Abstract
Objectives
In SSc patients, disease specific determinants that influence health-related quality of life (HRQoL) over time have not been described. We aim to, in patients with SSc, (i) evaluate if and how HRQoL changes over time, and (ii) assess how different SSc domains and functional impairments contribute to changes in HRQoL over time.
Methods
All SSc patients from the Leiden SSc cohort were included; patients with disease duration <24 months were classified as incident cases. HRQoL was assessed prospectively on an annual basis using the EQ-5D and the SF36. To assess baseline associations between clinical characteristics and HRQoL, linear regressions were performed. To identify possible associations between SSc characteristics and HRQoL change over time, linear mixed models were performed in both incident and prevalent cases.
Results
In total, 492 SSc patients were included (n = 202 incident cases), with a median follow-up duration of 3.4 years. At baseline, presence of organ involvement was independently associated with a worse SF36 physical component score and lower EQ-5D score. Over time, gastrointestinal symptoms, Raynaud and digital ulcers were independently associated with deterioration of HRQoL in both incident and prevalent cases. In prevalent cases, pulmonary arterial hypertension (PAH) was associated with a decrease in HRQoL over time. Worse functioning as measured by six-min walking distance, mouth-opening, finger-to-palm distance and grip-strength contributed significantly to deterioration of HRQoL over time.
Conclusion
In SSc, key clinical burdens that contribute to worsening of HRQoL over time include digital ulcers, Raynaud and gastrointestinal involvement. In addition, PAH is a significant burden in prevalent disease.
Keywords: systemic sclerosis, quality of life, impairment, organ involvement
Rheumatology key messages
In SSc, Raynaud symptoms, digital ulcers and gastro-intestinal complications have the largest impact on quality of life.
Of functional assessments, worsening of mouth-opening, six-minute walk test and hand-function contribute to decreasing quality of life.
In patients with longstanding disease, pulmonary arterial hypertension contributes to a decrease in health-related quality of life over time.
Introduction
SSc is a complex connective tissue disease characterized by deregulation of the immune system, vasculopathy and excessive collagen deposition leading to fibrosis of the skin and internal organs [1]. SSc is a heterogeneous disease, in which multiple manifestations are associated with considerable morbidity and mortality [2]. Two major clinical subtypes, namely, limited cutaneous (lcSSc) and diffuse cutaneous SSc (dcSSc), can be recognized according to the extent of skin involvement [3]. Given its severe and systemic character, health-related quality of life (HRQoL) is significantly affected in SSc patients both compared with the general population, and to patients with other rheumatic diseases or chronic conditions [4–6].
HRQoL is a patient reported outcome that includes domains related to physical, mental, emotional and social functioning. It focuses on the impact that health status has on quality of life. Several tools are available to evaluate HRQoL in SSc patients. Some are specific for distinct organ systems or manifestations, while others are generic and can be applied to SSc and to a broad spectrum of rheumatic and non-rheumatic diseases. Among the generic indices, the Short Form-36 (SF36) and the EuroQol Five-Dimensional descriptive system (EQ-5D) are widely used given their reliability and construct validity [7, 8]. The SF36 is a multidimensional questionnaire evaluating both physical and mental functioning. The EQ-5D is simple, quickly completed, and provides a multidimensional description of HRQoL. However, because the EQ-5D contains only a few questions, it could be considered simplistic and not capable of fully assessing individuals’ HRQoL. These patient-reported outcomes are frequently included as secondary endpoints in randomized trials, highlighting the importance of addressing HRQoL indices when the efficacy of novel therapies is investigated [9].
Previous studies have evaluated SSc-related HRQoL cross-sectionally [9–12]. Pain, dyspnea, digital ulcers (DU), RP and gastrointestinal (GI) manifestations have been shown to have a negative influence on HRQoL [9–12]. Most of the available evidence originates from studies with cross-sectional designs and focuses on one clinical characteristic. Other studies evaluated data from randomized controlled trials with a relatively short reassessment period [12–14]. Due to the chronic nature of SSc, it is of additional importance to assess which disease manifestations have largest impact on disease-related HRQoL longitudinally [4]. This is of additional importance for design of therapeutic trials where manifestations with the highest clinical burden should be taken into account. Therefore, using both the SF36 and EQ-5D, we evaluated the main determinants of HRQoL in a monocentric unselected cohort of SSc patients with prospective and longitudinal data available. First, we evaluated which factors are associated with HRQoL at first evaluation. Second, and as the main purpose of our study, we evaluated if and how HRQoL changes over time and how different SSc manifestations impact on HRQoL over time.
Methods
Study design and patients
For the current study, all SSc patients followed at the Leiden University Medical Center (LUMC) from the ongoing, prospective, observational SSc cohort were included (time period 2009–2019). Patients with disease duration <24 months were classified as incident cases. Patients had to fulfill the criteria of the ACR/EULAR 2013 for SSc [15] and had to have a clinical diagnosis of SSc. All patients undergo annual evaluation in the LUMC and clinical, laboratory, and imaging variables are systematically recorded in the research database; the Combined Care in SSc (CCISS, approved by the local Ethics Committee P09.003/SH/sh in Leiden) registry [16]. Questionnaires are collected on an annual basis. The cohort study is designed in accordance with the ethical principles of the Declaration of Helsinki. All patients gave written informed consent.
Health-related quality of life assessment
The Dutch version of the SF36 was used. Eight areas are covered in this questionnaire including: physical function, physical role, bodily pain, general health, vitality, social function, emotional role and mental health. The score ranges from 0 (poor health status) to 100 (good health status). Evidently, scores can be summarized in two global scores: the physical component score (PCS) and the mental component score (MCS) [17].
The EQ-5D is a generic tool consisting of five questions on mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with three potential answers (1 = no problem, 2 = moderate problem, 3 = severe problem) for each item. A sum utility score is calculated using nation-specific algorithms [18]. The Dutch tariff [19] was applied in the present study. Results vary from −0.59 to 1. Negative scores indicate a patient’s perception of a health status worse than death, while a score of 1 means perfect health. The second part of the questionnaire consists of a single visual analogue scale (VAS) through which patients are asked to rate their health of the day from 0 to 100. Higher values represent better health [20].
Patient characteristics and independent variables
For organ involvement, the following definitions were applied: DU were recorded as present when there was clear visible tissue breakdown. Both ischaemic and mechanical (results of microtrauma and increased skin tension) ulcers were included in this definition. Interstitial lung disease (ILD) was defined based on the combination of forced vital capacity (FVC) <70% and evidence for ILD on high-resolution computed tomography (HRCT). An experienced radiologist evaluated the HRCT for ground glass opacifications, reticulations and honeycombing. Pulmonary arterial hypertension (PAH) was defined as a mean pulmonary arterial pressure (mPAP) ≥25 mmHg at rest as assessed by right heart catheterization (RHC); including presence of pre-capillary PH, defined by a pulmonary capillary wedge pressure (PCWP) ≤15 mmHg and a pulmonary vascular resistance (PVR) >3 Wood units (WU) on RHC. All patients with suspicion for PAH were referred for RHC. To evaluate myocardial involvement, we used a modified Medsger score. The Medsger scale mainly relies on the left ventricular ejection fraction (LVEF) for determination of myocardial involvement [21]. However, the use of this parameter alone could lead to an underestimation of presence of myocardial involvement. Therefore, we used a combined value where patients had to have at least two of the following: arrhythmias (>2% ventricular or supraventricular arrhythmia, atrial fibrillation), conduction problems, decreased LVEF <54%, diastolic or systolic dysfunction, pericarditis or pericardial effusion. Myositis was defined based on a combination of creatine kinase (CK) measurements, proximal muscle weakness, if available, histology. Presence of gastrointestinal (GI) involvement was defined based on the composite of severe GI symptoms according to the University of California Los Angeles GI tract (UCLA-GIT) questionnaire and/or the presence of gastric antral vascular ectasia (GAVE), and/or faecal incontinence, and/or weight loss and/or >10% or parenteral nutrition [22, 23]. As the Dutch version of the UCLA-GIT was translated and validated in 2012, we do have some missing questionnaires in patients entering the cohort before 2012; baseline n = 143 missing (of 492), n = 74 missing (of 387) at 1 year follow-up, n = 26 missing (of 298) at 2 year follow-up.
In addition to specific organ dysfunction, the impact of functional assessments on HRQoL was evaluated. For this, handgrip strength measured in kilograms by a handheld dynamometer [24], finger range of motion measured by the standard finger-to-palm (FTP) method [25] (full fist closure was recorded as zero), mouth-opening measured by the maximal interincisal distance [26], and the six-min walking distance were evaluated in relation to HRQoL [27].
As sensitivity analyses, we evaluated the association between the Health Assessment Questionnaire (HAQ), and the SSc specific version Scleroderma HAQ (SHAQ) and HRQoL measured by the EQ-5D and SF36. The HAQ comprises 20 items divided into eight domains, with a final composite score ranging from 0 (no disability) to 3 (maximal disability). The sHAQ evaluates five additional domains (scored on a 0–100 VAS) assessing disability induced by SSc specific symptoms, including DU, RP, lung complaints, gastrointestinal symptoms and disease severity [28].
The SHAQ, SF36 and EQ-5D are frequently used to evaluate HRQoL in SSc. The SHAQ has been extensively validated in SSc [7, 8], and is designed to measure functional ability or disability in SSc. It is quickly completed by the patients; however, by definition, it does not investigate the psychological aspect of HRQoL [7]. The SF36 and EQ-5D include both physical and mental aspects of HRQoL.
Statistical analyses
Descriptive statistics were used to summarize baseline demographic and clinical characteristics of the included patients. Continuous variables are presented as mean (s.d.) or median [interquartile range (IQR)] and categorical variables are presented as counts and percentages. The association between HRQoL based on the EQ-5D or SF36 (dependent variable) and the independent variables (organ involvement and functional performance) were expressed as the beta (b) and the s.e., or as a P-value (considered significant when <0.05). The UCLA GIT questionnaire was collected on an annual basis since 2012, for the missing numbers we used next observation carried backward. Univariable and multivariable linear regression models were constructed for both incident and prevalent cases, and the following confounders were fixed in the multivariable model: age, smoking, socio-economic status (defined as International Standard Classification of Education criteria), cardiopulmonary comorbidities and disease duration. Additional relevant variables based in univariable analyses were also included. Global curves for our outcomes over time were evaluated for both the incident and the prevalent cases. Linear mixed-effect models were used to assess changes in HRQoL score (MCS, PCS and EQ-5D) over the observation time, to control for repeated measurements, and to identify SSc characteristics associating with change in HRQoL during follow-up. The mixed models were separately performed in the incident and prevalent cases to adjust for the different disease durations. Time and risk factors were fixed effects in the analyses. All models included random intercept and slope to account for the longitudinal aspect of the data, and a compound symmetry correlation matrix was used. We selected the most fitting variance-covariance structure with the aid of the Akaike’s score. The continuous predictors were mean centered to help interpreting the coefficients. The beta coefficient for each of individual independent variables of interest can be used to compare the strength of the effect of each variable on the dependent variable. For every 1-unit change in the predictor variable (independent), the outcome variable (dependent) will change by the beta coefficient value. Based on the number of tests performed, we corrected for multiple testing using the Bonferroni method. Statistical analyses were performed on SPSS version 26.
Results
Patient group
In total, 492 patients with SSc were included. Mean age was 55 years, 79% of the patients were female, and 24% had dcSSc. At baseline, median duration since first non-Raynaud symptom was 3 years. Of the 492 included patients, 202 patients could be included in the incident cohort (disease duration since non-Raynaud <24 months). The baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the included patients
| Total cohort | Incident | Prevalent | |
|---|---|---|---|
| n = 492 (100%) | n = 202 (41%) | n = 290 (59%) | |
| Female, n (%) | 390 (79) | 153 (76) | 237 (82) |
| Age, mean (s.d.) | 55 (14) | 53 (14) | 57 (14) |
| High education, n (%) | 101 (21) | 43 (21) | 58 (20) |
| Current smoker, n (%) | 79 (16) | 30 (15) | 49 (17) |
| Disease duration since NR, median (IQR) | 3.2 (0.8–10.3) | 0.7 (0.3–1.2) | 8 (5–15) |
| Follow-up duration, median (IQR) | 3.4 (2.0–6.2) | 3 (1–5) | 4 (2–7) |
| Disease characteristics | |||
| Diffuse cutaneous subset, n (%) | 118 (24) | 51 (25) | 67 (23) |
| Anti-centromere positive, n (%) | 194 (39) | 81 (40) | 113 (39) |
| Anti-topoisomerase positive, n (%) | 116 (24) | 55 (27) | 61 (21) |
| Digital Ulcers, n (%) | 62 (13) | 17 (8) | 45 (! 6) |
| Modified Rodnan Skin score, median (IQR) | 4 (0–6) | 3 (0–7) | 4 (2–6) |
| Organ involvement | |||
| Interstitial lung disease, n (%) | 183 (37) | 62 (31) | 121 (42) |
| FVC % of pred, mean (s.d.) | 97 (23) | 97 (25) | 98 (21) |
| DLCO % of pred, mean (s.d.) | 64 (24) | 65 (28) | 63 (22) |
| Pulmonary arterial hypertension, n (%) | 26 (5) | 8 (4) | 18 (6) |
| LVEF <54%, n (%) | 31 (6) | 11 (5) | 20 (7) |
| Renal crisis, n (%) | 14 (3) | 6 (3) | 8 (3) |
| Severe GI involvement, n (%) | 82 (16) | 35 (17) | 47 (16) |
| Myositis, n (%) | 8 (2) | 8 (4) | 0 (0) |
| Functional impairment | |||
| Six-minute walk test (m), mean (s.d.) | 395 (259) | 416 (260) | 377 (265) |
| Mouth opening (mm), mean (s.d.) | 31 (37) | 26 (45) | 33 (35) |
| Grip strength (kg), mean (s.d.) | 13 (36) | 11 (39) | 16 (31) |
| Finger-to-palm (cm), mean (s.d.) | 9.7 (22) | 9 (23) | 12 (20) |
| Medication at baseline | |||
| Mycophenolate mofetil, n (%) | 19 (4) | 7 (4) | 12 (4) |
| Methotrexate, n (%) | 68 (14) | 27 (13) | 41 (14) |
| Cyclophosphamide, n (%) | 11 (2) | 9 (5) | 2 (1) |
| Azathioprine, n (%) | 14 (3) | 6 (3) | 8 (3) |
| Hydroxychloroquine, n(%) | 22 (5) | 9 (5) | 13 (5) |
DLCO: diffusing capacity for carbon monoxide; FVC: forced vital capacity; GI: gastrointestinal; IQR: interquartile range; LVEF: left ventricular ejection fraction; n: number; pred: predicted.
Baseline associations with health-related quality of life (cross-sectional)
The SF36 mean scores of the total group were 62.6 (s.d. 22) on the MCS and 47.7 (s.d. 20) on the PCS, and the overall mean score on the EQ-5D was 0.66 (SD 0.26). Patients with diffuse cutaneous SSc (dcSSc), lower education level, shorter disease duration and cardiopulmonary comorbidities had worse quality of life as measured by SF36 and EQ-5D (Supplementary Table S1, available at Rheumatology online).
Of the evaluated organ systems in the incident cases, mRSS, Raynaud and GI symptoms were identified as independent determinants of HRQoL at baseline (Table 2), organ involvement as a composite variable also independently associated with the PCS of the SF36 (multivariable β −11.1, P < 0.001) and with the EQ-5D (multivariable β −0.12, P < 0.001; Table 2). In the prevalent cases (supplementary Table S2) only severe GI and Raynaud symptoms were found as independent determinants of HRQoL. Secondly, we evaluated associations between functional assessments and HRQoL at baseline in which positive associations between the six-min walk test and the PCS of SF36 (multivariable β 0.02, P = 0.001), MCS (multivariable β 0.02, P = 0.003), and the EQ-5D (multivariable β 0.13, P = 0.001) were identified in the incident cases (Table 2), these associations were not found in the prevalent cases (Supplementary Table S2, available at Rheumatology online).
Table 2.
Associations at baseline between clinical and functional assessments and HRQoL in incident SSc cases (n = 202)
| Univariable linear regression |
Multivariable linear regression |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCS |
PCS |
EQ-5D |
MCS |
PCS |
EQ-5D |
|||||||
| Predictors | B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P |
| mRSS > 15 | −8.04 (4.4) | 0.07 | −5.6 (3.8) | 0.001 | −0.006 (0.002) | 0.01 | −8.62 (4.6) | 0.06 | −16.23 (3.9) | <0.001 | −0.13 (0.05) | 0.01 |
| Digital ulcer | 0.13 (0.16) | 0.44 | −0.01 (0.15) | 0.91 | −0.001 (0.002) | 0.72 | 0.11 (0.17) | 0.50 | −0.004 (0.15) | 0.98 | −0.001 (0.002) | 0.70 |
| ILD | −1.01 (3.5) | 0.77 | −4.89 (3.1) | 0.12 | 0.004 (0.04) | 0.92 | −1.68 (3.6) | 0.64 | −4.92 (3.2) | 0.13 | −0.005 (0.04) | 0.90 |
| PAH | 2.38 (8.3) | 0.77 | −10.54 (7.4) | 0.16 | −0.028 (0.1) | 0.77 | – | – | – | – | – | – |
| Severe GI | −12.19 (4.2) | 0.004 | −8.99 (3.7) | 0.02 | −0.13 (0.05) | 0.01 | −11.86 (4.3) | 0.007 | −8.69 (3.9) | 0.03 | −0.12 (0.05) | 0.02 |
| Myositis | −3.31 (8.2) | 0.69 | −10.86 (7.4) | 0.14 | −0.14 (0.1) | 0.14 | – | – | – | – | – | – |
| Myocardial | 0.063 (3.7) | 0.98 | −1.06 (3.3) | 0.75 | 0.004 (0.04) | 0.93 | 0.7 (4.2) | 0.87 | 1.95 (3.8) | 0.61 | 0.003 (0.05) | 0.95 |
| Renal crisis | −8.37 (9.4) | 0.38 | −14.29 (8.4) | 0.09 | 0.40 (0.11) | 0.72 | – | – | – | – | – | – |
| Six-min walk test | 0.01 (0.006) | 0.02 | 0.023 (0.005) | <0.001 | 0.005 (0.0) | <0.002 | 0.02 (0.006) | 0.003 | 0.02 (0.006) | <0.001 | 0.13(0.002) | <0.001 |
| Mouth opening | 0.027 (0.04) | 0.46 | 0.012 (0.03) | 0.72 | −0.06 (0.0) | 0.41 | 0.03 (0.04) | 0.39 | 0.02 (0.03) | 0.65 | 0.00 (0.00) | 0.53 |
| Fingertip to palm | −0.21 (0.07) | 0.77 | − 0.13 (0.06) | 0.04 | −0.003 (0.001) | <0.001 | −0.02 (0.07) | 0.78 | −0.13 (0.06) | 0.04 | −0.003 (0.001) | 0.001 |
| Grip strength | 0.035 (0.04) | 0.41 | 0.012 (0.04) | 0.75 | −0.001 (0.0) | 0.26 | 0.04 (0.04) | 0.37 | 0.014 (0.04) | 0.72 | 0.0001 (0.00) | 0.36 |
| Raynauds VAS | −58.14 (19.19) | 0.003 | −46.95 (17.26) | 0.007 | −0.46 (0.23) | 0.04 | −58.68 (19.5) | 0.003 | −49.28 (17.4) | 0.005 | −0.46 (0.23) | 0.04 |
| Organ involvement | −6.75 (3.28) | 0.04 | −11.30 (2.8) | <0.001 | −0,11 (0.04) | 0.003 | −6.88 (3.3) | 0.04 | −11.13 (3.9) | <0.001 | −0.12 (0.04) | 0.002 |
GI: gastrointestinal; ILD: interstitial lung disease; MCS: mental component score; mRSS: modified Rodnan Skin Score; PAH: pulmonary arterial hypertension; PCS: physical component score; VAS: visual analogue scale. Multivariable linear regression adjusted for age, socio-economic status, comorbidities and smoking. Bold indicates significant associations after Bonferroni correction. We were underpowered to evaluate pulmonary arterial hypertension, myositis and renal crisis in this multivariable analysis.
HRQoL changes over time
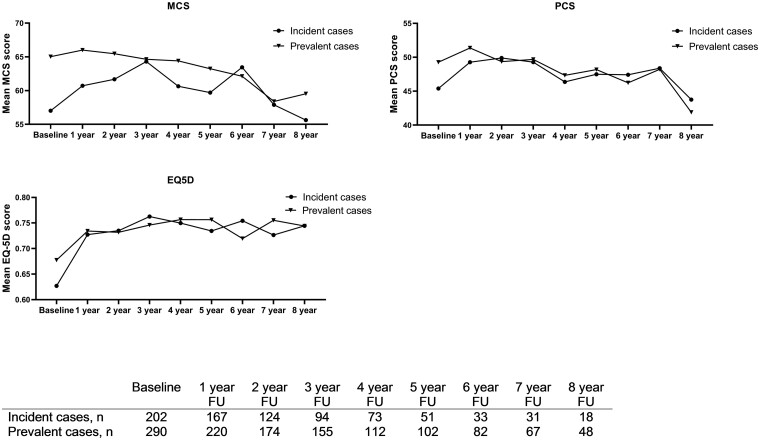
Evaluating the MCS, PCS and EQ-5D mean scores over time (follow-up period: 8 years) we found that the MCS (β −1.32, P < 0.001) and PCS (β −1.30, P < 0.001) worsened over time with respectively −1.32 and −1.30 points every year on a scale from 0–100, while the EQ-5D (β 0.01, P < 0.001) improved over time, although the extent of change was minimal (Supplementary File Table S3, available at Rheumatology online). The global curves of outcomes reflecting HRQoL (MCS, PCS and EQ-5D) over time for the incident and prevalent are shown in Fig. 1. Interestingly, incident cases showed worse HRQoL during the first two years of follow-up, but after two till three years of follow-up, the curves of the incident and prevalent cases were quite similar for the SF36 and the EQ-5D.
Fig. 1.
Mean scores over time of the EQ-5D and both component scores of the SF35 (MCS: mental component score; PCS: physical component score) in the incident and prevalent cases
Clinical characteristics and worsening of quality of life over time (longitudinal)
To identify SSc patients at risk for worsening of HRQoL, we assessed factors associating with HRQoL change over an 8-year follow-up period in both the incident and prevalent cohort including 1977 measurements for each of the questionnaires (in total, n = 775 inception cohort, n = 1202 prevalent cohort). In the multivariable linear mixed-effect models, Raynaud, GI symptoms and presence of DU were identified as independent risk factors for worsening of HRQoL over time in both cohorts (Table 3). In the incident cohort, also mRSS skin score and cardiac involvement were identified as risk factors for worsening of HRQoL over time. PAH was found to be an important risk factor for worsening of HRQoL in the prevalent cohort (PCS and EQ-5D). In the incident cases, Raynaud symptoms were independently associated with a change in physical (PCS) and general health status (EQ-5D). The mRSS score was independently associated with a worsening PCS and EQ-5D score. GI symptoms were independently associated with worsening of the PCS and the EQ-5D. Presence of DU was independently associated with worsening of the PCS.
Table 3.
Linear mixed model performed in inception and prevalent cohort
| Incident cohort |
Prevalent cohort |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCS adjusted |
PCS adjusted |
EQ-5D adjusted |
MCS adjusted |
PCS adjusted |
EQ-5D adjusted |
|||||||
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| mRSS> 15 | −2.7 (−7.3, 1.8) | 0.2 | −6.2 (−10.2, −2.2) | 0.002 | −0.06 (−0.1, −0.005) | 0.003 | 1.6 (−3.0, 6.2) | 0.5 | −1.06 ( −4.9, 2.7) | 0.6 | −0.06 (−0.1, −0.009) | 0.02 |
| ILD | 4.9 (0.3, 9.5) | 0.04 | 0.8 (−3.3, 4.9) | 0.7 | 0.02 (−0.04, 0.06) | 0.6 | 2.3 (−1.4, 6.03) | 0.2 | 1.2 (−2.1, 4.6) | 0.5 | −0.01 (−0.05, 0.03) | 0.5 |
| PAH | 2.4 (−7.0, 11.7) | 0.6 | 1.4 (−6.9, 9.7) | 0.7 | 0.01 (−0.07, 0.10) | 0.8 | −6.4 (−13.6, 0.79) | 0.08 | −9.6 (−16.0, −3.1) | 0.004 | −0.1 (−0.2, − 0.07) | <0.001 |
| GI | −3.07 (−6.5, 0.4) | 0.08 | −2.3 (−5.4, −0.7) | 0.002 | −0.05 (−0.09, −0.009) | 0.002 | −2.6 (−5.2, 0.10) | 0.06 | −2.07 (−4.2, −0.09) | 0.03 | − 0.05 (−0.07, −0.02) | 0.003 |
| Myositis | −1.4 (−8.6, 5.9) | 0.7 | −6.2 (−12.6, 0.1) | 0.05 | − 0.07 (−0.2, 0.01) | 0.09 | 1.5 (−16.2, 19.2) | 0.9 | 2.6 (−11.8, 17.0) | 0.7 | − 0.009 (−0.2, 0.2) | 0.9 |
| Cardiac | −22.2 (−34.7, −9.7) | 0.001 | −11.8 (−23.2, −0.3) | 0.04 | −0.2 (−0.2, −0.007) | 0.04 | 1.3 (−11.1, 13.7) | 0.8 | 0.6 (−10.5, 11.6) | 0.9 | − 0.09 (−0.2, 0.04) | 0.2 |
| Renal crisis | 1.2 (−10.5, 12.9) | 0.8 | 0.6 (−9.9, 11.2) | 0.9 | 0.1 (−0.02, 0.2) | 0.09 | 1.3 (−11.8, 14.4) | 0.8 | −5.6 (−17.9, 6.8) | 0.4 | 0.05 (−0.07, 0.2) | 0.4 |
| Digital ulcers | −0.1 (−4.9, 4.7) | 0.9 | −5.0 (−9.2, −0.8) | 0.002 | − 0.06 (−0.12, −0.006) | 0.03 | −2.8 (−5.5, −0.02) | 0.04 | −3.0 (−5.3, −0.8) | 0.009 | −0.02 (−0.05, 0.01) | 0.2 |
| VAS Raynaud | −0.04 (−0.1, 0.02) | 0.2 | − 0.09 (−0.2, 0.04) | <0.001 | −0.002 (−0.003, −0.001) | <0.001 | −0.1 (−0.2, −0.09) | <0.001 | −0.1 (−0.2, −0.09) | <0.001 | −0.002 (−0.002, −0.001) | <0.001 |
mRSS, GI, myocardial, DU and Raynaud symptoms are associated with HRQoL changes over time in incident cohort. PAH, GI, DU and Raynaud symptoms are associated with HRQoL changes over time in prevalent cohort. Linear mixed model to evaluate association between organ involvement and HRQoL over time adjusted for age, socio-economic status, comorbidities and smoking. In this table, the mains effect are shown. P-value cut-off after Bonferroni P <0.006. Bold indicates significant associations after Bonferroni correction. GI: gastrointestinal; ILD: interstitial lung disease; mRSS: modified Rodnan Skin Score; PAH: pulmonary arterial hypertension; VAS: visual analogue scale.
To evaluate which functional impairments affect worsening of HRQoL in SSc, we evaluated the six-min walk test, mouth opening, finger-to-palm and grip strength in multivariable linear mixed effect models (Table 4). All functional outcomes were independently associated with worsening HRQoL over time as measured by components of the SF36 in both the incident and the prevalent cases. Only the fingertip to palm distance showed a significant association with worsening of HRQoL as measured by EQ-5D in both cohorts. The largest difference between the incident and prevalent cases was observed in the results for the six-min walk test, which had a larger impact on HRQoL in the incident patients.
Table 4.
Linear mixed model; function impairment and HRQoL performed in incident and prevalent cohort
| Incident cohort |
Prevalent cohort |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCS adjusted |
PCS adjusted |
EQ-5D adjusted |
MCS adjusted |
PCS adjusted |
EQ-5D adjusted |
|||||||
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| 6MWT | 0.01 (0.007, 0.02) | <0.001 | 0.02 (0.010, 0.02) | <0.001 | 0.0001 (0.0004, 0.002) | 0.002 | 0.003 (−0.002, 0.008) | 0.21 | 0.005 (0.001, 0.009) | 0.01 | 0.0002 (−0.0007, 0.0003) | 0.5 |
| Mouth open | 0.05 (0.02, 0.07) | <0.001 | 0.02 (0.004, 0.04) | 0.02 | 0.00006 (−0.0003, 0.0002) | 0.64 | 0.03 (0.01, 0.05) | <0.001 | 0.02 (0.002, 0.03) | 0.02 | 0.0001 (−0.0002, 0.0002) | 0.9 |
| FTP | −0.07 (0.04, 0.10) | <0.001 | 0.03 (0.002, 0.06) | 0.03 | −0.0005 (−0.0008, −0.0001) | 0.009 | −0.08 (0.05, 0.11) | <0.001 | −0.06 (0.03, 0.08) | <0.001 | −0.0003 (−0.0006, −0.0001) | 0.04 |
| Grip strength | 0.06 (0.04, 0.09) | <0.001 | 0.04 (0.02, 0.06) | 0.001 | −0.0001 (−0.0004, 0.0002) | 0.46 | 0.04 (0.02, 0.06) | <0.001 | 0.02 (0.005, 0.04) | 0.01 | 0.00002 (−0.0002, 0.0002) | 0.9 |
Adjusted for age, socio-economic status, comorbidities and smoking. In this table both the main effect and the time coefficients are shown. P-value cut-off after Bonferroni P<0.013. Bold indicates significant associations after Bonferroni correction. 6MWT: six-min walk test; FTP: fingertip-to-palm; Mouth open: mouth opening.
As a sensitivity check to confirm that both SF36 and EQ-5D measurements capture global disability in SSc, we evaluated associations between the SHAQ and the SF36 and EQ-5D. All scores, both cross-sectionally and over time, showed significant and strong association with SHAQ (Table 5). Difference between the incident and prevalent cases were predominantly seen in the VAS digestive, which only showed a significant association with the SF36 in the prevalent cases (Table 5).
Table 5.
Linear mixed model HAQ and SF-36 and EQ5D performed in incident and prevalent cohort
| Incident cohort |
Prevalent cohort |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCS adjusted |
PCS adjusted |
EQ5D adjusted |
MCS adjusted |
PCS adjusted |
EQ5D adjusted |
|||||||
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| DIG VAS | −0.06 (−0.1, 00.002) | 0.05 | −0.05 (−0.1, 0.003) | 0.06 | −0.001 (−0.002, −0.0004) | 0.002 | −0.1 (−0.2, −0.09) | <0.001 | −0.09 (−0.1, −0.05) | <0.001 | −0.001 (−0.002, −0.001) | <0.001 |
| DU VAS | −0.09 (−0.2, −0.01) | 0.02 | −0.1 (−0.2, −0.05) | 0.001 | −0.002 (−0.002, −0.0006) | 0.001 | −0.1 (−0.2, −0.08) | <0.001 | −0.1 (−0.2, −0.07) | <0.001 | −0.001 (−0.002, −0.0008) | <0.001 |
| Pulm VAS | −0.1 (−0.2, −0.02) | 0.006 | −0.1 (−0.2, 0.07) | <0.001 | −0.001 (−0.002, −0.0001) | 0.02 | −0.1 (−0.2, −0.07) | <0.001 | −0.1 (−0.2, 0.09) | <0.001 | −0.001 (−0.002, −0.0008) | <0.001 |
| Severity VAS | −0.09 (−0.2, −0.03) | 0.002 | −0.2 (−0.2, −0.1) | <0.001 | −0.002 (−0.003, −0.001) | <0.001 | −0.1 (−0.2, −0.1) | <0.001 | −0.2 (−0.2, −0.7) | <0.001 | −0.003 (−0.002, −0.002) | <0.001 |
| HAQ-DI | −9.6 (−11.8, −7.3) | <0.001 | −12.3 (−13.2, −10.5) | <0.001 | −0.005 (−0.01, 0.00) | 0.05 | −1.7 (−2.6, −0.8) | <0.001 | −3.04 (−3.9, −2.1) | <0.001 | −0.006 (−0.008, −0.002) | <0.001 |
Adjusted for age, socio-economic status, comorbidities and smoking. In this table both the main effect and the time coefficients are shown. DIG: digestivus; DU: digital ulcera; HAQ DI: health assessment questionnaire disability index; Pulm: pulmonary; Severity: disease severity; VAS: visual analogue scale; bold indicates P-value<0.05.
Discussion
In this study, we aimed to explore which disease-specific characteristics are associated with change of HRQoL in patients with SSc over time. Over time, HRQoL in SSc slightly worsened, and key clinical burdens determining worsening of HRQoL included DU, Raynaud and GI involvement. In addition, functional impairment as reflected by worse walking distance, mouth opening, and hand function, independently impact on worsening of HRQoL. Some differences were found for the incident and prevalent cases, where skin score and six-min walk test had a larger influence on HRQoL in incident cases and PAH had a larger influence on HRQoL in prevalent cases.
Literature on longitudinal variations of HRQoL in SSc is scarce. Most published studies have been conducted on cross-sectional data. Our results on cross-sectional associations at baseline are largely in line with previous findings. Indeed, the extent of skin involvement has frequently been identified as one of the factors affecting HRQoL in SSc. Compared with lcSSc, patients with dcSSc had poorer HRQoL scores [4, 10, 29] and the degree of skin involvement measured by mRSS had a negative impact on HRQoL [5]. GI manifestations have also been studied. Both Franck-Larsson et al. [30] and Omair and Lee [31] showed that lower GI symptoms, and especially faecal incontinence, contributed negatively to HRQoL in patients with SSc, while Frantz et al. [9] revealed how GI complications were significantly associated with perception of disease severity. Our results are also in accordance with previous studies showing that Raynaud Phenomenon is one of the most common symptoms influencing HRQoL in SSc [32]. Although hands are frequently involved in SSc and dedicated programs have been developed to improve hand function [25], the impact of hand disability on HRQoL has not been extensively explored. In our study, we show that functional disability of the hand, measured by finger-to-palm distance and grip strength, significantly contributed to worsening of HRQoL over time.
It is important to note that severe GI involvement, and also RP significantly impact SSc-related HRQoL, both cross-sectionally and over time, while other disease manifestations including ILD did not seem to affect the patient’s perception of HRQoL. As elegantly hypothesized by Frantz et al. [9], caring physicians naturally focus their attention on life-threatening manifestations. Consequently, physicians might assume that these life-threatening manifestations will also considerably impact HRQoL from the patients’ perspective. Our results indicated that daily life of SSc patients is significantly affected by relatively less severe, but troublesome and difficult to control symptoms, including Raynaud’s and GI complaints.
Strikingly, in the multivariable analyses, presence of ILD did not impact HRQoL over time, which is the opposite of what one might have expected. We can only speculate about the explanation for this observation. For example, it is known that not all SSc-ILD patients actually experience symptoms attributable to ILD. However, by using a combined definition for presence of ILD, based on interstitial lung abnormalities on HRCT together with FVC % of predicted (<70), we aimed to identify patients with more severe, and clinically relevant ILD. Interestingly, the pulmonary VAS (of the HAQ) and the six-min walk test did show an association with change in SF36 and EQ-5D, indicating that indeed patients who actually experience dyspnoea do experience worse quality of life. This observation might indicate that the clinically applied definitions for SSc-ILD are not completely in line with symptoms as experienced by the patients. Another explanation might be that SSc-ILD patients are treated more aggressively and earlier in the disease course which might come to benefit of other disease manifestations as well, and consequently is beneficial for HRQoL [33]. Finally, patients with SSc-ILD might reflect a study population with more severe disease. In this subgroup, SSc-HRQol might be more severely affected from the start and, while over time, these patients learn to cope with their situation. Consequently, presence of ILD does not result in more deterioration of SSc-HRQoL over time.
There are some limitations to our study. The first limitation is intrinsic to the use of patient reported outcomes, which are self-administered questionnaires that largely dependent on patients’ perceptions and do not always reflect disease activity or severity of specific manifestations in an objective way. However, the used questionnaires are all validated in SSc [7, 28, 34, 35]. To confirm construct validity, a sensitivity analysis using SHAQ was performed that showed highly significant associations with SF36 and EQ-5D both cross-sectionally and over time. Second, consensus about the definition of specific SSc-related organ involvement is not unanimous among experts, as for example for cardiac complications. The definitions applied to define organ involvement have been largely based on the Medsger scale with adjustments where deemed necessary. Our work has major strengths too: most importantly, the longitudinal design. Few studies investigated determinants of HRQoL evolution over time in SSc patients. We were able to include patients with up to 8 years of follow-up. Secondly, most published articles investigate the effect of a specific disease manifestation, the effect of medication or a selected patients’ group. In our analyses, we included a heterogeneous and unselected population of 492 SSc patients in which 202 were incident cases, contributed data had a high rate of completeness and, in our opinion, we thus provide fairly generalizable results.
In conclusion, this study provides unique information about the most important determinants of HRQoL in SSc. Deeper knowledge of factors significantly influencing not only HRQoL, but also changes of HRQoL over time, is of relevance to tailor most appropriate treatment strategies. Moreover, a thorough understanding of HRQoL determinants may help caring physicians to identify the unmet needs of SSc patients and the areas where more vigorous pharmacological or non-pharmacological interventions are indicated. We confirm previous findings about the impact of RP, hand function, skin involvement and GI symptoms on daily life of SSc patients, but we also outline the possibility that these factors may predict further HRQoL deterioration. Our results suggest that major attention should be paid to GI symptoms, Raynaud and DU as possible predictors of worsening HRQoL. In addition, in patients with longstanding disease, PAH is importantly influencing HRQoL over time.
Supplementary Material
Acknowledgements
We would like to thank Xanthe Matthijssen for her statistical assistance.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: All authors have declared that they have no conflicts of interest.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Gabrielli A, Avvedimento EV, Krieg T.. Scleroderma. NEJM 2009;360:1989–2003. [DOI] [PubMed] [Google Scholar]
- 2. Denton CP, Khanna D.. Systemic sclerosis. Lancet 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 3. Wollheim FA. Classification of systemic sclerosis. Visions and reality. Rheumatology 2005;44:1212–6. [DOI] [PubMed] [Google Scholar]
- 4. Hudson M, Thombs BD, Steele R; for the Canadian Scleroderma Research Group et al. Quality of life in patients with systemic sclerosis compared to the general population and patients with other chronic conditions. J Rheumatol 2009;36:768–72. [DOI] [PubMed] [Google Scholar]
- 5. Park EH, Strand V, Oh YJ, Song YW, Lee EB.. Health-related quality of life in systemic sclerosis compared with other rheumatic diseases: a cross-sectional study. Arthritis Res Ther 2019;21:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullinger M, Quitmann J.. Quality of life as patient-reported outcomes: principles of assessment. Dialogues Clin Neurosci 2014;16:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gualtierotti R, Ingegnoli F, Scalone L. et al. Feasibility, acceptability and construct validity of EQ-5D in systemic sclerosis. Swiss Med Wkly 2016;146:w14394. [DOI] [PubMed] [Google Scholar]
- 8. Li L, Cui Y, Chen S. et al. The impact of systemic sclerosis on health-related quality of life assessed by SF-36: a systematic review and meta-analysis. Int J Rheum Dis 2018;21:1884–93. [DOI] [PubMed] [Google Scholar]
- 9. Frantz C, Avouac J, Distler O. et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: a large international survey. Semin. Arthritis Rheum 2016;46:115–23. [DOI] [PubMed] [Google Scholar]
- 10. Georges C, Chassany O, Toledano C. et al. Impact of pain in health related quality of life of patients with systemic sclerosis. Rheumatology 2006;45:1298–302. [DOI] [PubMed] [Google Scholar]
- 11. Baron M, Sutton E, Hudson M. et al. The relationship of dyspnoea to function and quality of life in systemic sclerosis. Ann Rheum Dis 2007;67:644–50. [DOI] [PubMed] [Google Scholar]
- 12. Matucci-Cerinic M, Krieg T, Guillevin L. et al. Elucidating the burden of recurrent and chronic digital ulcers in systemic sclerosis: long-term results from the DUO Registry. Ann Rheum Dis 2016;75:1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khanna D, Yan X, Tashkin DP. et al. Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum 2007;56:1676–84. [DOI] [PubMed] [Google Scholar]
- 14. Milio G, Corrado E, Genova C. et al. Iloprost treatment in patients with Raynaud's phenomenon secondary to systemic sclerosis and the quality of life: a new therapeutic protocol. Rheumatology 2006;45:999–1004. [DOI] [PubMed] [Google Scholar]
- 15. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meijs J, Schouffoer AA, Ajmone Marsan N. et al. Therapeutic and diagnostic outcomes of a standardised, comprehensive care pathway for patients with systemic sclerosis. RMD Open 2016;2:e000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sekhon S, Pope JCanadian Scleroderma Research GroupBaron M.. The minimally important difference in clinical practice for patient-centered outcomes including health assessment questionnaire, fatigue, pain, sleep, global visual analog scale, and SF-36 in scleroderma. J Rheumatol 2010;37:591–8. [DOI] [PubMed] [Google Scholar]
- 18. Rabin R, Gudex C, Selai C, Herdman M.. From translation to version management: a history and review of methods for the cultural adaptation of the EuroQol five-dimensional questionnaire. Value Health 2014;17:70–6. [DOI] [PubMed] [Google Scholar]
- 19. Lamers LM, McDonnell J, Stalmeier PF, Krabbe PF, Busschbach JJ.. The Dutch tariff: results and arguments for an effective design for national EQ-5D valuation studies. Health Econ 2006;15:1121–32. [DOI] [PubMed] [Google Scholar]
- 20. Kwakkenbos L, Fransen J, Vonk MC. et al. A comparison of the measurement properties and estimation of minimal important differences of the EQ-5D and SF-6D utility measures in patients with systemic sclerosis. Clin Exp Rheumatol 2013;31:50–6. [PubMed] [Google Scholar]
- 21. Meune C, Vignaux O, Kahan A, Allanore Y.. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010;103:46–52. [DOI] [PubMed] [Google Scholar]
- 22. Khanna D, Hays RD, Maranian P. et al. Reliability and validity of the University of California, Los Angeles Scleroderma clinical trial consortium gastrointestinal tract instrument. Arthritis Rheum 2009;61:1257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meijs J, Pors D, Vliet VT, Huizinga TW, Schouffoer AA.. Translation, cross-cultural adaptation, and validation of the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument (SCTC GIT) 2.0 into Dutch. Clin Exp Rheumatol 2014;32:S-41–8. [PubMed] [Google Scholar]
- 24. Mathiowetz V, Kashman N, Volland G. et al. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 1985;66:69–74. [PubMed] [Google Scholar]
- 25. Landim SF, Bertolo MB, Marcatto de Abreu MF. et al. The evaluation of a home-based program for hands in patients with systemic sclerosis. J Hand Ther 2019;32:313–21. [DOI] [PubMed] [Google Scholar]
- 26. Vitali C, Baldanzi C, Crispiatico V. et al. Effect of impairment-oriented and function-oriented exercises on mouth function in subjects with systemic sclerosis. Folia Phonatr LogoP 2020;72:389–401. [DOI] [PubMed] [Google Scholar]
- 27. Rizzi M, Radovanovic D, Santus P. et al. Usefulness of six-minute walk test in systemic sclerosis. Clin Exp Rheumatol 2018;36:161–7. [PubMed] [Google Scholar]
- 28. Steen VD, Medsger TA. Jr.. The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 1997;40:1984–91. [DOI] [PubMed] [Google Scholar]
- 29. Arat S, Verschueren P, De Langhe E. et al. The association of illness perceptions with physical and mental health in systemic sclerosis patients: an exploratory study. Musculoskeletal Care 2012;10:18–28. [DOI] [PubMed] [Google Scholar]
- 30. Franck-Larsson K, Graf W, Ronnblom A.. Lower gastrointestinal symptoms and quality of life in patients with systemic sclerosis: a population-based study. Eur J Gastroenterol Hepatol 2009;21:176–82. [DOI] [PubMed] [Google Scholar]
- 31. Omair MA, Lee P.. Effect of gastrointestinal manifestations on quality of life in 87 consecutive patients with systemic sclerosis. J Rheumatol 2012;39:992–6. [DOI] [PubMed] [Google Scholar]
- 32. Pauling JD, Saketkoo LA, Matucci-Cerinic M, Ingegnoli F, Khanna D.. The patient experience of Raynaud's phenomenon in systemic sclerosis. Rheumatology 2019;58:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giacomelli R, Liakouli V, Berardicurti O. et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int 2017;37:853–63. [DOI] [PubMed] [Google Scholar]
- 34. Cossutta R, Zeni S, Soldi A. et al. Evaluation of quality of life in patients with systemic sclerosis by administering the SF-36 questionnaire. Reumatismo 2002;54:122–7. [DOI] [PubMed] [Google Scholar]
- 35. Khanna D, Furst DE, Clements PJ. et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol 2005;32:832. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.