Abstract
Estimation of leaf nutrient composition of dominant plant species from contrasting habitats (i.e., karst and nonkarst forests) provides an opportunity to understand how plants are adapted to karst habitats from the perspective of leaf traits. Here, we measured leaf traits—specific leaf area (SLA), concentrations of total carbon ([TC]), nitrogen ([TN]), phosphorus ([TP]), calcium ([Ca]), magnesium ([Mg]), manganese ([Mn]), minerals ([Min]), soluble sugars, soluble phenolics, lipids, and organic acids ([OA])—and calculated water‐use efficiency (WUE), construction costs (CC), and N/P ratios, and searched for correlations between these traits of 18 abundant plant species in karst and nonkarst forests in southwestern China. Variation in leaf traits within and across the abundant species was both divergent and convergent. Leaf [TC], [Ca], [Min], [OA], and CC were habitat‐dependent, while the others were not habitat‐ but species‐specific. The correlations among [TN], [TP], SLA, [TC], CC, [Min], WUE, [OA], and CC were habitat‐independent, and inherently associated with plant growth and carbon allocation; those between [CC] and [Lip], between [Ca] and [Mg], and between [Mg] and [WUE] were habitat‐dependent. Habitat significantly affected leaf [Ca] and thus indirectly affected leaf [OA], [Min], and CC. Our results indicate that plants may regulate leaf [Ca] to moderate levels via adjusting leaf [OA] under both high and low soil Ca availability, and offer new insights into the abundance of common plant species in contrasting habitats.
Keywords: adaptation, calcium, Guizhou, mineral, nonkarst, nutrients
Variation in leaf traits within and across the common tree species was both divergent and convergent, and the causal effects of leaf traits were habitat‐dependent or habitat‐independent. Habitat had significant effects on leaf [Ca] and thus indirectly affected leaf [OA], [Min], and CC. Our study revealed leaf [OA] plays an important role in adjusting leaf concentrations and enhances adaptability of dominant plant species in karst habitats, offering new insights into the abundance of common tree species in contrasting habitats, which would be useful in karst forest restoration.
1. INTRODUCTION
Karst is a unique ecological system, which is defined as a landscape formed by dissolution of soluble rocks with rocky soils, caves, sinkholes, and lacking surface stream (Geekiyanage et al., 2019; Williams, 2008). Karst habitats are fragile and vulnerable, with high concentrations of calcium ([Ca]) and high pH in their shallow soils (Wei et al., 2018). One of the largest karst ecosystems is located in subtropical mountainous regions of southern and southwestern China (Yuan, 1991), exhibiting remarkably high species richness and endemism, contributing significantly to the floristic diversity of China (Wei et al., 2018), due to the fine‐scale heterogeneity of hydrogeology, topography, and associated water availability influenced by a monsoon climate (Guo et al., 2017). Being important hot spots of biodiversity and endemism (Clements et al., 2006), the karst ecosystems in China are under threats from human disturbance and global change which weaken their stability and accelerate rocky desertification (Lian et al., 2015; Tian et al., 2017).
A variety of plant functional traits have been considered to be related to dynamics of plant communities and functions of forest ecosystems (Boukili & Chazdon, 2017; He et al., 2019; Kunstler et al., 2016). Some plant traits are associated with factors that drive plant diversity and community assembly (Adler et al., 2013; Kunstler et al., 2016); similarity of leaf traits may increase competition among coexisting dominant tree species (Kraft et al., 2015). Many important leaf traits are used to assess plant adaptability and growth in different environments. For example, a high specific leaf area (SLA, Balachowski & Volaire, 2018; Hamann et al., 2018; Lambers & Poorter, 1992) and high leaf nutrient concentrations (Lambers & Poorter, 1992; Zhang et al., 2018) reflect a high capacity for plant growth. Some plant chemicals (secondary metabolites) are helpful to enhance adaptability to stressful environments. Soluble phenolics (SP, Karabourniotis et al., 2014) are related to plant defense under biotic stresses; soluble sugars (SS) serve as osmotic solutes to acclimate to water deficits (Galiano et al., 2017; Kuang et al., 2017); organic acids (OA) are important to sustain cellular functions under drought (Farooq et al., 2009).
The coordination among different leaf traits, which are shaped by evolution (Firn et al., 2019), allows plant adaptation to diverse habitats (Ahrens et al., 2020; Firn et al., 2019; Gratani & Bombelli, 2000; Moreira & Pearse, 2017; Wright et al., 2004, 2005). Correlations among leaf traits are used to interpret the biodiversity–ecosystem functions (Cronin & Schoolmaster, 2018; Schoolmaster et al., 2020) and can be used to track how plants respond to environmental change (Cronin & Schoolmaster, 2018). For example, plants can decrease SLA to increase water‐use efficiency (WUE) in habitats with low water availability (Maxwell et al., 2018) and synthesize OA to abate the adverse effects of excess leaf [Ca] (Kinzel, 1983).
Comparison of the differences in leaf traits and their effects on dominant plant species from contrasting habitats gives an opportunity to understand how dominant plants adapt to different habitats (Geekiyanage et al., 2018). Soil properties, such as soil pH values, water availability, and Ca concentrations, significantly differ between karst and nonkarst habitats (Hao et al., 2015), and these can substantially affect plant growth (Burstrom, 1968; Kinzel, 1983). However, there are a few plant species abundant in both karst and nonkarst forests in southwestern China (Zhu et al., 1998), despite species composition being notably different between the habitats (Fu et al., 2015). In this study, we measured or calculated 15 leaf traits including SLA, concentrations of leaf total carbon ([TC]), nitrogen ([TN]), phosphorus ([TP]), magnesium ([Mg]), manganese ([Mn]), minerals ([Min]), lipids ([Lip]), soluble sugars ([SS]), [SP], [Ca], [OA], leaf construction costs (CC), water‐use efficiency (WUE), and N/P ratios of 18 abundant plant species common to both karst and nonkarst forests in southwestern China. We aimed to test whether (a) leaf traits and their correlations may differ between the two habitats owing to the differences in soil characteristics (Donoghue, 2008; Mori et al., 2019); and (b) leaf traits acclimate to the unique soil parameters, for example, high soil [Ca] and pH values (Hao et al., 2015).
2. MATERIALS AND METHODS
2.1. Study site, species, and sampling
This study was conducted in Guizhou Province of southwestern China (103°36′–109°35′E, 24°37′–29°13′N), which has a typical karst distribution accounting for approximately 74% of its total area (Zhu, 1993). Characterized by a plateau monsoon humid climate, Guizhou has a mean annual temperature and a mean annual precipitation of 15.5°C and 1,400 mm, respectively, and has typical subtropical karst forests (Tian et al., 2017). Soils in karst forests in this province are generally developed from dolomite and/or limestone, with pH values varying from 6.3 to 7.8 (Wang et al., 2004), and soil [Ca] of 10.6 ± 6.3 mg/g (Zhang et al., 2014). In this study, the karst and nonkarst forests were uniformly selected, with soils developed from limestone and from granite, respectively.
Leaves of 18 common plant species abundant in both karst and nonkarst forests throughout this province (Huang et al., 1988) (Table 1) were sampled for analysis. Each species was sampled from six forests (three karst and three nonkarst). A minimum of three mature trees per species were sampled per forest. At least 10 mature, fully expanded, and healthy leaves were collected per tree. To minimize the influence of tree age, the individual trees of the species were of similar accounts of growth rings, which was determined by tree core extracted using an increment borer (Ф 5.15 mm, Haglöf, Sweden), in both karst and nonkarst habitats. After sampling, the leaves were stored in ice bags and transported back to the laboratory.
TABLE 1.
The 18 dominant plant species abundant in both karst and nonkarst habitats throughout Guizhou Province. Species names follow Flora of China, available online at www. efloras.org
| Species | Family | Leaf type | Life form |
|---|---|---|---|
| Broussonetia papyrifera (Linnaeus) L'Heritier ex Ventenat | Moraceae | Deciduous | Tree |
| Celtis sinensis Pers. | Ulmaceae | Deciduous | Tree |
| Debregeasia orientalis C. J. Chen | Urticaceae | Deciduous | Shrub |
| Hovenia acerba Lindl. | Rhamnaceae | Deciduous | Tree |
| Camptotheca acuminata Decne. | Nyssaceae | Deciduous | Tree |
| Clerodendrum mandarinorum Diels | Verbenaceae | Deciduous | Shrub |
| Liquidambar formosana Hance | Hamamelidaceae | Deciduous | Tree |
| Ligustrum lucidum Ait. | Oleaceae | Evergreen | Tree |
| Platycarya strobilacea Sieb. et Zucc. | Juglandaceae | Deciduous | Tree |
| Populus adenopoda Maxim. | Salicaceae | Deciduous | Tree |
| Robinia pseudoacacia L. | Fabaceae | Deciduous | Tree |
| Betula luminifera H. Winkl. | Betulaceae | Deciduous | Tree |
| Litsea cubeba (Lour.) Pers. | Lauraceae | Deciduous | Shrub |
| Lindera glauca (Sieb. et Zucc.) Bl. | Lauraceae | Deciduous | Shrub |
| Litsea mollis Hemsl. | Lauraceae | Deciduous | Shrub |
| Quercus fabri Hance | Fagaceae | Deciduous | Tree |
| Triadica sebifera (Linnaeus) Small | Euphorbiaceae | Deciduous | Tree |
| Toxicodendron vernicifluum (Stokes) F. A. Barkl. | Anacardiaceae | Deciduous | Tree |
2.2. Leaf trait measurements
In the laboratory, the leaves were cleaned and divided into two parts. One part was used to measure leaf area (Li–COR LI‐3000C, Inc., Lincoln, Nebraska, USA) and then dried to constant weight (65℃ for 72 hr) for SLA calculation. The other part was freeze‐dried, ground, and then used for chemical analyses. Leaf [TC] and [TN] were measured with an elemental analyzer (Isoprime 100, Elementar Isoprime, South Manchester, UK). Leaf [TP] was determined via molybdenum–antimony colorimetry after digestion by sulfuric acid (Murphy & Riley, 1962).
The concentrations of chemical compounds, including leaf [OA], [Min], [TSC], [Lip], [SS], and [SP], were measured according to Poorter et al. (1997) and Blainski et al. (2013). Briefly, a part of leaf powder, about 1.0 g, was extracted with a mixture of chloroform:methanol:water (2:2:1; v:v:v). The chloroform phase was used to determine leaf [Lip] from the residue weighed after evaporation (Poorter et al., 1997). The water/methanol phase was used to determine [SS] and [SP] using the anthrone and Folin–Ciocalteu method, respectively (Poorter et al., 1997). Leaf [lignin] was determined after chloroform:methanol:water extraction and 3% HCl extraction (Poorter et al., 1997).
Concentrations of leaf ([]) were determined according to Cataldo et al. (1975). Another part of leaf powder, about 0.10 g, was combusted in a muffle furnace at 550°C for 6 hr. The ashes after combusted consist of minerals (all mineral nutrients in leaves), oxides (derived from OA), and nitrate (Poorter et al., 1997). Leaf ash concentrations ([Ash]) and ash alkalinity were determined acidimetrically. Then, we calculated leaf [OA] and [Min] based on[], [Ash], and ash alkalinity via the following equations:
Leaf CC was calculated according to Poorter et al. (1997):
Water‐use efficiency of the plant species was calculated based on leaf δ13C values (Ehleringer & Cerling, 1995; Farquhar et al., 1982), which were determined using a mass spectrometer (Thermo Finnigan, North Pod Waltham, Massachusetts, USA). Leaf [Ca], [Mg], and [Mn] were determined by atomic absorption spectroscopy (ContrAA700, Analytik Jena AG, Jena, Germany) after digestion using a Microwave Reaction System (Multiwave 3000, Anton Paar, Graz, Austria).
2.3. Statistical analyses
All statistical analyses were conducted using R software (version 4.0.2). The units and key statistic summary of each leaf trait are provided in Table 2. Prior to multivariate analysis, the traits were checked for approximate normality (Shapiro–Wilk test). Those that did not follow normality were log10‐ ([TP], SLA, [Ca], [Mg], [Min], [SP]), square‐ ([SS], [OA]), or box‐cox– (CC, [TC]) transformed, and then, all leaf traits standardized to a mean of 0 and SD of 1. Differences in leaf traits between the habitats were tested using general linear mixed effect models (GLMEMs), with species and individuals as the random effects (Crawley, 2007), and determined using Tukey's HSD post hoc tests and conducted by the lsmeans function in lsmeans package after processing GLMEMs (Lenth, 2016). The correlations between leaf traits across the habitats were estimated by the pc function and idaFast function in R package pcalg (Kalisch et al., 2012). Briefly, we used the pc function to estimate the equivalence class of a directed acyclic graph (DAG) based on the PC algorithm; then, we used the idaFast function to calculate the coefficient of each pathway in DAG. Pearson's coefficients (r) were calculated to test the correlations of the traits between the habitats. We tested the paths of effects of habitat on those traits that differed between habitats via structural equation model (SEM) by the sem function in lavaan package. Briefly, we built an a priori model using these leaf traits affected by habitat. After running the a priori model, all nonsignificant paths were removed (p > .05) and we ran this new model again. The ratio of chi‐square to degrees of freedom (chi‐square/DF, ≤ 2, p > .05), comparative fit index (CFI, ≥0.95), and root mean squared error of approximation (RMSEA, 0 ≤ RMSEA ≤ 0.05) were used to assess the goodness of the final model when chi‐square/DF ≤2 (p > .05) (Schermelleh‐Engel et al., 2003). The significance was set at p < .05.
TABLE 2.
Abbreviations, units, means, standard deviation (SD), minimum, and maximum values of each leaf trait in this study
| Leaf trait | Abbreviation | Unit | Statistical summary [mean ± SD (min–max)] | |
|---|---|---|---|---|
| Karst | Nonkarst | |||
| Leaf carbon concentration | [TC] | mg/g | 454 ± 28 (372–499) | 459 ± 37 (288–504) |
| Leaf nitrogen concentration | [TN] | mg/g | 25.5 ± 6.19 (8.40–37.7) | 25.5 ± 6.43 (15.2–46.8) |
| Leaf phosphorus concentration | [TP] | mg/g | 1.40 ± 0.42 (0.64–2.23) | 1.50 ± 0.49 (0.70–2.61) |
| Leaf N to P ratio | N/P | unitless | 19.0 ± 4.73 (7.10–29.6) | 18.1 ± 4.77 (9.31–31.2) |
| Leaf calcium concentration | [Ca] | mg/g | 5.13 ± 3.63 (0.71–13.5) | 3.68 ± 2.56 (0.59–11.7) |
| Leaf manganese concentration | [Mg] | mg/g | 0.57 ± 0.38 (0.12–1.74) | 0.56 ± 0.32 (0.11–1.69) |
| Leaf magnesium concentration | [Mn] | mg/g | 0.20 ± 0.42 (0.01–2.55) | 0.29 ± 0.47 (0.00–2.34) |
| Leaf mineral concentration | [Min] | mg/g | 37.2 ± 24.0 (6.24–104) | 32.1 ± 24.9 (3.48–117) |
| Leaf lipid concentration | [Lip] | mg/g | 79.2 ± 21.5 (25.2–145) | 78.2 ± 23.5 (2.90–139) |
| Leaf soluble phenolic concentration | [SP] | mg/g | 42.6 ± 33.3 (4.80–134) | 40.1 ± 34.6 (7.29–148) |
| Leaf soluble sugar concentration | [SS] | mg/g | 114 ± 65.0 (0.86–345) | 102 ± 64.1 (8.03–283) |
| Leaf organic acid concentration | [OA] | mg/g | 105 ± 43.1 (8.76–192) | 88.8 ± 44.2 (23.8–209) |
| Specific leaf area | SLA | m2/kg | 14.9 ± 4.28 (6.26–23.8) | 15.2 ± 4.05 (8.27–25.2) |
| Water‐use efficiency | WUE | μmol/mol | 56.9 ± 16.4 (23.9–90.6) | 52.9 ± 15.3 (16–83.2) |
| Leaf construction cost | CC | g glucose/g | 1.34 ± 0.15 (0.90–1.56) | 1.38 ± 0.20 (0.49–1.65) |
3. RESULTS
3.1. Variations of leaf traits
All the studied leaf traits were affected by species (Figure 1). The variations in the leaf traits were inconsistent among species (Figure 2). Furthermore, five leaf traits ([TC], [Ca], [Min], [OA], and CC) were affected by habitat, and two leaf traits ([Ca] and N/P ratios) were affected by the interaction of habitat and species (Figure 1). We quantitatively assessed the degree of departure from the y = x line with values in karst habitats plotted against those in nonkarst habitats and found these five leaf traits affected by habitat substantially departed from this line across plant species (Figure 3a, e, h, l, o). In addition, leaf CC and [TC] were significantly lower in karst habitats than in nonkarst habitats, while leaf [Ca], [Min], and [OA] were significantly higher in the former than in the latter (Figure 4a, e, h, l, o).
FIGURE 1.
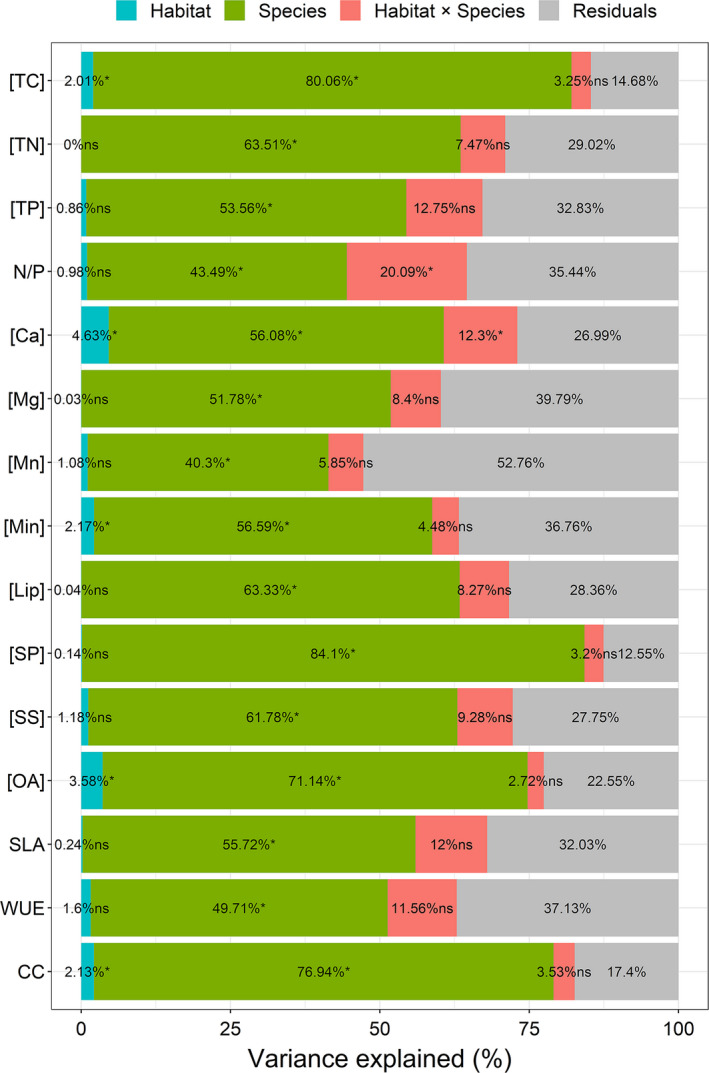
Variance explained for each leaf trait investigated in this study. * and ns indicate significance and nonsignificance, respectively, in two ANOVA analyses at p < .05. Abbreviations of all leaf traits are provided in Table 2
FIGURE 2.

Variations (chi‐square) of the studied leaf traits within tree species from karst to nonkarst habitats (Kruskal test). Squares with a significant difference (p < .05) are filled. Bel, Betula luminifera; Brp, Broussonetia papyrifera; Caa, Camptotheca acuminata; Ces, Celtis sinensis; Clm, Clerodendrum mandarinorum; Deo, Debregeasia orientalis; Hoa, Hovenia acerba; Lil, Ligustrum lucidum; Lig, Lindera glauca; Lif, Liquidambar formosana; Lic, Litsea cubeba; Lim, Litsea mollis; Pls, Platycarya strobilacea; Poa, Populus adenopoda; Quf, Quercus fabri; Rop, Robinia pseudoacacia; Sas, Sapium sebiferum; Tov, Toxicodendron vernicifluum. Abbreviations of all leaf traits are provided in Table 2
FIGURE 3.
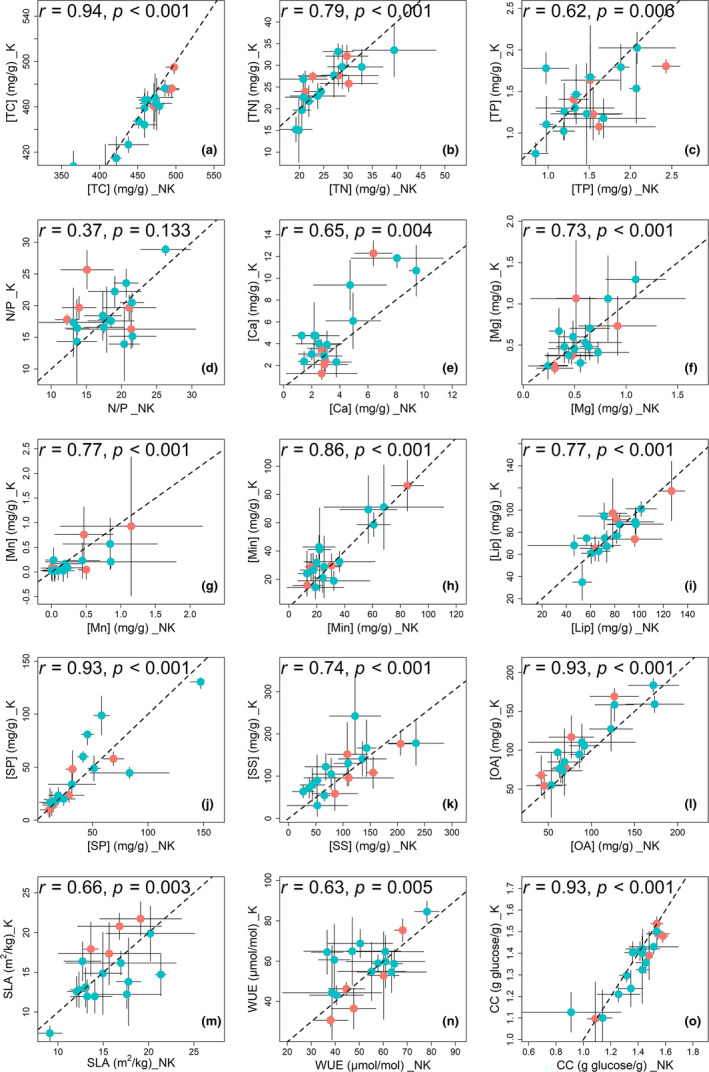
Correlations (Pearson's correlation coefficients, r) between the traits of 18 common tree species from karst (K) and nonkarst (NK) habitats. The abbreviations of the tree species are indicated in Table 1. Abbreviation of all leaf traits is provided in Table 2
FIGURE 4.

Differences in leaf traits of the dominant plant species between karst and nonkarst habitats. Different lowercase letters indicate significant differences between habitats based on linear mixed effect models (post hoc Tukey test, p < .05). The absence of lowercase letters indicates that the effect of habitat was not significant. Boxes in each boxplot show the first and third quartiles and the median; the upper and lower whiskers indicate the largest and smallest values away from 1.5*IQR (interquartile range) of the third quartiles and first quartiles, respectively; black points in each figure are values that fell outside the whiskers. Abbreviation of all leaf traits is provided in Table 2
3.2. Effects of habitat on the correlations among leaf traits
The correlations among leaf traits were either habitat‐independent or habitat‐dependent for the common trees (Figure 5a, b). In both habitats, the correlations between [TC] and CC (positive), [TN] and SLA (positive), [OA] and [Min] (positive), [OA] and [Ca] (positive), [Min] and [CC] (negative), [TN] and [TP] (positive), and SLA and WUE (negative) were significant (Figure 5a, b). However, in karst habitats, leaf CC was additionally correlated with leaf [Lip] (positively), and WUE was additionally correlated with leaf [Mg] (positively), which was not found in nonkarst habitats. In nonkarst habitats, leaf [Ca] was positively correlated with leaf [Mg] (Figure 5b).
FIGURE 5.

Correlations of the studied leaf traits derived from the idaFast function in karst (a) and nonkarst (b) habitats. The lines (both dashed and solid ones) linking two traits denote significant correlations (p < .05, black for positive and red for negative), and the effect size is shown by number close to the line. The dashed and solid lines indicate that correlations are uniform and different, respectively, between both habitats. A missing edge between two traits indicates no causal effects. Abbreviation of all leaf traits is provided in Table 2
3.3. Pathways via which habitat affected leaf trait variations
The effects of habitat on leaf [OA], [Min], and CC were indirect, via affecting leaf [Ca] (Figure 6a). In addition, leaf CC was decreased by both leaf [Ca], [Min], and [OA], but lower for species from karst than those from nonkarst habitats (Figure 6a, b). Furthermore, leaf [Ca] presented a stronger impact on leaf CC than leaf [OA] and [Min] (Figure 6a).
FIGURE 6.

(a) structural equation model (SEM) paths of the effects of habitat on leaf traits; (b) the standardized total effects of habitat, leaf [Ca], [Min], and [OA] on leaf construction costs (CC); (c) correlations between leaf traits of 18 dominant tree species. These blank squares indicate that the correlations between leaf traits are nonsignificant (p > .05); and (d) the a priori modeling of SEM. We removed leaf carbon concentrations when performing SEM, because of the high correlation between leaf [TC] and CC (r = 0.96, p < .001, c). Abbreviation of all leaf traits are provided in Table 2
4. DISCUSSION
4.1. Intraspecific and interspecific variations in leaf traits
A variety of leaf traits reflects adaptation of plants to a specific environment (Hazen et al., 2018), but some exhibit substantial phenotypic plasticity in many plants (Bjorkman et al., 2018; Russo & Kitajima, 2016). In the present study, however, we only observed that habitat had significant effects on plant leaf [TC], [Min], [OA], [Ca], and leaf CC (Figures 1 and 4), but species had significant effects on all leaf traits studied here (Figure 1). The inconsistency between habitats and across the common plants in this study (Figures 1, 2, and 4) segregates the importance of intraspecific (Kraft et al., 2014) and interspecific variations (Albert et al., 2011). Although the dissimilarity of leaf traits decreases competition among coexisting plant species (Kraft et al., 2015), the fine‐scale diversity of hydrogeology, topography, and associated water availability influenced by a monsoon climate (Guo et al., 2017) meets the demand of the dominant plant species for resource acquisition, both in karst and nonkarst habitats.
The differences in soil conditions, especially soil [Ca] and soil water availability, between karst and nonkarst forests (Hao et al., 2015; Wei et al., 2018), may have contributed to the divergence in leaf [Ca], [Min], [OA], [TC], and CC (Figure 4). Our results imply that the different soil properties between karst and nonkarst habitats may have limited impact on most leaf traits in our study, which may be due to similar environmental conditions (Tian et al., 2017), such as adequate soil [TN] and [TP] for plant growth in both habitats in southwestern China (assessed by leaf N/P ratios, karst: 19 ± 4.7; nonkarst: 18 ± 4.8, Geekiyanage et al., 2019), and moderate nutrient retrieval by plant growth in karst habitats (Liu et al., 2015). Part of our first aim of this study is to assess whether leaf traits are affected by habitat and species. We found that five leaf traits were significantly affected by habitat, while all leaf traits were affected by species.
4.2. The importance of specific correlations between leaf traits in plant adaptation
Either independent or dependent effects of habitat on the correlations between leaf traits (Figure 5a, b) suggest that combinations of leaf traits, both convergence and divergence, are important for plant adaptation. The leaf traits with habitat‐independent relationships were [TC], [TN], [TP], SLA, CC, [OA], [Ca], [Min], and WUE, implying their importance for resource acquisition and resistance to environmental stress in both karst and nonkarst habitats (Figure 5). Leaf [TN], [TP], and SLA are important traits reflecting plant growth, and CC is associated with plant's carbon budget (Lambers & Poorter, 1992; Lambers et al., 1998; Liu et al., 2016). The positive bidirectional influence between leaf [TN] and [TP] and the positive effect of [TN]/[TP] on plant SLA support the contention that high leaf [TN] and [TP] enhance plant growth (Lambers & Poorter, 1992).
Physiologically, a high WUE tends to be associated with a low SLA (Wellstein et al., 2017), leaf [TN], and [TP] (Prieto et al., 2018; Wright et al., 2005), thus maintaining C, N, and P acquisition and utilization. There are three mechanisms that may explain the negative correlations between SLA and WUE: (a) CO2 supply at sites of carboxylation may be decreased due to a longer internal CO2 diffusion pathway in thicker leaves (Hultine & Marshall, 2000; Prieto et al., 2018); (b) densely packed mesophyll may reduce the conductance of mesophyll to CO2 in thicker leaves (Prieto et al., 2018; Tomás et al., 2013); and (c) more enzymes related to photosynthesis in thicker leaves may increase the demand for CO2 (Hultine & Marshall, 2000; Prieto et al., 2018). The negative correlations between leaf nutrients (N and P) and WUE may result from plants enhancing mass flow of nutrients by increasing transpiration and enhancing uptake of mobile nutrients, and plants with high leaf nutrient concentrations increasing stomatal conductance and photosynthetic activity (Field et al., 1983; Prieto et al., 2018). Although there were some habitat‐dependent correlations between leaf traits, for example, positive correlation between leaf [Lip] and CC and between leaf [Mg] and WUE in karst habitats and negative correlations between [TP] and [SP] in nonkarst habitats, we assume that the habitat‐independent correlations of leaf traits are evolutionary outcomes of natural selection, since successful trait combinations are appropriate for plant growth in specific habitat (Ahrens et al., 2020; Firn et al., 2019; Moreira & Pearse, 2017). These habitat‐independent correlations may explain why dominant species grow well in both karst and nonkarst habitats.
The similar effect sizes but opposite directions, between leaf [OA] and leaf [Ca] and [Min] (0.72 vs. 0.67, 0.72 vs. 0.74) in karst vs. nonkarst habitats, suggest the importance of leaf [OA] for plant adaptation through adjusting leaf [Ca] (Triplett et al., 1980). We speculate that both the effects of [Ca] on [OA] in karst habitats and of [OA] on [Ca] in nonkarst habitats are to maintain leaf [Ca] at moderate levels, which can benefit plant growth (Figure 7). Generally, plants growing in karst habitats accumulate OA in leaves to maintain ion balance and to decline the restriction of excess [Ca] on plant growth (Figure 7, White & Broadley, 2003), while those growing in nonkarst habitats need an amount of Ca to maintain normal physiological functions, for example, preventing an efflux of potassium and decreasing turgor (Bressan et al., 1998; Burstrom, 1968). Therefore, plants in nonkarst habitats might enhance Ca via increasing OA in leaves (Figure 7). The role of leaf OA regulating the level of Ca may allow species to dominate in both karst and nonkarst habitats.
FIGURE 7.
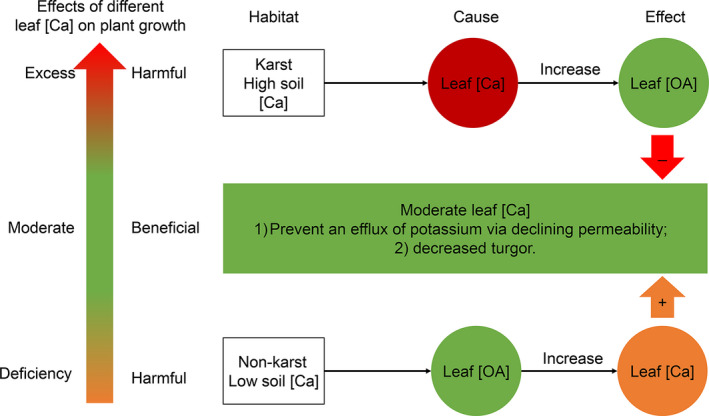
A conceptual model explaining causal effects between the concentrations of leaf calcium ([Ca]) and organic acids ([OA])
4.3. High leaf [OA] is a consequence of high leaf [Ca] in karst
Calcium is a plant macronutrient, while abundant Ca has adverse effects, for example, affecting ion uptake by roots (Kinzel, 1983). We found that the effect of habitat on leaf traits was associated with the differences in leaf [Ca] between the habitats (Figure 6), indicating plants are substantially affected by high soil [Ca]. Therefore, the significantly higher leaf [Ca], [Min], and [OA] in karst than in nonkarst habitats (Figure 4) partly reflects the effects of habitat properties, especially high soil [Ca] (Hao et al., 2015; Wei et al., 2018). The high leaf [OA] was likely a consequence of high leaf [Ca] in karst habitats (Triplett et al., 1980). The significantly lower leaf CC ([TC]) in karst habitats than in nonkarst habitats (Figure 4) likely reflects the accumulation of cheap compound (e.g., OA) and minerals (Figure 6a, b; Lambers & Poorter, 1992; Poorter & Bergkotte, 1992). The results suggest that leaf traits of dominant species in karst habitats are mainly affected by leaf [Ca], which may affect plant's adaptability to karst habitats. Considering plant species in this study were sampled from relatively fertile locations in both karst and nonkarst habitats, more studies of plant species only dominating in karst habitats should be conducted to adequately understand how plants adapt to karst habitats.
5. CONCLUSIONS
We quantitatively assessed the variations and causal effects of leaf traits of plant species common in both karst and nonkarst habitats. We showed that the variations in leaf traits within and across the common plant species were both divergent and convergent between the habitats, and the correlations between leaf traits were either dependent or independent of habitat. Leaf [OA] was affected by leaf [Ca] and [Min] in karst habitats, while leaf [Ca] and [Min] were affected by leaf [OA] in nonkarst habitats. The high leaf [OA] of dominant species may be associated with decreasing adverse effects of high [Ca] in karst habitats. Our results provide insights into the functioning of plant species common both in karst and nonkarst forests. Further studies are warranted to evaluate the physiological effects of leaf [OA] and [Ca] on plant adaptability in karst habitats.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Songbo Tang: Visualization (equal); Writing‐review & editing (equal). Jianfeng Liu: Visualization (equal); Writing‐review & editing (equal). Hans Lambers: Writing‐review & editing (equal). Lingling Zhang: Methodology (equal); Writing‐review & editing (equal). Zhanfeng Liu: Writing‐review & editing (equal). Yutong Lin: Investigation (equal); Writing‐review & editing (equal). Yuanwen Kuang: Conceptualization (lead); Funding acquisition (lead); Writing‐original draft (equal); Writing‐review & editing (equal).
ACKNOWLEDGMENTS
This study was supported by the subproject of the National Major Scientific Research Project of China (No. 2013CB956701) and Youth Innovation Promotion Association of Chinese Academy of Sciences.
Tang, S. , Liu, J. , Lambers, H. , Zhang, L. , Liu, Z. , Lin, Y. , & Kuang, Y. (2021). Increase in leaf organic acids to enhance adaptability of dominant plant species in karst habitats. Ecology and Evolution, 11, 10277–10289. 10.1002/ece3.7832
DATA AVAILABILITY STATEMENT
Data are available from the Dryad Digital Repository https://doi.org/10.5061/dryad.x95x69pjc (Tang et al., 2021).
REFERENCES
- Adler, P. B. , Fajardo, A. , Kleinhesselink, A. R. , & Kraft, N. J. B. (2013). Trait‐based tests of coexistence mechanisms. Ecology Letters, 16(10), 1294–1306. 10.1111/ele.12157 [DOI] [PubMed] [Google Scholar]
- Ahrens, C. W. , Andrew, M. E. , Mazanec, R. A. , Ruthrof, K. X. , Challis, A. , Hardy, G. , Byrne, M. , Tissue, D. T. , & Rymer, P. D. (2020). Plant functional traits differ in adaptability and are predicted to be differentially affected by climate change. Ecology and Evolution, 10(1), 232–248. 10.1002/ece3.5890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, C. H. , Grassein, F. , Schurr, F. M. , Vieilledent, G. , & Violle, C. (2011). When and how should intraspecific variability be considered in trait‐based plant ecology? Perspectives in Plant Ecology Evolution and Systematics, 13(3), 217–225. 10.1016/j.ppees.2011.04.003 [DOI] [Google Scholar]
- Balachowski, J. A. , & Volaire, F. A. (2018). Implications of plant functional traits and drought survival strategies for ecological restoration. Journal of Applied Ecology, 55(2), 631–640. 10.1111/1365-2664.12979 [DOI] [Google Scholar]
- Bjorkman, A. D. , Myers‐Smith, I. H. , Elmendorf, S. C. , Normand, S. , Rüger, N. , Beck, P. S. A. , Blach‐Overgaard, A. , Blok, D. , Cornelissen, J. H. C. , Forbes, B. C. , Georges, D. , Goetz, S. J. , Guay, K. C. , Henry, G. H. R. , HilleRisLambers, J. , Hollister, R. D. , Karger, D. N. , Kattge, J. , Manning, P. , … Weiher, E. (2018). Plant functional trait change across a warming tundra biome. Nature, 562(7725), 57–62. 10.1038/s41586-018-0563-7 [DOI] [PubMed] [Google Scholar]
- Blainski, A. , Lopes, G. C. , & de Mello, J. C. P. (2013). Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules, 18(6), 6852–6864. 10.3390/molecules18066852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukili, V. K. , & Chazdon, R. L. (2017). Environmental filtering, local site factors and landscape context drive changes in functional trait composition during tropical forest succession. Perspectives in Plant Ecology Evolution and Systematics, 24, 37–47. 10.1016/j.ppees.2016.11.003 [DOI] [Google Scholar]
- Bressan, R. A. , Hasegawa, P. M. , & Pardo, J. M. (1998). Plants use calcium to resolve salt stress. Trends in Plant Science, 3(11), 411–412. 10.1016/s1360-1385(98)01331-4 [DOI] [Google Scholar]
- Burstrom, H. (1968). Calcium and plant growth. Biological Reviews, 43, 287–316. 10.1111/j.1469-185X.1968.tb00962.x [DOI] [Google Scholar]
- Cataldo, D. A. , Haroon, M. , Schrader, L. E. , & Youngs, V. L. (1975). Rapid colorimetric determination of nitrate in plant‐tissue by nitration of salicylic‐acid. Communications in Soil Science and Plant Analysis, 6(1), 71–80. 10.1080/00103627509366547 [DOI] [Google Scholar]
- Clements, R. , Sodhi, N. S. , Schilthuizen, M. , & Ng, P. K. L. (2006). Limestone karsts of southeast Asia: Imperiled arks of biodiversity. BioScience, 56(9), 733–742. 10.1641/0006-3568(2006)56[733:lkosai]2.0.co;2 [DOI] [Google Scholar]
- Crawley, M. J. (2007). Mixed‐effects models. In Crawley M. J. (Ed.), The R book (pp. 627–660). Wiley. 10.1002/9780470515075.ch19 [DOI] [Google Scholar]
- Cronin, J. P. , & Schoolmaster, D. R. Jr (2018). A causal partition of trait correlations: Using graphical models to derive statistical models from theoretical language. Ecosphere, 9(9), e02422. 10.1002/ecs2.2422 [DOI] [Google Scholar]
- Donoghue, M. J. (2008). A phylogenetic perspective on the distribution of plant diversity. Proceedings of the National Academy of Sciences of the United States of America, 105, 11549–11555. 10.1073/pnas.0801962105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer, J. R. , & Cerling, T. E. (1995). Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiology, 15(2), 105–111. 10.1093/treephys/15.2.105 [DOI] [PubMed] [Google Scholar]
- Farooq, M. , Wahid, A. , Kobayashi, N. , Fujita, D. , & Basra, S. M. A. (2009). Plant drought stress: Effects, mechanisms and management. Agronomy for Sustainable Development, 29(1), 185–212. 10.1051/agro:2008021 [DOI] [Google Scholar]
- Farquhar, G. D. , Oleary, M. H. , & Berry, J. A. (1982). On the relationship between carbon isotope discrimination and the inter‐cellular carbon‐dioxide concentration in leaves. Australian Journal of Plant Physiology, 9(2), 121–137. 10.1071/pp9820121 [DOI] [Google Scholar]
- Field, C. , Merino, J. , & Mooney, H. A. (1983). Compromises between water‐use efficiency and nitrogen‐use efficiency in 5 species of California evergreens. Oecologia, 60(3), 384–389. 10.1007/bf00376856 [DOI] [PubMed] [Google Scholar]
- Firn, J. , McGree, J. M. , Harvey, E. , Flores‐Moreno, H. , Schütz, M. , Buckley, Y. M. , Borer, E. T. , Seabloom, E. W. , La Pierre, K. J. , MacDougall, A. M. , Prober, S. M. , Stevens, C. J. , Sullivan, L. L. , Porter, E. , Ladouceur, E. , Allen, C. , Moromizato, K. H. , Morgan, J. W. , Harpole, W. S. , … Risch, A. C. (2019). Leaf nutrients, not specific leaf area, are consistent indicators of elevated nutrient inputs. Nature Ecology & Evolution, 3(3), 400–407. 10.1038/s41559-018-0790-1 [DOI] [PubMed] [Google Scholar]
- Fu, T. G. , Chen, H. S. , Zhang, W. , Nie, Y. P. , Gao, P. , & Wang, K. L. (2015). Spatial variability of surface soil saturated hydraulic conductivity in a small karst catchment of southwest China. Environmental Earth Sciences, 74(3), 2381–2391. 10.1007/s12665-015-4238-5 [DOI] [Google Scholar]
- Galiano, L. , Timofeeva, G. , Saurer, M. , Siegwolf, R. , Martinez‐Vilalta, J. , Hommel, R. , & Gessler, A. (2017). The fate of recently fixed carbon after drought release: Towards unravelling C storage regulation in Tilia platyphyllos and Pinus sylvestris . Plant Cell and Environment, 40(9), 1711–1724. 10.1111/pce.12972 [DOI] [PubMed] [Google Scholar]
- Geekiyanage, N. , Goodale, U. M. , Cao, K. , & Kitajima, K. (2018). Leaf trait variations associated with habitat affinity of tropical karst tree species. Ecology and Evolution, 8(1), 286–295. 10.1002/ece3.3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geekiyanage, N. , Goodale, U. M. , Cao, K. , & Kitajima, K. (2019). Plant ecology of tropical and subtropical karst ecosystems. Biotropica, 51(5), 626–640. 10.1111/btp.12696 [DOI] [Google Scholar]
- Gratani, L. , & Bombelli, A. (2000). Correlation between leaf age and other leaf traits in three Mediterranean maquis shrub species: Quercus ilex, Phillyrea latifolia and Cistus incanus . Environmental and Experimental Botany, 43(2), 141–153. 10.1016/s0098-8472(99)00052-0 [DOI] [Google Scholar]
- Guo, Y. , Wang, B. , Mallik, A. U. , Huang, F. , Xiang, W. , Ding, T. , Wen, S. , Lu, S. , Li, D. , He, Y. , & Li, X. (2017). Topographic species‐habitat associations of tree species in a heterogeneous tropical karst seasonal rain forest, China. Journal of Plant Ecology, 10(3), 450–460. 10.1093/jpe/rtw057 [DOI] [Google Scholar]
- Hamann, E. , Kesselring, H. , & Stocklin, J. (2018). Plant responses to simulated warming and drought: A comparative study of functional plasticity between congeneric mid and high elevation species. Journal of Plant Ecology, 11(3), 364–374. 10.1093/jpe/rtx023 [DOI] [Google Scholar]
- Hao, Z. , Kuang, Y. , & Kang, M. (2015). Untangling the influence of phylogeny, soil and climate on leaf element concentrations in a biodiversity hotspot. Functional Ecology, 29(2), 165–176. 10.1111/1365-2435.12344 [DOI] [Google Scholar]
- Hazen, R. F. , Moody, K. N. , & Blum, M. J. (2018). Neutral and non‐neutral factors shape an emergent plant‐antagonist interaction. Evolutionary Ecology, 32(2–3), 265–285. 10.1007/s10682-018-9935-6 [DOI] [Google Scholar]
- He, P. , Wright, I. J. , Zhu, S. , Onoda, Y. , Liu, H. , Li, R. , Liu, X. , Hua, L. , Oyanoghafo, O. O. , & Ye, Q. (2019). Leaf mechanical strength and photosynthetic capacity vary independently across 57 subtropical forest species with contrasting light requirements. New Phytologist., 223(2), 607–618. 10.1111/nph.15803 [DOI] [PubMed] [Google Scholar]
- Huang, W. , Tu, Y. , & Yang, L. (1988). Vegetation of Guizhou. Guizhou People's Publishing House. [Google Scholar]
- Hultine, K. R. , & Marshall, J. D. (2000). Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia, 123(1), 32–40. 10.1007/s004420050986 [DOI] [PubMed] [Google Scholar]
- Kalisch, M. , Maechler, M. , Colombo, D. , Maathuis, M. H. , & Buehlmann, P. (2012). Causal inference using graphical models with the R package pcalg. Journal of Statistical Software, 47(11), 1–26. [Google Scholar]
- Karabourniotis, G. , Liakopoulos, G. , Nikolopoulos, D. , Bresta, P. , Stavroulaki, V. , & Sumbele, S. (2014). "Carbon gain vs. water saving, growth vs. defense": Two dilemmas with soluble phenolics as a joker. Plant Science, 227, 21–27. 10.1016/j.plantsci.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Kinzel, H. (1983). Influence of limestone, silicates and soil pH on vegetation. In Lange O. L., Nobel P. S., Osmond C. B., & Ziegler H. (Eds.), Physiological plant ecology III: Responses to the chemical and biological environment (pp. 201–244). Springer, Berlin Heidelberg. [Google Scholar]
- Kraft, N. J. B. , Crutsinger, G. M. , Forrestel, E. J. , & Emery, N. C. (2014). Functional trait differences and the outcome of community assembly: An experimental test with vernal pool annual plants. Oikos, 123(11), 1391–1399. 10.1111/oik.01311 [DOI] [Google Scholar]
- Kraft, N. J. B. , Godoy, O. , & Levine, J. M. (2015). Plant functional traits and the multidimensional nature of species coexistence. Proceedings of the National Academy of Sciences of the United States of America, 112(3), 797–802. 10.1073/pnas.1413650112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang, Y. , Xu, Y. , Zhang, L. , Hou, E. , & Shen, W. (2017). Dominant trees in a subtropical forest respond to drought mainly via adjusting tissue soluble sugar and proline content. Frontiers in Plant Science, 8, 802. 10.3389/fpls.2017.00802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunstler, G. , Falster, D. , Coomes, D. A. , Hui, F. , Kooyman, R. M. , Laughlin, D. C. , Poorter, L. , Vanderwel, M. , Vieilledent, G. , Wright, S. J. , Aiba, M. , Baraloto, C. , Caspersen, J. , Cornelissen, J. H. C. , Gourlet‐Fleury, S. , Hanewinkel, M. , Herault, B. , Kattge, J. , Kurokawa, H. , … Westoby, M. (2016). Plant functional traits have globally consistent effects on competition. Nature, 529(7585), 204–U174. 10.1038/nature16476 [DOI] [PubMed] [Google Scholar]
- Lambers, H. , & Poorter, H. (1992). Inherent variation in growth‐rate between higher‐plants ‐ a search for physiological causes and ecological consequences. Advances in Ecological Research, 23, 187–261. 10.1016/S0065-2504(08)60148-8 [DOI] [Google Scholar]
- Lambers, H. , Poorter, H. , & Van Vuuren, M. I. (1998). Inherent variation in plant growth. Backhuys Publishers. [Google Scholar]
- Lenth, R. V. (2016). Least‐squares means: The R package lsmeans. Journal of Statistical Software, 69(1), 1–33. 10.18637/jss.v069.i01 [DOI] [Google Scholar]
- Lian, Y. Q. , You, G. J. Y. , Lin, K. R. , Jiang, Z. C. , Zhang, C. , & Qin, X. Q. (2015). Characteristics of climate change in southwest China karst region and their potential environmental impacts. Environmental Earth Sciences, 74(2), 937–944. 10.1007/s12665-014-3847-8 [DOI] [Google Scholar]
- Liu, H. , Liu, W. , Wang, W. , Chai, J. , & Tao, J. (2015). Leaf traits and nutrient resorption of major woody species in the karst limestone area of Chongqing. Acta Ecologica Sinica, 35(12), 4071–4080. (In Chinese with English abstract). [Google Scholar]
- Liu, N. , Guo, Q. F. , Ren, H. , & Sun, Z. Y. (2016). Schima superba outperforms other tree species by changing foliar chemical composition and shortening construction payback time when facilitated by shrubs. Scientific Reports, 6, 10780. 10.1038/srep19855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell, T. M. , Silva, L. C. R. , & Horwath, W. R. (2018). Integrating effects of species composition and soil properties to predict shifts in montane forest carbon‐water relations. Proceedings of the National Academy of Sciences of the United States of America, 115(18), E4219–E4226. 10.1073/pnas.1718864115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, X. , & Pearse, I. S. (2017). Leaf habit does not determine the investment in both physical and chemical defenses and pair‐wise correlations between these defensive traits. Plant Biology, 19(3), 354–359. 10.1111/plb.12537 [DOI] [PubMed] [Google Scholar]
- Mori, G. B. , Schietti, J. , Poorter, L. , & Fernandez Piedade, M. T. (2019). Trait divergence and habitat specialization in tropical floodplain forests trees. PLoS One, 14(2), e0212232. 10.1371/journal.pone.0212232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, J. , & Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36. 10.1016/S0003-2670(00)88444-5 [DOI] [Google Scholar]
- Poorter, H. , & Bergkotte, M. (1992). Chemical‐composition of 24 wild‐species differing in relative growth‐rate. Plant Cell and Environment, 15(2), 221–229. 10.1111/j.1365-3040.1992.tb01476.x [DOI] [Google Scholar]
- Poorter, H. , Van berkel, Y. , Baxter, R. , Den hertog, J. , Dijkstra, P. , Gifford, R. M. , Griffin, K. L. , Roumet, C. , Roy, J. , & Wong, S. C. (1997). The effect of elevated CO2 on the chemical composition and construction costs of leaves of 27 C3 species. Plant Cell and Environment, 20(4), 472–482. 10.1046/j.1365-3040.1997.d01-84.x [DOI] [Google Scholar]
- Prieto, I. , Querejeta, J. I. , Segrestin, J. , Volaire, F. , & Roumet, C. (2018). Leaf carbon and oxygen isotopes are coordinated with the leaf economics spectrum in Mediterranean rangeland species. Functional Ecology, 32(3), 612–625. 10.1111/1365-2435.13025 [DOI] [Google Scholar]
- Russo, S. E. , & Kitajima, K. (2016). The ecophysiology of leaf lifespan in tropical forests: Adaptive and plastic responses to environmental heterogeneity. In Goldstein G., & Santiago L. S. (Eds.), Tropical tree physiology: Adaptations and responses in a changing environment (pp. 357–383). Springer International Publishing. [Google Scholar]
- Schermelleh‐Engel, K. , Moosbrugger, H. , & Müller, H. (2003). Evaluating the fit of structural equation models: Tests of significance and descriptive goodness‐of‐fit measures. Methods of Psychological Research, 8(2), 23–74. [Google Scholar]
- Schoolmaster, D. R. Jr , Zirbel, C. R. , & Cronin, J. P. (2020). A graphical causal model for resolving species identity effects and biodiversity–ecosystem function correlations. Ecology, 101(8), e03070. 10.1002/ecy.3070 [DOI] [PubMed] [Google Scholar]
- Tang, S. B. , Liu, J. F. , Lambers, H. , Zhang, L. L. , Lin, Y. T. , & Kuang, Y. W. (2021). Data from: Increase of leaf organic acids to enhance adaptability of dominant plant species in karst habitats. Dryad, Dataset. 10.5061/dryad.x95x69pjc [DOI] [PMC free article] [PubMed]
- Tian, Y. C. , Bai, X. Y. , Wang, S. J. , Qin, L. Y. , & Li, Y. (2017). Spatial‐temporal changes of vegetation cover in Guizhou Province. Southern China. Chinese Geographical Science, 27(1), 25–38. 10.1007/s11769-017-0844-3 [DOI] [Google Scholar]
- Tomás, M. , Flexas, J. , Copolovici, L. , Galmés, J. , Hallik, L. , Medrano, H. , Ribas‐Carbó, M. , Tosens, T. , Vislap, V. , & Niinemets, Ü. (2013). Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models. Journal of Experimental Botany, 64(8), 2269–2281. 10.1093/jxb/ert086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett, E. W. , Barnett, N. M. , & Blevins, D. G. (1980). Organic‐acids and ionic balance in xylem exudate of wheat during nitrate or sulfate absorption. Plant Physiology, 65(4), 610–613. 10.1104/pp.65.4.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.‐J. , Li, R.‐L. , Sun, C.‐X. , Zhang, D.‐F. , Li, F.‐Q. , Zhou, D.‐Q. , Xiong, K.‐N. , & Zhou, Z.‐F. (2004). How types of carbonate rock assemblages constrain the distribution of karst rocky desertified land in Guizhou Province, PR China: Phenomena and mechanisms. Land Degradation & Development, 15(2), 123–131. 10.1002/ldr.591 [DOI] [Google Scholar]
- Wei, X. , Deng, X. , Xiang, W. , Lei, P. , Ouyang, S. , Wen, H. , & Chen, L. (2018). Calcium content and high calcium adaptation of plants in karst areas of southwestern Hunan. China. Biogeosciences, 15(9), 2991–3002. 10.5194/bg-15-2991-2018 [DOI] [Google Scholar]
- Wellstein, C. , Poschlod, P. , Gohlke, A. , Chelli, S. , Campetella, G. , Rosbakh, S. , Canullo, R. , Kreyling, J. , Jentsch, A. , & Beierkuhnlein, C. (2017). Effects of extreme drought on specific leaf area of grassland species: A meta‐analysis of experimental studies in temperate and sub‐Mediterranean systems. Global Change Biology, 23(6), 2473–2481. 10.1111/gcb.13662 [DOI] [PubMed] [Google Scholar]
- White, P. J. , & Broadley, M. R. (2003). Calcium in plants. Annals of Botany, 92(4), 487–511. 10.1093/aob/mcg16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, P. W. (2008). The role of the epikarst in karst and cave hydrogeology: A review. International Journal of Speleology, 37(1), 1–10. 10.5038/1827-806X.37.1.1 [DOI] [Google Scholar]
- Wright, I. J. , Reich, P. B. , Cornelissen, J. H. C. , Falster, D. S. , Garnier, E. , Hikosaka, K. , Lamont, B. B. , Lee, W. , Oleksyn, J. , Osada, N. , Poorter, H. , Villar, R. , Warton, D. I. , & Westoby, M. (2005). Assessing the generality of global leaf trait relationships. New Phytologist, 166(2), 485–496. 10.1111/j.1469-8137.2005.01349.x [DOI] [PubMed] [Google Scholar]
- Wright, I. J. , Reich, P. B. , Westoby, M. , Ackerly, D. D. , Baruch, Z. , Bongers, F. , Cavender‐Bares, J. , Chapin, T. , Cornelissen, J. H. C. , Diemer, M. , Flexas, J. , Garnier, E. , Groom, P. K. , Gulias, J. , Hikosaka, K. , Lamont, B. B. , Lee, T. , Lee, W. , Lusk, C. , … Villar, R. (2004). The worldwide leaf economics spectrum. Nature, 428(6985), 821–827. 10.1038/nature02403 [DOI] [PubMed] [Google Scholar]
- Yuan, D. (1991). Karst of China. Geological Publishing House. [Google Scholar]
- Zhang, H. , Chen, H. Y. H. , Lian, J. , John, R. , Ronghua, L. I. , Liu, H. , Ye, W. , Berninger, F. , & Ye, Q. (2018). Using functional trait diversity patterns to disentangle the scale‐dependent ecological processes in a subtropical forest. Functional Ecology, 32(5), 1379–1389. 10.1111/1365-2435.13079 [DOI] [Google Scholar]
- Zhang, Z. H. , Hu, B. Q. , & Hu, G. (2014). Spatial heterogeneity of soil chemical properties in a subtropical karst forest. Southwest China. Scientific World Journal, 2014, 473651. 10.1155/2014/473651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H. , Wang, H. , & Baogui, L. (1998). The structure, species composition and diversity of the limestone vegetation in Xishuangbanna, SW China. Gardens' Bulletin (Singapore), 50(1), 5–30. [Google Scholar]
- Zhu, S. Q. (1993). Study on the Karst Forest Ecosystem. Guizhou Science and Technology Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Dryad Digital Repository https://doi.org/10.5061/dryad.x95x69pjc (Tang et al., 2021).


