To the editor:
Several of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines use an mRNA lipid nanoparticle-encapsulated platform. In experimental models, the induced antibody titers are higher, and T- and B-cell responses are enhanced, compared to the level with traditional vaccines. Possibly, due to this higher efficacy, more and more kidney-specific side effects of mRNA vaccines related to immune-mediated glomerular disease are being reported.1, 2, 3 Because of similar type I interferon and proinflammatory cytokine pathways in systemic lupus erythematodes and coronavirus disease 2019 (COVID-19), either potentiated or dysregulated immune responses to SARS-CoV-2 vaccines can be suspected to trigger disease activity in previously stable lupus.4
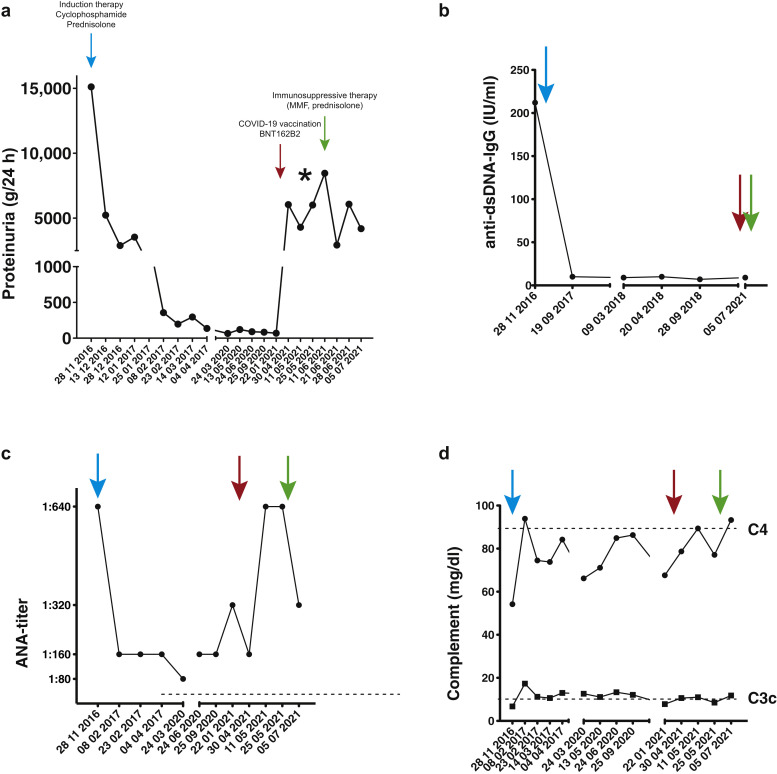
We present the case of a 42-year-old female patient who was diagnosed with lupus nephritis class V in 2016 on renal biopsy (Figure 1 g and h) after she developed typical butterfly rash and nephrotic syndrome with proteinuria of 6 g/d (Figure 2 a). At diagnosis, anti-dsDNA-IgG levels were 210 IU/ml, the antinuclear antibody (ANA) titer result was 1:640, and both complement C3 and C4 were slightly reduced (Figure 2b–d). Renal function was not impaired in 2016. She received induction treatment with cyclophosphamide and steroids and went into full remission (Figure 2a). Maintenance therapy consisted of hydroxychloroquine 200 mg qd until 2021. On April 21, 2021, she received the first dose of the COVID-19 mRNA vaccine BNT162b2, which she tolerated well. One week later, the proteinuria increased from 0.07 g/d to 6 g/d, and 5 weeks later it increased to 8.4 g/d (Figure 2a). She developed nephrotic syndrome with hyperlipoproteinemia and hypalbuminemia. Renal function was again not impaired. There were no other obvious triggers present. Complement was slightly below normal, as it had been in previous analyses (Figure 2d). ANA titers increased to 1:640, but there was no spike of anti-dsDNA-IgG (Figure 2b and c). Hematuria and skin abnormalities were absent, but she was feeling fatigued and complained about an unspecific weakness. Renal biopsy (Figure 1a–f) demonstrated lupus nephritis class V and II with slight focal and segmental mesangial hypercellularity (up to 5 cells per mesangial field) without any irregularities of the basement membranes (no spikes or double contours). The modified activity score (International Society of Nephrology/Renal Pathology Society/National Institutes of Health [ISN/RPS/NIH]) was 0/24, and the chronicity score was 0/12.5 Spontaneous remission did not occur in follow-up measurements, and as nephrotic proteinuria persisted for 7 weeks, we initiated immunosuppressive therapy with mycophenolate mofetil (1 g bid) and prednisolone (60 mg qd). As can be seen in Figure 2a, proteinuria declined initially, and the patient reported substantial improvement of her general well-being and the absence of foamy urine. Proteinuria increased again the following week, but with a tendency toward improvement of the absolute amount in the next measurements. ANA titers, which increased after vaccination, also declined after the start of therapy. Anti-DNA-antibody levels did not increase after the vaccination, and the slightly-below-normal C3c-levels increased (Figure 2b–d).
Figure 1.
Representative micrographs showing normal glomeruli by conventional histology (periodic acid–Schiff reaction) except for (a) slight focal and segmental mesangial hypercellularity and (b) granular immunoreactivity for IgG and (c) C3c along glomerular capillary walls and withinthemesangium. (d–h) Ultrastructure (contrasted with osmium tetroxide and tannic acid) reveals endothelial tubuloreticular inclusions (black arrows) and global subepithelial electron-dense immune deposits (white arrows) amounting to >80% of all capillary loops as well as moderate (40%) loss of podocyte foot processes. (g,h) First biopsy from 2016, for comparison. (a) Periodic acid–Schiff reaction; (b,c) DAB immunohistochemistry. (a–c) Original magnification ×400. Bar = 50 μm. Ultrastructure: bars = (d) 100 nm, (e,g) 500 nm, and (f,h) 1000 nm. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Figure 2.
(a) Time course of proteinuria with end of induction therapy (blue arrow), coronavirus disease 2019 (COVID-19) vaccination (red arrow), and start of immunosuppressive therapy (green arrow). ∗Time of biopsy and detection of spike-specific IgG antibody after the first vaccination with BNT162b2. Serological parameters (b) anti-dsDNA-IgG, (c) antinuclear antibody (ANA), and (d) complement C3c and C4 are plotted in relation to induction therapy, 2016 (blue); vaccination (red); and start of immunosuppression (green). Dotted lines mark normal limits. Note that the dates are different in the graphs because values were not obtained at each visit. MMF, mycophenolate mofetil.
The patient had already developed an antibody response against the spike protein of SARS-CoV-2 (Figure 2); thus, we decided to postpone the second vaccination in light of declining incidence numbers. When to proceed with the second vaccination remains to be determined, as full remission of the proteinuria has not been achieved yet.
To our knowledge, our case report is the first to describe a biopsy-proven relapse of lupus nephritis class V and II. New-onset minimal change glomerulopathies3 , 6 and other forms of glomerulonephritis (e.g., de novo IgAN,2 relapse IgA nephropathy,1 and even anti–glomerular basement membrane glomerulonephritis)7 have been described as sequelae of mRNA COVID-19 vaccination. This case adds yet another piece of evidence that relapse in immune-mediated disease might be induced by COVID-19 mRNA vaccine. Although the mechanisms triggering these relapses are still elusive, stringent postvaccination surveillance for renal function, proteinuria, and serologic markers for immune disease is essential in this vulnerable patient population.
References
- 1.Negrea L., Rovin B.H. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021;99:1487. doi: 10.1016/j.kint.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan H.Z., Tan R.Y., Choo J.C.J. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100:469–471. doi: 10.1016/j.kint.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebedev L., Sapojnikov M., Wechsler A. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang W., Askanase A.D., Khalili L., Merrill J.T. SARS-CoV-2 vaccines in patients with SLE. Lupus Sci Med. 2021;8 doi: 10.1136/lupus-2021-000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajema I.M., Wilhelmus S., Alpers C.E. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Maas R.J., Gianotten S., van der Meijden W.A.G. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:312. doi: 10.1053/j.ajkd.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacker A., Kung V., Andeen N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021;100:471–472. doi: 10.1016/j.kint.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]