Abstract
Objectives
To compare patient-reported outcome (PRO) domains between three arthritis phenotypes [undifferentiated arthritis (UA), autoantibody-negative RA (RA−) and autoantibody-positive RA (RA+)] at diagnosis, after 2 years and over time.
Methods
All UA (n = 130), RA− (n = 176) and RA+ (n = 331) patients from the tREACH trial, a stratified single-blinded trial with a treat-to-target approach, were used. PRO comparisons between phenotypes at baseline and after 2 years were performed with analysis of variance, while a linear mixed model compared them over time. Effect sizes were weighted against the minimal clinically important differences (MCIDs) for each PRO.
Results
RA− patients had a higher disease burden compared with RA+ and UA. At baseline and after 2 years, RA− patients had more functional impairment and a poorer Physical Component Summary (PCS) compared with the other phenotypes, while they only scored worse for general health and morning stiffness duration at baseline. The MCIDs were exceeded at baseline, except for functional ability between RA+ and UA, while after 2 years only the MCID of the PCS was exceeded by RA− compared with UA and RA. After 2 years the PROs of all phenotypes improved, but PROs measuring functioning were still worse compared with the general population, even when patients had low disease activity.
Conclusion
RA− patients had the highest disease burden of all phenotypes. Although most patients have low disease activity after treatment, all clinical phenotypes still have a similar significant impact on patients’ lives, which is mainly physical. Therefore it is important to assess and address PROs in daily practice because of persistent disease burden despite low disease activity.
Trial registration
ISRCTN26791028.
Keywords: rheumatoid arthritis, autoantibodies, patient-reported outcomes, early arthritis, clinical phenotypes
Rheumatology key messages
The difference in impact on patients’ lives between the different (rheumatoid) arthritis phenotypes is unclear.
RA− patients had the highest disease burden when compared with the other clinical phenotypes.
Assessing and addressing patient-relevant outcomes in daily practice is important because of persistent disease burden.
Introduction
Rheumatologists frequently assess new-onset inflammatory arthritis, but recognizing the underlying disease can be challenging, especially in an early stage [1, 2]. Early arthritis can be divided into three clinical phenotypes: undifferentiated arthritis (UA), autoantibody-negative RA (RA−) and autoantibody-positive RA (RA+).
Although the aforementioned phenotypes have a different prognosis, (initial) treatment is similar for each phenotype [1–7]. Fortunately, the prognosis has improved enormously due to early intensive DMARD strategies and a treat-to-target management approach [1, 2]. However, in the past, the emphasis was more on clinical outcomes and less on patient-relevant outcomes, but today the delivery of healthcare is shifting towards a patient-centred care (PCC) approach. PCC focuses on outcomes that are meaningful and valuable to the individual patient [8–11]. It is already known that arthritis has a significant impact on patients’ lives, but it is not completely clear whether this impact differs between the different phenotypes and which domains are influenced most [7, 9, 12].
Recently the International Consortium for Health Outcome Measurement internationally agreed upon which domains were most relevant for inflammatory arthritis patients and determined a standard set of patient-reported outcome (PRO) measures [10, 13]. This is in agreement with previously developed core outcome sets of the OMERACT, ACR/EULAR and the Combination of Methotrexate and Etanercept in Early Rheumatoid Arthritis trial (COMET) [14]. The patient-relevant domains for inflammatory arthritis are pain, fatigue, activity limitation, overall emotional and physical health impact, work/school/housework ability and productivity [10, 13]. Although these domains are to a certain extent addressed in previous literature, they have never been addressed all at once [9, 13, 15, 16].
Moreover, literature comparing PROs between the aforementioned phenotypes is sparse. To our knowledge, Boer et al. [12] compared PROs between RA− and RA+ patients at baseline and over time, while Nordberg et al. [7, 17] compared them at baseline and after 2 years, but not over time. Moreover, both studies did not include UA patients, nor did they address all domains.
Therefore the aim of this study was to compare all patient-relevant outcome domains for inflammatory arthritis between three clinical phenotypes (UA, RA− and RA+) at diagnosis, after 2 years and over time.
Methods
Patients
For this study we used data from the tREACH trial, which was a multicentre, stratified, single-blinded, randomized controlled trial with a treat-to-target approach that compared different initial treatment strategies [18, 19]. The tREACH trial has been described in detail elsewhere [19]. All patients gave written informed consent before inclusion and medical ethics committees at each participating centre approved the tREACH study protocol (MEC-2006-252). The inclusion and follow-up period has already ended and all data have been collected.
All patients enrolled in the tREACH trial were selected for this study. Patients who fulfilled the 1987 and/or 2010 criteria for RA were classified as autoantibody negative (RA−) or autoantibody positive (RA+) depending on the absence or presence of one or more autoantibodies. Patients with one or more swollen joints who did not fulfil the 1987 and/or 2010 classification criteria for RA were classified as undifferentiated arthritis (UA). Our dataset contains 130 UA, 176 RA− and 331 RA+ patients.
Study design
The tREACH trial had a treat-to-target approach aiming for low disease activity (DAS <2.4) [20]. Treatment alterations could occur every 3 months depending on the disease activity, and in case of very active disease, based on the rheumatologists’ insight, an earlier visit could be planned.
Patients received either initial MTX, including DMARD combination therapies with or without glucocorticoid bridging therapy (MTX+), HCQ or NSAIDs/glucocorticoids (no DMARDs). Treatment was intensified in case of still active disease (DAS ≥2.4). Treatment intensifications occurred in the following order: triple DMARD therapy, consisting of MTX, SSZ and HCQ; MTX + etanercept (50 mg/week, s.c.); MTX + adalimumab (40 mg/2 weeks, s.c.) and MTX + abatacept (500–1000 mg/4 weeks, i.v., weight dependent).
Tapering of medication occurred if the DAS was <1.6 at two consecutive visits. Medication was gradually discontinued, except for HCQ and naproxen, which were immediately stopped. In case of a flare (DAS ≥2.4) during tapering, full treatment was restarted, according to the stage in the protocol.
Data collection procedure
Visits occurred every 3 months and at each visit the DAS and the following PROs were collected: quality of life, functional ability, general health (GH), pain, morning stiffness duration, fatigue and productivity loss. Health status and anxiety and depression were collected every 6 months.
Quality of life was measured with the European Quality of Life 5-Dimensions 3-Levels (EQ-5D-3L) questionnaire. Higher scores represent a higher quality of life [21]. For functional ability, the HAQ disability index (HAQ-DI) was used and higher scores indicate poorer function [22–24]. GH was measured on a 0–100 mm visual analogue scale (VAS), where higher scores reflect a poorer health status [25, 26]. Pain and morning stiffness were measured with a Likert scale and are interpreted the same as GH [27]. Fatigue was measured with the Fatigue Assessment Scale (FAS) and higher scores reflect more fatigue [22]. For anxiety and depression the Hospital Anxiety and Depression Scale (HADS) was used and a score >7 represents a possible anxiety disorder or depression [28, 29]. Health status was measured with the 36-item Short Form Health Survey (SF-36), which assesses eight domains on a scale of 0–100, with higher scores indicating better health status. The SF-36 addresses the following domains: physical functioning (PF), role physical (RP), bodily pain (BP), GH perceptions, vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH). These domains are also combined into a Physical Component Summary (PCS) score and Mental Component Summary (MCS) score [25, 30–32]. Productivity loss includes absenteeism, i.e. sick leave, and presenteeism, i.e. working while sick, in the last 3 months. For both outcomes, proportions of occurrence over the entire working population are given [27].
Data analysis procedure
Analysis of variance (ANOVA) was used to compare the difference in each PRO (11 in total) between the three phenotypes (i.e. UA, RA− and RA+) at diagnosis and after 2 years. In case of a significant difference, statistical comparisons between the phenotypes were made by Student’s t test, chi-squared test or Wilcoxon rank sum test, when appropriate. Furthermore, the effect sizes were weighted against the minimal clinically important difference (MCID) for each PRO, except for morning stiffness duration due to an unknown MCID [21–23, 25, 27–29, 33].
Additionally, a linear mixed model (LMM) with an unstructured covariance matrix was used to compare each PRO over time in which the arthritis phenotype, time, age, gender, initial treatment and baseline DAS and PRO were the covariates. Within the LMM, the RA+ patients were set as the reference.
Besides the aforementioned analyses, we also assessed whether PROs after 2 years of follow-up differed between patients with and without active disease (respectively DAS ≥2.4 and DAS <2.4) stratified for clinical phenotype (see Supplementary Table S1, available at Rheumatology online). Finally, we compared the RA patients who fulfilled the 1987 criteria with those who fulfilled the 2010 criteria, stratified for RA phenotype (see Supplementary Table S2, available at Rheumatology online).
The dropout ratios for UA, RA− and RA+ patients were 31% (40/130), 27% (48/176) and 24% (81/331), respectively (see Supplementary Fig. S1, available at Rheumatology online). Multiple imputations with chained equations (MICE), with 40 imputations, were used to handle these missing data. Imputation regression models were constructed for the DAS and all PROs, in which treatment, gender, age and the corresponding measures at baseline were the independent variables.
Although dropout ratios were similar between groups, we hypothesized that the reason for dropout might be different for the UA group compared with the RA groups. We therefore performed a sensitivity analysis using only complete cases and patients who are in DMARD-free remission (DFR) from the UA group and compared them with the other two phenotypes to ensure that our findings were valid.
Furthermore, to correct for multiple testing, a Bonferroni correction was applied for all ANOVAs and LMMs. The calculated P-values were corrected by multiplying the P-value with the 33 performed tests. If a significant Bonferroni-corrected P-value was acquired with the ANOVA, additional tests were performed without adjustment. A P-value ≤0.05 was still considered statistically significant with the aforementioned approach. Statistical analyses were done in Stata version 15.1 (StataCorp, College Station, TX, USA.
Results
Patients
The baseline characteristics of the 130 UA, 176 RA− and 331 RA+ patients are given in Table 1. Age, gender and symptom duration were equally divided among the clinical phenotypes (Table 1).
Table 1.
Baseline characteristics for all arthritis subsets
| Characteristics | UA (n = 130) | RA− (n = 176) | RA+ (n = 331) |
|---|---|---|---|
| Demographics | |||
| Age, years, mean (s.d.) | 50 (14) | 55 (15) | 53 (14) |
| Sex, female, n (%) | 88 (68) | 118 (67) | 225 (68) |
| Disease characteristics | |||
| Symptom duration, days, median (IQR) | 136 (77–223) | 129 (89–198) | 146 (84–213) |
| RF positivity, n (%) | 6 (5) | 0 (0) | 261 (79) |
| ACPA positivity, n (%) | 6 (5) | 0 (0) | 261 (79) |
| Erosive disease, n (%)a | 0 (0) | 25 (14) | 41 (12) |
| DAS44, mean (s.d.) | 2.34 (0.67) | 3.42 (1.00) | 3.19 (0.87) |
| SJC44, median (IQR) | 3 (2–4) | 7 (4–12) | 6 (3–11) |
| ESR, mm/h, median (IQR) | 14 (7–26) | 16 (9–31) | 23 (13–39) |
| Initial treatment | |||
| MTX+, n (%) | 37 (28) | 80 (45) | 250 (76) |
| csDMARDs, n (%) | 38 (29) | 49 (28) | 66 (20) |
| No DMARDs, n (%) | 55 (42) | 47 (27) | 15 (5) |
Erosive disease is defined as having an erosion score >1 in three separate joints [34].
csDMARDs, HCQ therapy; MTX+, all MTX treatment strategies, including combination therapies, with or without glucocorticoid bridging therapy; No DMARDs, NSAIDs or glucocorticoids.
Clinical outcomes
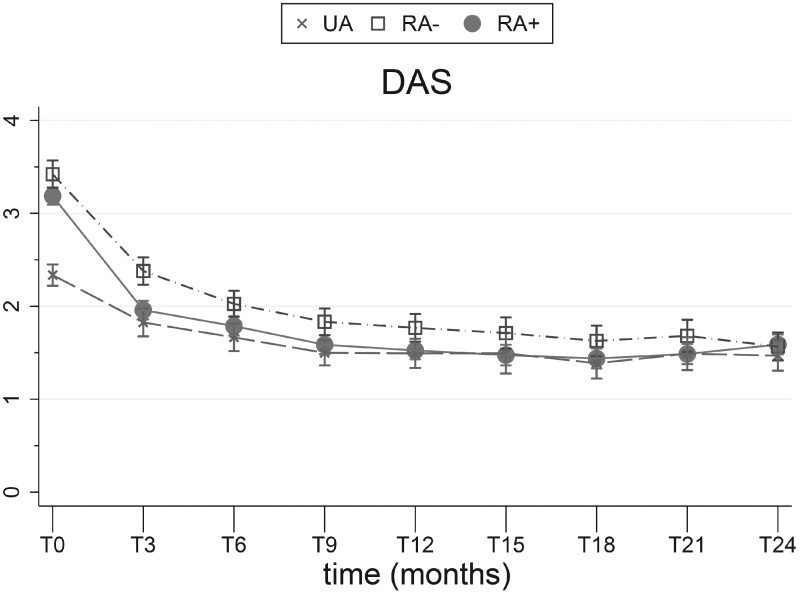
Disease activity was higher in RA patients compared with UA patients, including the 44-joint swollen joint count (SJC44). At baseline, the mean DAS was 2.34 (s.d. 0.67), 3.42 (1.00) and 3.19 (0.87) for UA, RA− and RA+ patients, respectively, while the median SJC44 was 3 [interquartile range (IQR) 2–4], 7 (4–12) and 6 (3–11), respectively (Table 1, Fig. 1). After 2 years the DAS was comparable between all phenotypes (Fig. 1).
Fig. 1.
DAS
Error bars indicate 95% CIs and IQRs for given means.
Quality of life
Quality of life (EQ-5D-3L) did not differ between the phenotypes at baseline and after 2 years (Table 2, Fig. 2A). However, the EQ-5D-3L over time was lower for RA− compared with RA+ patients [β = −0.03 (95% CI −0.04, −0.01), P = 0.033] (Table 3, Fig. 2A).
Table 2.
Difference between phenotypes at baseline and after 2 years
| PROs | Time | UA (n = 130) | RA− (n = 176) | RA+ (n = 331) | ANOVA P-value | Adjusted P-value | Significant difference between subsets | Effect sizes vs MCIDa |
|---|---|---|---|---|---|---|---|---|
| Quality of lifeb | T0 | 0.69 (0.19) | 0.63 (0.24) | 0.64 (0.23) | .039 | 1.27 | ||
| (EQ-5D-3L, MCID ≥0.04) [21] | T24 | 0.80 (0.16) | 0.76 (0.16) | 0.80 (0.14) | 117 | |||
| Functional abilityc | T0 | 0.63 | 1.00 | 0.75 | .000 | .000 | RA−vs UA P = .000 | 0.37 |
| (HAQ-DI, MCID ≥0.22) [22–24] | (0.25-0.88) | (0.63-1.50) | (0.38-1.25) | RA+vs UA P = .000 | 0.12 | |||
| RA+vs RA−P = .003 | −0.25 | |||||||
| T24 | 0.49 | 0.65 | 0.52 | .000 | RA−vs UA P = .000 | 0.16 | ||
| (0.25–0.64) | (0.50–1.03) | (0.30–0.75) | .000 | RA+vs RA−P = .000 | −0.13 | |||
| General healthc | T0 | 39 (25–58) | 52 (34–67) | 50 (30–68) | .001 | .026 | RA−vs UA P = .001 | 13 |
| (VAS, MCID ≥10) [25, 26] | RA+vs UA P = .001 | 11 | ||||||
| T24 | 19 (9–32) | 22 (10–48) | 19 (8–40) | .107 | ||||
| Painc | T0 | 5 (3–7) | 6 (4–8) | 5 (3–8) | .006 | .182 | ||
| (10-point Likert, MCID ≥1) [35] | T24 | 2 (1–4) | 3 (1–5) | 2 (1–3) | .069 | |||
| Morning stiffness durationc | T0 | 1 (1–2) | 2 (1–4) | 2 (1–4) | .000 | .010 | RA−vs UA P = .000 | 1 |
| (7-point Likert) | RA+vs UA P = .018 | 1 | ||||||
| RA+vs. RA−.017 | 0 | |||||||
| T24 | 1 (0–1) | 1 (0–2) | 0 (0–1) | .022 | .726 | |||
| Fatigueb | T0 | 21.0 (7) | 22.6 (7) | 22.0 (7) | .152 | |||
| (FAS, MCID ≥10) [22] | T24 | 19.5 (7) | 21.8 (7) | 20.3 (6) | .023 | .749 | ||
| Anxietyb | T0 | 5.5 (3.6) | 6.0 (3.9) | 5.6 (3.8) | .571 | |||
| (HADS, MCID ≥1.7) [28, 29] | T24 | 4.4 (2.8) | 4.6 (3.5) | 4.0 (2.6) | .142 | |||
| Depressionb | T0 | 4.1 (3.6) | 4.9 (3.8) | 4.5 (3.6) | .168 | |||
| (HADS, MCID ≥1.7) [28, 29] | T24 | 2.9 (2.3) | 3.2 (2.9) | 2.7 (2.3) | .192 | |||
| Health status PCSb | T0 | 43.0 (6) | 39.9 (6) | 40.5 (6) | .000 | .000 | RA−vs UA P = .000 | −3.1 |
| (SF-36, MCID ≥2.5) [25, 30–33] | RA+vs UA P = .000 | −2.5 | ||||||
| T24 | 45.4 (8) | 39.6 (10) | 43.2 (9) | .000 | .000 | RA−vs UA P = .000 | −5.8 | |
| RA+vs UA P = .039 | −2.2 | |||||||
| RA+vs RA−P = .001 | 3.6 | |||||||
| Health status MCSb | T0 | 45.2 (7) | 45.6 (6) | 45.4 (7) | .816 | |||
| (SF-36, MCID ≥5) [25, 30–33] | T24 | 54.2 (9) | 54.7 (9) | 54.8 (8) | .826 | |||
| Productivity loss | T3 | −10% | −7% | −6% | .050 | 1.45 | ||
| (presenteeism, MCID ≥10) [26] | T24 | −5% | −10% | −4% | .044 | |||
Effect sizes in bold exceed the MCID.
Reported mean (s.d.).
Reported median (IQR).
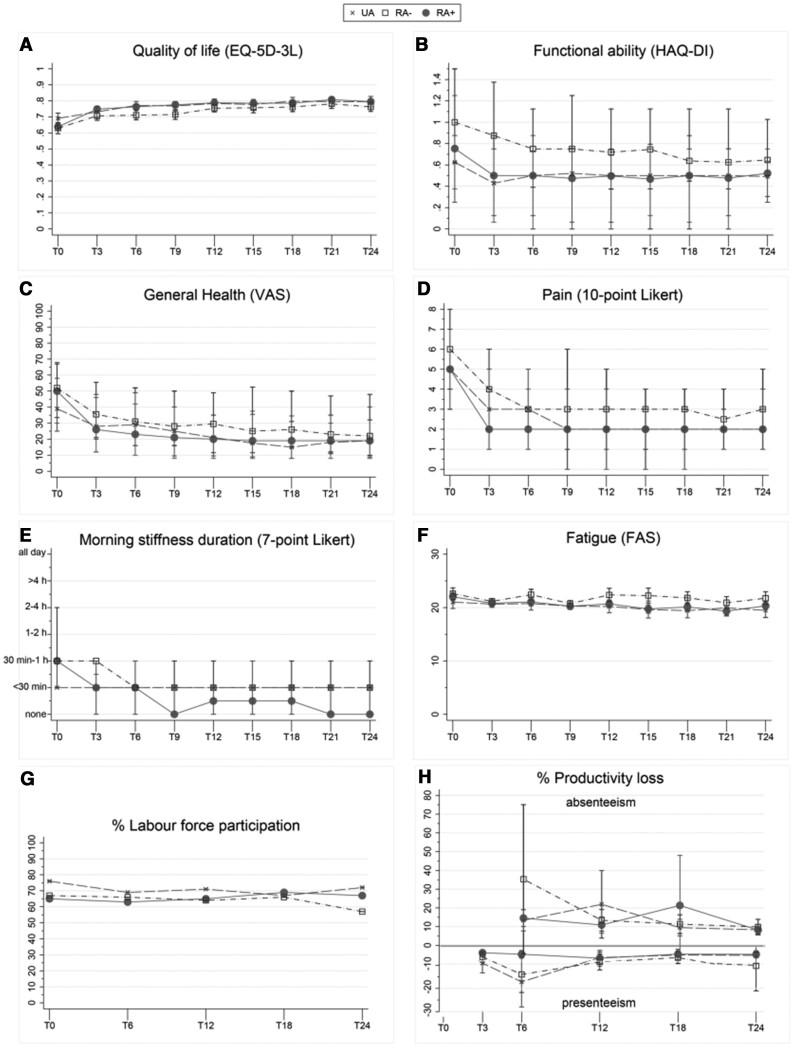
Fig. 2.
PRO measures
Higher scores indicate a higher disease burden, except for quality of life, paid work and presenteeism. For quality of life and fatigue, error bars indicate 95% CIs for given means. For functional ability, GH, pain and morning stiffness, error bars indicate IQRs for given medians. Absenteeism, sick leave occurrence in the last 6 months; presenteeism, working while sick.
Table 3.
PROs over time for all arthritis subsets
| PROs over time | UA (n = 130) |
RA− (n = 176) |
RA+ (n = 331) | ||
|---|---|---|---|---|---|
| β (95% CI) | Adjusted P-value | β (95% CI) | Adjusted P-value | ||
| Quality of life (EQ-5D-3L) | −0.01 (−0.03, 0.01 | −0.03 (−0.04, −0.01) | 0.033 | Ref | |
| Functional ability (HAQ-DI) | 0.11 (0.04, 0.18) | 0.16 (0.11, 0.22) | 0.000 | Ref | |
| General health (VAS) | 2.94 (0.02, 5.85) | 5.66 (3.27, 8.04) | 0.000 | Ref | |
| Pain (10-point Likert) | 0.35 (0.03, 0.67) | 0.51 (0.25, 0.77) | 0.000 | Ref | |
| Morning stiffness duration (7-point Likert) | 0.20 (0.02, 0.39) | 0.29 (0.13, 0.44) | 0.000 | Ref | |
| Fatigue (FAS) | 0.15 (−0.41, 0.71) | 0.60 (0.14, 1.06) | Ref | ||
| Anxiety (HADS) | 0.24 (0.01, 0.46) | 0.23 (0.05, 0.42) | Ref | ||
| Depression (HADS) | 0.17 (−0.05, 0.38) | 0.21 (0.03, 0.38) | Ref | ||
| PCS (SF-36) | −0.76 (−2.10, 0.58) | −1.76 (−2.86, −0.66) | Ref | ||
| MCS (SF-36) | −0.57 (−1.82, 0.67) | −0.90 (−1.92, 0.13) | Ref | ||
| Productivity loss (presenteeism) | −1.46 (−4.15, 1.23) | −3.25 (−5.43, −1.07) | Ref | ||
Functional ability (HAQ-DI)
At baseline and after 2 years, RA− patients had the most functional impairment [at baseline, median 1.00 (IQR 0.63–1.50); after 2 years, 0.65 (0.50–1.03)], followed by RA+ [at baseline, 0.75 (0.38–1.25); after 2 years, 0.52 (0.30–0.75)] and UA [at baseline, 0.63 (0.25–0.88); after 2 years, 0.49 (0.25–0.64)] (Table 2, Fig. 2B). The MCID was only exceeded (≥0.22) at baseline when RA− was compared with UA and RA+ (Table 2). Functional impairment over time was also more severe for RA− compared with RA+ [β = 0.16 (95% CI 0.11, 0.22), P = 0.000] (Table 3, Fig. 2B).
GH
At baseline, GH was worse for RA− [median 52 (IQR 34–67)] and RA+ [50 (30–68)] compared with UA [39 (25–58)], which also exceeded the MCID (≥10; Table 2, Fig. 2C). No significant differences were seen after 2 years (Table 2, Fig. 2C). GH over time was poorer for RA− compared with RA+ patients [β = 5.66 (95% CI 3.27, 8.04), P = 0.000] (Table 3, Fig. 2C).
Pain
No significant differences in pain were seen between all phenotypes at baseline or after 2 years (Table 2, Fig. 2D). Pain over time was more severe for RA− compared with RA+ [β = 0.51 (95% CI 0.25, 0.77), P = 0.000] (Table 3, Fig. 2D).
Morning stiffness duration
No significant differences in morning stiffness duration were seen at baseline or after 2 years (Table 2, Fig. 2E). Morning stiffness duration over time was more severe for RA− compared with RA+ [β = 0.29 (95% CI 0.13, 0.44), P = 0.000] (Tables 2 and 3, Fig. 2E).
Fatigue
No significant differences in fatigue were seen between all phenotypes at baseline, after 2 years or over time (Tables 2 and 3, Fig. 2F).
Anxiety and depression (HADS)
At baseline, a possible anxiety disorder (HADS >7) was seen in 28% (n = 36), 30% (n = 52) and 28% (n = 92) of UA, RA− and RA+ patients, respectively, which decreased to 8% (n = 11), 13% (n = 22) and 6% (n = 21), respectively, after 2 years. In addition, depression (HADS >7) at baseline was seen in 16% (n = 21), 22% (n = 39) and 19% (n = 63) of UA, RA− and RA+ patients, respectively, which decreased to 3% (n = 4), 8% (n = 14) and 3% (n = 10), respectively, after 2 years. Anxiety and depression scores showed no significant differences between all phenotypes at baseline, after 2 years or over time (Tables 2 and 3).
Health status
At baseline, the SF-36 PCS was lower for RA− [mean 39.9 (s.d. 6)] and RA+ [40.5 (6)] compared with UA patients [43.0 (6)]. In addition, after 2 years the PCS was lower for RA− [mean 39.6 (s.d. 10)] and RA+ [43.2 (9)] compared with UA [45.4 (8)] and also for RA− [39.6 (10)] compared with RA+ [43.2 (9)]. MCIDs at baseline and after 2 years were exceeded (≥2.5) when the phenotypes were compared with each other, except for the comparison between RA+ and RA− at baseline and RA+ and UA after 2 years (Table 2). The PCS showed no significant difference between phenotypes over time (Table 3). The MCS showed no significant differences between phenotypes at baseline, after 2 years or over time (Tables 2 and 3).
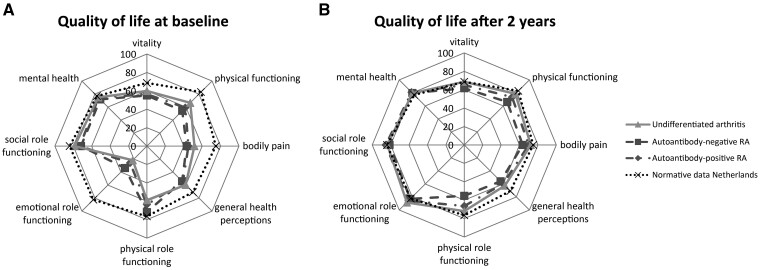
At baseline, all phenotypes had lower scores at each SF-36 domain compared with the general Dutch population norms (Fig. 3A) [36]. After 2 years, the physical components and GH perceptions were still lower compared with the general Dutch population norms, which is irrespective of the phenotype, but they did improve compared with baseline (Fig. 3B) [36].
Fig. 3.
Quality of life radar charts of baseline and 2 year scores across all SF-36 domains
(A) Baseline and (B) 2 year scores of quality of life, measured with the SF-36 domains, for all arthritis subsets compared with general Dutch population norms [34].
Productivity loss
Labour force participation (LFP), which is defined as the number of people currently employed divided by the total working population in the age group between 15 and 75 years, was 76%, 67% and 65% for UA, RA− and RA+ patients, respectively, at baseline. Due to an annual unemployment rate of 2%, 5% and 1%, the LFP rate decreased to 72%, 57% and increased to 67%, respectively, after 2 years (Fig. 2G). Presenteeism, i.e. working while sick, occurred in 10% of UA, 7% of RA− and 6% of RA+ patients after 3 months and changed to 5%, 10% and 4%, respectively, after 2 years (Table 2, Fig. 2H). In the second year of follow-up, 35% (n = 46) of UA, 30% (n = 53) of RA− and 27% (n = 91) of RA+ patients took sick leave with an average duration of 6, 8 and 8 absence days, respectively. No significant differences in productivity loss were seen between the three phenotypes at 3 months, after 2 years or over time (Tables 2 and 3, Fig. 2H).
Active (DAS ≥2.4) vs non-active (DAS <2.4) disease
Overall, 85% of patients were in low disease activity after 2 years of follow-up. All PROs were worse for patients with active disease after 2 years compared with those with low disease activity, except for morning stiffness, productivity loss, fatigue, anxiety and MCS. More importantly, the patients with low disease activity still had poorer PROs compared with the general population. Poorer outcomes were seen in EQ-5D-3L (0.78–0.81 vs ≥0.897), HAQ (0.42–0.63 vs ≤0.25), PCS (41.3–46.5 vs ≥50) and productivity loss (6–14% vs 3.4% (see Supplementary Table S1, available at Rheumatology online).
1987 vs 2010 classification criteria for RA
No significant differences in PROs were seen between patients who fulfilled the 1987 criteria for RA compared with those who fulfilled the 2010 criteria at baseline and after 2 years (see Supplementary Table S2, available at Rheumatology online).
Sensitivity analyses
Our sensitivity analyses comparing the original UA analyses with complete cases and UA patients in DMARD-free remission (DFR) showed similar results, except for functional ability (HAQ) and productivity loss. At baseline the HAQ was a median of 0.63 (IQR 0.25–0.88), 0.50 (0.13–0.88) and 0.50 (0.13–0.88) in the original UA analyses, the complete cases and UA patients in DFR, respectively. After 2 years the HAQ was a median of 0.49 (IQR 0.25–0.64), 0.44 (0.13–1.00) and 0.00 (0.00–0.25), respectively. At 3 months and after 2 years, presenteeism also occurred more often in the original analysis (10% at 3 months and 5% after 2 years) compared with patients who were in DFR (7% at 3 months and 0% after 2 years) (see Supplementary Table S3, available at Rheumatology online).
Discussion
Patient-relevant outcomes are gaining wide acceptance in daily care, but data on their influenceability and differences within three RA phenotypes, i.e. UA, RA− and RA+ patients, are sparse. RA− patients had a higher disease burden compared with the other phenotypes with regard to quality of life, functional ability, GH, pain and morning stiffness. At baseline and after 2 years, RA− patients showed more functional impairment and a poorer PCS compared with the other phenotypes, while they only scored worse for GH and morning stiffness at baseline. For the aforementioned PROs, differences the MCIDs were exceeded at baseline, except for functional ability for RA+ and UA, while after 2 years only the MCID of PCS was exceeded for RA− compared with UA and RA+. After 2 years the PROs of all the phenotypes improved, but PROs measuring functioning were still worse compared with the general population.
This is the first study that compared all recommended PROs in UA, RA− and RA+ [7, 12, 17]. These three phenotypes are based on differences in clinical prognosis, with the best prognosis for UA followed by RA− and RA+ [1, 2]. However, whether these differences also exist from a patient perspective is not well-known. The highest disease burden was found in RA− followed by RA+ and UA patients who had a similar disease burden. Although most arthritis patients, irrespective of phenotype, had low disease activity after treatment, all phenotypes still had a similar significant impact on patients’ lives, mainly visible in outcomes measuring functioning and GH, when compared with the general population [36]. For example, the EQ-5D-3L was 0.69, 0.63 and 0.64 at baseline and 0.80, 0.76 and 0.80 after 2 years for UA, RA− and RA+, respectively, which is lower compared with the general Dutch norm of ≥0.897 and similar to other Dutch RA trials [37–39]. Also, more functional impairment was seen—0.63, 1.00 and 0.75 at baseline and 0.49, 0.65 and 0.52 after 2 years for UA, RA− and RA+, respectively—compared with the normative value of ≤0.25 [40–44]. Even though the tREACH trial used a treat-to-target approach and upholds current guidelines, the PROs were worse compared with the general population even when patients had low disease activity [19, 45].
Although the literature suggests that the prognosis is best for UA followed by RA− and RA+, the disease burden is equal (UA) or even higher (RA−) compared with RA+ [2]. Furthermore, no differences are seen in the DAS after 2 years of follow-up, which does not support the possible difference in clinical prognosis. Unfortunately, we do not have data on radiographic progression after 2 years, but we do have the 1 year data for RA− and RA+, which showed no significant differences in the modified total Sharp score for RA− [median 0 (IQR 0–1.50)] and RA+ [1 (0–3)] [19, 45]. However, we do think that sustained DFR would be the most suitable outcome to validate the possible difference in prognosis between the three clinical phenotypes, but the literature regarding this topic is scarce [46, 47]. Therefore, insights into the difference in (sustained) DFR between the aforementioned clinical phenotypes would be interesting for future research.
Anxiety and depression should also be taken into account at the initiation of treatment, as they are often present at diagnosis and may affect outcomes in the short and long term [48]. Depression may have a negative impact on treatment outcomes and can also become chronic, especially if it takes too long to reach low disease activity [49–51]. Therefore it is important to identify patients with depressive symptoms at an early stage.
As a result of the (chronic) impact of these three phenotypes on patients’ lives, there are also societal consequences [52]. The LFP rate was lower for RA patients (62%) compared with the general population (69%) [53]. The annual unemployment rate was only higher in RA− patients (5% vs 3.4%) [53]. Compared with the general Dutch population, the average number of absence days was only higher in RA patients (8 vs 7 days) [54]. Due to the lower LFP rate at baseline and higher sick leave days during follow-up, it is of the utmost importance to recognize and treat (persistent) arthritis as early as possible and to strive for remission within 6 months, because both are associated with sustained worker productivity [55–57].
One of the strengths of our study is the extensive PRO data, which is also a limitation because of multiple testing. However, we applied a Bonferroni correction to handle multiple testing and also compared effect sizes with known MCIDs from the literature [21–23, 25, 27–29, 33]. Furthermore, PRO domain differences were consistent between the different measures and no contradictory results were found. Our results are also comparable to previous literature. However, previous literature did not include all three phenotypes, nor did it address all domains [7, 12].
A limitation of measuring PROs could be that these self-reported outcomes might be susceptible to recall bias and non-response and are therefore difficult to interpret on a population level. In our cohort, the dropout rates were higher for UA [31% (40/130)] compared with RA− [27% (48/176)] and RA+ [24% (81/331)]. Also, the reason for dropout might not be the same for each group. For example, UA patients might drop out due to inactive disease, while RA− patients might drop out because they are unsatisfied with their treatment due to chronic disability despite low disease activity. If dropout reasons between groups were indeed skewed, then this might lead to worse PROs in especially the UA group. Therefore we performed sensitivity analyses in which we compared UA patients who were in DFR and those with complete data with the other phenotypes. Similar results were seen, except for functional ability and productivity loss, which were worse in our original analysis compared with the sensitivity analyses. This implies a slightly lower disease burden on these domains for patients who dropped out. However, we still think our results are valid because only small differences were seen in two domains and functional ability scores were still in the same 95% CIs.
To conclude, RA− patients had the highest disease burden when compared with the other phenotypes (i.e. RA+ and UA patients). Although most arthritis patients, irrespective of the clinical phenotype, had low disease activity after treatment, all phenotypes still had a similar significant impact on patients’ lives, mainly visible in the outcomes measuring functioning and GH, when compared with the general population. Therefore it is important to assess and address patient-relevant outcomes in daily practice because patients still have a continuous high disease burden despite reaching low disease activity.
Supplementary Material
Acknowledgements
We would like to thank the participating patients in the tREACH trial for their willingness to contribute to the study and for their cooperation. Furthermore, we would like to thank all rheumatologists from the following participating centres: Erasmus MC, Rotterdam; Maasstad ziekenhuis, Rotterdam; Sint Fransiscus Gasthuis & Vlietland, Rotterdam and Schiedam; Albert Schweitzer ziekenhuis, Dordrecht; Admiraal de Ruyter ziekenhuis, Goes en Vlissingen; and Zorgsaam ziekenhuis, Terneuzen. We also thank all study nurses, laboratory personnel, co-investigators and other persons who were involved with the tREACH trial. All authors contributed to the conception or design of the trial; the acquisition, analysis or interpretation of data for the work; drafting or revision of the manuscript; and final approval of the manuscript for publication. All authors contributed to refinement of the article.
Funding: The tREACH trial was supported by an unrestricted grant from Pfizer (WI229707). Pfizer had no involvement in the study design; the collection, analysis and interpretation of data; writing of the report; and the decision to submit for publication. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication. Data management was sponsored by the Dutch Arthritis Society (16-3-101).
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Combe B, Landewe R, Daien CI. et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017;76:948–59. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewe RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 3. Ajeganova S, Huizinga TW.. Rheumatoid arthritis: seronegative and seropositive RA: alike but different? Nat Rev Rheumatol 2015;11:8–9. [DOI] [PubMed] [Google Scholar]
- 4. Akdemir G, Verheul MK, Heimans L. et al. Predictive factors of radiological progression after 2 years of remission-steered treatment in early arthritis patients: a post hoc analysis of the IMPROVED study. RMD Open 2016;2:e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi ST, Lee KH.. Clinical management of seronegative and seropositive rheumatoid arthritis: a comparative study. PLoS One 2018;13:e0195550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farragher TM, Goodson NJ, Naseem H. et al. Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum 2008;58:359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nordberg LB, Lillegraven S, Lie E. et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD-naive patients classified according to the 2010 ACR/EULAR criteria. Ann Rheum Dis 2017;76:341–5. [DOI] [PubMed] [Google Scholar]
- 8. Gossec L. Patient-reported outcomes in rheumatoid arthritis: why are they important and how should they be assessed? Turk J Rheumatol 2010;25:99–104. [Google Scholar]
- 9. Kalyoncu U, Dougados M, Daures JP, Gossec L.. Reporting of patient-reported outcomes in recent trials in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2009;68:183–90. [DOI] [PubMed] [Google Scholar]
- 10. Oude Voshaar MAH, Das Gupta Z, Bijlsma JWJ. et al. The International Consortium for Health Outcome Measurement (ICHOM) set of outcomes that matter to people living with inflammatory arthritis: consensus from an international working group. Arthritis Care Res (Hoboken) 2019;71:1556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heller JE, Shadick NA.. Outcomes in rheumatoid arthritis: incorporating the patient perspective. Curr Opin Rheumatol 2007;19:101–5. [DOI] [PubMed] [Google Scholar]
- 12. Boer AC, Boonen A, van der Helm van Mil AHM.. Is anti-citrullinated protein antibody-positive rheumatoid arthritis still a more severe disease than anti-citrullinated protein antibody-negative rheumatoid arthritis? A longitudinal cohort study in rheumatoid arthritis patients diagnosed from 2000 onward. Arthritis Care Res (Hoboken) 2018;70:987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Consortium for Health Outcomes Measurement. Inflammatory Arthritis Data Collection Reference Guide. https://ichom.org/files/medical-conditions/inflammatory-arthritis/inflammatory-arthritis-reference-guide.pdf (3 August 2020, date last accessed).
- 14. van Tuyl LH, Boers M.. Patient-reported outcomes in core domain sets for rheumatic diseases. Nat Rev Rheumatol 2015;11:705–12. [DOI] [PubMed] [Google Scholar]
- 15. Hiligsmann M, Rademacher S, Kaal KJ, Bansback N, Harrison M.. The use of routinely collected patient-reported outcome measures in rheumatoid arthritis. Semin Arthritis Rheum 2018;48:357–66. [DOI] [PubMed] [Google Scholar]
- 16. Kilic L, Erden A, Bingham CO, Gossec L, Kalyoncu U.. The reporting of patient-reported outcomes in studies of patients with rheumatoid arthritis: a systematic review of 250 articles. J Rheumatol 2016;43:1300–5. [DOI] [PubMed] [Google Scholar]
- 17. Nordberg LB, Lillegraven S, Aga AB. et al. Comparing the disease course of patients with seronegative and seropositive rheumatoid arthritis fulfilling the 2010 ACR/EULAR classification criteria in a treat-to-target setting: 2-year data from the ARCTIC trial. RMD Open 2018;4:e000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Claessen SJ, Hazes JM, Huisman MA. et al. Use of risk stratification to target therapies in patients with recent onset arthritis; design of a prospective randomized multicenter controlled trial. BMC Musculoskelet Disord 2009;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Jong PH, Hazes JM, Han HK. et al. Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann Rheum Dis 2014;73:1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Heijde DM, van ’t Hof M, van Riel PL, van de Putte LB.. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20:579–81. [PubMed] [Google Scholar]
- 21. Luo N, Johnson J, Coons SJ.. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care 2010;48:365–71. [DOI] [PubMed] [Google Scholar]
- 22. Hazes JM, Taylor P, Strand V. et al. Physical function improvements and relief from fatigue and pain are associated with increased productivity at work and at home in rheumatoid arthritis patients treated with certolizumab pegol. Rheumatology (Oxford) 2010;49:1900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Redelmeier DA, Lorig K.. Assessing the clinical importance of symptomatic improvements. An illustration in rheumatology. Arch Intern Med 1993;153:1337–42. [PubMed] [Google Scholar]
- 24. Siegert CEH, Vleming L-J, Van-Denbroucke JP, Cats A.. Measurement of disability in Dutch rheumatoid arthritis patients. Clin Rheumatol 1984;3:305–9. [DOI] [PubMed] [Google Scholar]
- 25. Strand V, Gossec L, Proudfoot CWJ. et al. Patient-reported outcomes from a randomized phase III trial of sarilumab monotherapy versus adalimumab monotherapy in patients with rheumatoid arthritis. Arthritis Res Ther 2018;20:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nikiphorou E, Radner H, Chatzidionysiou K. et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther 2016;18:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson JK, Zimmerman L, Caplan L, Michaud K.. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S14–36. [DOI] [PubMed] [Google Scholar]
- 28. Lemay KR, Tulloch HE, Pipe AL, Reed JL.. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev 2019;39:E6–E11. [DOI] [PubMed] [Google Scholar]
- 29. Puhan MA, Frey M, Buchi S, Schunemann HJ.. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 31. Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A.. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997;36:551–9. [DOI] [PubMed] [Google Scholar]
- 32. Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 33. Smolen JS, Emery P, Ferraccioli GF. et al. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomised, placebo-controlled trial. Ann Rheum Dis 2015;74:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Heijde D, van der Helm-van Mil AH, Aletaha D. et al. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria. Ann Rheum Dis 2013;72:479–81. [DOI] [PubMed] [Google Scholar]
- 35. Strand V, van Vollenhoven RF, Lee EB. et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology 2016;55:1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aaronson NK, Muller M, Cohen PDA. et al. Translation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations. J Clin Epidemiol 1998;51:1055–68. [DOI] [PubMed] [Google Scholar]
- 37. Versteegh MM, Vermeulen KM, Evers SMAA. et al. Dutch tariff for the five-level version of EQ-5D. Value Health 2016;19:343–52. [DOI] [PubMed] [Google Scholar]
- 38. ter Wee MM, Coupe VM, den Uyl D. et al. Cost-utility of COBRA-light versus COBRA therapy in patients with early rheumatoid arthritis: the COBRA-light trial. RMD Open 2017;3:e000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Hout WB, Goekoop-Ruiterman YP, Allaart CF. et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:291–9. [DOI] [PubMed] [Google Scholar]
- 40. Krishnan E, Sokka T, Häkkinen A, Hubert H, Hannonen P.. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum 2004;50:953–60. [DOI] [PubMed] [Google Scholar]
- 41. Strand V, Schiff M, Tundia N. et al. Effects of upadacitinib on patient-reported outcomes: results from SELECT-BEYOND, a phase 3 randomized trial in patients with rheumatoid arthritis and inadequate responses to biologic disease-modifying antirheumatic drugs. Arthritis Res Ther 2019;21:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heimans L, Akdemir G, Boer KV. et al. Two-year results of disease activity score (DAS)-remission-steered treatment strategies aiming at drug-free remission in early arthritis patients (the IMPROVED study). Arthritis Res Ther 2016;18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Konijn NPC, van Tuyl LHD, Boers M. et al. Similar efficacy and safety of initial COBRA-light and COBRA therapy in rheumatoid arthritis: 4-year results from the COBRA-light trial. Rheumatology (Oxford) 2017;56:1586–96. [DOI] [PubMed] [Google Scholar]
- 44. van Dongen H, van Aken J, Lard LR. et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2007;56:1424–32. [DOI] [PubMed] [Google Scholar]
- 45. Luurssen-Masurel N, Weel AEAM, Hazes JMW, de Jong PHP.. Towards stratified treatment of rheumatoid arthritis. Int J Clin Rheumatol 2020;15:73–82. [Google Scholar]
- 46. Verstappen M, van Mulligen E, de Jong PHP, van der Helm-Van Mil AHM.. DMARD-free remission as novel treatment target in rheumatoid arthritis: a systematic literature review of achievability and sustainability. RMD Open 2020;6:e001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ajeganova S, Huizinga T.. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis 2017;9:249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matcham F, Norton S, Scott DL, Steer S, Hotopf M.. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology (Oxford) 2016;55:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hider SL, Tanveer W, Brownfield A, Mattey DL, Packham JC.. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology (Oxford) 2009;48:1152–4. [DOI] [PubMed] [Google Scholar]
- 50. Kekow J, Moots R, Khandker R. et al. Improvements in patient-reported outcomes, symptoms of depression and anxiety, and their association with clinical remission among patients with moderate-to-severe active early rheumatoid arthritis. Rheumatology (Oxford) 2011;50:401–9. [DOI] [PubMed] [Google Scholar]
- 51. Margaretten M, Julian L, Katz P, Yelin E.. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumatol 2011;6:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burton W, Morrison A, Maclean R, Ruderman E.. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occup Med (Lond) 2006;56:18–27. [DOI] [PubMed] [Google Scholar]
- 53.Volksgezondheidenzorg.info. Participatie cijfers en context arbeidsparticipatie. https://www.volksgezondheidenzorg.info/onderwerp/participatie/cijfers-context/arbeidsparticipatie#node-werkloosheid-naar-leeftijd-en-geslacht (3 August 2020, date last accessed).
- 54.Volksgezondheidenzorg.info. Ziekteverzuim cijfers en context huidige situatie. https://www.volksgezondheidenzorg.info/onderwerp/ziekteverzuim/cijfers-context/huidige-situatie#node-verzuimduur-naar-leeftijd-en-geslacht (3 August 2020, date last accessed).
- 55. van Vilsteren M, Boot CRL, Knol DL. et al. Productivity at work and quality of life in patients with rheumatoid arthritis. BMC Musculoskelet Disorders 2015;16:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gignac MA, Cao X, Lacaille D, Anis AH, Badley EM.. Arthritis-related work transitions: a prospective analysis of reported productivity losses, work changes, and leaving the labor force. Arthritis Rheum 2008;59:1805–13. [DOI] [PubMed] [Google Scholar]
- 57. Kim D, Kaneko Y, Takeuchi T.. Importance of obtaining remission for work productivity and activity of patients with rheumatoid arthritis. J Rheumatol 2017;44:1112–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.