CFS − group (Fig. 2).
Serum NfL levels were normal in all patients, except one (ME/CFS + , woman, age 54; 28.5 pg/mL, normal value < 17.5 pg/mL) (Mariotto et al. 2019) and did not differ comparing ME/CFS + and ME/CFS − groups.
Discussion
We identified a subset of 10 patients (i.e., 27% of the included patients), who developed ME/CFS-like symptoms of fatigue, sleep disturbances, pain, mood changes, and subjective cognitive complaints persisting over 6 months after SARS-CoV-2 infection recovery. Our figures appear slightly lower than that previously reported after SARS infection, i.e., ME/CFS-like symptoms in 40% of survivors 1 year after infection (Lam et al. 2009), but higher than that reported in a recent study aimed to assess psychological morbidities among COVID-19 survivors, i.e., ME/CFS-like symptoms in 14.2% of survivors 6 months after infection onset (Simani et al. 2021). These discrepancies across studies might be explained by different ME/CFS criteria and severity of the two coronavirus infections.
In accordance with another study on persistent fatigue after COVID-19 (Ahmed et al. 2020), we did not find significant difference in clinical/inflammatory features of SARS-CoV-2 acute infection comparing ME/CFS-like patients to those without ME/CFS-like symptoms. Although most of the patients were not severely ill, this finding suggests that ME/CFS-like symptoms may be independent from SARS-CoV-2 infection severity.
COVID-19 survivors underwent a multidimensional assessment that showed worse sleep, fatigue pain, depressive symptoms, subjective cognitive complaints, and dyspnea in ME/CFS-like group.
More severe mood alterations were found in ME/CFS + than ME/CFS- patients, in line with a recent prospective study on COVID-19 survivors (Rass et al. 2021).
ME/CFS + patients reported higher subjective cognitive complaints than the ME/CFS- group, even in the absence of overt cognitive dysfunction. The subjective–objective cognitive mismatch suggests that ME/CFS-like cognitive complaints may fall within the functional cognitive disorder spectrum (Teodoro et al. 2018). Alternatively, the tools we used could be not calibrated to capture subtle cognitive impairment.
Serum NfL levels were normal except in one ME/CFS-like patient, and we found no differences when comparing ME/CFS + vs. ME/CFS − . This finding suggests that axonal damage does not seem to underlie ME/CFS-like symptoms in SARS-CoV-2 survivors and requires confirmation in larger studies.
Lung function was normal and not significantly different in ME/CFS + vs. ME/CFS − groups. Of interest, ME/CFS-like patients perceived baseline Borg scale dyspnea as more marked than those without ME/CFS-like symptoms. This result appears consistent with a previous study reporting altered dyspnea perception in the absence of objective changes of respiratory distress during a rebreathing test in patients with fibromyalgia and ME/CFS, suggesting a possible functional nature of this symptom (Van den Bergh et al. 2017).
Fatigue, pain, and excessive interoceptive monitoring in ME/CFS may produce a shift from externally directed attention to subjective complaints resulting in perceiving cognitive and motor tasks as extremely effortful (Teodoro et al. 2018).
Our results suggest that ME/CFS-like symptoms in patients with COVID-19 may fall under functional neurological syndromes; i.e., a set of disorders characterized by abnormal symptom experiences probably related to an imbalance between somatic bottom-up perception and attentional, affective, and memory top-down processes (Van Den Houte et al. 2018).
Strengths of this study are the 6-month follow-up data and the multidimensional assessment encompassing immunologic, axonal, and lung markers. Limitations include its observational nature, small sample size, the absence of a pre-COVID-19 evaluation that prevented conclusions on the cause-effect relationship, no age- and sex-matched control group of patients without COVID-19 to check for psychological distress caused by the pandemic itself, and no data on inflammatory features at 6-month follow-up. Whether these symptoms might be a consequence of SARS-CoV-2 infection or unmasking of previous latent predisposition is an open question that should be addressed in future studies.
Despite our data should be considered preliminary, they raise concern on a possible future ME/CFS-like pandemic long after SARS-CoV-2 infection recovery, irrespectively of its severity and inflammatory features. Future larger studies with longer follow-ups are needed to explore ME/CFS-like symptom prevalence following SARS-CoV-2 infection.
Author contribution
EM: design of the study, acquisition, interpretation of the data, draft and revision of the manuscript for important intellectual content. SM, DG, GD, SB, AF, SZ, DG: acquisition, interpretation of the data, revision of the manuscript for important intellectual content. EC, SF: design of the study, acquisition, interpretation of the data, revision of the manuscript for important intellectual content. ST: design of the study, acquisition, analysis and interpretation of the data, draft and revision of the manuscript for important intellectual content, study supervision. All authors read and approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
Consent to participate was obtained from included patients, and the study was approved by the Ethics Committee of Verona University Hospital (Approval #2785CESC Verona-Rovigo).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elisa Mantovani, Email: elisa.mantovani@univr.it.
Stefano Tamburin, Email: stefano.tamburin@univr.it.
References
- Aamodt AH, Høgestøl EA, Popperud TH, Holter JC, Dyrhol-Riise AM, Tonby K, Stiksrud B, Quist-Paulsen E, Berge T, Barratt-Due A, Aukrust P, Heggelund L, Blennow K, Zetterberg H, Harbo HF. Blood neurofilament light concentration at admittance: a potential prognostic marker in COVID-19. J Neurol. 2021;20:1–10. doi: 10.1007/s00415-021-10517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, Eyre L, Breen A, O'Connor R, Jones A, Sivan M (2020) Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med 52:jrm00063. 10.2340/16501977-2694 [DOI] [PubMed]
- Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles AC, Speight N, Vallings R, Bateman L, Baumgarten-Austrheim B, Bell DS, Carlo-Stella N, Chia J, Darragh A, Jo D, Lewis D, Light AR, Marshall-Gradisnik S, Mena I, Mikovits JA, Miwa K, Murovska M, Pall ML, Stevens S. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid CVB, Knudsen CS, Parkner T. Reference interval and preanalytical properties of serum neurofilament light chain in Scandinavian adults. Scand J Clin Lab Invest. 2020;80:291–295. doi: 10.1080/00365513.2020.1730434. [DOI] [PubMed] [Google Scholar]
- Johnston S, Brenu EW, Staines D, Marshall-Gradisnik S. The prevalence of chronic fatigue syndrome/myalgic encephalomyelitis: a meta-analysis. Clin Epidemiol. 2013;5:105–110. doi: 10.2147/CLEP.S39876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MH, Wing YK, Yu MW, Leung CM, Ma RC, Kong AP, So WY, Fong SY, Lam SP. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- Lee SH, Shin HS, Park HY, Kim JL, Lee JJ, Lee H, Won SD, Han W. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East Respiratory Syndrome survivors. Psychiatry Investig. 2019;16:59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotto S, Gajofatto A, Zuliani L, Zoccarato M, Gastaldi M, Franciotta D, Cantalupo G, Piardi F, Polo A, Alberti D, Sartori S, Zanusso G, Agrò L, Demurtas R, Sechi G, Sechi E, Monaco S, Ferrari S. Serum and CSF neurofilament light chain levels in antibody-mediated encephalitis. J Neurol. 2019;266:643–1648. doi: 10.1007/s00415-019-09306-z. [DOI] [PubMed] [Google Scholar]
- Mariotto S, Savoldi A, Donadello K, Zanzoni S, Bozzetti S, Carta S, Zivelonghi C, Alberti D, Piraino F, Minuz P, Girelli D, Crisafulli E, Romano S, Marcon D, Marchi G, Gottin L, Polati E, Zanatta P, Monaco S, Tacconelli E, Ferrari S. Nervous system: subclinical target of SARS-CoV-2 infection. J Neurol Neurosurg Psychiatry. 2020;91:1010–1012. doi: 10.1136/jnnp-2020-323881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS, ERS Task Force, Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Rass V, Beer R, Josef Schiefecker A, Kofler M, Lindner A, Mahlknecht P, Heim B, Limmert V, Sahanic S, Pizzini A, Sonnweber T, Tancevski I, Loeffler-Ragg J, Scherfler C, Zamarian L, Bellmann-Weiler R, Weiss G, Djamshidian A, Kiechl S, Seppi K, Pfausler B, Helbok R. Neurological outcome and quality of life three months after COVID-19: a prospective observational cohort study. Eur J Neurol. 2021 doi: 10.1111/ene.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. As Their Numbers Grow, COVID-19 “Long Haulers” Stump Experts. JAMA. 2020 doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- Simani L, Ramezani M, Darazam IA, Sagharichi M, Aalipour MA, Ghorbani F, Pakdaman H. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J Neurovirol. 2021;27:154–159. doi: 10.1007/s13365-021-00949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro T, Edwards MJ, Isaacs JD. A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: systematic review. J Neurol Neurosurg Psychiatry. 2018;89:1308–1319. doi: 10.1136/jnnp-2017-317823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, O'Connor L, Leavy D, O'Brien K, Dowds J, Sugrue JA, Hopkins D, Martin-Loeches I, Ni Cheallaigh C, Nadarajan P, McLaughlin AM, Bourke NM, Bergin C, O'Farrelly C, Bannan C, Conlon N. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh O, Witthöft M, Petersen S, Brown RJ. Symptoms and the body: taking the inferential leap. Neurosci Biobehav Rev. 2017;74:185–203. doi: 10.1016/j.neubiorev.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Van Den Houte M, Bogaerts K, Van Diest I, De Bie J, Persoons P, Van Oudenhove L, Van den Bergh O. Perception of induced dyspnea in fibromyalgia and chronic fatigue syndrome. J Psychosom Res. 2018;106:49–55. doi: 10.1016/j.jpsychores.2018.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.