Abstract
Many organisms can reproduce both asexually and sexually. For cyclical parthenogens, periods of asexual reproduction are punctuated by bouts of sexual reproduction, and the shift from asexual to sexual reproduction has large impacts on fitness and population dynamics. We studied populations of Daphnia dentifera to determine the amount of investment in sexual reproduction as well as the factors associated with variation in investment in sex. To do so, we tracked host density, infections by nine different parasites, and sexual reproduction in 15 lake populations of D. dentifera for 3 years. Sexual reproduction was seasonal, with male and ephippial female production beginning as early as late September and generally increasing through November. However, there was substantial variation in the prevalence of sexual individuals across populations, with some populations remaining entirely asexual throughout the study period and others shifting almost entirely to sexual females and males. We found strong relationships between density, prevalence of infection, parasite species richness, and sexual reproduction in these populations. However, strong collinearity between density, parasitism, and sexual reproduction means that further work will be required to disentangle the causal mechanisms underlying these relationships.
Keywords: density, ephippia, multiparasite, parasitism, pathogens, phenology, Red Queen
Lake populations of Daphnia varied substantially in investment in sex, with some populations reproducing entirely asexually throughout the study period and others shifting almost entirely to sexual reproduction by late autumn. We found that higher Daphnia density and parasitism were associated with greater investment in sex.

1. INTRODUCTION
A major challenge in evolutionary biology is explaining variation in reproductive strategies—especially why so many organisms reproduce sexually (Lively & Morran, 2014; Neiman et al., 2017; Otto, 2009). Sexual reproduction has several potential drawbacks, including the “twofold cost” of sex (Neiman et al., 2017; Otto, 2009; Stelzer, 2011), challenges in finding a mate, acquisition of sexually transmitted infections, and shuffling of alleles that worked well in a parent (Kokko, 2020; McLeod & Day, 2014; Otto, 2009). At the same time, sexual reproduction also has advantages, including providing an opportunity to purge deleterious mutations and producing novel genotypes that can avoid infection by parasites (Jaenike, 1978; Kondrashov, 1984; Lively, 2010; Muller, 1964). However, framing reproduction as a dichotomy between (entirely) sexual and (entirely) asexual ignores the abundance of organisms that combine the two (Gerber et al., 2018; Kokko, 2020). By being able to shift between sexual and asexual reproduction, cyclical parthenogens are often described as experiencing the “best of both worlds” (Kokko, 2020), gaining the benefits of sexual reproduction while also avoiding its costs. However, this ability to shift between these two modes of reproduction raises a new question: how much to invest in asexual versus sexual reproduction?
When considering investment in sexual reproduction, it is important to consider that sexual reproduction in cyclical parthenogens is often associated with dormancy (Gerber & Kokko, 2018; Gerber et al., 2018; Kokko, 2020; Walsh, 2013). Sexual reproduction thus not only affords the benefits of creating novel genotypes and purging mutational load (Cáceres et al., 2009), but also can allow a lineage to escape through time, potentially waiting out harsh conditions. Given the strong spatial and temporal variation in biotic and abiotic conditions that exists in nature, it is perhaps not surprising that populations of cyclical parthenogens can vary substantially in the degree to which they reproduce sexually (Walsh, 2013)—as seen, for example, in studies of Daphnia populations (e.g., Gerber et al., 2018; Johnson et al., 2009; Tessier & Cáceres, 2004; Walsh & Post, 2012).
Prior research on Daphnia, a dominant member of pond and lake food webs, has identified a variety of factors that contribute to asexual versus sexual reproduction, including predation, parasitism, crowding, resource limitation, and changing abiotic conditions (Gerber et al., 2018; Haltiner et al., 2020; Stross & Hill, 1965; Walsh, 2013). A potential role of parasitism in sexual reproduction in Daphnia has received particular attention in recent years. Sexually produced Daphnia offspring are more fit against contemporaneous parasites (Auld et al., 2016; Ebert et al., 2007), and more susceptible genotypes are more likely to shift to sexual reproduction (Duncan et al., 2006; Mitchell et al., 2004). Moreover, studies on two different Daphnia–parasite systems found the production of males was more likely in the presence of parasites (Hite et al., 2017; Roth et al., 2008) and, in a third, sexual reproduction was higher in years with more infection by a chytrid parasite (Johnson et al., 2009).
A potential role of parasites in driving sexual reproduction has also been studied in other systems, including plants (Busch et al., 2004), Caenorhabditis elegans (Lynch et al., 2018; Morran et al., 2011; Slowinski et al., 2016), and snails (Ben‐Ami & Heller, 2005; Dagan et al., 2013; Schrag et al., 1994), most notably the New Zealand freshwater snail Potamopyrgus antipodarum (e.g., Gibson et al., 2018; Lively, 1987; Lively & Dybdahl, 2000). Asexual P. antipodarum are most common in habitats with no or low levels of infection by virulent parasites (King et al., 2009; McKone et al., 2016). Moreover, male snails were more common when a virulent parasite was common (Vergara et al., 2013) and asexual snails tended to have higher levels of infection (Vergara et al., 2014), though a more recent study found the opposite pattern (asexual snails having lower levels of infection, perhaps because they have become rare (Gibson & Lively, 2019)). This prior work on P. antipodarum demonstrates the value of studies comparing levels of parasitism and sexual reproduction in natural populations.
In this study, we explored the prevalence of sexual reproduction in lake populations of Daphnia dentifera (Figure 1) and whether particular lakes have consistently high levels of sexual reproduction across years. We then asked what factors are associated with the amount of sexual reproduction. We were particularly interested in the degree to which the prevalence of sexual reproduction in a population is related to the level of parasitism and/or to overall population density. We explored this by tracking sexual reproduction, density, and infections by multiple parasites in 15 D. dentifera populations over 3 years to better understand variation in sexual reproduction in this dominant member of lake food webs. We found that parasitism and density were both associated with sexual reproduction, but strong correlations between parasitism, density, and sexual reproduction highlight the need for additional work to uncover the mechanisms driving these patterns.
FIGURE 1.

An uninfected (left) and a Pasteuria‐infected (right) Daphnia dentifera. The uninfected animal has asexual embryos developing in her brood pouch
2. MATERIALS
2.1. Study system
Daphnia dentifera is a dominant zooplankton species in lakes in the Midwestern United States, feeding on phytoplankton and serving as prey to small fish and invertebrate predators (Tessier & Woodruff, 2002). Daphnia often switch to sexual reproduction at particular times of the year, when it becomes less costly (Gerber et al., 2018); the species we focused on, D. dentifera, shifts to sexual reproduction in autumn (Duffy et al., 2008). During sexual reproduction, female Daphnia create clones that are males and haploid resting eggs, which the males then fertilize (Ebert, 2005). The resting eggs (encased in a chitinous envelope called an ephippium) are released by the sexually reproducing females and remain dormant before later hatching, ideally when environmental conditions have improved (Hairston, 1996).
Daphnia dentifera occurs at varying densities across our 15 study lakes in Southeast Michigan, USA, and is infected by a suite of parasites (Duffy et al., 2010). We tracked D. dentifera population sizes through time, as well as infections of nine microparasites (Duffy et al., 2010, 2015; Green, 1974; Lu et al., 2020; Wolinska et al., 2008): Metschnikowia bicuspidata (fungus), Pasteuria ramosa (bacterium), Spirobacillus cienkowskii (bacterium), Blastulidium paedophthorum (oomycete), Gurleya vavrai (microsporidian), Larssonia obtusa (microsporidian), Caullerya mesnili (ichthyosporean), an undescribed microsporidian gut parasite (“MicG”), and an unknown Saprolegnia‐like oomycete (“spider”).
2.2. Field sampling
We studied host and parasite communities in 15 lakes in Southeast Michigan, USA (Table S1), over 3 years (2014–2016). We sampled lakes roughly once every 2 weeks from mid‐July to mid‐November each year (usually nine sampling events per year). In addition, we intensively sampled four of the study sites (Gosling, North, Pickerel, and Sullivan Lakes) every 3 days during 2016 for a study focused on population dynamics. For each lake, on each sampling date, we collected three replicate vertical tows from the bottom of the lake with a 153‐μm Wisconsin plankton net and sampled from three different locations in each lake. This yielded three replicate samples per lake per sampling day, each of which contained one tow from each of the three locations within the lake. We used one of these samples to quantify infection prevalence and investment in sex. To quantify infection prevalence, we visually diagnosed parasite infections in live hosts under a dissection microscope at 20–50× magnification using dark field microscopy (or under a compound microscope at 200–400× magnification for early‐stage infections). As Daphnia are mostly transparent, many parasite infections are visibly detectable with this method. We also identified individuals as juvenile females, asexually reproducing females, sexually reproducing females, or males based on morphological differences (Brooks, 1957). For this sample where we quantified infection prevalence and investment in sex, we randomly subsampled the collected hosts, surveying at least 200 D. dentifera individuals for possible parasite infections or surveying all individuals when fewer than 200 individuals were present. We preserved the other two replicate samples in 90% ethanol. Later, we estimated the density of each host species by randomly subsampling and counting one of these samples (which combined one tow from each of the three locations in the lake) to estimate the density of each host species. We counted at least two subsamples from each lake‐date; if the total density of the two subsamples were not within 80% of each other, additional subsamples were counted. The subsamples were averaged yielding a single density estimate per lake‐date, with density calculated as the number of hosts throughout the water column for a given surface area of the lake (number of hosts per m2 of lake surface).
2.3. Statistical analysis
We explored relationships between density, parasitism, and investment in sex. For density, we integrated the total density of D. dentifera for each lake in a year over all sampling dates (i.e., we calculated the area under the curve with day on the x‐axis and host density on the y‐axis) and then took the log of that value. We analyzed two metrics related to parasitism: (a) integrated prevalence, determined by integrating the proportion of hosts infected with any parasite across sampling events within a lake and year, and (b) parasite species richness, calculated by tallying the number of parasite species observed infecting D. dentifera in a particular lake in a given year. Analyses with mean host density and parasitism yielded qualitatively similar results (Figure S1).
We also analyzed two metrics related to investment in sex: (a) the maximum investment in sex in the population as either the percent sexual ((males + ephippial females)/(total population)) or the percent sexual adults ((males + ephippial females)/(males + adult females)) and (b) integrated investment in sex, which, similar to the above metrics, was determined by integrating the proportion of hosts that were sexually reproducing (ephippial females or males) across sampling events within a lake and year. When determining the maxima, we only used samples that included at least 15 D. dentifera so that we could have greater confidence in the estimate of the investment in sex.
We plotted and analyzed data in R version 4.0.5. We analyzed whether lakes varied in investment in sex using a generalized linear model. The response variable was the number of sexual and number of asexual individuals observed on the day with the maximum percent sexual for that lake and year; because of overdispersion of the data, we used a quasibinomial error distribution. Because of limitations on mixed models and quasidistributions, our model included lake and year as fixed effects.
In addition to determining whether populations differed in the degree to which they reproduced sexually, we were also interested in assessing whether variation in investment in sex was associated with density or parasitism. We did not use a time series approach for this, because, based on our prior work on this system, we knew that investment in sex is strongly seasonal. Moreover, because sexual reproduction is associated with dormancy in this system, density would be expected to decrease as a result of sexual reproduction, even if high density had initially triggered investment in sex. Finally, we do not have any information on potential time lags that might occur between parasitism and investment in sex, especially given the presence of maternal and grandmaternal effects in Daphnia (e.g., Little et al., 2003; Lynch & Ennis, 1983; Poulsen et al., 2021) and the ability of parasite spores to persist outside the host (Duffy & Hunsberger, 2018; King et al., 2013). As a result, our analyses focused on integrated metrics of density, parasitism, and sexual reproduction, as well as parasite species richness across the entire sampling season. We calculated correlations between sexual reproduction (measured as the integrated investment in sexual reproduction) and (a) integrated D. dentifera density, (b) parasite species richness, and (c) integrated prevalence of infection. In order to check for collinearity, we also calculated correlations between integrated density, parasite species richness, and integrated prevalence of infection. Finally, we used a model selection approach to compare different possible models for investment in sexual reproduction. For all of these models, integrated investment in sexual reproduction was the response variable. These models included different combinations of integrated D. dentifera density, parasite species richness, integrated prevalence of infection, and year as independent variables. We created various submodels and then used model selection and Akaike information criteria (AIC) to compare 15 different models (as detailed in Table 1 in Section 3, below).
TABLE 1.
Model selection results from linear models with total integrated sexual reproduction as the response variable
| Model | AIC | ΔAIC | AIC weight | |
|---|---|---|---|---|
| 1 | Sex ~ log(int. density) + parasite SR | 253.10 | 0.00 | 0.264 |
| 2 | Sex ~ log(int. density) | 253.12 | 0.01 | 0.262 |
| 3 | Sex ~ log(int. density) + int. inf. prev. | 253.83 | 0.73 | 0.183 |
| 4 | Sex ~ log(int. density) * int. inf. prev. | 255.81 | 2.71 | 0.068 |
| 5 | Sex ~ parasite SR | 256.26 | 3.15 | 0.055 |
| 6 | Sex ~ log(int. density) + year | 256.68 | 3.58 | 0.044 |
| 7 | Sex ~ parasite SR + int. inf. prev. | 256.94 | 3.83 | 0.039 |
| 8 | Sex ~ log(int. density) + int. inf. prev. + year | 257.72 | 4.61 | 0.026 |
| 9 | Sex ~ log(int. density) + int. inf. prev. + parasite SR + year | 257.78 | 4.67 | 0.026 |
| 10 | Sex ~ log(int. density) + int. inf. prev. * year | 258.01 | 4.91 | 0.023 |
| 11 | Sex ~ log(int. density) *int. inf. prev. + year | 259.69 | 6.59 | 0.010 |
| 12 | Sex ~ int. inf. prev. | 270.72 | 17.62 | 3.95E−05 |
| 13 | Sex ~ int. inf. prev. + year | 270.77 | 17.67 | 3.85E−05 |
| 14 | Sex ~ int. inf. prev. * year | 270.89 | 17.79 | 3.62E−05 |
| 15 | Sex ~ year | 278.04 | 24.93 | 1.02E−06 |
Models are arranged by AIC score. “Int. density” indicates integrated Daphnia dentifera density, “int. inf. prev.” indicates integrated infection prevalence, and “parasite SR” indicates parasite species richness.
3. RESULTS
There was substantial variation in investment in sex, density, and parasite prevalence in the study populations of D. dentifera (Figure 2). Sexual reproduction was seasonal, with male and ephippial female production beginning as early as late September and generally increasing through November (black lines in Figure 2). In some lakes and years, we never observed any males or ephippial females, whereas in others, populations shifted to nearly all sexual. Lakes that had higher investment in sex in 1 year tended to also have high investment in sex in the other 2 years (Figure 3a,c; maximum investment in sex in the total population: lake: F = 4.02, p = 0.0008).
FIGURE 2.
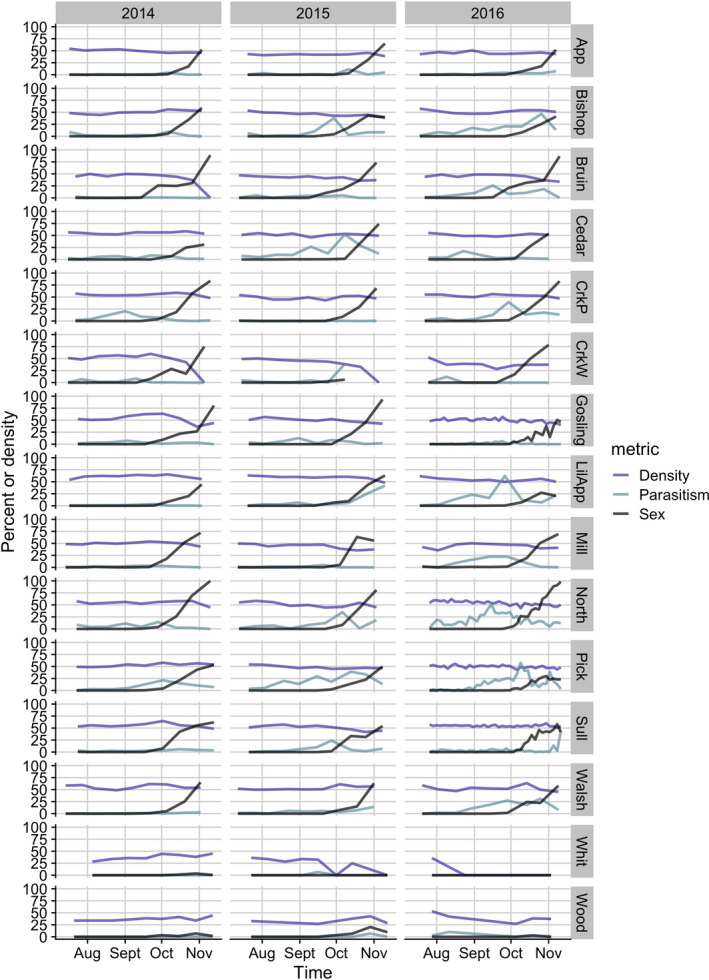
There was considerable variation in the density of Daphnia dentifera (purple lines), the percentage of D. dentifera reproducing sexually (black lines), as well as in the percent infected with at least one parasite (ocean blue lines) across lakes and time. The percent sexual was derived from the ratio of males and ephippial females out of the total population counted. Percent infected was calculated as the percent of D. dentifera with any parasitic infection, including coinfections. Density is (LN (#D. dentifera m−2 + 1)) multiplied by 5 in order to improve the visibility
FIGURE 3.
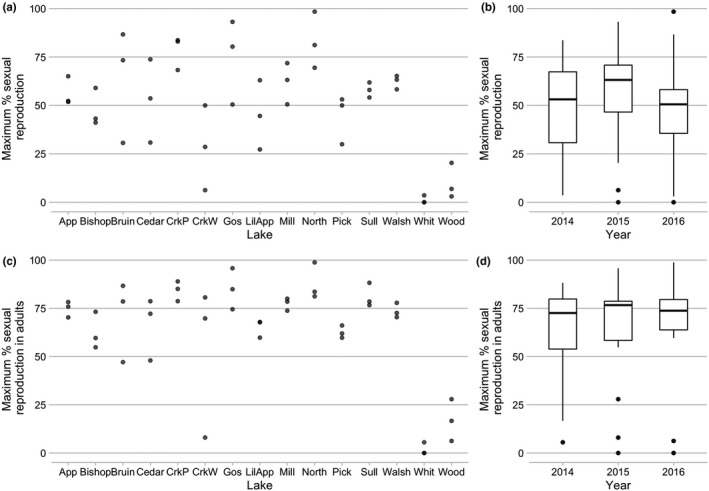
Variation in the maximum percentage of the total population (a & b) or of adults (c & d) reproducing sexually. In a & c, the data are plotted by lake, with three values per lake (one for 2014, 2015, and 2016); points are partially transparent to facilitate visualization of overlapping points. In b & d, the data are plotted by year, with 15 values per year (one for each lake). For a & b, the percent sexual was calculated as (males + ephippial females)/(total population); for c & d, the percent sexual adults was calculated as (males + ephippial females)/(males + adult females). These values were only calculated for lake‐dates for which the sample contained at least 15 individuals
There was also substantial variation in the prevalence of parasites (ocean blue lines in Figure 2) across lakes. In some lakes and years, there was very little parasitism; in other lakes and years infection prevalence exceeded 50% at the peak of infections. Density was generally fairly consistent within lakes over time (purple lines in Figure 2), but populations crashed to near or below detection limits in some lakes and years.
Investment in sexual reproduction by D. dentifera was strongly associated with the log of integrated D. dentifera density (Figure 4a; r = 0.637, p < 0.0001) and parasite species richness (Figure 4b; r = 0.602, p < 0.0001); it was also associated with the integrated prevalence of infection (Figure 4d; r = 0.350, p = 0.019). The log of integrated D. dentifera density, parasite species richness, and integrated prevalence of infection were also correlated with one another (density and parasite species richness: Figure 4c; r = 0.791, p < 0.0001; prevalence of infection and density: Figure 4e; r = 0.359, p = 0.015; and prevalence of infection and parasite species richness: Figure 4f; r = 0.371, p = 0.012). Comparing the AICs of models incorporating different possible drivers of variation in investment in sex suggests the importance of density and/or parasitism: All top models (ΔAIC < 4.0) included one or more of log of integrated density, parasite species richness, and integrated prevalence of infection as a predictor of sexual reproduction (Table 1).
FIGURE 4.
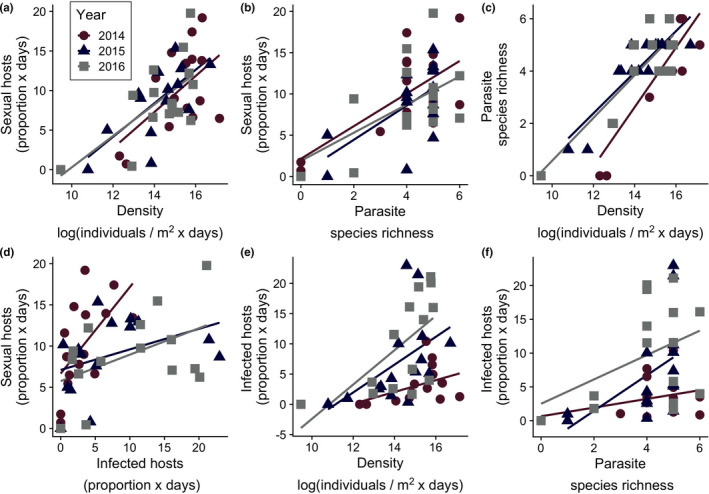
There are strong relationships between density, parasitism, and sexual reproduction, but collinearity makes it challenging to determine underlying drivers of these correlations. Panels show relationships between the log of integrated Daphnia dentifera density, parasite species richness, integrated infection prevalence, and investment in sexual reproduction, with separate lines shown for each year. The areal density of D. dentifera, the proportion of infected D. dentifera, and the proportion of male and sexual female D. dentifera values were each separately integrated across sampling events to obtain a single value (each point represents a single lake in a given year)
The strength of the relationship between the integrated prevalence of individual parasites and the integrated prevalence of sexual reproduction varied across parasites (Figure 5; Table 2). The correlation between B. paedophthorum, an oomycete that attacks developing embryos, was the strongest and similar to the correlation between total parasitism and sex (r = 0.350, p = 0.0186). The relationship between the most common parasite, the parasitic castrator Pasteuria ramosa, and sexual reproduction was less strong (Figure 5; Table 2).
FIGURE 5.
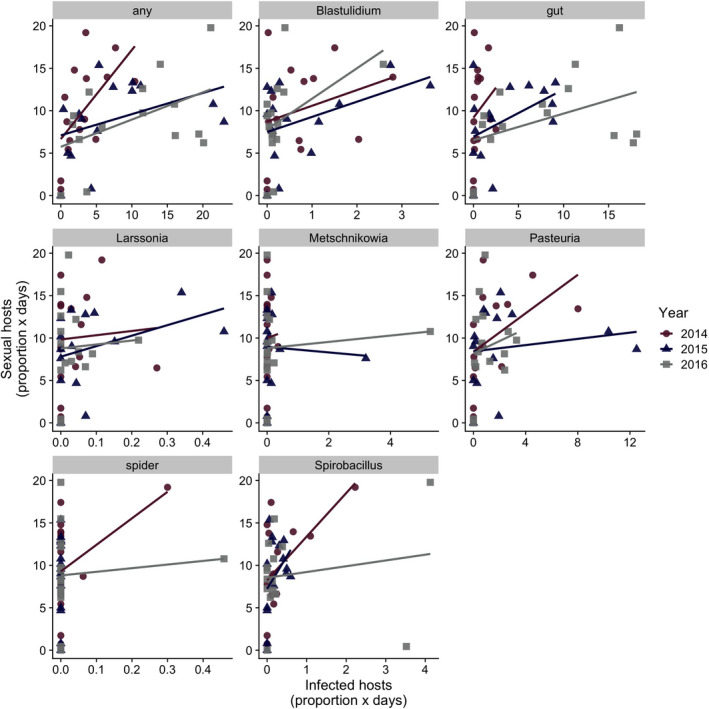
Total integrated sexual hosts compared to integrated infected hosts with different parasite species. “Gut” parasites are a combination of two parasite species (which were not initially distinguished): Caullerya mesnili (ichthyosporean) and an undescribed microsporidian gut parasite (“MicG”). Gurleya vavrai is not plotted because we did not observe any Daphnia dentifera infected with G. vavrai during this study
TABLE 2.
Summary of the virulent effects and prevalence of the five most common parasites in this study, as well as the correlation between the integrated prevalence of each parasite and the integrated prevalence of sexual reproduction (as shown in Figure 5)
| Parasite | Parasite virulence | Parasite prevalence | Correlation between integrated prevalence and sexual reproduction | ||||
|---|---|---|---|---|---|---|---|
| Impact on reproduction | Impact on lifespan | Median | Mean | Max | r | p | |
| Pasteuria ramosa | Castrating | Low | 1.9% | 4.9% | 36.5% | 0.218 | 0.150 |
| Metschnikowia bicuspidata | Moderate | High | 0.0% | 0.9% | 14.0% | 0.018 | 0.908 |
| Spirobacillus cienkowskii | Castrating | Very high | 0.5% | 1.7% | 20.1% | 0.256 | 0.090 |
| Blastulidium paedophthorum | Castrating | None detected | 0.9% | 2.2% | 11.2% | 0.369 | 0.013 |
| Gut | Variable | High for C. mesnili, none detected for MicG | 7.3% | 13.5% | 57.8% | 0.231 | 0.127 |
Information on virulence in Daphnia dentifera comes from prior studies (Auld et al., 2012; Duffy & Hall, 2008; Duffy et al., 2015; Rogalski et al., 2021; Wale et al., 2019). “Gut” parasites are the ichthyosporean Caullerya mesnili and a microsporidian currently known as “MicG” (Rogalski et al., 2021; GenBank accession MH635259). Parasite prevalences come from the 15 lake populations and 3 years that were the focus of the present study. The correlation was calculated between the integrated prevalence of each particular parasite and the integrated prevalence of sexual individuals (Figure 5).
4. DISCUSSION
We found substantial variation in investment in sexual reproduction in natural populations of D. dentifera, with some populations remaining entirely asexual and others becoming almost entirely sexual in autumn. That variation was fairly consistent across years, with lakes that had high investment in sex 1 year also tending to have high investment in sex in the other 2 years. We found strong relationships between density, parasitism, and sexual reproduction in this system, suggesting that density and/or parasitism might be linked with investment in sex in these populations. However, strong collinearity in the underlying data means that further work will be required to disentangle the drivers of these relationships.
Our findings are consistent with earlier studies that found density to be an important factor influencing the shift from asexual to sexual reproduction in cyclical parthenogens like Daphnia and rotifers (Berg et al., 2001; Gilbert, 2020; Haltiner et al., 2020; Larsson, 1991; Stelzer & Snell, 2003; Stross & Hill, 1965). One possible explanation for this association is that, in many cyclical parthenogens, sexual reproduction is associated with the production of long‐lasting resting stages, meaning sexual reproduction may serve as a means of temporal dispersal when faced with strong competition in dense populations (Gerber et al., 2018; Gilbert, 2020). High densities also reduce the relative costs of sexual reproduction; as populations approach carrying capacity, asexual reproduction is less beneficial, reducing the opportunity costs of sexual reproduction (Burt, 2000; Gerber et al., 2018).
We also found that parasitism was positively correlated with sexual reproduction in D. dentifera. Prior work has especially focused on the bacterial parasite Pasteuria ramosa and investment in sex. Pasteuria is highly virulent (Auld et al., 2012; Ebert et al., 2000) and can reach quite high prevalence (Duncan & Little, 2007). It also shows very strong host–parasite genotype specificity, with parasite infectivity (and host susceptibility) being determined by host (and parasite) genotype (Carius et al., 2001; Ebert et al., 2016). One would expect this matching mechanism to favor genetic recombination (and it does in Auld et al., 2016), which could, in turn, drive Red Queen dynamics, where reciprocal evolutionary dynamics arise from selection of two antagonists on one another. Indeed, one of the best examples of Red Queen dynamics comes from the Daphnia–Pasteuria system (Decaestecker et al., 2007). In our present study, Pasteuria was the second most common of the nine parasites that we tracked (after “gut” parasites; Table 2). The overall relationship between Pasteuria infection levels and investment in sex in D. dentifera was consistent with that of the combined infection levels and investment in sex (Figure 5), but was not significant. Instead, the strongest correlation was between the integrated prevalence of an oomycete that attacks developing embryos, B. paedophthorum. Overall, prior work in Daphnia suggested that parasites might favor sexual reproduction in hosts; our work expands this by showing that the prevalence of sexually reproducing individuals in natural lake populations is associated with parasitism (as well as density).
Intriguingly, there was a strong positive relationship between parasite species richness (the number of parasite taxa observed over the summer and fall in a particular lake) and the amount of sexual reproduction (Figure 4b). An earlier study on hermaphroditic snails found that male outcrossing ability correlated with an index that combined trematode prevalence and species richness (Schrag et al., 1994); similar to our study, that study found a correlation between species richness and prevalence (in the snail study, the prevalence of one particular trematode) that made it hard to disentangle their relative effects. Looking at a much larger scale, a study on plants found that species that are attacked by more fungal pathogens have higher outcrossing rates, as compared to species that are attacked by fewer pathogens (Busch et al., 2004). Collectively, these results suggest additional research on parasite species richness and sexual reproduction is warranted.
We focused on the influences of parasitism and density on investment in sex. An interesting avenue for future research would be to consider, in addition to density and parasitism, the impacts of resources and predators, which have also been shown to influence shifts to sexual reproduction in Daphnia (Walsh, 2013). However, doing so becomes logistically challenging. While it is relatively straightforward to quantify the abundance of invertebrate predators such as Chaoborus larvae, directly quantifying the rate of fish predation is challenging, though body size can be used as a proxy (Brooks & Dodson, 1965; Kitchell & Kitchell, 1980). Similarly, directly quantifying resource quality can be challenging, since chlorophyll levels in a lake do not strongly correlate with the resources experienced by Daphnia (Tessier & Woodruff, 2002). However, the average clutch size (known as the “egg ratio”) of uninfected hosts can be used as an indicator of resource levels as experienced by Daphnia (Kerfoot et al., 1988; Threlkeld, 1979) so, similar to predation, it is possible to use proxies to assess resource levels. Thus, future studies that measure invertebrate predators, Daphnia body size, and Daphnia egg ratio in addition to the factors we measured in this study would give greater insight into the factors driving variation in investment in sex.
It would also be interesting for future research to consider the potential impacts of abiotic factors on sexual reproduction in lake Daphnia populations. In particular, temperature and light are known cues for Daphnia reproductive cycles (Stross & Hill, 1965). This work should consider not only direct impacts of those abiotic factors on sexual reproduction, but also the potential for indirect effects. Prior studies in this system have shown that habitat structure (including light and thermal structure) can have a range of direct and indirect effects on parasitism (Penczykowski et al., 2014; Shaw et al., 2020; Strauss et al., 2016), and it is possible (perhaps even likely) that the same is true for investment in sex.
Shifts from asexual to sexual reproduction in cyclical parthenogens have large impacts on fitness (Gerber et al., 2018) and population dynamics. We found that wild D. dentifera populations varied greatly in the degree to which they invested in sexual reproduction, with some remaining entirely asexual and others shifting almost entirely to sexual reproduction. Host density and parasitism were strongly predictive of the frequency of sexual females and males in these populations, providing evidence in support of links between parasitism, density, and sexual reproduction.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Camden D. Gowler: Conceptualization (equal); Data curation (lead); Formal analysis (equal); Investigation (equal); Methodology (equal); Visualization (equal); Writing‐original draft (equal). Mary A. Rogalski: Investigation (equal); Methodology (equal); Writing‐review & editing (supporting). Clara L. Shaw: Investigation (equal); Methodology (equal); Writing‐review & editing (supporting). Katherine K. Hunsberger: Investigation (equal); Methodology (equal); Writing‐review & editing (supporting). Meghan A. Duffy: Conceptualization (lead); Formal analysis (equal); Funding acquisition (lead); Project administration (lead); Visualization (equal); Writing‐original draft (equal).
Supporting information
Figure S1
Table S1
Supplementary Material
ACKNOWLEDGMENTS
We are extremely grateful to Rebecca Bilich for her assistance with field sampling and to Curt Lively, Piet Spaak, and an anonymous reviewer for feedback on an earlier version of this manuscript. This work was supported by NSF grant DEB‐1305836 to MAD and by the Moore Foundation (GBMF9202; DOI: https://doi.org/10.37807/GBMF9202).
Gowler, C. D. , Rogalski, M. A. , Shaw, C. L. , Hunsberger, K. K. , & Duffy, M. A. (2021). Density, parasitism, and sexual reproduction are strongly correlated in lake Daphnia populations. Ecology and Evolution, 11, 10446–10456. 10.1002/ece3.7847
DATA AVAILABILITY STATEMENT
Data and associated code are available at Dryad: https://doi.org/10.5061/dryad.pzgmsbcm6.
REFERENCES
- Auld, S. K. J. R. , Hall, S. R. , & Duffy, M. A. (2012). Epidemiology of a Daphnia‐multiparasite system and its implications for the Red Queen. PLoS One, 7, e39564.– 10.1371/journal.pone.0039564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld, S. K. J. R. , Tinkler, S. K. , & Tinsley, M. C. (2016). Sex as a strategy against rapidly evolving parasites. Proceedings of the Royal Society B: Biological Sciences, 283, 20162226. 10.1098/rspb.2016.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Ami, F. , & Heller, J. (2005). Spatial and temporal patterns of parthenogenesis and parasitism in the freshwater snail Melanoides tuberculata . Journal of Evolutionary Biology, 18, 138–146. 10.1111/j.1420-9101.2004.00791.x [DOI] [PubMed] [Google Scholar]
- Berg, L. , Pálsson, S. , & Lascoux, M. (2001). Fitness and sexual response to population density in Daphnia pulex . Freshwater Biology, 46, 667–677. [Google Scholar]
- Brooks, J. L. (1957). The systematics of North American Daphnia . Memoirs of the Connecticut Academy of Arts & Sciences, 13, 1–180. [Google Scholar]
- Brooks, J. L. , & Dodson, S. I. (1965). Predation, body size, and composition of plankton. Science, 150, 28–35. [DOI] [PubMed] [Google Scholar]
- Burt, A. (2000). Sex, recombination, and the efficacy of selection – Was Weismann right? Evolution, 54, 337–351. [DOI] [PubMed] [Google Scholar]
- Busch, J. W. , Neiman, M. , & Koslow, J. M. (2004). Evidence for maintenance of sex by pathogens in plants. Evolution, 58, 2584–2590. 10.1111/j.0014-3820.2004.tb00886.x [DOI] [PubMed] [Google Scholar]
- Cáceres, C. E. , Hartway, C. , & Paczolt, K. A. (2009). Inbreeding depression varies with investment in sex in a facultative parthenogen. Evolution, 63, 2474–2480. 10.1111/j.1558-5646.2009.00707.x [DOI] [PubMed] [Google Scholar]
- Carius, H. J. , Little, T. J. , & Ebert, D. (2001). Genetic variation in a host‐parasite association: Potential for coevolution and frequency‐dependent selection. Evolution, 55, 1136–1145. 10.1111/j.0014-3820.2001.tb00633.x [DOI] [PubMed] [Google Scholar]
- Dagan, Y. , Liljeroos, K. , Jokela, J. , & Ben‐Ami, F. (2013). Clonal diversity driven by parasitism in a freshwater snail. Journal of Evolutionary Biology, 26, 2509–2519. 10.1111/jeb.12245 [DOI] [PubMed] [Google Scholar]
- Decaestecker, E. , Gaba, S. , Raeymaekers, J. A. M. , Stoks, R. , Van Kerckhoven, L. , Ebert, D. , & De Meester, L. (2007). Host–parasite “Red Queen” dynamics archived in pond sediment. Nature, 450, 870–873. 10.1038/nature06291 [DOI] [PubMed] [Google Scholar]
- Duffy, M. A. , Brassil, C. E. , Hall, S. R. , Tessier, A. J. , Cáceres, C. E. , & Conner, J. K. (2008). Parasite‐mediated disruptive selection in a natural Daphnia population. BMC Evolutionary Biology, 8, 80. 10.1186/1471-2148-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, M. A. , Cáceres, C. E. , Hall, S. R. , Tessier, A. J. , & Ives, A. R. (2010). Temporal, spatial, and between‐host comparisons of patterns of parasitism in lake zooplankton. Ecology, 91, 3322–3331. 10.1890/09-1611.1 [DOI] [PubMed] [Google Scholar]
- Duffy, M. A. , & Hall, S. R. (2008). Selective predation and rapid evolution can jointly dampen effects of virulent parasites on Daphnia populations. American Naturalist, 171, 499–510. [DOI] [PubMed] [Google Scholar]
- Duffy, M. A. , & Hunsberger, K. K. (2018). Infectivity is influenced by parasite spore age and exposure to freezing: Do shallow waters provide Daphnia a refuge from some parasites? Journal of Plankton Research, 41, 12–16. [Google Scholar]
- Duffy, M. A. , James, T. Y. , & Longworth, A. (2015). Ecology, virulence, and phylogeny of Blastulidium paedophthorum, a widespread brood parasite of Daphnia spp. Applied and Environment Microbiology, 81, 5486–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, A. B. , & Little, T. J. (2007). Parasite‐driven genetic change in a natural population of Daphnia . Evolution, 61, 796–803. 10.1111/j.1558-5646.2007.00072.x [DOI] [PubMed] [Google Scholar]
- Duncan, A. B. , Mitchell, S. E. , & Little, T. J. (2006). Parasite‐mediated selection and the role of sex and diapause in Daphnia . Journal of Evolutionary Biology, 19, 1183–1189. 10.1111/j.1420-9101.2006.01085.x [DOI] [PubMed] [Google Scholar]
- Ebert, D. (2005). Ecology, epidemiology, and evolution of parasitism in Daphnia . National Library of Medicine. [Google Scholar]
- Ebert, D. , Altermatt, F. , & Lass, S. (2007). A short term benefit for outcrossing in a Daphnia metapopulation in relation to parasitism. Journal of the Royal Society, Interface, 4, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert, D. , Duneau, D. , Hall, M. D. , Luijckx, P. , Andras, J. P. , Du Pasquier, L. , & Ben‐Ami, F. (2016). Chapter five – A population biology perspective on the stepwise infection process of the bacterial pathogen Pasteuria ramosa in Daphnia . In Rollinson D., & Stothard J. R. (Eds.), Advances in parasitology (pp. 265–310). Academic Press. [DOI] [PubMed] [Google Scholar]
- Ebert, D. , Lipsitch, M. , & Mangin, K. L. (2000). The effect of parasites on host population density and extinction: Experimental epidemiology with Daphnia and six microparasites. American Naturalist, 156, 459–477. [DOI] [PubMed] [Google Scholar]
- Gerber, N. , & Kokko, H. (2018). Abandoning the ship using sex, dispersal or dormancy: Multiple escape routes from challenging conditions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 373, 20170424. 10.1098/rstb.2017.0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, N. , Kokko, H. , Ebert, D. , & Booksmythe, I. (2018). Daphnia invest in sexual reproduction when its relative costs are reduced. Proceedings of the Royal Society B: Biological Sciences, 285, 20172176. 10.1098/rspb.2017.2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, A. K. , Delph, L. F. , Vergara, D. , & Lively, C. M. (2018). Periodic, parasite‐mediated selection for and against sex. American Naturalist, 192, 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, A. K. , & Lively, C. M. (2019). Chapter 2‐Genetic diversity and disease spread: Epidemiological models and empirical studies of a snail–trematode system. In Wilson K., Fenton A., & Tompkins D. (Eds.), Wildlife disease ecology: Linking theory to data and application (pp. 32‐57). British Ecological Society, Cambridge University Press. [Google Scholar]
- Gilbert, J. J. (2020). Variation in the life cycle of monogonont rotifers: Commitment to sex and emergence from diapause. Freshwater Biology, 65, 786–810. 10.1111/fwb.13440 [DOI] [Google Scholar]
- Green, J. (1974). Parasites and epibionts of Cladocera. The Transactions of the Zoological Society of London, 32, 417–515. 10.1111/j.1096-3642.1974.tb00031.x [DOI] [Google Scholar]
- Hairston, N. G. Jr (1996). Zooplankton egg banks as biotic reservoirs in changing environments. Limnology and Oceanography, 41, 1087–1092. 10.4319/lo.1996.41.5.1087 [DOI] [Google Scholar]
- Haltiner, L. , Hänggi, C. , Spaak, P. , & Dennis, S. R. (2020). Sex in crowded places: Population density regulates reproductive strategy. Hydrobiologia, 847, 1727–1738. 10.1007/s10750-019-04143-7 [DOI] [Google Scholar]
- Hite, J. L. , Penczykowski, R. M. , Shocket, M. S. , Griebel, K. A. , Strauss, A. T. , Duffy, M. A. , Cáceres, C. E. , & Hall, S. R. (2017). Allocation, not male resistance, increases male frequency during epidemics: A case study in facultatively sexual hosts. Ecology, 98, 2773–2783. 10.1002/ecy.1976 [DOI] [PubMed] [Google Scholar]
- Jaenike, J. (1978). An hypothesis to account for the maintenance of sex within populations. Evolutionary Theory, 3, 191–194. [Google Scholar]
- Johnson, P. T. J. , Ives, A. R. , Lathrop, R. C. , & Carpenter, S. R. (2009). Long‐term disease dynamics in lakes: Causes and consequences of chytrid infections in Daphnia populations. Ecology, 90, 132–144. [DOI] [PubMed] [Google Scholar]
- Kerfoot, W. C. , Levitan, C. , & DeMott, W. R. (1988). Daphnia‐phytoplankton interactions: Density‐dependent shifts in resource quality. Ecology, 69, 1806–1825. 10.2307/1941159 [DOI] [Google Scholar]
- King, K. C. , Auld, S. , Wilson, P. J. , James, J. , & Little, T. J. (2013). The bacterial parasite Pasteuria ramosa is not killed if it fails to infect: Implications for coevolution. Ecology and Evolution, 3, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, K. C. , Delph, L. F. , Jokela, J. , & Lively, C. M. (2009). The geographic mosaic of sex and the Red Queen. Current Biology, 19, 1438–1441. 10.1016/j.cub.2009.06.062 [DOI] [PubMed] [Google Scholar]
- Kitchell, J. A. , & Kitchell, J. F. (1980). Size‐selective predation, light transmission, and oxygen stratification – Evidence from the recent sediments of manipulated lakes. Limnology and Oceanography, 25, 389–402. [Google Scholar]
- Kokko, H. (2020). When synchrony makes the best of both worlds even better: How well do we really understand facultative sex? American Naturalist, 195, 380–392. 10.1086/706812 [DOI] [PubMed] [Google Scholar]
- Kondrashov, A. S. (1984). Deleterious mutations as an evolutionary factor. 1. The advantage of recombination. Genetical Research, 44, 199–217. 10.1017/S0016672300026392 [DOI] [PubMed] [Google Scholar]
- Larsson, P. (1991). Intraspecific variability in response to stimuli for male and ephippia formation in Daphnia pulex . Hydrobiologia, 225, 281–290. 10.1007/BF00028406 [DOI] [Google Scholar]
- Little, T. J. , O'Connor, B. , Colegrave, N. , Watt, K. , & Read, A. F. (2003). Maternal transfer of strain‐specific immunity in an invertebrate. Current Biology, 13, 489–492. 10.1016/S0960-9822(03)00163-5 [DOI] [PubMed] [Google Scholar]
- Lively, C. M. (1987). Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature, 328, 519–521. 10.1038/328519a0 [DOI] [Google Scholar]
- Lively, C. M. (2010). A review of Red Queen models for the persistence of obligate sexual reproduction. Journal of Heredity, 101(Suppl 1), S13–S20. 10.1093/jhered/esq010 [DOI] [PubMed] [Google Scholar]
- Lively, C. M. , & Dybdahl, M. F. (2000). Parasite adaptation to locally common host genotypes. Nature, 405, 679–681. 10.1038/35015069 [DOI] [PubMed] [Google Scholar]
- Lively, C. M. , & Morran, L. T. (2014). The ecology of sexual reproduction. Journal of Evolutionary Biology, 27, 1292–1303. 10.1111/jeb.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Ocaña‐Pallarès, E. , López‐Escardó, D. , Dennis, S. R. , Monaghan, M. T. , Ruiz‐Trillo, I. , Spaak, P. , & Wolinska, J. (2020). Revisiting the phylogenetic position of Caullerya mesnili (Ichthyosporea), a common Daphnia parasite, based on 22 protein‐coding genes. Molecular Phylogenetics and Evolution, 151, 106891. [DOI] [PubMed] [Google Scholar]
- Lynch, M. , & Ennis, R. (1983). Resource availability, maternal effects, and longevity. Experimental Gerontology, 18, 147–165. [DOI] [PubMed] [Google Scholar]
- Lynch, Z. R. , Penley, M. J. , & Morran, L. T. (2018). Turnover in local parasite populations temporarily favors host outcrossing over self‐fertilization during experimental evolution. Ecology and Evolution, 8, 6652–6662. 10.1002/ece3.4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone, M. , Gibson, A. , Cook, D. , Freymiller, L. , Mishkind, D. , Quinlan, A. , York, J. , Lively, C. , & Neiman, M. (2016). Fine‐scale association between parasites and sex in Potamopyrgus antipodarum within a New Zealand lake. New Zealand Journal of Ecology, 40, 330–333. 10.20417/nzjecol.40.40 [DOI] [Google Scholar]
- McLeod, D. V. , & Day, T. (2014). Sexually transmitted infection and the evolution of serial monogamy. Proceedings of the Royal Society B: Biological Sciences, 281, 20141726. 10.1098/rspb.2014.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, S. E. , Read, A. F. , & Little, T. J. (2004). The effect of a pathogen epidemic on the genetic structure and reproductive strategy of the crustacean Daphnia magna: Parasitic infection, sex and diversity. Ecology Letters, 7, 848–858. 10.1111/j.1461-0248.2004.00639.x [DOI] [Google Scholar]
- Morran, L. T. , Schmidt, O. G. , Gelarden, I. A. , Parrish, R. C. 2nd , & Lively, C. M. (2011). Running with the Red Queen: Host‐parasite coevolution selects for biparental sex. Science, 333, 216–218. 10.1126/science.1206360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, H. J. (1964). The relation of recombination to mutational advance. Mutation Research, 106, 2–9. 10.1016/0027-5107(64)90047-8 [DOI] [PubMed] [Google Scholar]
- Neiman, M. , Lively, C. M. , & Meirmans, S. (2017). Why sex? A pluralist approach revisited. Trends in Ecology & Evolution, 32, 589–600. 10.1016/j.tree.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Otto, S. P. (2009). The evolutionary enigma of sex. American Naturalist, 174(Suppl. 1), S1–S14. 10.1086/599084 [DOI] [PubMed] [Google Scholar]
- Penczykowski, R. M. , Hall, S. R. , Civitello, D. J. , & Duffy, M. A. (2014). Habitat structure and ecological drivers of disease. Limnology and Oceanography, 59, 340–348. 10.4319/lo.2014.59.2.0340 [DOI] [Google Scholar]
- Poulsen, R. , De Fine Licht, H. H. , Hansen, M. , & Cedergreen, N. (2021). Grandmother's pesticide exposure revealed bi‐generational effects in Daphnia magna . Aquatic Toxicology, 236, 105861. 10.1016/j.aquatox.2021.105861 [DOI] [PubMed] [Google Scholar]
- Rogalski, M. A. , Stewart Merrill, T. , Gowler, C. D. , Cáceres, C. E. , & Duffy, M. A. (2021). Context dependent host‐symbiont interactions: Shifts along the parasitism‐mutualism continuum. Authorea. 10.22541/au.162454903.33763984/v1 [DOI] [PubMed] [Google Scholar]
- Roth, O. , Ebert, D. , Vizoso, D. B. , Bieger, A. , & Lass, S. (2008). Male‐biased sex‐ratio distortion caused by Octosporea bayeri, a vertically and horizontally‐transmitted parasite of Daphnia magna . International Journal for Parasitology, 38, 969–979. [DOI] [PubMed] [Google Scholar]
- Schrag, S. J. , Mooers, A. O. , Ndifon, G. T. , & Read, A. F. (1994). Ecological correlates of male outcrossing ability in a simultaneous hermaphrodite snail. American Naturalist, 143, 636–655. 10.1086/285624 [DOI] [Google Scholar]
- Shaw, C. L. , Hall, S. R. , Overholt, E. P. , Cáceres, C. E. , Williamson, C. E. , & Duffy, M. A. (2020). Shedding light on environmentally transmitted parasites: Lighter conditions within lakes restrict epidemic size. Ecology, 101, e03168. 10.1002/ecy.3168 [DOI] [PubMed] [Google Scholar]
- Slowinski, S. P. , Morran, L. T. , Parrish, R. C. II , Cui, E. R. , Bhattacharya, A. , Lively, C. M. , & Phillips, P. C. (2016). Coevolutionary interactions with parasites constrain the spread of self‐fertilization into outcrossing host populations. Evolution, 70, 2632–2639. 10.1111/evo.13048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer, C.‐P. (2011). The cost of sex and competition between cyclical and obligate parthenogenetic rotifers. American Naturalist, 177, E43–E53. 10.1086/657685 [DOI] [PubMed] [Google Scholar]
- Stelzer, C. P. , & Snell, T. W. (2003). Induction of sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by a density‐dependent chemical cue. Limnology and Oceanography, 48, 939–943. [Google Scholar]
- Strauss, A. T. , Shocket, M. S. , Civitello, D. J. , Hite, J. L. , Penczykowski, R. M. , Duffy, M. A. , Cáceres, C. E. , & Hall, S. R. (2016). Habitat, predators, and hosts regulate disease in Daphnia through direct and indirect pathways. Ecological Monographs, 86, 393–411. [Google Scholar]
- Stross, R. G. , & Hill, J. C. (1965). Diapause induction in Daphnia requires two stimuli. Science, 150, 1462–1464. 10.1126/science.150.3702.1462 [DOI] [PubMed] [Google Scholar]
- Tessier, A. J. , & Cáceres, C. E. (2004). Differentiation in sex investment by clones and populations of Daphnia . Ecology Letters, 7, 695–703. 10.1111/j.1461-0248.2004.00627.x [DOI] [Google Scholar]
- Tessier, A. J. , & Woodruff, P. (2002). Cryptic trophic cascade along a gradient of lake size. Ecology, 83, 1263–1270. 10.1890/0012-9658(2002)083[1263:CTCAAG]2.0.CO;2 [DOI] [Google Scholar]
- Threlkeld, S. T. (1979). The midsummer dynamics of two Daphnia species in wintergreen lake, Michigan. Ecology, 60, 165–179. 10.2307/1936478 [DOI] [Google Scholar]
- Vergara, D. , Jokela, J. , & Lively, C. M. (2014). Infection dynamics in coexisting sexual and asexual host populations: Support for the Red Queen hypothesis. American Naturalist, 184, S22–S30. 10.1086/676886 [DOI] [PubMed] [Google Scholar]
- Vergara, D. , Lively, C. M. , King, K. C. , & Jokela, J. (2013). The geographic mosaic of sex and infection in lake populations of a New Zealand snail at multiple spatial scales. American Naturalist, 182, 484–493. 10.1086/671996 [DOI] [PubMed] [Google Scholar]
- Wale, N. , Turrill, M. L. , & Duffy, M. A. (2019). A colorful killer: Daphnia infected with the bacterium Spirobacillus cienkowskii exhibit unexpected color variation. Ecology, 100, e02562. [DOI] [PubMed] [Google Scholar]
- Walsh, M. R. (2013). The link between environmental variation and evolutionary shifts in dormancy in zooplankton. Integrative and Comparative Biology, 53, 713–722. 10.1093/icb/ict035 [DOI] [PubMed] [Google Scholar]
- Walsh, M. R. , & Post, D. M. (2012). The impact of intraspecific variation in a fish predator on the evolution of phenotypic plasticity and investment in sex in Daphnia ambigua . Journal of Evolutionary Biology, 25, 80–89. 10.1111/j.1420-9101.2011.02403.x [DOI] [PubMed] [Google Scholar]
- Wolinska, J. , King, K. C. , Vigneux, F. , & Lively, C. M. (2008). Virulence, cultivating conditions, and phylogenetic analyses of oomycete parasites in Daphnia . Parasitology, 135, 1667–1678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Supplementary Material
Data Availability Statement
Data and associated code are available at Dryad: https://doi.org/10.5061/dryad.pzgmsbcm6.


