Abstract
Objectives
According to guidelines, clinical arthritis is mandatory for diagnosing RA. However, in the absence of clinical synovitis, imaging-detected subclinical synovitis is increasingly used instead and is considered as a starting point for DMARD therapy. To search for evidence we studied the natural course of arthralgia patients with subclinical synovitis from three longitudinal cohorts and determined the frequencies of non-progression to clinically apparent inflammatory arthritis (IA) (i.e. ‘false positives’).
Methods
Subclinical synovitis in the hands or feet of arthralgia patients was visualized with US (two cohorts; definition: greyscale ≥2 and/or power Doppler ≥1) or MRI (one cohort; definition: synovitis score ≥1 by two readers). Patients were followed for 1 year on for IA development; two cohorts also had 3 year data. Analyses were stratified for ACPA.
Results
Subclinical synovitis at presentation was present in 36%, 41% and 31% in the three cohorts. Of the ACPA-positive arthralgia patients with subclinical synovitis, 54%, 44% and 68%, respectively, did not develop IA. These percentages were even higher in the ACPA-negative arthralgia patients: 66%, 85% and 89%, respectively. Similar results were seen after 3 years of follow-up.
Conclusion
Replacing clinical arthritis with subclinical synovitis to identify RA introduces a high false-positive rate (44–89%). These data suggest an overestimation regarding the value of ACPA positivity in combination with the presence of subclinical synovitis in patients with arthralgia, which harbours the risk of overtreatment if DMARDs are initiated in the absence of clinical arthritis.
Keywords: RA, anti-citrullinated antibodies (biomarkers), MRI, ultrasonography, outcome assessment health care
Rheumatology key messages
A total of 44–68% of ACPA-positive arthralgia patients with subclinical synovitis did not progress to inflammatory arthritis.
Replacing the entry criterion in the 2010 criteria for RA with subclinical synovitis did not diminish the aforementioned false-positive rate.
This natural course suggests that initiating DMARD treatment in arthralgia patients would result in considerable overtreatment.
Introduction
Early start with DMARDs has become key in the treatment of RA because of its association with improved disease outcomes [1]. It has also fuelled research that aims to identify patients at risk for RA in the symptomatic phase preceding clinically apparent arthritis in the hope that even earlier treatment may prevent the development of RA. At present, clinically apparent arthritis is mandatory for diagnosing RA and according to current guidelines is the regular starting point for DMARD treatment [1].
However, this basic notion seems to be shifting in some places. A recent Dutch study showed that rheumatologists are increasingly willing to initiate ‘preventive’ treatment in the absence of clinical arthritis [2]. Likewise, a survey in the UK demonstrated that up to 73% of consulting rheumatologists would start DMARD treatment in ACPA-positive patients with musculoskeletal symptoms and power Doppler on US in the absence of clinically apparent arthritis [3].
Subclinical synovitis has indeed been consistently reported as a predictor for RA development; however, not all patients with this feature will develop RA [4, 5]. Although we and others have published about predictive models, the risk of patients with subclinical synovitis progressing to RA, especially in the presence of ACPA, cannot be easily deduced from these studies, while this is the clinical situation where DMARDs are increasingly considered in clinical practice. Therefore the question remains how often DMARD treatment in such patients would be correct and how frequently patients will be overtreated, as they would not have developed RA in the absence of DMARD treatment.
It is also suggested to apply the 2010 classification criteria for RA in patients with subclinical inflammation, thus replacing the entry criterion of clinical arthritis with that of subclinical synovitis. It is then conceptualized that subclinical synovitis and ≥6 points allow for an earlier classification of RA and could result in less overtreatment than treatment of subclinical synovitis alone.
Therefore we set out to search for evidence of the natural course and determined in arthralgia patients with subclinical synovitis from three longitudinal cohorts the frequencies of non-progression to clinically apparent inflammatory arthritis (IA; i.e. patients who could be considered as ‘false positives’) both in the presence and absence of ACPA. Furthermore, we explored if applying the 2010 criteria in patients with subclinical synovitis in the absence of clinical arthritis, thus broadening the entry criterion, diminished the false-positive rate.
Methods
Cohorts
Data from three independent Dutch cohorts of arthralgia patients with ≥1 year of follow-up for IA development were used. The cohorts have been described previously [6–8]. Details of cohorts and imaging are presented in Supplementary Data S1, available at Rheumatology online.
In short, cohort 1 is the SONAR (Sonographic evaluation of hands, shoulders and feet in patients presenting with inflammatory arthralgia to identify subclinical arthritis) study, a multicentre observational inflammatory arthralgia cohort. At baseline a bilateral US was performed of MCP joints 2–5, MTP joints 2–5 and the wrists. Subclinical synovitis was defined as greyscale (GS) ≥2 and/or power Doppler (PD) ≥1.
Cohort 2 is the clinically suspect arthralgia (CSA) cohort. Patients underwent contrast-enhanced 1.5 T MRI of the wrists, MCP joints 2–5 and MTP joints 2–5. Scans were independently scored by two trained readers for subclinical synovitis according to the Rheumatoid Arthritis MRI Score and a synovitis score ≥1 by both readers was used as the cut-off [9].
Cohort 3 is the seropositive arthralgia cohort, which included patients positive for ACPA and/or RF. A bilateral US of the wrists, MCP joints 2 and 3 and MTP joints 2, 3 and 5 was performed at baseline, according to a predefined US protocol [4, 7]. The definition of subclinical synovitis was similar to that in the SONAR study. In all three cohorts the imaging examiners were blinded to the clinical details and the treating rheumatologists were blinded to the imaging results.
Ethics
For cohort 1 (SONAR study), written informed consent was obtained from the participants according to the Declaration of Helsinki. The study was approved by the medical ethics committee of Erasmus University Medical Center, Rotterdam, The Netherlands (MEC-2010-353) and was assessed for feasibility by the local ethical bodies of Maasstad Hospital and Vlietland Hospital. For cohort 2 (CSA cohort), patient consent was obtained from all participants. This study was approved by the local medical ethics committee of Leiden University Medical Center. For cohort 3 (Amsterdam cohort), signed informed consent was obtained from all patients prior to inclusion. This study was approved by the Slotervaart ethics committee (U/1740/0327). Signed informed consent was obtained from all patients prior to inclusion.
Outcome
The primary outcome of all three cohorts was development of IA after 1 year, determined by physical examination of the treating rheumatologist. In cohorts 2 and 3 the outcome was also assessed after 3 years. Importantly, DMARD treatment (including glucocorticoid injections) was not initiated in the phase of arthralgia and only prescribed after a patient had developed clinically apparent arthritis.
Analysis
The true- and false-positive rates were determined. These were respectively the percentages of patients that developed and did not develop IA from all patients with a positive test. Analyses were stratified for ACPA status. For our second aim we applied the 2010 criteria at baseline in patients with subclinical synovitis. The entry criterion that requires the presence of clinical arthritis was replaced by the presence of one or more joints with subclinical synovitis in patients with arthralgia. The item ‘number of involved joints’ was solely based on the tender joint count (44 joints) and not by imaging.
Several sensitivity analyses were performed. First, the abovementioned analyses in cohort 2 and 3 were repeated when IA was assessed after 3 years of follow-up. Second, the definition of subclinical synovitis was evaluated in three ways. Because it is known that power Doppler could be a stronger predictor, progression to IA was shown for patients who had GS ≥2 or PD ≥ 1 separately [6, 10]. In addition, multiple imaging studies in the general population showed that symptom-free persons can have inflammatory features [11, 12]. Because this could affect the false-positive rate, analyses were repeated when features found in the general population were considered in the definition of the presence of subclinical synovitis. MRI-detected subclinical synovitis was considered present if it occurred in <5% of the healthy population of the same age category in the same joint (see Supplementary Data S1, available at Rheumatology online for further explanation) [11, 13]. Similarly, the definition of US-detected subclinical synovitis included the results from a large US study carried out on a symptom-free population [12]. Based on these results, the cut-off value for MTP joints 2 and 3 was adjusted and subclinical synovitis was considered present in MTP joints 2 and 3 if GS ≥3 and/or PD ≥1, while the cut-off in the MCP joints, wrist and MTP joints 4 and 5 remained unchanged (GS ≥2 and/or PD ≥1). Finally, although the threshold for US-detectable subclinical synovitis (GS ≥2 and/or PD ≥1) is most frequently used in the current literature [4, 6, 10], we also evaluated the effect of a more stringent threshold (GS ≥3 and/or PD ≥2) on the false-positive rate. Stata software version 15 (StataCorp, College Station, TX, USA) and SPSS version 25 (IBM, Armonk, NY, USA) were used.
Results
Baseline characteristics
A total of 166, 473 and 162 patients were included in cohorts 1, 2 and 3, respectively. Table 1 presents the baseline characteristics. The percentage of ACPA positives was 22% in cohort 1, 14% in cohort 2 and 56% in cohort 3. At baseline, 36%, 41% and 31% of patients had subclinical synovitis, respectively. After 1 year, 22%, 15% and 18%, respectively, had developed IA.
Table 1.
Baseline characteristics of arthralgia-patients included in the three cohorts, also stratified for ACPA status
| Characteristics | All arthralgia patients |
ACPA positive |
ACPA negative |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 166) | Cohort 2 (n = 473) | Cohort 3 (n = 162) | Cohort 1 (n = 37) | Cohort 2 (n = 64) | Cohort 3 (n = 90) | Cohort 1 (n = 129) | Cohort 2 (n = 409) | Cohort 3 (n = 72) | |
| Age, years, mean (s.d.) | 45 (12) | 44 (13) | 51 (11) | 45 (11) | 48 (13) | 51 (11) | 45 (12) | 43 (13) | 52 (11) |
| Female, n (%) | 136 (82) | 366 (77) | 120 (74) | 32 (86) | 52 (81) | 67 (74) | 104 (81) | 314 (77) | 53 (74) |
| Symptom duration, weeks, median (IQR) | 29 (19–40) | 19 (9–44) | 57 (26–157) | 28 (17–40) | 24 (13–53) | 52 (26–137) | 29 (20–39) | 18 (9–41) | 83 (30–209) |
| TJC44, median (IQR) | 5 (3–8) | 5 (2–9) | 1 (0–5) | 4 (2–7) | 3 (1–7) | 1 (0–5) | 5 (3–8) | 5 (2–10) | 1 (0–5) |
| ACPA positivity, n (%) | 37 (22) | 64 (14) | 90 (56) | NA | NA | NA | NA | NA | NA |
| RF positivity, n (%) | 49 (30) | 95 (20) | 119 (74) | 22 (59) | 49 (77) | 47 (52) | 27 (21) | 46 (11) | 72 (100) |
| Increased CRP, n (%) | 39 (23) | 101 (22) | 12 (7) | 11 (30) | 20 (32) | 8 (9) | 28 (22) | 81 (20) | 4 (6) |
| Presence of local subclinical synovitisa, n (%) | 60 (36) | 193 (41) | 50 (31) | 13 (35) | 36 (56) | 31 (34) | 47 (36) | 157 (38) | 19 (26) |
Presence of US- (cohort 1 and cohort 3) or MRI- (cohort 2) detected subclinical synovitis. Joints screened for cohorts 1 and 2: MCP 2–5, radiocarpal, intercarpal, radioulnar (cohort 2) and MTP 2–5. Joints screened for cohort 3: MCP 2–3; MTP 2, 3 and 5 and wrist.
IQR: interquartile range; TJC44: 44-joint tender joint count; NA: not applicable.
False-positive rates
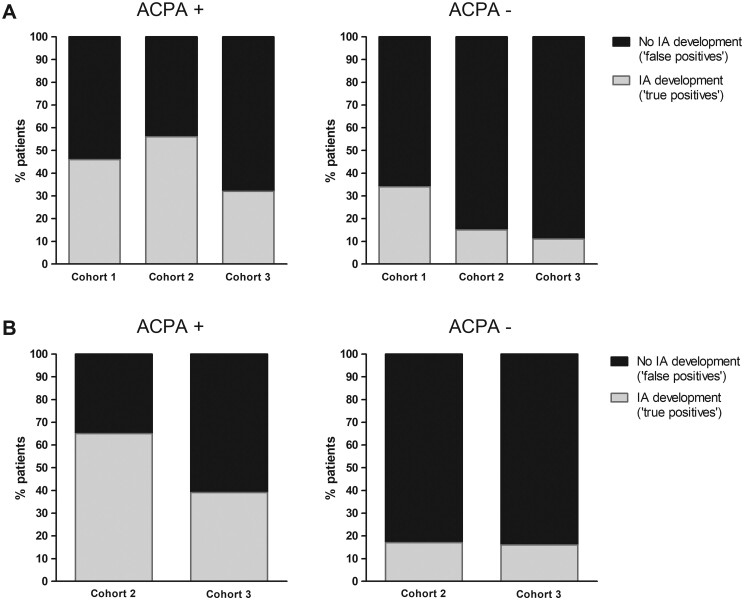
Of the ACPA-positive patients with subclinical synovitis, 54%, 44% and 68% did not develop IA in cohorts 1, 2 and 3, respectively (Fig. 1A). In the ACPA-negative patients with subclinical synovitis, 66%, 85% and 89%, respectively, did not progress to IA (Fig. 1A).
Fig. 1.
Percentage of progression and non-progression to inflammatory arthritis in arthralgia patients with subclinical synovitis at baseline
(A) ACPA-positive patients (cohort 1, n = 37; cohort 2, n = 64; cohort 3, n = 90). Patients with subclinical synovitis at baseline (cohort 1, n = 13; cohort 2, n = 36; cohort 3, n = 31). Of these, 6, 20 and 10 patients, respectively, developed IA after 1 year of follow-up. ACPA-negative patients (cohort 1, n = 129; cohort 2, n = 409; cohort 3, n = 72). Patients with subclinical synovitis at baseline (cohort 1, n = 47; cohort 2, n = 157; cohort 3, n = 19). Of these 16, 23 and 2 patients, respectively, developed IA after 1 year of follow-up. (B) ACPA-positive patients (cohort 2, n = 43; cohort 3, n = 90). Patients with subclinical synovitis at baseline (cohort 2, n = 26; cohort 3, n = 31). Of these, 17 and 12 patients, respectively, developed IA after 3 years of follow-up. ACPA-negative patients (cohort 2, n = 292; cohort 3, n = 72). Patients with subclinical synovitis at baseline (cohort 2, n = 121; cohort 3, n = 19). Of these, 20 and 3 patients, respectively, developed IA after 3 years of follow-up.
Evaluation of the use of subclinical synovitis as an entry criterion for the 2010 criteria
The analyses were also performed within arthralgia patients in whom subclinical synovitis was used as an entry criterion and who also had ≥6 points on the 2010 criteria (imaging was not used to evaluate the number of involved joints). Within the ACPA-positive patients, 45%, 37% and 63% did not progress to IA (Supplementary Fig. S1A, available at Rheumatology online). Within the ACPA-negative patients, 67%, 82% and 89% did not progress. Hence in both ACPA groups, the false-positive rates did not decrease when the 2010 criteria were used in arthralgia patients with subclinical synovitis.
Sensitivity analyses
First, analyses were repeated for cohorts 2 and 3 with IA development after 3 years of follow-up and similar false-positive rates were observed (Fig. 1B). Also, the results of the use of the 2010 criteria in patients with subclinical synovitis after 3 years were similar (Supplementary Fig. S1B, available at Rheumatology online).
Second, the results for progression to IA were shown separately for patients having GS ≥2 and patients having PD ≥1. No important differences were seen in patients with GS ≥2 compared with the main analysis (Supplementary Fig. S2A/S3A, available at Rheumatology online). The false-positive rate for PD did decrease somewhat in subgroups of cohort 1 and 3 compared with the main analyses, but remained substantial (Supplementary Fig. S2B/S3B, available at Rheumatology online).
Additionally, imaging findings observed in symptom-free persons were considered in the definition of subclinical synovitis. When using a more stringent definition for MRI-detected synovitis, 37% of ACPA-positive patients and 80% of ACPA-negative patients with subclinical synovitis did not progress to IA after 1 year (Supplementary Fig. S4, available at Rheumatology online). Also, when a more stringent definition for US-detected synovitis was used, a considerable proportion of ‘false positives’ remained, as 50% of the ACPA-positive and 71% of the ACPA-negative patients with subclinical synovitis did not progress to IA after 1 year (Supplementary Fig. S5, available at Rheumatology online).
Finally, an even more stringent threshold for subclinical synovitis was studied (GS ≥3 and/or PD ≥2). Although the number of patients with arthralgia that had subclinical synovitis according to this definition decreased, the high false-positive rates persisted (Supplementary Fig. S6, available at Rheumatology online).
Discussion
Although daily practice most likely differs per region, there is an increasing tendency to start DMARD treatment in arthralgia patients with subclinical synovitis, at least in some places [3]. This is based on the assumption that the clinical presentation of subclinical synovitis in ACPA-positive arthralgia is equivalent to imminent RA. The lack of evidence for this notion prompted us to perform a study in multiple cohorts. We observed that replacing clinical arthritis with subclinical synovitis for identification of IA introduced a high false-positive rate, as 44–68% of ACPA-positive and 66–89% of ACPA-negative arthralgia patients with subclinical synovitis did not develop IA. These results on the natural disease course of arthralgia patients with subclinical synovitis imply that starting DMARD treatment in these patients would lead to considerable overtreatment, as they would not progress to IA without DMARD therapy. Another argument is the lack of evidence that starting DMARD treatment in this phase will prevent the development of RA. However, this is currently being investigated in several trials and is outside the scope of this study [14].
Although the inclusion criteria of the three cohorts were somewhat different, the primary results were comparable and this strengthens the validity of the results.
US and MRI are both suitable for detecting subclinical inflammation in arthralgia [5, 6, 10]. Although MRI is more sensitive than US, the decrease in sensitivity (with MRI as reference) is less for the detection of synovitis than for tenosynovitis and osteitis [15, 16]. Interestingly, the results for the false-positive rates of the two imaging modalities were not importantly different. However, for clinical purposes, MRI can be less attractive compared with US since it is less easily available, is more expensive and requires intravenous contrast administration [15]. With respect to US, a limitation is that different machines were used in the two US studies. Nonetheless, the results were comparable and different machines are also used in daily clinical practice.
Ideally the definition of subclinical inflammation incorporates correction for the symptom-free population to prevent false-positive findings [11, 12]. For MRI, reference values were available and considered. For US we used the results of Padovano et al. [12] and the results with and without correction were similar. In cohort 3, the false-positive rate decreased slightly but remained considerable. This suggests that signs of inflammation found in the normal population do not explain the observed false-positive rates.
The 2010 criteria are intended for classification and not for diagnosis/treatment start. Furthermore, to prevent false-positive classifications, the 2010 criteria should only be applied in case of a clinical diagnosis of RA with one or more clinical swollen joints. Nonetheless, in the ‘pre-RA field’ it is suggested that applying the 2010 criteria to patients with subclinical inflammation can be helpful. Previous studies that evaluated imaging as entry criterion for the 2010 criteria were done in patients with clinically apparent arthritis or in a mixed population with arthralgia and arthritis [17, 18]. Our data from three cohorts with arthralgia patients and subclinical synovitis revealed that a high proportion of patients with subclinical synovitis and ≥6 points did not develop IA/RA. Consequently there is currently no evidence to change the entry criterion from clinical synovitis to subclinical synovitis, as the false-positive rate remained substantial.
Furthermore, the additional benefit of applying imaging in the 2010 criteria in patients with clinical arthritis to determine the number of involved joints has also been studied [13]. This is different from the current research where imaging-detected subclinical synovitis replaces the entry criterion of clinical arthritis.
In clinical practice, rheumatologists may be inclined to start DMARDs in ACPA-positive arthralgia patients with subclinical synovitis. The current data from three cohorts suggest that ACPA positivity in combination with subclinical synovitis is overestimated in its ability to indicate the future development of IA/RA. Also, in our sensitivity analysis where more stringent definitions of subclinical synovitis were used, high false-positive rates remained. Altogether, this emphasizes the need for other biomarkers, in addition to ACPA and subclinical synovitis, to enhance risk stratification in patients with arthralgia. For example, imaging-detected tenosynovitis has been shown to be a better predictor than imaging-detected synovitis [5, 19]. Combining imaging with other predictors (e.g. clinical, genetic and serological data) will presumably result in higher positive predictive values and true positive rates [14, 19].
A recent study on long-term outcomes of arthralgia patients with subclinical inflammation that did not progress to IA showed that 33–38% of these patients, including those with ACPA positivity, had symptom resolution [20]. Interestingly, this was also associated with a reduction in subclinical inflammation, illustrating that a combination of symptoms, inflammation and presence of autoantibodies can be self-limiting.
In conclusion, our results showed that the presence of subclinical synovitis and ACPA positivity is not equal to RA development. Therefore, in our view, further observational studies on the natural disease course are necessary to derive accurate and validated risk stratification for patients presenting with arthralgia. Thus, when randomized clinical trials show that treatment of arthralgia patients prevents progression to IA, we can apply this treatment to the right patients and avoid significant overtreatment.
Funding: The research leading to these results has received funding from the Dutch Arthritis Foundation (SONAR cohort, CSA Leiden cohort, Amsterdam cohort), the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme (starting grant, agreement no. 714312; CSA Leiden cohort) and an investigator-initiated grant from Pfizer (SONAR cohort).
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Combe B, Landewe R, Daien CI. et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017;76:948–59. [DOI] [PubMed] [Google Scholar]
- 2. Van Boheemen L, Bolt JW, Ter Wee M. et al. Persons at risk of rheumatoid arthritis or axial spondyloarthritis have different perceptions on preventive intervention than rheumatologists. Ann Rheum Dis 2020;79(Suppl 1):269–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mankia K, Briggs C, Emery P.. How are rheumatologists managing anticyclic citrullinated peptide antibodies-positive patients who do not have arthritis? J Rheumatol 2020;47:305–6. [DOI] [PubMed] [Google Scholar]
- 4. van de Stadt LA, Bos WH, Meursinge Reynders M. et al. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: a prospective cohort study. Arthritis Res Ther 2010;12:R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Steenbergen HW, Mangnus L, Reijnierse M, Huizinga TW, van der Helm-van Mil AH.. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis 2016;75:1824–30. [DOI] [PubMed] [Google Scholar]
- 6. van der Ven M, van der Veer-Meerkerk M, Ten Cate DF. et al. Absence of ultrasound inflammation in patients presenting with arthralgia rules out the development of arthritis. Arthritis Res Ther 2017;19:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Beers-Tas MH, Blanken AB, Nielen MMJ. et al. The value of joint ultrasonography in predicting arthritis in seropositive patients with arthralgia: a prospective cohort study. Arthritis Res Ther 2018;20:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Steenbergen HW, van Nies JA, Huizinga TW, Bloem JL. et al. Characterising arthralgia in the preclinical phase of rheumatoid arthritis using MRI. Ann Rheum Dis 2015;74:1225–32. [DOI] [PubMed] [Google Scholar]
- 9. Ostergaard M, Peterfy C, Conaghan P. et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol 2003;30:1385–6. [PubMed] [Google Scholar]
- 10. Nam JL, Hensor EM, Hunt L. et al. Ultrasound findings predict progression to inflammatory arthritis in anti-CCP antibody-positive patients without clinical synovitis. Ann Rheum Dis 2016;75:2060–7. [DOI] [PubMed] [Google Scholar]
- 11. Mangnus L, van Steenbergen HW, Reijnierse M, van der Helm-van Mil AH.. Magnetic resonance imaging-detected features of inflammation and erosions in symptom-free persons from the general population. Arthritis Rheumatol 2016;68:2593–602. [DOI] [PubMed] [Google Scholar]
- 12. Padovano I, Costantino F, Breban M, D’Agostino MA.. Prevalence of ultrasound synovial inflammatory findings in healthy subjects. Ann Rheum Dis 2016;75:1819–23. [DOI] [PubMed] [Google Scholar]
- 13. Boer AC, Boeters DM, van der Helm-van Mil AHM.. The use of MRI-detected synovitis to determine the number of involved joints for the 2010 ACR/EULAR classification criteria for rheumatoid arthritis – is it of additional benefit? Ann Rheum Dis 2018;77:1125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Steenbergen HW, da Silva JAP, Huizinga TWJ, van der Helm-van Mil AHM.. Preventing progression from arthralgia to arthritis: targeting the right patients. Nat Rev Rheumatol 2018;14:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohrndorf S, Boer AC, Boeters DM. et al. Do musculoskeletal ultrasound and magnetic resonance imaging identify synovitis and tenosynovitis at the same joints and tendons? A comparative study in early inflammatory arthritis and clinically suspect arthralgia. Arthritis Res Ther 2019;21:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wakefield RJ, O’Connor PJ, Conaghan PG. et al. Finger tendon disease in untreated early rheumatoid arthritis: a comparison of ultrasound and magnetic resonance imaging. Arthritis Rheum 2007;57:1158–64. [DOI] [PubMed] [Google Scholar]
- 17. Nakagomi D, Ikeda K, Okubo A. et al. Ultrasound can improve the accuracy of the 2010 American College of Rheumatology/European League against rheumatism classification criteria for rheumatoid arthritis to predict the requirement for methotrexate treatment. Arthritis Rheum 2013;65:890–8. [DOI] [PubMed] [Google Scholar]
- 18. Tamai M, Kita J, Nakashima Y. et al. Combination of MRI-detected bone marrow oedema with 2010 rheumatoid arthritis classification criteria improves the diagnostic probability of early rheumatoid arthritis. Ann Rheum Dis 2014;73:2219–20. [DOI] [PubMed] [Google Scholar]
- 19. Matthijssen XME, Wouters F, Boeters DM. et al. A search to the target tissue in which RA-specific inflammation starts: a detailed MRI study to improve identification of RA-specific features in the phase of clinically suspect arthralgia. Arthritis Res Ther 2019;21:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ten Brinck RM, Boeters DM, van Steenbergen HW, van der Helm-van Mil AHM.. Improvement of symptoms in clinically suspect arthralgia and resolution of subclinical joint inflammation: a longitudinal study in patients that did not progress to clinical arthritis. Arthritis Res Ther 2020;22:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.