Abstract
Rationale: There is an urgent need for improved understanding of the mechanisms and clinical characteristics of acute respiratory distress syndrome (ARDS) due to coronavirus disease (COVID-19).
Objectives: To compare key demographic and physiologic parameters, biomarkers, and clinical outcomes of COVID-19 ARDS and ARDS secondary to direct lung injury from other etiologies of pneumonia.
Methods: We enrolled 27 patients with COVID-19 ARDS in a prospective, observational cohort study and compared them with a historical, pre–COVID-19 cohort of patients with viral ARDS (n = 14), bacterial ARDS (n = 21), and ARDS due to culture-negative pneumonia (n = 30). We recorded clinical demographics; measured respiratory mechanical parameters; collected serial peripheral blood specimens for measurement of plasma interleukin (IL)-6, IL-8, and IL-10; and followed patients prospectively for patient-centered outcomes. We conducted between-group comparisons with nonparametric tests and analyzed time-to-event outcomes with Kaplan-Meier and Cox proportional hazards models.
Results: Patients with COVID-19 ARDS had higher body mass index and were more likely to be Black, or residents of skilled nursing facilities, compared with those with non–COVID-19 ARDS (P < 0.05). Patients with COVID-19 had lower delivered minute ventilation compared with bacterial and culture-negative ARDS (post hoc P < 0.01) but not compared with viral ARDS. We found no differences in static compliance, hypoxemic indices, or carbon dioxide clearance between groups. Patients with COVID-19 had lower IL-6 levels compared with bacterial and culture-negative ARDS at early time points after intubation but no differences in IL-6 levels compared with viral ARDS. Patients with COVID-19 had longer duration of mechanical ventilation but similar 60-day mortality in both unadjusted and adjusted analyses.
Conclusions: COVID-19 ARDS bears several similarities to viral ARDS but demonstrates lower minute ventilation and lower systemic levels of IL-6 compared with bacterial and culture-negative ARDS. COVID-19 ARDS was associated with longer dependence on mechanical ventilation compared with non–COVID-19 ARDS. Such detectable differences of COVID-19 do not merit deviation from evidence-based management of ARDS but suggest priorities for clinical research to better characterize and treat this new clinical entity.
Keywords: pneumonia, acute respiratory distress syndrome, SARS-CoV-2, COVID-19
The coronavirus disease (COVID-19) pandemic has created formidable challenges for healthcare systems and the medical research community globally. Respiratory infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can lead to severe pneumonia and acute respiratory distress syndrome (ARDS), requiring support with mechanical ventilation in 10–20% of hospitalized patients (1, 2). Whereas ARDS is a heterogeneous clinical entity resulting from various direct or indirect pulmonary insults, the severe hypoxemic respiratory failure of COVID-19 represents a syndrome caused by a single, novel pathogen, which has nonetheless led to an unprecedented (in volume and time concentration) presentation of hypoxemic patients admitted to intensive care units (ICUs) in many areas worldwide.
Intensivists around the world have been striving to treat patients with COVID-19 while learning about the potential unique features of disease caused by this novel pathogen. In parallel, a vigorous debate has erupted in the academic literature as well as in both conventional and social media regarding the clinical phenotyping and recommended management strategies for patients with severe COVID-19 (3 –9). A central question revolves around whether ARDS due to SARS-CoV-2 infection has a unique phenotype that should alter existing, evidence-based management strategies for ARDS, particularly those related to ventilator management (10, 11). Although many studies have provided data from patients with COVID-19 (1, 4, 6, 12 –14), few have directly compared key variables between COVID-19 and non–COVID-19 ARDS. Furthermore, prior comparative studies have focused on single variables such as respiratory mechanics (15) or biomarker data (16). Therefore, we sought to characterize key demographic and physiologic parameters, biomarkers, and clinical outcomes of COVID-19 ARDS and directly compare them with a well-characterized research cohort of patients with ARDS with viral, bacterial, and culture-negative etiologies of direct lung injury at two associated academic referral medical centers (17).
Methods
Patients
From April 4 through September 15, 2020, we prospectively enrolled hospitalized patients in ICUs at the University of Pittsburgh Medical Center Presbyterian and Shadyside hospitals with documented SARS-CoV-2 infection into the University of Pittsburgh ALIR (Acute Lung Injury Registry) and Biospecimen Repository (protocol STUDY19050099) (17, 18). Patient enrollment to the ongoing ALIR study was initiated following institutional approval and biosafety infrastructure upgrades for handling biospecimens with SARS-CoV-2 (19). In this analysis, we included patients aged 18–90 years that met ARDS diagnostic criteria based on the Berlin Definition (20), as determined by consensus of at least three board-certified intensivists. Exclusion criteria were preexisting chronic respiratory failure due to neuromuscular or neurologic disease, presence of tracheostomy, inability to obtain consent, prisoner status, comfort-measures-only status, and blood hemoglobin <8 g/dl. Written informed consent was provided by all participants or their legally authorized representatives.
Historical (Non–COVID-19) ARDS Control Subjects
For comparisons with COVID-19 ARDS, we included patients previously enrolled in the ALIR study (before April 2020) that met diagnostic criteria for ARDS (20). To ensure homogeneity of the comparison groups, we included only patients with ARDS secondary to direct lung injury from viral, bacterial, or culture-negative pneumonia based on available clinical microbiologic workup. We excluded patients with ARDS attributed to coinfection with both viral and bacterial lung pathogens.
Clinical Data Extraction
We collected baseline (day of intubation) variables for demographics and comorbid conditions, serial (postintubation Days 1, 6, 11, and 16) data on physiologic parameters of gas exchange and ventilatory mechanics, and laboratory test results from the electronic medical records. We evaluated baseline severity of illness by calculating modified Sequential Organ Failure Assessment scores (not including the neurologic component). We recorded administered conventional therapies for gas exchange support often used in ARDS (e.g., prone positioning or neuromuscular blockade), as well as experimental or off-label therapies used specifically for COVID-19 (e.g., hydroxychloroquine or remdesivir). We followed patients prospectively for duration of mechanical ventilation, ventilator-free days by Day 28, and 60-day mortality.
Biomarker Analysis
We collected serial blood samples (postenrollment Days 1, 5, and 10 when subjects remained in the ICU), which were centrifuged for plasma separation and stored at −80°C until conduct of experiments. For patients with COVID-19, we measured plasma biomarkers with a V-Plex human biomarker multiplex assay (MesoScale Diagnostics). We used data on interleukin (IL)-6, IL-8, and IL-10 levels, which were the three cytokines available for comparisons in the historical ARDS samples, previously measured with a customized Luminex assay (R&D Systems) (17). To assess for potential systematic bias between the assays, we performed de novo analysis of 14 historical ARDS samples using the MesoScale platform (online supplement). To account for variability of sample acquisition from timing of intubation, we grouped available samples in three time intervals after intubation (early [0–4 d], middle [5–10 d], and late [>10 d]) (17) and performed comparisons between COVID-19 and non–COVID-19 ARDS within each time interval.
Statistical Analyses
We compared continuous and categorical variables between COVID-19 and non–COVID-19 ARDS categories with nonparametric tests (Kruskal-Wallis or Fisher’s tests, as appropriate). Following a global test for differences between all comparison groups, we conducted pairwise comparisons and adjusted post hoc P values with a conservative Benjamini-Hochberg test for multiple testing. For serially collected variables of respiratory mechanics, we examined for longitudinal evolution with linear regression models adjusted for time. For the clinical outcomes of duration of mechanical ventilation, ventilator-free days, and 60-day mortality, we built regression models comparing COVID-19 versus non–COVID-19 ARDS, adjusted for age, sex, and nursing home residence before admission. Similarly, for the time-to-event outcomes of 60-day survival and liberation from mechanical ventilation, we constructed Kaplan-Meier curves and built Cox proportional hazards models adjusted for age, sex, and nursing home residence.
Results
Clinical Characteristics of Patients with COVID-19 and Non–COVID-19 ARDS
We prospectively enrolled 27 patients with COVID-19 ARDS and compared them with 65 previously enrolled patients with non–COVID-19 ARDS, including viral ARDS (n = 14, including 8 cases of influenza, 3 of rhinovirus, 2 of non–SARS-COV-2 coronavirus, and 1 of respiratory syncytial virus), bacterial ARDS (n = 21), and culture-negative ARDS (i.e., cases with clinically suspected pneumonia with negative microbiologic workup, n = 30), as characterized in Figure E3 in the online supplement.
Enrollment of patients with COVID-19 occurred in two temporally distinct waves (with the first wave from April to June 2020 and the second wave from July to September 2020, Figure E4). Notably, 8/27 (29.6%) patients with COVID-19 were residents of skilled nursing facilities before hospital admission, all of whom were enrolled during the first wave (April to June 2020) (Table E1), consistent with the temporo-spatial epidemiology of the COVID-19 outbreak in Western Pennsylvania. No other baseline clinical variables were significantly different between the first and second waves of enrolled patients with COVID-19. Overall, patients with COVID-19 had a similar age distribution compared with those with non–COVID-19 ARDS but had higher body mass index and were more likely to be Black (Table 1). We did not identify any differences between patients with COVID-19 and those with non–COVID-19 ARDS in baseline comorbidities, including diabetes mellitus, chronic obstructive pulmonary disease, and history of immune suppression, or baseline severity of illness as captured by modified Sequential Organ Failure Assessment scores.
Table 1.
Clinical characteristics of 27 ICU patients with ARDS due to COVID-19 pneumonia compared with historical patients with ARDS
| COVID-19 (n = 27) |
Viral ARDS (n = 14) |
Bacterial ARDS (n = 21) |
Culture-Negative ARDS (n = 30) | |
|---|---|---|---|---|
| Age, median (IQR), yr | 63 (58–74) | 61 (43–73) | 57 (35–65) | 56 (51–64) |
| Sex, female, n (%) | 13 (48.1) | 10 (71.4) | 11 (52.4) | 15 (50.0) |
| Race, n (%) | ||||
| White | 18 (66.7) | 13 (92.9) | 19 (90.5) | 30 (100.0) |
| Black | 9 (33.3) | 1 (7.1) | 2 (9.5) | 0 (0.0) |
| Body mass index, median (IQR), kg/m2 | 34.0 (29.8–40.2) | 29.7 (27.3–34.8) | 25.2 (20.8–29.6) | 32.3 (26.5–36.4) |
| H/o chronic disease, n (%) | ||||
| Diabetes mellitus | 13 (48.1) | 5 (35.7) | 9 (42.9) | 9 (30.0) |
| Chronic renal failure | 4 (14.8) | 1 (7.1) | 4 (19.0) | 5 (16.7) |
| COPD | 7 (25.9) | 4 (28.6) | 5 (23.8) | 6 (20.9) |
| Immune suppression | 3 (11.1) | 4 (28.6) | 5 (23.8) | 8 (26.7) |
| Skilled nursing facility before admission, n (%) | 8 (29.6) | 0 (0.0) | 3 (12.5) | 0 (0.0) |
| Modified SOFA score, median (IQR)* | 7.0 (4.0–8.0) | 5.5 (4.0–6.0) | 8.0 (5.0–10.0) | 7.0 (5.0–10.0) |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; H/o = history of; ICU = intensive care unit; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment.
Modified SOFA scores were calculated on the day of intubation without including the neurologic score of SOFA, given that all patients were intubated and mechanically ventilated; therefore, the maximum score is 20.
Patients with COVID-19 ARDS frequently received rescue maneuvers for gas exchange, and they were more likely to undergo prone positioning (70.4%, P < 0.01), continuous neuromuscular blockade (74.1%, P < 0.01), and extracorporeal membrane oxygenation (28%, P < 0.01) compared with patients with non–COVID-19 ARDS (Table 2). Glucocorticoid administration in patients with COVID-19 (51.9%) was not significantly higher than that in patients with non–COVID-19 ARDS (Table 2); however, glucocorticoid use was significantly increased during the second wave (100% vs. 13.3% in the first wave, P < 0.01, Table E1), following the release of the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial results in June 2020 (21). Remdesivir was administered in about half of patients with COVID-19 (55.5%) but with increased use during the second wave (90.9%; first wave 33.3%, P = 0.01). Convalescent plasma was administered to 10 patients (37.0%) overall, and hydroxychloroquine to two patients (7.4%) during the first wave.
Table 2.
Notable therapies administered to patients with COVID-19 ARDS or historical ARDS control subjects
| COVID-19 ARDS (n = 27) |
Viral ARDS (n = 14) |
Bacterial ARDS (n = 21) | Culture-Negative ARDS (n = 30) | P Value | |
|---|---|---|---|---|---|
| Gas exchange rescue | |||||
| Prone positioning | 19 (70.4) | 4 (28.6) | 1 (4.8) | 2 (6.7) | <0.01 |
| Neuromuscular blockade | 20 (74.1) | 4 (28.6) | 6 (28.6) | 8 (26.7) | <0.01 |
| Inhaled vasodilators | 5 (18.5) | 0 (0.0) | 2 (9.5) | 4 (13.3) | 0.37 |
| ECMO | 7 (28.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <0.01 |
| ARDS supportive care | |||||
| Therapeutic anticoagulation | 10 (37.0) | 6 (42.9) | 8 (38.1) | 10 (33.3) | 0.94 |
| Continuous renal replacement therapy | 7 (25.9) | 3 (21.4) | 5 (23.8) | 7 (23.3) | 0.99 |
| Corticosteroids | 14 (51.9) | 8 (57.1) | 7 (33.3) | 9 (30.0) | 0.19 |
| COVID-19 therapeutics | |||||
| Hydroxychloroquine | 2 (13.3) | N/A | N/A | N/A | N/A |
| Remdesivir | 15 (55.5) | N/A | N/A | N/A | N/A |
| Convalescent plasma | 10 (37.0) | N/A | N/A | N/A | N/A |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease; ECMO = extracorporeal membrane oxygenation; N/A = not applicable.
Data are shown as n (%).
Comparison of Gas Exchange and Respiratory Parameters
The median ratio of arterial oxygen tension/pressure to fraction of inspired oxygen for patients with COVID-19 ARDS on the day of intubation was 120.0 (interquartile range, 72.0–147.5), which was similar in patients with non–COVID-19 ARDS (Figure 1A). No difference in applied positive end-expiratory pressures (PEEP) between COVID-19 and non–COVID-19 ARDS was found (data not shown). However, patients with COVID-19 received lower minute ventilation (8.7 [7.0–10.7] L/min) compared with patients with bacterial (12.0 [9.6–14.0] L/min; P < 0.01) and culture-negative ARDS (11.3 [9.2–13.5] L/min; post hoc P < 0.05) (Figure 1B), without significant differences in CO2 clearance. We found no significant change of delivered minute ventilation throughout serial examination of recorded values (postintubation Days 1, 6, 11, and 16, data not shown). Thus, patients with COVID-19 ARDS and viral ARDS required less minute ventilation to clear arterial carbon dioxide tension/pressure than the bacterial and culture-negative counterparts, possibly owing to lower fraction of dead-space ventilation in viral etiologies of ARDS.
Figure 1.
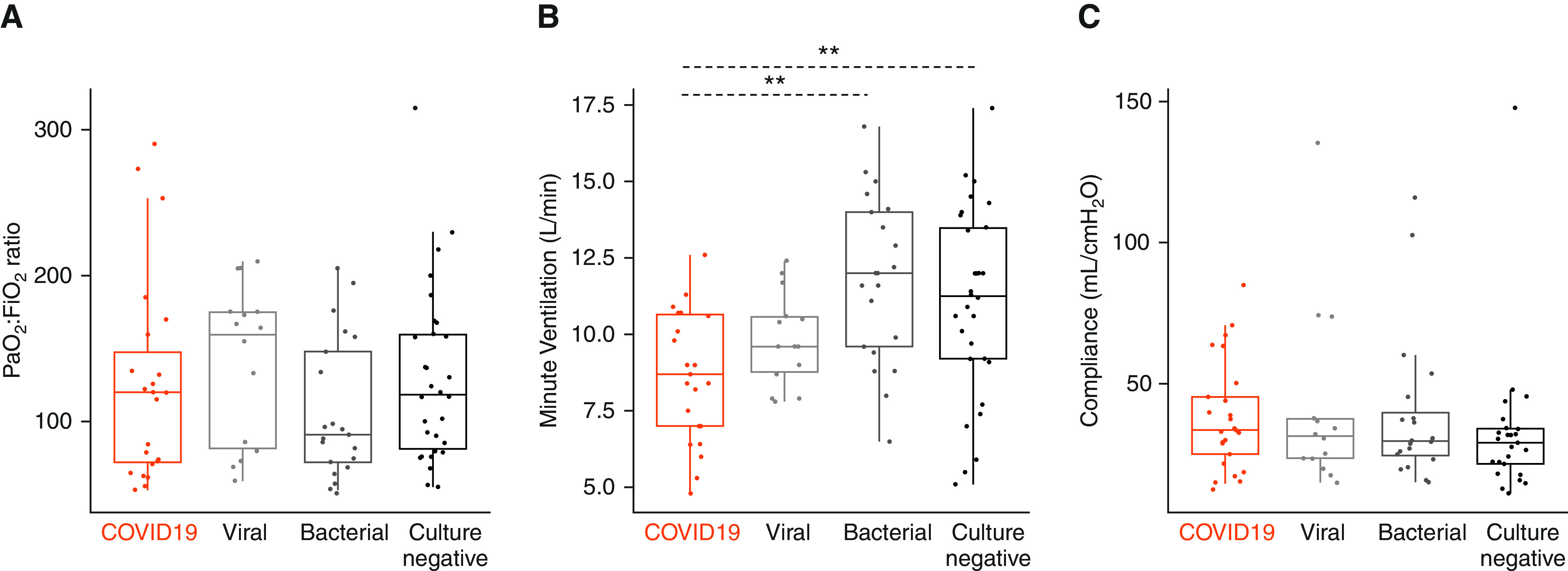
Comparison of select mechanical ventilation parameters between COVID-19 ARDS and non–COVID-19 ARDS cohorts obtained on the day of intubation. (A) Baseline PaO2:Fi O2 ratio. (B) Minute ventilation (L/min) and (C) static compliance of the respiratory system (ml/cm H2O) obtained during the first 24 hours of intubation. Statistical analysis was by Kruskal-Wallis test with Benjamini-Hochberg post hoc test for multiple comparisons of historical groups in relation to the COVID-19 group; asterisks represent post hoc statistical relationships. **P < 0.01. ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease; Fi O2= fraction of inspired oxygen; PaO2= arterial oxygen tension/pressure.
Static compliance of the respiratory system (calculated as ratio of tidal volume over the pressure difference between measured plateau pressure and PEEP) on the day of intubation was similar in patients with COVID-19 (33.7 [25.1–45.4] ml/cm H2O) and non–COVID-19 ARDS (Figure 1C). Notably, patients with COVID-19 ARDS during the first wave of enrollment had higher baseline compliance than patients during the second wave (Figure E5), with significant decrement in longitudinal measurements of compliance for first-wave patients (P for trend over time <0.05). When considering all patients with COVID-19 combined versus those with non–COVID-19 ARDS, we found neither significant differences between groups in longitudinal measurements of compliance nor any evidence for declining compliance during the course of mechanical ventilation (Figure E6).
Comparison of Inflammatory Plasma Biomarker Levels
We analyzed IL-6, IL-8, and IL-10 levels, as these were the three cytokines available in both COVID-19 and non–COVID-19 ARDS groups. To account for differences in time from intubation to sample collection, we compared cytokine levels between COVID-19 ARDS and non-COVID-19 ARDS etiologies at early (0–4 d), middle (5–10 d), and late (>10 d) time intervals after intubation (Figure 2). IL-6 levels were markedly lower in COVID-19 ARDS (median 18.9 [9.0–30.2] pg/ml) compared with bacterial (259.4 [142.5–3,317.0] pg/ml, P < 0.0001) and culture-negative ARDS (134.5 [39.8–414.9] pg/ml, P < 0.01) groups at the early time interval (Figure 2A), as well as when all non–COVID-19 ARDS groups were combined (P < 0.0001, Figure E7). There were no significant differences in IL-6 levels at subsequent time intervals between COVID-19 and specific etiologies of non–COVID-19 ARDS (Figure 2A). However, we do note lower IL-6 levels in patients with COVID-19 ARDS compared with the combined non–COVID-19 ARDS group at the late time interval (>10 d after intubation, P < 0.05, Figure E7). Similarly, IL-8 levels were lower in COVID-19 ARDS (14.1 [8.1–30.3] pg/ml) at the early time interval compared with bacterial ARDS (36.8 [22.5–153.1] pg/ml, P = 0.02) without any other significant differences at later time intervals (Figure 2B). No significant differences in IL-10 levels between COVID-19 and non–COVID-19 ARDS groups were noted (Figures 2C and E7). Sensitivity analyses for the cytokine measurement assays showed that the observed differences in IL-6 levels between COVID-19 and non–COVID-19 were robust to adjustments for potential differences from the two assays used (Online Supplement).
Figure 2.
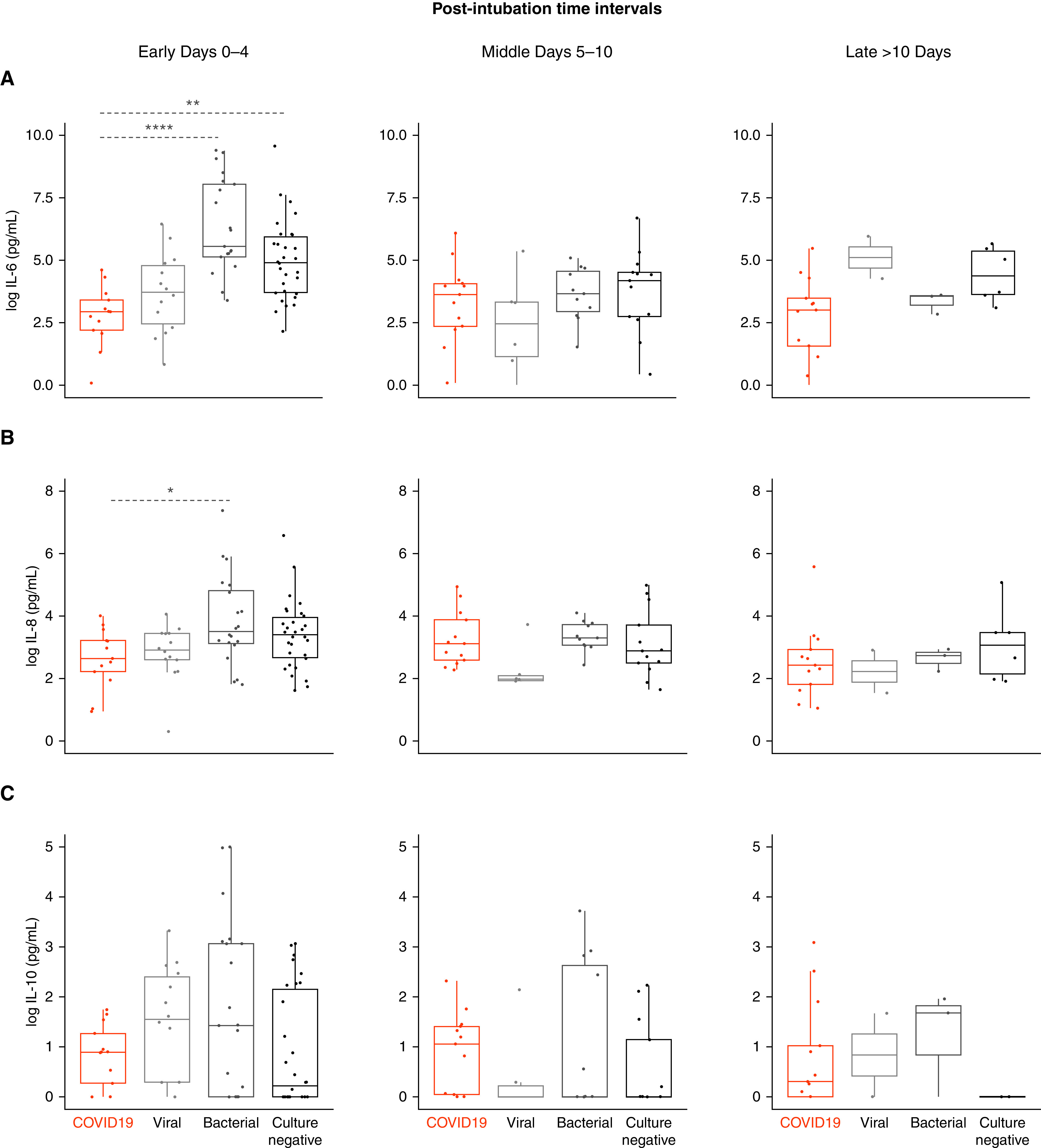
Comparison of select plasma cytokine levels between COVID-19 ARDS and non–COVID-19 ARDS cohorts. Log-transformed plasma levels of (A) IL-6 (pg/ml), (B) IL-8 (pg/ml), and (C) IL-10 (pg/ml) at early (0–4 d), middle (5–10 d), and late (>10 d) time intervals after intubation in patients with COVID-19 ARDS (n = 27 patients) quantified by multiplex assay compared with historical cohorts including ARDS due to viral pneumonia (n = 14 patients), bacterial pneumonia (n = 21 patients), and culture-negative etiology (n = 30 patients) quantified by prior multiplex assay. Statistical analysis was by Kruskal-Wallis test with Benjamini-Hochberg post hoc test for multiple comparisons of historical groups in relation to the COVID-19 group; asterisks represent post hoc statistical relationships. *P < 0.05, **P < 0.01, and ****P < 0.0001. ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease; IL = interleukin.
Clinical Outcomes
Unadjusted and adjusted comparisons for clinical outcomes are provided in Table 3. Patients with COVID-19 had fewer ventilator-free days up to Day 28 (median 0 [0–13.0] d) compared with those with non–COVID-19 ARDS (median 12 [0–23] d, unadjusted P < 0.05), but the effect of COVID-19 was not significant following adjustment for age, sex, and nursing home residence before admission. There was no significant difference in 60-day mortality. In time-to-event analysis for liberation from mechanical ventilation (Figures 3A and E8A), COVID-19 ARDS was associated with slower liberation from mechanical ventilation after adjustment for age, sex, and nursing home residence in a Cox proportional hazards model (adjusted hazard ratio, 0.48; 95% confidence interval [95% CI], 0.24–0.98; P < 0.05). We found no significant differences in unadjusted or adjusted analyses for 60-day survival (Figures 3B and E8B) between COVID-19 and non–COVID-19 ARDS (adjusted hazard ratio, 0.71; 95% CI, 0.33–1.56; P = 0.39).
Table 3.
Clinical outcomes of mechanically ventilated patients with COVID-19 ARDS and historical control subjects
| COVID-19 (n = 27) |
Viral ARDS (n = 14) |
Bacterial ARDS (n = 21) | Culture-Negative ARDS (n = 30) |
P Value* | Adjusted P Value† | |
|---|---|---|---|---|---|---|
| Duration of mechanical ventilation, median (IQR), d | 13.5 (8.0–18.0) | 7.5 (2.5–14.8) | 8.0 (5.0–25.0) | 7.0 (5.3–9.8) | 0.06 | 0.86 |
| VFDs, median (IQR), d | 0 (0–13.0) | 20.50 (11.8–24.5) | 0 (0–20.0) | 17.0 (0–21.8) | 0.02 | 0.19 |
| 60-d mortality, n (%)‡ | 12 (44.4) | 3 (21.4) | 8 (38.1) | 12 (40.0) | 0.54 | 0.84 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease; IQR = interquartile range; VFDs = ventilator-free days.
Unadjusted statistical comparisons between the four groups were performed by Fisher exact test for 60-day mortality and Kruskal-Wallis test for duration of mechanical ventilation and VFDs.
Adjusted statistical comparisons between COVID-19 and non–COVID-19 ARDS (viral, bacterial, or culture negative) were performed with regression models (linear for duration of mechanical ventilation, zero-inflated binomial for VFDs, and logistic for 60-d mortality), adjusted for the confounding effects of age, sex, and nursing home residence before admission.
60-day mortality data did not include one subject with COVID-19 who remained hospitalized at the time of analysis.
Figure 3.
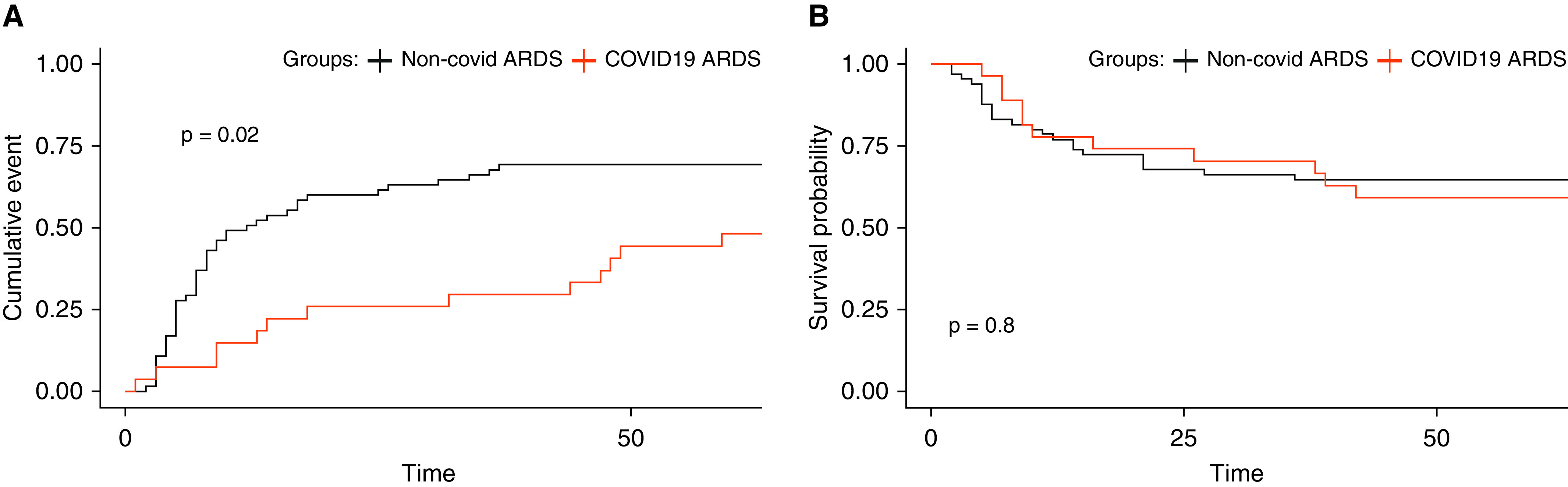
Comparison of time to ventilator liberation and survival at 60 days between COVID-19 and non–COVID-19 ARDS cohorts. Kaplan-Meier curves of (A) probability of liberation from mechanical ventilation up to 60 days after intubation during COVID-19 ARDS compared with non–COVID-19 ARDS. A Cox proportional hazards model revealed a hazard ratio of 0.48 (95% confidence interval, 0.24–0.98; P < 0.05) after adjustment for age, sex, and nursing home residence, and (B) probability of 60-day survival from date of intensive care unit admission during COVID-19 ARDS compared with non–COVID-19 ARDS. A Cox proportional hazards model revealed a hazard ratio of 0.71 (95% confidence interval, 0.33–1.56; P = 0.39) after adjustment for age, sex, and nursing home residence. ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease.
Discussion
Our systematic comparison of patients with COVID-19 ARDS and patients with ARDS in the pre–COVID-19 era requiring ICU admission in our institution revealed important similarities and dissimilarities between the syndromes, including demographics, respiratory physiology parameters, systemic IL-6 levels, and clinical outcomes. The first key dissimilarity we demonstrate is a disproportionate impact of COVID-19 on Black patients compared with historical ARDS cohorts. Prior reports have shown increased rates of hospitalization among Black patients with COVID-19 compared with non-Black patients in other major metropolitan regions in the United States (22, 23). The disproportionate impact of COVID-19 on Black communities may result from socioeconomic risk factors associated with systemic discrimination, such as higher likelihood of employment in essential service occupations with increased risk of infectious exposure (22, 23). We speculate that the higher proportion of Black patients with COVID-19 ARDS in our cohort is reflective of the increased burden of disease in the local Black community (https://www.alleghenycounty.us/Health-Department/Resources/COVID-19/COVID-19.aspx).
The second key dissimilarity we report pertains to the decreased levels of IL-6 in patients with COVID-19 compared with historical bacterial and culture-negative ARDS groups. This finding is consistent with other reports that note significantly increased levels of IL-6 in patients enrolled in ARDS clinical trials before COVID-19 compared with early reports from the COVID-19 pandemic (24). With direct comparisons of data collected in our institution, we illustrate a >10-fold lower level of IL-6 in COVID-19 compared with bacterial ARDS, but we note similar IL-6 levels in COVID-19 and viral ARDS. We identified a smaller scale difference in IL-8 levels between COVID-19 and bacterial ARDS at the early time interval only, and no differences in IL-10 levels. Such markedly different levels of IL-6 compared with other etiologies of ARDS are consistent with accumulating data that have challenged the earlier concept of an IL-6–related “cytokine storm” in COVID-19, as well as a recent clinical trial that showed no benefit for IL-6 inhibition in hypoxemic patients (24 –27). Our analyses of systemic IL-6 levels cannot rule out the possibility of IL-6–mediated mechanisms in lung tissue microenvironments. The markedly lower IL-6 levels in the systemic circulation do not support the plausibility for a generalizable therapeutic benefit from anti–IL-6 therapies in most critically ill patients with COVID-19, but there may be a subgroup with relatively higher IL-6 inflammation that could benefit from IL-6 inhibition. Ongoing studies examining the role of cytokines associated with ARDS phenotypes can offer further insight on the most critical pathways involved in COVID-19 ARDS pathogenesis (17, 18, 28, 29).
Finally, we note a prolonged duration of mechanical ventilation during COVID-19 ARDS compared with non–COVID-19 ARDS. We report a median of 13.5 days of mechanical ventilation, which is similar to other reports during COVID-19 (12) but is nearly twice the median ventilator duration of the LUNG-SAFE (Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE) trial (30). There are multiple potential reasons for the increased duration of ventilation such as changes in individual practice patterns to limit occupational exposures to SARS-CoV-2, as well as the limited functional baseline of many patients affected by the first wave of COVID-19 (8 of 15 of first-wave patients were nursing home residents). However, we speculate that prolonged ventilatory dependence results from the protracted course of SARS-CoV-2, which is supported by a report from New York City that quantified a median of 18 days’ duration of mechanical ventilation (1). We are unaware of systematic differences in rates of bacterial coinfection during COVID-19 ARDS compared with ARDS due to other viral pathogens that might explain the increased duration of mechanical ventilation during COVID-19 ARDS. We describe 60-day mortality rates similar to other reports of in-hospital mortality during moderate and severe ARDS (30).
Despite these key dissimilarities between COVID-19 and non–COVID-19 ARDS, we note that most clinical parameters we examined were similar, which should caution against systematic deviation from evidence-based management strategies for ARDS. For example, many have suggested that COVID-19 ARDS is a separate clinical phenotype distinct from ARDS in the pre–COVID-19 era with a characteristic “low-elastance” ARDS phenotype during SARS-CoV-2 pneumonia (6, 7). However, this notion has been rebutted by empiric demonstration of compliance levels in patients with COVID-19 that were similar in distribution to historical values (4, 12). We describe a baseline static respiratory compliance of 33.7 ml/cm H2O, which is similar to compliance values reported for both non–COVID-19 (31, 32) and COVID-19 ARDS (4, 6, 12). Interestingly, we found significant differences in compliance between patients enrolled during the first and second waves of the COVID-19 outbreak in our region, with first-wave patients exhibiting higher levels of compliance on the day of intubation followed by a significant decline in longitudinal compliance measurements. These differences in respiratory mechanics could result from clinical management variability during the evolution of the pandemic. In the first months of the pandemic, many endorsed early intubation practices for patients with severe COVID-19, with the cited rationale of preventing nosocomial spread of aerosolized SARS-CoV-2 particles and concern for high clinical failure rates with noninvasive respiratory support. With increased recognition of the physiologic principles governing COVID-19 ARDS and expanding clinical experience, there has been greater acceptance of extended trials of noninvasive support (positive pressure or high-flow oxygen delivery) with the objective of avoiding intubation (33, 34). Therefore, the early reports for recognition of a “low-elastance”/“high compliance” phenotype of COVID-19 ARDS (6, 7) may be reflective of the evolving practice patterns or demographics of the population affected by severe COVID-19. We further note a recent report that described elevated compliance and lung gas volume during COVID-19 ARDS compared with non–COVID-19 ARDS to support the novelty of COVID-19 ARDS as a clinical entity (35). However, in that report, nearly 50% of the “matched” patients with non–COVID-19 ARDS had indirect lung injury as the etiology of ARDS. Our analyses focused exclusively on patients with pneumonia as the risk factor for ARDS, which may offer improved rigor and directness in the comparisons between COVID-19 and non–COVID-19 ARDS. For example, we illustrate a requirement for lower minute ventilation (median 8.7 L/min) in our patient cohort with COVID-19 ARDS, similar to other reports (36), but without any significant difference in the efficiency of arterial carbon dioxide tension/pressure clearance compared with non–COVID-19 ARDS. The differences in minute ventilation were accounted for by higher minute ventilation administered in patients with bacterial and culture-negative ARDS, whereas patients with other viral etiologies of ARDS in our cohort had similar minute ventilation requirements as COVID-19 ARDS (10, 30). Overall, we noted more frequent use of prone positioning and neuromuscular blockade in COVID-19 ARDS compared with non–COVID-19 ARDS, but we did not have available data (e.g., provider surveys or large-scale temporal patterns at our institution) to understand the reasons for the wider adoption of these management practices in COVID-19 ARDS.
Our study has several limitations. First, we describe small numbers of patients with COVID-19 ARDS. Furthermore, our report is limited to two affiliated academic medical center hospitals, which impacts the generalizability of the results. However, these limitations are mitigated by our efforts to contextualize findings of COVID-19 against non–COVID-19 ARDS and highlight important differences and similarities. Nonetheless, we caution against overinterpretation of identified different features of COVID-19 ARDS, as such observations do not justify deviation from evidence-based management practices for ARDS that have emerged over decades of carefully conducted clinical trials.
In conclusion, we suggest that COVID-19 ARDS has important similarities and differences compared with other pathogen-mediated etiologies of ARDS in several key clinical, physiologic, and biomarker variables. The clinical and biological heterogeneity within COVID-19 ARDS underscores the importance of further efforts to identify and therapeutically target subphenotypes in COVID-19 and related critical illness (5, 17, 18, 28, 37).
Acknowledgments
Acknowledgment
The authors thank the patients and patient families that have enrolled in the University of Pittsburgh Acute Lung Injury Registry. They also thank the physicians, nurses, respiratory therapists, and other staff at the University of Pittsburgh Medical Center Presbyterian and Shadyside Hospital intensive care units for assistance with coordination of patient enrollment and collection of patient samples. They thank Heather Michael, John Ries, and Michelle Busch at the Center for Medicine and the Microbiome at the University of Pittsburgh for assistance with processing clinical samples.
Footnotes
Supported by Career Development Award Number IK2 BX004886 from the U.S. Department of Veterans Affairs Biomedical Laboratory R&D Service (W.B.); the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers K23 HL129987 (G.D.K.), K23GM122069 (F.A.S.), P01HL114453 (P.R., J.S.L., B.J.M.), and R01 HL136143, R01 HL142084, and K24 HL143285 (J.S.L.); the University of Pittsburgh Clinical and Translational Science Institute COVID-19 Pilot Grant Program (G.D.K.); the University of Pittsburgh Medical Center Immune Therapy and Transplant Center (A.M.); the University of Pittsburgh Medical Center (D.A.A.V.); and a Hillman Postdoctoral Fellowship for Innovative Cancer Research (A.R.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Department of Veterans Affairs, or any other sponsoring agency.
Author Contributions: W.B. and G.D.K. conceived, designed, analyzed, and interpreted the data and wrote the manuscript. H.Y. performed critical data analysis and interpretation and wrote the manuscript. F.A.S., T.S., C.D., N.A.-Y., R.S.D., N.B., C.S., and B.R.R. performed critical data collection and analysis and revised the work for important intellectual content. A.S., C.J.W., C.L., A.R.C., C.C., F.S., and T.C.B. performed critical experiments, interpreted the data, and revised the work for important intellectual content. D.A.A.V., P.R., A.R., Y.Z., J.S.L., B.M., B.J.M., and A.M. designed and interpreted the data and revised the work for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bos LDJ, Paulus F, Vlaar APJ, Beenen LFM, Schultz MJ. Subphenotyping acute respiratory distress syndrome in patients with COVID-19: consequences for ventilator management. Ann Am Thorac Soc. 2020;17:1161–1163. doi: 10.1513/AnnalsATS.202004-376RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bos LDJ, Sinha P, Dickson RP. The perils of premature phenotyping in COVID-19: a call for caution. Eur Respir J. 2020;56:2001768. doi: 10.1183/13993003.01768-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 8. Waterer GW, Rello J, Wunderink RG. COVID-19: first do no harm. Am J Respir Crit Care Med. 2020;201:1324–1325. doi: 10.1164/rccm.202004-1153ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17:787–789. doi: 10.1513/AnnalsATS.202004-325IP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11. Sahetya SK, Mancebo J, Brower RG. Fifty years of research in ARDS. vt selection in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:1519–1525. doi: 10.1164/rccm.201708-1629CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbeta E, Motos A, Torres A, Ceccato A, Ferrer M, Cilloniz C, et al. Covid Clinic Critical Care Group. SARS-CoV-2-induced acute respiratory distress syndrome: pulmonary mechanics and gas-exchange abnormalities. Ann Am Thorac Soc. 2020;17:1164–1168. doi: 10.1513/AnnalsATS.202005-462RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schenck EJ, Hoffman K, Goyal P, Choi J, Torres L, Rajwani K, et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17:1158–1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haudebourg A-F, Perier F, Tuffet S, de Prost N, Razazi K, Mekontso Dessap A, et al. Respiratory mechanics of COVID-19- versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202:287–290. doi: 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson JG, Simpson LJ, Ferreira A-M, Rustagi A, Roque J, Asuni A, et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5:140289. doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kitsios GD, Yang L, Manatakis DV, Nouraie M, Evankovich J, Bain W, et al. Host-response subphenotypes offer prognostic enrichment in patients with or at risk for acute respiratory distress syndrome. Crit Care Med. 2019;47:1724–1734. doi: 10.1097/CCM.0000000000004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bain W, Li H, van der Geest R, Moore SR, Olonisakin TF, Ahn B, et al. Increased alternative complement pathway function and improved survival during critical illness. Am J Respir Crit Care Med. 2020;202:230–240. doi: 10.1164/rccm.201910-2083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bain W, Lee JS, Watson AM, Stitt-Fischer MS. Practical guidelines for collection, manipulation and inactivation of SARS-CoV-2 and COVID-19 clinical specimens. Curr Protoc Cytom. 2020;93:e77. doi: 10.1002/cpcy.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 21. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adegunsoye A, Ventura IB, Liarski VM. Association of Black race with outcomes in COVID-19 disease: a retrospective cohort study. Ann Am Thorac Soc. 2020;17:1336–1339. doi: 10.1513/AnnalsATS.202006-583RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 25. Hedrick TL, Murray BP, Hagan RS, Mock JR. COVID-19: clean up on IL-6. Am J Respir Cell Mol Biol. 2020;63:541–543. doi: 10.1165/rcmb.2020-0277LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitsios GD, Yang H, Yang L, Qin S, Fitch A, Wang X-H, et al. Respiratory tract dysbiosis is associated with worse outcomes in mechanically ventilated patients. Am J Respir Crit Care Med. 2020;202:1666–1677. doi: 10.1164/rccm.201912-2441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 31. Henderson WR, Chen L, Amato MBP, Brochard LJ. Fifty years of research in ARDS. respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:822–833. doi: 10.1164/rccm.201612-2495CI. [DOI] [PubMed] [Google Scholar]
- 32. Panwar R, Madotto F, Laffey JG, Van Haren FMP. LUNG SAFE Investigators and the ESICM Trials Group. Compliance phenotypes in early acute respiratory distress syndrome before the COVID-19 pandemic. Am J Respir Crit Care Med. 2020;202:1244–1252. doi: 10.1164/rccm.202005-2046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201:1319–1320. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiumello D, Busana M, Coppola S, Romitti F, Formenti P, Bonifazi M, et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020;46:2187–2196. doi: 10.1007/s00134-020-06281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu X, Liu X, Xu Y, Xu Z, Huang Y, Chen S, et al. Ventilatory ratio in hypercapnic mechanically ventilated patients with COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1297–1299. doi: 10.1164/rccm.202002-0373LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. ARDS Network. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


