Abstract
Lymphoma development is the most serious complication of SS and the main factor impacting on mortality rate in patients with this condition. Lymphomas in SS are most commonly extranodal non-Hodgkin B-cell lymphomas of the mucosa-associated lymphoid tissue and frequently arise in salivary glands that are the target of a chronic inflammatory autoimmune process. Extensive work on lymphomagenesis in SS has established that the progression towards B-cell lymphoma is a multistep process related to local chronic antigenic stimulation of B cells. These neoplastic B cells in SS frequently derived from autoreactive clones, most commonly RF-producing B cells, which undergo uncontrolled proliferation and malignant escape. In this review, we highlight the most important recent findings that have enhanced our understanding of lymphoma development in SS, with particular reference to the close link between autoimmunity and lymphomagenesis. We also discuss how the identification of key factors involved in B-cell malignancies may impact on our ability to identify at early stages patients at increased risk of lymphoma with potential significant repercussions for the clinical management of SS patients. Finally, we identified the most promising areas of current and further research with the potential to provide novel basic and translational discoveries in the field. The questions of finding new biomarkers, developing a validated score for predicting lymphoma occurrence and assessing if a better control of disease activity will decrease the risk of lymphoma in primary SS will be the enthralling questions of the next few years.
Keywords: Sjogren’s syndrome, lymphoma, MALT, BAFF, germinal centre
Rheumatology key messages
Development of lymphoma in SS involves local chronic antigenic stimulation of autoimmune B cells.
The same pathophysiologic processes involved in SS also drive SS-associated lymphomagenesis.
B-cell activation, disease activity and decrease immunosurveillance are the key predictive factors of SS- associated lymphomas.
Introduction
Primary SS (pSS) is a systemic autoimmune disease characterized by lymphocytic infiltrates of salivary and tear glands leading to oral and ocular dryness. Dryness, fatigue and pain are present in all patients. Apart from these disabling symptoms, 30% to 50% of the patients develop systemic manifestations including renal, pulmonary or neurological manifestations [1, 2]. Among these systemic involvements, occurrence of lymphoma is one of the most severe complications. Investigation of the pathophysiology of lymphomagenesis is a key line of research. First, it might give us tools for an earlier and better detection of high-risk patients. Second, it might give clues for a better understanding of pathophysiology of both mechanisms of autoimmunity and of lymphomagenesis outside the context of auto-immunity. Third, it might lead to the identification of new treatment targets in autoimmune diseases. This review will propose an update concerning lymphoma development in pSS patients focusing on epidemiology, management of this complication and mechanisms leading to lymphomagenesis.
Epidemiology and clinical aspects
Epidemiology
Autoimmunity promotes the risk of lymphoma. More precisely, patients suffering from RA, lupus and pSS are exposed to an increased risk of B-cell lymphoma. pSS is the autoimmune disease associated with the highest risk, ranging from 7 to 15 compared with the general population [3–6]. B-cell lymphomas occur in ∼5% to 10% of pSS patients and it has been recently reported that the risk increases by 2.2% per year of age [7].
Histological findings
Most lymphomas in pSS are of B-cell lineage and of low- or intermediate-grade malignancy. Lymphoma mainly develops from marginal zone B cells, leading to marginal zone lymphoma and especially mucosa-associated lymphoid tissue (MALT) lymphoma (MALT-L) in 65% of the cases [8, 9]. Among the other lymphoma sub-types that have been found to be increased, lympho-plasmacytoid lymphomas are probably marginal zone lymphoma with plasma-cell differentiation and diffuse large B-cell lymphoma may be, at least in some cases, the transformation of previous low-grade lymphomas [10–12]. MALT-L are localized mainly within salivary glands (SG) (parotid and sub-mandibular), the target organ of the disease, but also in other mucosal sites such as orbits, naso-pharynx, stomach, thyroid and lung. MALT-L in pSS have classical characteristics: B phenotype of MALT-L is confirmed by panB markers such as CD19, CD20, CD22, CD79a and CD79b. Monotypic Ig of IgM isotype is often found at the membrane with a predominance of the κ light chain [13]. However, classical oncogenic events are not likely to frequently promote lymphomagenesis in the context of pSS. Translocations involving MALT1 gene have been shown to play a role in MALT-L development in general (t(11; 18) (q21; 21) and t(14; 18) (q32; q21)). In comparison, these translocations are rather infrequent in MALT-L developing from SG (prevalence of 27% and 15% respectively) [14].
Clinical aspects
The most frequent symptom leading to suspicions of lymphoma in a pSS patient is the occurrence of a unilateral, fixed and hard parotid gland enlargement. Differentiating clinically a lymphoma from a benign polyclonal parotid hyperplasia is not so easy. Hyperplasia is frequent in pSS patients but is frequently bilateral, going up and down. Complementary examinations may help. Parotid gland MRI may be used [15]. Salivary gland ultrasound with Doppler has been shown to be useful for pSS diagnosis and in trained hands it could give the possibility of driving biopsy during the same procedure [16]. More recently, the usefulness of 18F-FDG Positron Emission Tomography has been assessed in a study assessing 45 pSS patients with active disease, including 15 with lymphoma [17]. In this retrospective study, having a standardized uptake value max of parotid gland ⩾ 4.7 and/or the presence of focal pulmonary lesions was highly suggestive of lymphoma.
Low-grade non-Hodgkin B-cell lymphomas complicating pSS are characterized by an indolent course, small tumour burden, normal lactate dehydrogenase and β2-microglobulin levels and an excellent performance status. B symptoms are absent and bone marrow involvement is rare.
The management of MALT-L is not consensual. Several strategies have been proposed including a wait and see strategy. However, lymphomagenesis in pSS is the final step of persistent polyclonal B-cell activation, transformed into monoclonality and then into lymphoma. In this context, treating the low-grade lymphoma may be the best way to control the chronic B-cell activation and possibly to avoid the risk of transformation into high-grade lymphoma with poorer prognosis. Treatment classically includes rituximab (RTX) associated with either alkylating agents or fludarabine [18]. Several studies have demonstrated the efficacy and safety of the combination of RTX with bendamustine in low-grade B-cell lymphomas including mantle cell lymphomas and extra-gastric MALT-L [19, 20]. RTX with bendamustine has been recently assessed in MALT-L complicating pSS. This strategy was likely to be safe and efficient and interestingly seemed to be associated with a control of activity of the autoimmune process [21]. The overall prognostic of MALT-L occurring in pSS patients is excellent. Disease activity assessed by the EULAR SS disease activity index and the International Prognostic Index have been shown to be interesting predictive tools for outcomes of event-free survival and overall survival [8].
From predictors to pathogenesis of lymphoma
Classical predictors
As described above, the risk of lymphoma is limited to a fraction of pSS patients. Thus, definition of predictors allowing identifications of this subset of patients at high risk is challenging. Several studies have identified predictors of lymphoma development in pSS (Table 1).
Table 1.
Classical predictors of lymphoma in SS patients
| Predictive factors | References |
|---|---|
| Clinical | |
| Permanent swelling of SG | [22–24] |
| Adenopathy | [25, 26] |
| Purpura | [4, 26, 27] |
| Paraclinical | |
| Cryoglobulinemia | [26, 28] |
| Lymphopenia | [4, 29] |
| Low C4 | [4, 26, 27, 29] |
| Monoclonal component | [25] |
| GC-like structures within SG, FS | [30, 31] |
GC: germinal centres; SG: salivary glands; FS: focus score.
Clinical and biological predictors have been identified and confirmed by several groups. To sum up, the main clinical predictors include permanent swelling of SG, splenomegaly and lymphadenopathies, skin involvement and especially palpable purpura [18]. Biological predictors are cryoglobulinemia, lymphopenia (CD4), low complement levels and a monoclonal component in serum or urine [18]. Interestingly, disease activity assessed by the EULAR SS disease activity index was found to predict lymphoma development with a dose effect [9]. Several groups have aimed to define a predictive score of lymphoma based on these predictors. Fragkioudaki et al. [32] have built a score including salivary gland enlargement, lymphadenopathy, Raynaud phenomenon, anti-Ro/SSA and/or anti-La/SSB autoantibodies, RF positivity, monoclonal component, and low C4. Presence of ⩽2 of these factors was associated with low probability of lymphoma occurrence (3.8%) whereas the probability reached 100% if all seven factors were present. Similarly, Quartuccio et al. focused on patients with salivary gland enlargement [33]. They define four biomarkers (low C4, cryoglobulinemia, anti-SSB antibodies and leukopenia) and found that the positivity of one or no biomarker provided a negative predictive value for lymphoma of about 90% in this subgroup of patients.
In addition to these predictors that can be easily checked in clinical practice, five significant advances have been made in understanding the mechanisms leading to the transformation of the B-cell clone. These predictors lead to a better understanding of the origin of lymphoma complicating pSS.
Activity of the disease
Lymphomagenesis occurring in patients with autoimmune diseases results from chronic activation of autoimmune system. This over-activation is associated with a more active disease. In patients with RA, it has been demonstrated that the risk of B-cell lymphoma is higher in patients with the highest cumulative disease activity [34]. In pSS, it is now demonstrated that disease activity assessed by the EULAR SS disease activity index predicts lymphoma development with a dose effect [9].
RF reactivity and chronic antigenic stimulation
Cryoglobulinemia, a cold-precipitable IC with RF activity, is a well-accepted predictor of lymphoma. However, the detection of cryoglobulins can be challenging. Recently, RF itself, independently of cryoglobulinemia, has been found to be a predictor of lymphoma in pSS patients [9]. This could have a practical impact, as RF is easier to measure in clinical practice. Moreover, the relevance of RF is supported by the evidence that lymphoma B cells in pSS patients could be RF B cells stimulated by ICs. In fact, lymphomatous B cells from two pSS patients with salivary-gland lymphoma expressed an IgM-k with RF activity [35]. Similarly, the study of the repertoire of mature B-cell lymphomas has shown that 41% of MALT-L occurring in SG expressed B Cell receptors with strong CDR3 homology to RF, whereas only 18% of MALT-L occurring in the stomach presented this homology, which was never found in pulmonary MALT-L [36]. More recently, it has been demonstrated that homology to RF was a rare event among clonotypes expressed by B cells infiltrating SG from pSS. However, when present, it was likely to represent a strong advantage supporting lymphomatous escape [37]. The role of the IC in stimulating these RF B cells is probably crucial in the transformation of B cells leading to lymphoma. Thus, a first step of lymphomagenesis in pSS may be the accumulation of IC leading to continuous stimulation of RF B. These B cells, if not completely controlled, may evolve to lymphomatous cells. Interestingly, it could be argued that the over-representation of MALT compared with nodal lymphoma could be linked to a variability of the efficacy of mechanisms of control depending on the microenvironment: increased mucosal production of ICs stimulating RF B cells coupled with a less efficient immunosurveillance of these continuously stimulated RF B cells in epithelial mucosal tissue compared with lymph nodes or bone marrow could explain the increased risk of MALT-L in pSS patients.
Germinal centre-like structures and lymphomagenesis
As described above, MALT-L development in pSS appears to be the result of local antigen-driven B cell selection, which allows the proliferative advantage and eventually malignant escape of B cells frequently bearing an RF-reactive B-cell receptor. Antigen-driven B cell selection physiologically takes place in secondary lymphoid organs in structures defined as germinal centres (GC) where the process of affinity maturation via somatic hypermutation of the variable Ig heavy and light chain genes happens. Developments in the last 15 years in the field of SG histopathology have led to the appreciation that a high degree of heterogeneity exists in terms of immune cell infiltration and organization in the SG of pSS patients ranging from sparse T cells to highly organized B-cell rich follicles. Ectopic GC-like structures, which can be defined as aggregates of T and B organized in segregated areas associated with the development of follicular dendritic cell networks (Fig. 1), can be detected in around 20–40% of patients [38]. There is conclusive evidence that ectopic GCs in SS allow affinity maturation of GC B cells via the process of locally-driven somatic Ig variable heavy and light chain gene hypermutation [39]. Of interest, malignant B-cell clones in MALT-L display a high degree of somatically hypermutated Ig genes in line with a post-GC origin. Ig gene hypermutation and lymphomagenesis seems to be a highly related process in SS as off-target mutations in key proto-oncogenes regulating B-cell proliferation, a process known as aberrant somatic hypermutation, promote genetic instability and malignant escape in MALT-L [40]. Physiological and aberrant somatic hypermutation have both been related to the expression of a nucleic acid editing enzyme, activation-induced cytidine deaminase encoded by the AICDA gene [40, 41], which can be abundantly found in ectopic GC in the SG of SS patients and MALT-L [42]. Of interest, in parotid MALT-L activation-induced cytidine deaminase is restricted to GC suggesting that these structures are critically involved in the genetic instability associated with lymphomagenesis. Both processes of ectopic GC and lymphoma might be underlined by common genetic predisposition as indicated by the association of C-X-C chemokine receptor type 5 (CXCR5) gene polymorphisms, a unique receptor for the GC-associated chemokine CXCL13, with pSS but also with non-Hodgkin lymphoma (NHL) in independent studies outside of the context of autoimmunity [43].
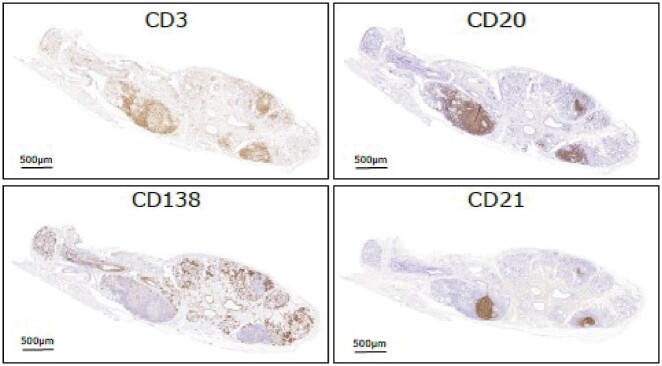
Fig. 1.
Immunohistochemical features of ectopic GCs in the SG of SS
Representative microphotographs depicting sequential immunohistochemistry staining for CD3 (T cells), CD20 (B cells), CD21 (follicular dendritic cell networks) and CD138 (plasma cells) identifying ectopic GC in labial salivary gland biopsy of SS patients. GC: germinal centres; SG: salivary glands.
Based on the above evidence, several studies have investigated the potential role of gland histopathology and ectopic GCs as predictors of lymphoma progression in pSS patients, yielding inconclusive data. A retrospectively study on minor SG from pSS patients showed a significantly higher focus score (FS) in patients developing NHL [30]. The FS quantifies the number of inflammatory cell foci containing at least 50 mononuclear cells per 4 mm2. A threshold of FS ⩾ 3 (which is strongly associated with the presence of ectopic GCs) had a positive predictive value of 16% for lymphoma, and a negative predictive value of 98%. Highly infiltrated minor SG with a FS >3 were an independent predictor of NHL development.
In a first report in a small retrospective cohort of SS patients with MALT-L, we observed a higher prevalence of ectopic GCs, defined by the presence of CD21+ follicular dendritic cells networks containing GC B cells, in labial salivary gland biopsies of SS patients who later developed parotid MALT-T compared with the general SS population (75% vs 33%) [42]. A subsequent large study estimated a 16-fold higher risk of developing NHL in GC-positive SS patients in comparison with GC-negative patients. GCs were identified by haematoxylin & eosin (H&E) staining in labial salivary gland biopsies and a biopsy was considered GC positive in the presence of a clearly visible lighter area in a densely packed dark foci (lymphocytic cell clusters of at least 50 mononuclear cells) within otherwise normal salivary gland epithelium, containing cells usually present in classical GCs (follicular dendritic cells, centrocytes, centroblasts and macrophages). At diagnosis, 25% of pSS patients had GC-like structures in their SG and 4% developed NHL, with a median onset of 7 years from the initial diagnostic salivary gland biopsy. Six of seven patients had GC-like structures at diagnosis; the remaining patient was GC negative at the time of diagnosis [31]. More recently, a retroprospective study confirmed that the presence of ectopic GC-like structures was associated with a higher risk (7.8-fold) of NHL occurrence, together with other independent clinical and biological predictors, such as parotid enlargement, sensorimotor neuropathy, splenomegaly, cryoglobulin and low C4 serum levels [44]. Similar to the previous study, GC-like structures were identified on the basis of H&E staining on minor SG. In addition, the presence of these structures was confirmed with immunohistochemistry showing a low expression of Bcl-2 and a high GC B-cell associated transcription factor, Bcl6 reactivity. In contrast with these observations, Johnsen et al. did not observe any difference in the prevalence of GC-like structures (again identified by H&E and CD21 staining) in labial gland biopsies of pSS with different forms of NHL [45]. Surprisingly, the prevalence of GC was higher in patients without lymphoma compared with those with lymphoma (46% vs 33%) exhibited GCs. Similarly, a more recent study that restricted the analysis to SS patients with parotid MALT lymphomas, reported GC-like structures in two out of 11 minor SG from pSS-patients with parotid MALT lymphoma and four out of 22 pSS without lymphoma (4/22) by H&E, with Bcl6 staining not adding much to the identification of GC-like structures [46]. To sum up, studies that did not find an association between GC-like structures and lymphoma in pSS shared methodological characteristics including their case-control design (instead of cross-sectional) and a small sample size. Both these features may contribute to the observed discrepancy.
Overall, although promising, the clinical utility of GC-like structures in the SG of pSS patients as predictors of the evolution towards lymphoma requires further longitudinal studies in larger population together with an emphasis on consensus guidelines to standardize the assessment of ectopic GC-like structures, as discussed in the final section of this review.
B cell cytokines
Cytokines promoting B-cell stimulation could also be involved in lymphomagenesis. Given its role in pSS pathogeny [47] and in lymphomatous escape, B-cell-activating factor of the tumour-necrosis-factor family (BAFF) could also participate in the development of lymphoma in pSS patients [48]. In pSS, BAFF level is increased in patients with current or previous lymphoma vs patients without lymphoma [1]. Moreover, BAFF levels correlated with disease activity and were associated with clonal B-cell expansion in SG [49]. BAFF levels have been found to be increased in pSS patients even years after treatment and remission of lymphoma [1]. Hence, serum BAFF levels may not be directly related to lymphoma but may be constitutionally linked to genetics. Polymorphisms in BAFF loci have been found to be associated with increased BAFF levels [50, 51] even if the association between single nucleotide polymorphism (SNP) of BAFF and/or BAFF receptor and pSS remain controversial [51–53]. Other cytokines such as receptor for colony stimulating factor 1-related tyrosine kinase 3 ligand [54] or IL14 could also participate in chronic B-cell activation and lymphoma development [55]. In addition, serum levels of CXCL13 and CCL11, two chemokines that are linked to the development of the GC like structures, have been shown to be associated with lymphoma occurrence in pSS [56].
Genetic control of immune activation
SG in pSS patients form a micro-environment promoting chronic B-cell stimulation. In the absence of efficient treatment able to stop chronic stimulation by ICs, check-points of autoimmune B-cell activation are crucial and any dysfunction, even slight, could favor lymphomatous escape. The demonstration of the impact of A20 dysfunction illustrates this concept. TNFAIP3 gene encodes the A20 protein, which is a central gatekeeper of NF-kB activation. A20 is a good candidate given the role of somatic deletion of TNFAIP3 in MALT lymphoma [57] and the association of several SNPs of TNFAIP3 in autoimmune diseases [58]. In addition, A20 was found to be downregulated in SG from pSS patients vs controls, and the cells with downregulated A20 expression showed enhanced NF-kB activities [57]. It has been demonstrated that rs2230926, a coding SNP of TNFAIP3 responsible for a missense mutation, was not associated with pSS alone but with the occurrence of lymphoma in pSS patients [59, 60]. Then, we demonstrated that functional abnormalities of TNFAIP3 either slight and germline or dramatic and somatic were present in 75% of pSS patients with pSS-associated MALT lymphoma. More recently, absent or weak immune reactivity of A20 has been described in SG from pSS patients with lymphoma, confirming the role of loss of control in lymphomatous escape of autoimmune B cells [61].
The role of inflammation beyond specific B-cell activation could also be discussed even if systemic inflammation does not seem to promote lymphomagenesis per se, as supported by the lack of over risk of lymphoma in auto-inflammatory diseases. However, it has been recently proposed that inflammasome could be involved in lymphomas complicating pSS as described by Baldini et al. [62]. In this study, P2X7 receptor (P2X7R) and components of the inflammasome (NLRP3, caspase-1 and IL-18) were shown to be increased in SG biopsy in patients who would develop lymphoma. This up-regulation was associated with the presence of GC and was restricted to epithelial cells. From this, it could be hypothesized that specific features of epithelial cells could also participate in the lymphomatous escape of B cells.
More recently, the role of epigenetic has also been assessed. SNPs within the methylene-tetrahydrofolate reductase gene have been shown to be associated with occurrence of lymphoma in pSS patients [63]. Whether this statistical association could have functional basis needs to be demonstrated but it could be hypothesized that genetic modification of methylene-tetrahydrofolate reductase could impact DNA methylation and genomic stability. Micro-RNA could also be involved. It has been recently demonstrated that decreased levels of miR200b-5p within salivary gland biopsy was predictive of lymphoma development [64]. The functional basis of this association needs to be further explored.
The 2019 proposed scenario for lymphomagensis in pSS
There is little doubt that significant progress in the understanding of the key mechanisms underlying lympomagenesis in SS has been made in the past decades (Fig. 2). However, several unknown aspects regarding B-cell lymphomas remain to be elucidated and lymphoma still represent an unmet clinical need in SS. The fundamental hypothesis of the multistep evolution of lymphomas in SS whereby B cells undergo an antigen-driven selection allowing the progression from poly- to oligo- to monoclonal proliferation brings several corollaries of potential translational relevance to the clinical management of SS patients.
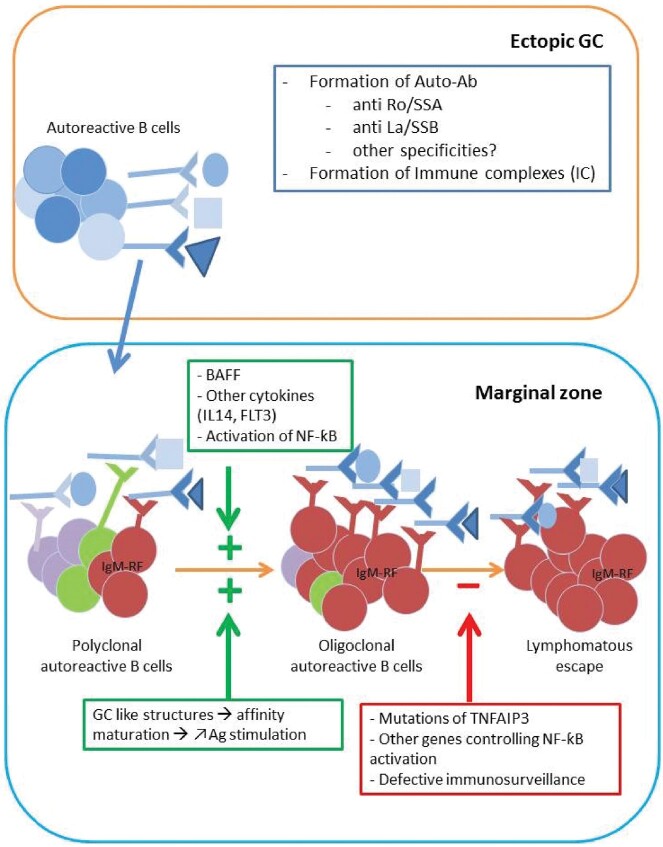
Fig. 2.
The 2019 proposed scenario for lymphomagensis in pSS
The development of lymphoma in pSS is a multi-step process that is centered on chronic antigenic stimulation. Formation of auto-Ab is favored within GC-like structures leading to the presence of ICs at high concentrations at epithelial sites. These latter stimulate the expansion of RF-reactive B cells. Affinity maturation within GC-like structures gives a selective advantage to auto-immune clones. The activation of these autoreactive B cells is amplified by several factors including BAFF via paracrine and/or autocrine secretion, cytokines such as FLT-3 or IL-14 and the over-activation of the NF-κB pathway. Loss of immune control of genetic origin (TNFAIP3 mutations) and a less effective anti-tumor immunosurveillance at mucosal sites might precipitate the lymphomatous escape. GC: germinal centres; pSS: primary SS.
The first and foremost is the possibility to identify and validate genetic, immunological, histopathological and/or clinical predictors in order to recognize early the subset of SS patients at higher risk of lymphoma development. The use of SG histopathology and in particular the role of GC-like structures has so far yielded interesting but conflicting data, mostly due to the heterogeneity of the SS population enrolled, the nature and size of the cohort studied and most importantly the highly variable definition of GC used, as discussed above. Current efforts are directed to reach an international consensus on salivary gland histopathology and the immunohistological definition of GC [65, 66] which is the first step required prior to the validation of histopathology in longitudinal observational studies. Although there is a strong rationale for genetic susceptibility as a critical factor predisposing to B cell lymphomas in SS, as discussed earlier in this review, the role of genetic (and epigenetic) and the identification of other candidate susceptibility genes/loci remains an area of active investigation. Once again, given the relatively low prevalence of lymphoma in SS, we believe that only a coordinated effort from the scientific community will be able to significant advance the field allowing, for example, genome-wide association studies to be performed, which have yielded breakthrough progress in other areas.
The identification of the close pathogenic link between autoimmunity and lymphomagenesis in SS has also opened a series of key questions and areas for further work. One such critical aspect is the clarification of the preferential selection of RF-producing B cells and the understanding of the mechanisms underlying their proliferative advantage and clonal expansion. Very recently, the role of CD21 low B cells has been studied. These B cells are found in conditions of persistent immune activation. Glauzy et al. [22] have recently demonstrated that clonal lymphoproliferations in pSS patients preferentially accumulate in this compartment. In addition, Bende et al. have demonstrated that these subsets were polyreactive and show RF activity. The down-regulation of CD21 might be a negative control for chronic B Cell receptor [23]. In this regard, the study of cross-activation of the B-cell receptor with other key pathways regulating B-cell survival and activation such as TLRs and BAFFR could yield novel information. An equally promising approach based on the evidence of the antigen-driven process driving lymphomagenesis is the prompt identification of common B-cell precursors of malignant B-cell clones using high throughput variable heavy and light Ig gene sequencing in diagnostic SG biopsies linked with the generation of recombinant monoclonal antibodies to identify key (auto)antigens that could allow an early stratification of patients at higher risk of future progression. Additionally, this approach would also allow investigating the nature of the other (auto)antigens recognized by malignant B cells, as RF reactivity only accounts for <50% of parotid MALT-L.
The ultimate goal of future research, however, which follows the validation of clinical tools and biomarkers able to predict early in the disease SS patients at high risk of lymphoma, is the administration of therapeutics able to prevent such evolution by blocking key pathways involved in SS lymphomagenesis. In this regard, the rapid intensification of therapeutic testing in randomized clinical trials in SS patients with novel biologics, with particular focus on those interfering with B/T cell costimulation and those blocking BAFF-dependent B-cell activation and survival, could provide such tools in the not so distant future. However, as mentioned above, given the low incidence of lymphomas in SS, in order to provide a meaningful clinical impact on patients with SS, large, multicentre and prospective studies are needed. There is hope that flagship international efforts currently underway such as the EU-funded Horizon2020 HarmonicSS (http://harmonicss.eu) and IMI-NECESSITY consortia will provide such a platform for the further advancement of this field. Waiting for these new advances, how can we prevent lymphoma in pSS patients? RTX is currently the only B-cell targeted therapy and could have a preventive effect. Hydroxychloroquine might offer another way to control B-cell activation. It is able to reduce B-cell activation as observed by reduced gammaglobulins after exposure [24, 67, 68]. Assessing the impact of long-term exposure to RTX or hydroxychloroquine on the risk of lymphoma is a challenging task, regarding the high number of patients and the long duration of the study time needed for this type of study. It is the reason why we have to develop intermediate end-points that could be a good proxy for the risk of further development of lymphoma. In this regard, setting up and validating a composite clinico-biological score based on seven simple variables and predictive of lymphoma occurrence, called SCOLYSS, is in progress in the context of the HARMONICSS and NECESSITY projects. The questions of finding new biomarkers, developing a validated score for predicting lymphoma occurrence and assessing if a better control of disease activity will decrease the risk of lymphoma in pSS will be the enthralling questions of the next few years.
Acknowledgements
This work was support by the following research grants: Horizon2020 EU-funded HarmonicSS (to M.B. and X.M.), the Arthritis Research UK (grants 21753 to EP and 21268 to MB) and the Fondation pour la Recherche Médicale DEQ20150934719: Sjögren’s syndrome and Autoimmunity-associated Lymphomas: SAIL (X.M.).
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Gottenberg JE, Seror R, Miceli-Richard C. et al. Serum levels of beta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren’s syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS One 2013;8:e59868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brito-Zerón P, Ramos-Casals M; EULAR-SS task force group. Advances in the understanding and treatment of systemic complications in Sjögren’s syndrome. Curr Opin Rheumatol 2014;26:520–7. [DOI] [PubMed] [Google Scholar]
- 3. Weng M-Y, Huang Y-T, Liu M-F, Lu T-H.. Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjogren’s syndrome in Taiwan. Ann Rheum Dis 2012;71:524–7. [DOI] [PubMed] [Google Scholar]
- 4. Theander E, Henriksson G, Ljungberg O. et al. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 2006;65:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnsen SJ, Brun JG, Gøransson LG. et al. Risk of non-Hodgkin’s lymphoma in primary Sjögren’s syndrome: a population-based study. Arthritis Care Res 2013;65:816–21. [DOI] [PubMed] [Google Scholar]
- 6. Liang Y, Yang Z, Qin B, Zhong R.. Primary Sjogren’s syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis 2014;73:1151–6. [DOI] [PubMed] [Google Scholar]
- 7. Chiu Y-H, Chung C-H, Lin K-T. et al. Predictable biomarkers of developing lymphoma in patients with Sjögren syndrome: a nationwide population-based cohort study. Oncotarget 2017;8:50098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papageorgiou A, Ziogas DC, Mavragani CP. et al. Predicting the outcome of Sjogren’s syndrome-associated non-hodgkin’s lymphoma patients. PLoS One 2015;10:e0116189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nocturne G, Virone A, Ng W-F. et al. Rheumatoid factor and disease activity are independent predictors of lymphoma in primary Sjögren’s Syndrome. Arthritis Rheumatol 2016;68:977–85. [DOI] [PubMed] [Google Scholar]
- 10. Ekström Smedby K, Vajdic CM, Falster M. et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maeshima AM, Taniguchi H, Toyoda K. et al. Clinicopathological features of histological transformation from extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue to diffuse large B-cell lymphoma: an analysis of 467 patients. Br J Haematol 2016;174:923–31. [DOI] [PubMed] [Google Scholar]
- 12. Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM.. Malignant lymphoma in primary Sjögren’s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren’s Syndrome. Arthritis Rheum 1999;42:1765–72. [DOI] [PubMed] [Google Scholar]
- 13. Royer B, Cazals-Hatem D, Sibilia J. et al. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood 1997;90:766–75. [PubMed] [Google Scholar]
- 14. Streubel B, Simonitsch-Klupp I, Müllauer L. et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia 2004;18:1722–6. [DOI] [PubMed] [Google Scholar]
- 15. Makula E, Pokorny G, Kiss M. et al. The place of magnetic resonance and ultrasonographic examinations of the parotid gland in the diagnosis and follow-up of primary Sjögren’s syndrome. Rheumatology 2000;39:97–104. [DOI] [PubMed] [Google Scholar]
- 16. Theander E, Mandl T.. Primary Sjögren’s syndrome: diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res 2014;66:1102–7. [DOI] [PubMed] [Google Scholar]
- 17. Keraen J, Blanc E, Besson F. et al. Usefulness of 18F-FDG Positron Emission Tomography (PET) for the diagnosis of lymphoma in primary Sjögren’s syndrome. Arthritis Rheumatol 2019;doi:10.1002/art.40829. [DOI] [PubMed] [Google Scholar]
- 18. Nocturne G, Mariette X.. Sjögren Syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol 2015;168:317–27. [DOI] [PubMed] [Google Scholar]
- 19. Rummel MJ, Niederle N, Maschmeyer G. et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203–10. [DOI] [PubMed] [Google Scholar]
- 20. Salar A, Domingo-Domenech E, Panizo C. et al. First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma (MALT2008-01): a multicentre, single-arm, phase 2 trial. Lancet Haematol 2014;1:e104–11. [DOI] [PubMed] [Google Scholar]
- 21. Demaria L, Henry J, Seror R. et al. Rituximab-Bendamustine (R-Benda) in MALT lymphoma complicating primary Sjögren syndrome (pSS). Br J Haematol 2019;184:472–5. [DOI] [PubMed] [Google Scholar]
- 22. Glauzy S, Boccitto M, Bannock JM. et al. Accumulation of antigen-driven lymphoproliferations in complement receptor 2/CD21-/low B cells from patients with Sjögren’s syndrome. Arthritis Rheumatol 2018;70:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bende RJ, van Noesel CJM.. Rheumatoid factor reactivity of expanded CD21-/low B cells in Sjögren’s Syndrome patients: comment on the article by Glauzy et al. Arthritis Rheumatol 2019;71:169–70. [DOI] [PubMed] [Google Scholar]
- 24. Gottenberg J-E, Ravaud P, Puéchal X. et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA 2014;312:249–58. [DOI] [PubMed] [Google Scholar]
- 25. Anaya JM, McGuff HS, Banks PM, Talal N.. Clinicopathological factors relating malignant lymphoma with Sjögren’s syndrome. Semin Arthritis Rheum 1996;25:337–46. [DOI] [PubMed] [Google Scholar]
- 26. Nishishinya MB, Pereda CA, Muñoz-Fernández S. et al. Identification of lymphoma predictors in patients with primary Sjögren’s syndrome: a systematic literature review and meta-analysis. Rheumatol Int 2015;35:17–26. [DOI] [PubMed] [Google Scholar]
- 27. Ioannidis JPA, Vassiliou VA, Moutsopoulos HM.. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum 2002;46:741–7. [DOI] [PubMed] [Google Scholar]
- 28. Tzioufas AG, Boumba DS, Skopouli FN, Moutsopoulos HM.. Mixed monoclonal cryoglobulinemia and monoclonal rheumatoid factor cross-reactive idiotypes as predictive factors for the development of lymphoma in primary Sjögren’s syndrome. Arthritis Rheum 1996;39:767–72. [DOI] [PubMed] [Google Scholar]
- 29. Solans-Laqué R, López-Hernandez A, Bosch-Gil JA. et al. Risk, predictors, and clinical characteristics of lymphoma development in primary Sjögren’s syndrome. Semin Arthritis Rheum 2011;41:415–23. [DOI] [PubMed] [Google Scholar]
- 30. Risselada AP, Kruize AA, Goldschmeding R. et al. The prognostic value of routinely performed minor salivary gland assessments in primary Sjögren’s syndrome. Ann Rheum Dis 2014;73:1537–40. [DOI] [PubMed] [Google Scholar]
- 31. Theander E, Vasaitis L, Baecklund E. et al. Extended report lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann Rheum Dis 2011;70:1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fragkioudaki S, Mavragani CP, Moutsopoulos HM.. Predicting the risk for lymphoma development in Sjogren syndrome: an easy tool for clinical use. Medicine 2016;95:e3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quartuccio L, Isola M, Baldini C. et al. Biomarkers of lymphoma in Sjögren’s syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J Autoimmun 2014;51:75–80. [DOI] [PubMed] [Google Scholar]
- 34. Baecklund E, Iliadou A, Askling J. et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006;54:692–701. [DOI] [PubMed] [Google Scholar]
- 35. Martin T, Weber JC, Levallois H. et al. Salivary gland lymphomas in patients with Sjögren’s syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum 2000;43:908–16. [DOI] [PubMed] [Google Scholar]
- 36. Bende RJ, Aarts WM, Riedl RG. et al. Among B cell non-Hodgkin’s lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J Exp Med 2005;201:1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bende RJ, Slot LM, Hoogeboom R. et al. Stereotypic rheumatoid factors that are frequently expressed in mucosa-associated lymphoid tissue-type lymphomas are rare in the labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheumatol 2015;67:1074–83. [DOI] [PubMed] [Google Scholar]
- 38. Bombardieri M, Lewis M, Pitzalis C.. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat Rev Rheumatol 2017;13:141–54. [DOI] [PubMed] [Google Scholar]
- 39. Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C.. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren’s syndrome. J Clin Invest 1998;102:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deutsch AJA, Aigelsreiter A, Staber PB. et al. MALT lymphoma and extranodal diffuse large B-cell lymphoma are targeted by aberrant somatic hypermutation. Blood 2007;109:3500–4. [DOI] [PubMed] [Google Scholar]
- 41. Muramatsu M, Kinoshita K, Fagarasan S. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000;102:553–63. [DOI] [PubMed] [Google Scholar]
- 42. Bombardieri M, Barone F, Humby F. et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjögren’s syndrome. J Immunol 2007;179:4929–38. [DOI] [PubMed] [Google Scholar]
- 43. Song H, Tong D, Cha Z, Bai J.. C-X-C chemokine receptor type 5 gene polymorphisms are associated with non-Hodgkin lymphoma. Mol Biol Rep 2012;39:8629–35. [DOI] [PubMed] [Google Scholar]
- 44. Sène D, Ismael S, Forien M. et al. Ectopic germinal center-like structures in minor salivary gland biopsy tissue predict lymphoma occurrence in patients with primary Sjögren’s syndrome. Arthritis Rheumatol 2018;70:1481–8. [DOI] [PubMed] [Google Scholar]
- 45. Johnsen SJ, Berget E, Jonsson MV. et al. Evaluation of germinal center-like structures and B cell clonality in patients with primary Sjögren syndrome with and without lymphoma. J Rheumatol 2014;41:2214–22. [DOI] [PubMed] [Google Scholar]
- 46. Haacke EA, van der Vegt B, Vissink A. et al. Germinal centres in diagnostic labial gland biopsies of patients with primary Sjögren’s syndrome are not predictive for parotid MALT lymphoma development. Ann Rheum Dis 2017;76:1781–4. [DOI] [PubMed] [Google Scholar]
- 47. Nocturne G, Mariette X.. B cells in the pathogenesis of primary Sjögren syndrome. Nat Rev Rheumatol 2018;14:133–45. [DOI] [PubMed] [Google Scholar]
- 48. Yang S, Li J-Y, Xu W.. Role of BAFF/BAFF-R axis in B-cell non-Hodgkin lymphoma. Crit Rev Oncol Hematol 2014;91:113–22. [DOI] [PubMed] [Google Scholar]
- 49. Quartuccio L, Salvin S, Fabris M. et al. BLyS upregulation in Sjogren’s syndrome associated with lymphoproliferative disorders, higher ESSDAI score and B-cell clonal expansion in the salivary glands. Rheumatology 2013;52:276–81. [DOI] [PubMed] [Google Scholar]
- 50. Steri M, Orrù V, Idda ML. et al. Overexpression of the cytokine BAFF and autoimmunity risk. N Engl J Med 2017;376:1615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gottenberg J-E, Sellam J, Ittah M. et al. No evidence for an association between the -871 T/C promoter polymorphism in the B-cell-activating factor gene and primary Sjögren’s syndrome. Arthritis Res Ther 2006;8:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nezos A, Papageorgiou A, Fragoulis G. et al. B-cell activating factor genetic variants in lymphomagenesis associated with primary Sjogren’s syndrome. J Autoimmun 2014;51:89–98. [DOI] [PubMed] [Google Scholar]
- 53. Papageorgiou A, Mavragani CP, Nezos A. et al. A BAFF receptor His159Tyr mutation in Sjögren’s syndrome-related lymphoproliferation. Arthritis Rheumatol 2015;67:2732–41. [DOI] [PubMed] [Google Scholar]
- 54. Tobón GJ, Renaudineau Y, Hillion S. et al. The Fms-like tyrosine kinase 3 ligand, a mediator of B cell survival, is also a marker of lymphoma in primary Sjögren’s syndrome. Arthritis Rheum 2010;62:3447–56. [DOI] [PubMed] [Google Scholar]
- 55. Shen L, Zhang C, Wang T. et al. Development of autoimmunity in IL-14alpha-transgenic mice. J Immunol 2006;177:5676–86. [DOI] [PubMed] [Google Scholar]
- 56. Nocturne G, Seror R, Fogel O. et al. CXCL13 and CCL11 serum levels and lymphoma and disease activity in primary Sjögren’s syndrome. Arthritis Rheumatol 2015;67:3226–33. [DOI] [PubMed] [Google Scholar]
- 57. Sisto M, Lisi S, Lofrumento DD. et al. A failure of TNFAIP3 negative regulation maintains sustained NF-κB activation in Sjögren’s syndrome. Histochem Cell Biol 2011;135:615–25. [DOI] [PubMed] [Google Scholar]
- 58. Musone SL, Taylor KE, Nititham J. et al. Sequencing of TNFAIP3 and association of variants with multiple autoimmune diseases. Genes Immun 2011;12:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nocturne G, Boudaoud S, Miceli-Richard C. et al. Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjogren’s syndrome. Blood 2013;122:4068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nocturne G, Tarn J, Boudaoud S. et al. Germline variation of TNFAIP3 in primary Sjögren’s syndrome-associated lymphoma. Ann Rheum Dis 2016;75:780–3. [DOI] [PubMed] [Google Scholar]
- 61. Johnsen SJ, Gudlaugsson E, Skaland I. et al. Low protein A20 in minor salivary glands is associated with lymphoma in primary Sjögren’s syndrome. Scand J Immunol 2016;83:181–7. [DOI] [PubMed] [Google Scholar]
- 62. Baldini C, Santini E, Rossi C, Donati V, Solini A.. The P2X7 receptor-NLRP3 inflammasome complex predicts the development of non-Hodgkin’s lymphoma in Sjogren’s syndrome: a prospective, observational, single-centre study. J Intern Med 2017;282:175–86. [DOI] [PubMed] [Google Scholar]
- 63. Fragkioudaki S, Nezos A, Souliotis VL. et al. MTHFR gene variants and non-MALT lymphoma development in primary Sjogren’s syndrome. Sci Rep 2017;7:7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kapsogeorgou EK, Papageorgiou A, Protogerou AD, Voulgarelis M, Tzioufas AG.. Low miR200b-5p levels in minor salivary glands: a novel molecular marker predicting lymphoma development in patients with Sjögren’s syndrome. Ann Rheum Dis 2018;77:1200–7. [DOI] [PubMed] [Google Scholar]
- 65. Fisher BA, Jonsson R, Daniels T. et al. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren’s syndrome. Ann Rheum Dis 2017;76:1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kroese FGM, Haacke EA, Bombardieri M.. The role of salivary gland histopathology in primary Sjögren’s syndrome: promises and pitfalls. Clin Exp Rheumatol 2018;112:222–33. [PubMed] [Google Scholar]
- 67. Fox RI, Dixon R, Guarrasi V, Krubel S.. Treatment of primary Sjögren’s syndrome with hydroxychloroquine: a retrospective, open-label study. Lupus 1996;5(Suppl 1):S31–6. [PubMed] [Google Scholar]
- 68. Kruize AA, Hené RJ, Kallenberg CG. et al. Hydroxychloroquine treatment for primary Sjögren’s syndrome: a two year double blind crossover trial. Ann Rheum Dis 1993;52:360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]