Abstract
Background & aims
Indirect calorimetry (IC) is the gold-standard for determining measured resting energy expenditure (mREE) in critical illness. When IC is not available, predicted resting energy expenditure (pREE) equations are commonly utilized, which often inaccurately predict metabolic demands leading to over- or under-feeding. This study aims to longitudinally assess mREE via IC in critically ill patients with SARS-CoV-2 (COVID-19) infection throughout the entirety of, often prolonged, intensive care unit (ICU) stays and compare mREE to commonly utilized pREE equations.
Methods
This single-center prospective cohort study of 38 mechanically ventilated COVID-19 patients from April 1, 2020 to February 1, 2021. The Q-NRG® Metabolic Monitor was used to obtain IC data. The Harris-Benedict (HB), Mifflin St-Jeor (MSJ), Penn State University (PSU), and weight-based equations from the American Society of Parenteral and Enteral Nutrition – Society of Critical Care Medicine (ASPEN-SCCM) Clinical Guidelines were utilized to assess the accuracy of common pREE equations and their ability to predict hypo/hypermetabolism in COVID-19 ICU patients.
Results
The IC measures collected revealed a relatively normometabolic or minimally hypermetabolic mREE at 21.3 kcal/kg/d or 110% of predicted by the HB equation over the first week of mechanical ventilation (MV). This progressed to significant and uniquely prolonged hypermetabolism over successive weeks to 28.1 kcal/kg/d or 143% of HB predicted by MV week 3, with hypermetabolism persisting to MV week 7. Obese individuals displayed a more truncated response with significantly lower mREE versus non-obese patients in MV week 1 (19.5 ± 1.0 kcal/kg/d vs 25.1 ± 1.8 kcal/kg/d, respectively; p < 0.01), with little change in weeks 2–3 (19.5 ± 1.5 kcal/kg/d vs 28.0 ± 2.0 kcal/kg/d; p < 0.01). Both ASPEN-SCCM upper range and PSU pREE equations provided close approximations of mREE yet, like all pREE equations, occasionally over- and under-predicted energy needs and typically did not predict late hypermetabolism.
Conclusions
Study results show a truly unique metabolic response in COVID-19 ICU patients, characterized by significant and prolonged, progressive hypermetabolism peaking at 3 weeks’ post-intubation, persisting for up to 7 weeks in ICU. This pattern was more clearly demonstrated in non-obese versus obese patients. This response is unique and distinct from any previously described model of ICU stress response in its prolonged hypermetabolic nature. This data reaffirms the need for routine, longitudinal IC measures to provide accurate energy targets in COVID-19 ICU patients. The PSU and ASPEN-SCCM equations appear to yield the most reasonable estimation to IC-derived mREE in COVID-19 ICU patients, yet still often over-/under-predict energy needs. These findings provide a practical guide for caloric prescription in COVID-19 ICU patients in the absence of IC.
Keywords: Critical care, Intensive care unit, Nutrition status, Indirect calorimetry, SARS-CoV-2, Energy expenditure
1. Introduction
Currently, limited data exist for understanding and determining the energy needs of critically ill, mechanically ventilated, SARS-CoV-2 (COVID-19) infected patients. The few studies published suggest an overall initial normometabolic state that may subsequently transition to significant hypermetabolism following intubation when measured by indirect calorimetry (IC) [[1], [2], [3]]. This paper extends the findings of previously reported data to 7 weeks post mechanical ventilation (MV) initiation examining the metabolic response to COVID-19 critical illness and providing novel and urgently needed comparisons between multiple predicted resting energy expenditure (pREE) equations used routinely in clinical practice [2]. IC remains the gold-standard for obtaining measured resting energy expenditure (mREE) and is the recommended means to determine energy requirements in critically ill patients per multiple societal guidelines, including in COVID-19 patients [[4], [5], [6], [7]]. However, the routine use of IC in clinical practice has traditionally come with inherent limitations, including time and clinical resource constraints and concerns surrounding the accuracy, inconvenience, and challenge of obtaining measurements [[8], [9], [10], [11]]. Furthermore, obtaining IC measurements in the COVID-19 patient population is more complex given their tenuous respiratory status, while infection control precautions provide additional restrictions, limiting staff exposure to these patients and reducing aerosolization procedures [7,12,13].
Because of these limitations, registered dietitians (RDs) and other critical care providers need practical tools for estimating the REE in critically ill COVID-19 patients. However, commonly used pREE equations have historically demonstrated inaccuracies in non-COVID-19 intensive care unit (ICU) patients versus IC-derived mREE [[14], [15], [16], [17]]. Given the inaccuracies of pREE equations, all critically ill patients, including critically ill COVID-19 patients, are at greater risk for under- and over-feeding, both of which have been associated with increased mortality, hospital length of stay (LOS), and complication rates [18]. To address the challenges of accurately estimating REE, the International Multicentric Study Group for Indirect Calorimetry championed a project leading to the development of an accurate, affordable, reliable, and user-friendly IC to measure REE in hospitalized patients [19]. The Q-NRG® Metabolic Monitor (Q-NRG) is a newly defined IC device that provides highly accurate measurements of REE, developed by a process where gas exchange simulations were tested and validated against mass spectrometry gas analysis [19]. The Q-NRG device is now United States Food and Drug Administration approved and available worldwide [20].
Utilizing the Q-NRG device, we undertook a study to better understand and describe the metabolic response to COVID-19 in critically ill patients requiring MV for up to 7 weeks. Further, we compared the metabolic response to COVID-19 to previously described models of the metabolic stress response to injury. Finally, we evaluated the accuracy of pREE equations versus IC-derived mREE to provide clinicians with practical guidance for the caloric prescription if longitudinal IC measures are not available throughout ICU LOS.
2. Materials & methods
2.1. Study population and design
The Longitudinal Energy Expenditure and Metabolic Effects in Patients with COVID-19 (LEEP-COVID) study is a prospective longitudinal cohort study of critically ill, adult patients infected by the SARS-CoV-2 virus, beginning on April 1, 2020. Patients with COVID-19 disease, ≥18 years of age, admitted to a Duke University Medical Center ICU, and already receiving or expected to require MV for >48 h were included in this study. Patients were excluded if their expected duration of ICU LOS or survival was <24 h or if they had an implantable cardiac device (pacemaker, defibrillator, etc.). This study was approved by the Duke Health Institutional Review Board in Durham, North Carolina, and patients were enrolled after obtaining a waiver of informed consent.
2.2. Measurements and study variables
Demographic and anthropometric data were collected at the time of ICU admission while clinical and medication data were collected upon hospital discharge. mREE data were obtained using the Q-NRG® Metabolic Monitor (COSMED Rome, Italy). Patients were temporarily excluded from IC assessment under the following conditions: FiO2 >70%, hemodynamic instability, positive end-expiratory pressure (PEEP) > 16 mmHg, on active venovenous extracorporeal membrane oxygenation (VV ECMO), or per ICU attending clinical judgment. IC data were selected from 10 to 30 min intervals which met steady-state conditions, defined by a variance of V02 and VC02 by <10% as per published validation data for the Q-NRG device [19]. Measurements not meeting these criteria were excluded from the final analysis. All values for mREE collected were categorized into four time periods based on the days following initiation of MV: MV week 1 (days 1–7), MV week 2 (days 8–14), MV week 3 (days 15–21), and MV weeks 4–7 (days 22–49). MV weeks 4–7 were combined for analysis due to a lower sample size. Patients with multiple IC measurements were averaged over weekly intervals. IC measurements were collected only during the period of MV and were not collected on some days or weeks if patients developed IC assessment exclusion criteria (i.e. FiO2 requirement >70%). IC assessments were no longer performed when patients no longer required MV or expired.
Calculations of pREE were performed on the day of IC measurement and multiple, weekly pREE values were averaged. Four commonly utilized pREE equations were chosen for comparative analysis to IC measurements: Harris-Benedict (HB), Mifflin St. Jeor (MSJ), Penn State University Equations (PSU 2003b and PSU 2010), and the American Society of Parenteral and Enteral Nutrition - Society of Critical Care Medicine (ASPEN-SCCM) Clinical Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient (Table 1 ) [5,14,[21], [22], [23]]. Anthropometric and clinical data used for pREE equations were correlated with admission height and weight recorded in the medical record, the maximum temperature (Tmax) on the day of IC measurement, and the minute ventilation (VE) recorded at the time of IC testing. All pREE calculations were performed according to patient sex, age, and body mass index (BMI) if applicable as displayed in Table 1. For the purposes of this study, ASPEN-SCCM Clinical Guidelines calculations were performed with both the lower- and upper-end of ranges of the ASPEN-SCCM weight-based equations to compare to IC-derived mREE [5]. The lower-end of these equations was used given closer correlation with the conditions by which pREE is calculated from in the HB and MSJ equations. The upper-end of the range of the weight-based equations was used to compare to the IC-derived mREE given the preliminary hypothesized hypermetabolic response in COVID-19 [[1], [2], [3]].
Table 1.
Predictive energy equations.
| Mifflin St. Jeor (MSJ) |
| Men: RMR = 10 x weight + 6.25 x height – 5 x age + 5 |
| Womena: RMR = 10 x weight + 6.25 x height – 5 x age – 161 |
| Harris Benedict (HB) |
| Men: RMR = 66.47 + 13.75 x weight + 5.0 x height – 6.75 x age |
| Women: RMR = 665.09 + 9.56 x weight + 1.84 x height – 4.67 x age |
| ASPEN/SCCM |
| ASPEN/SCCM Lower-End of Range |
| BMI <30 kg/m2: RMR = 25 kcal/kg x admission weight |
| BMI 30–50 kg/m2: RMR = 11 kcal/kg/0.65 = 16.9 kcal/kg/d x admission weight |
| BMI >50 kg/m2: RMR = 22 kcal/kg/0.65 x IBW |
| ASPEN/SCCM Upper-End of Range |
| BMI <30 kg/m2: RMR = 30 kcal/kg x admission weight |
| BMI 30–50 kg/m2: RMR = 14 kcal/kg/0.65 = 21.5 kcal/kg/d x admission weight |
| BMI >50 kg/m2: RMR = 25 kcal/kg/0.65 x IBW |
| Penn State (PSU) |
| Penn State 2003 b |
| RMR = MSJ x 0.96 + VE x 31 + Tmax x 167 - 6212 |
| Penn State 2010 (for BMI >30 kg/m2and > 60 years of age) |
| RMR = MSJ x 0.71 + VE x 64 + Tmax x 85–3085 |
pREE – Predicted Resting Energy Expenditure; RMR – Resting Metabolic Rate; ASPEN – American Society of Parenteral and Enteral Nutrition; SCCM – Society of Critical Care Medicine; IBW – Ideal Body Weight – measured by the Hamwi equation; VE – minute ventilation; Tmax – maximum temperature in past 24 h.
All units of measure are as follows: height – cm; weight – kg; age – years; VE – L/min; Tmax - °C.
Use sex-specific MSJ equations.
2.3. Statistical analysis
Descriptive statistics were used to report demographic, anthropometric, clinical, and medication data. Final kcal/kg/d data were calculated by dividing pREE or mREE kcals by kg of admission weight. Bland–Altman plots were used to display differences between methods for determining REE by MV week. Y-axes display differences between methods measured in kcal/kg/d while X-axes depict mean REE [24]. Two-sided t-tests were used to analyze differences between IC-derived mREE by MV week and mREE and pREE for all predictive energy equations among obese and non-obese groups. MV weeks 2 and 3 were combined to avoid statistical error due to a low sample size when comparing obese and non-obese groups. Data are reported as mean ± standard error of the mean (SEM), and a 2-sided p-value of <0.05 determined significance.
3. Results
Data from a total of 38 patients from April 1, 2020, to February 1, 2021, were included in the final analysis. Over a maximum 7-week time period during which patients required MV, patients were lost to follow-up due to either death (26%) or to being weaned from MV (63%). The majority of patients were male (61%), Black (47%), non-Hispanic (71%), and obese (BMI > 30 kg/m2) (58%). Upon admission, the median age was 61 years old and mean BMI was 31.8 ± 1.4 kg/m2. On average, patients spent 20.3 ± 2.8 days on MV while ICU LOS was 25.2 ± 2.8 days. Common COVID-19 treatments in this population are described in Table 2 .
Table 2.
Demographics, anthropometrics, and clinical data in COVID-19 ICU patients.
| (a) Demographics (n = 38) | |
| Age – years, median (range) | 61 (25–88) |
| Male sex, n (%) | 23 (61%) |
| Race, n (%) | |
| Black | 18 (47%) |
| White | 7 (18%) |
| Other | 13 (34%) |
| Ethnicity, n (%) | |
| Hispanic | 11 (29%) |
| (b) Anthropometrics (n = 38) | |
| Weight - kg | 88.1 ± 4.0 |
| BMI - kg/m2 | 31.8 ± 1.4 |
| Obese, n (%) | 22 (58%) |
| (c) Clinical Data (n = 37)a | |
| Duration of MV - days | 20.3 ± 2.8 |
| LOS - days | 33.7 ± 3.8 |
| ICU LOS - days | 25.2 ± 2.8 |
| Hospital Mortality (n, %) | 12 (32%) |
| (d) Medication data (n = 38) | |
| Remdesivir, n (%) | 30 (79%) |
| Insulin, n (%) | 29 (76%) |
| Steroids, n (%) | 24 (63%) |
Data are means ± SEM unless otherwise indicated.
LOV – Length on Ventilator; LOS – Length of Stay; ICU – Intensive Care Unit; SEM – Standard Error of Mean.
1 patient remains admitted.
3.1. Measured resting energy expenditure via indirect calorimetry
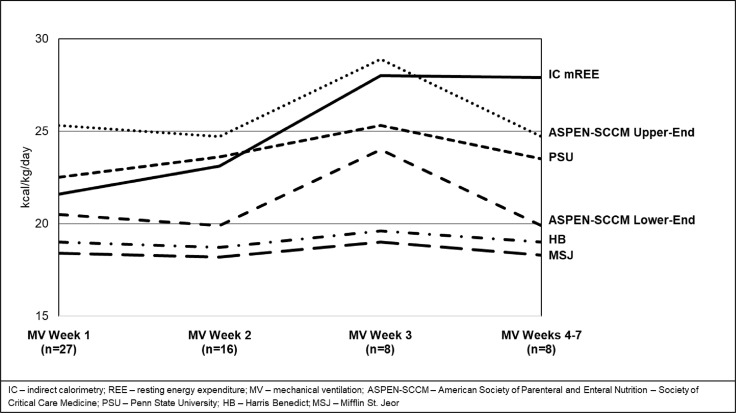
A total of 97 IC measurements collected during MV weeks 1–7 produced an average mREE of 23.2 ± 1.0 kcal/kg/d. Among all patients, the average mREE increased by 6.4 kcal/kg/d between MV weeks 1 and 3. In MV weeks 4–7, IC measurements averaged 27.9 ± 2.1 kcal/kg/d. The curve created by sequential mREE values demonstrates a truly unique and prolonged pattern of metabolic response to stress and critical illness versus historically described patterns in ICU and trauma patients [25]. Using a predictive equation as a reference point, mREE in MV week 1 appeared to be relatively normometabolic or minimally hypermetabolic at 110% of the HB pREE equation increasing by an average of 6.7 kcal/kg or 122% of HB pREE in MV week 2 and by an additional 4.9 kcal/kg in MV week 3–142% of the HB equation (Table 3 ).
Table 3.
IC-derived mREE by mechanical ventilation week in COVID-19 ICU patients.
| MV Week 1 (n = 27) | MV Week 2 (n = 16) | MV Week 3 (n = 8) | MV Weeks 4–7 (n = 8) | |
|---|---|---|---|---|
| IC mREE in kcal/kg/da | 21.6 ± 1.1 | 23.1 ± 2.4 | 28.0 ± 1.9 | 27.9 ± 2.1 |
| % of Harris Benedict | 113.1 ± 4.3 | 122.3 ± 9.9 | 142.1 ± 9.8 | 147.2 ± 10.5 |
Data are means ± SEM.
IC – Indirect Calorimetry; mREE – Measured Resting Energy Expenditure; SEM – Standard Error of Mean.
Kilograms reflects patients' admission weight.
Another aspect of COVID-19's unique metabolic stress response was demonstrated when IC measurements in obese COVID-19 patients showed significantly lower mREE at 19.5 ± 1.0 kcal/kg/d compared to their non-obese counterparts 25.1 ± 1.8 kcal/kg/d in MV week 1 (p < 0.01). In MV weeks 2–3, the mREE for obese patients held steady at 19.5 ± 1.5 kcal/kg, while the longitudinal response in non-obese patients increased by 2.9 kcal/kg to 28.0 ± 2.0 kcal/kg (Fig. 2 ). This greater increase in metabolic demands among non-obese COVID-19 patients is alternatively displayed through a 17% increase in the degree of hypermetabolism (from 117% to 134% of that predicted by the HB equation) compared to a 4% increase in the obese group (Table 4 ).
Fig. 2.
IC-derived measured REE compared to multiple commonly used predictive REE equations by mechanical ventilation week.
Table 4.
IC-derived mREE in obese vs non-obese COVID-19 ICU patients by mechanical ventilation week.
| MV Week 1 | Non-Obesea(n=10) | Obeseb(n=17) | p value |
|---|---|---|---|
| IC mREE - kcal/kg/dc | 25.1 ± 1.8 | 19.5 ± 1.0 | <0.01 |
| % of Harris Benedict |
117.3 ± 9.1 |
110.6 ± 4.4 |
0.51 |
|
MV Week 2–3 |
Non-Obesea(n=10) |
Obeseb(n=10) |
|
| IC mREE - kcal/kg/dc | 28.0 ± 2.0 | 19.5 ± 1.5 | <0.01 |
| % of Harris Benedict | 133.6 ± 9.4 | 115.2 ± 8.7 | 0.17 |
Data are means ± SEM.
MV – Mechanical Ventilation; IC – Indirect Calorimetry; mREE – Measured Resting Energy Expenditure; SEM – Standard Error of Mean.
BMI <30 kg/m2.
BMI >30 kg/m2.
Kilograms reflects patients' admission weight.
3.2. Comparison of indirect calorimetry measured resting energy expenditure to predictive energy equations
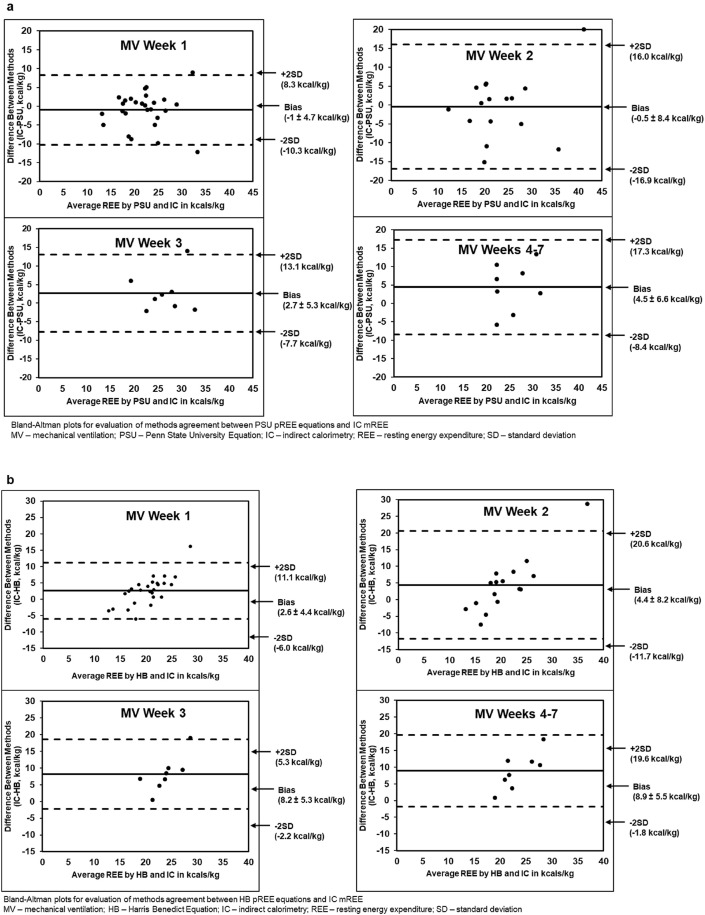
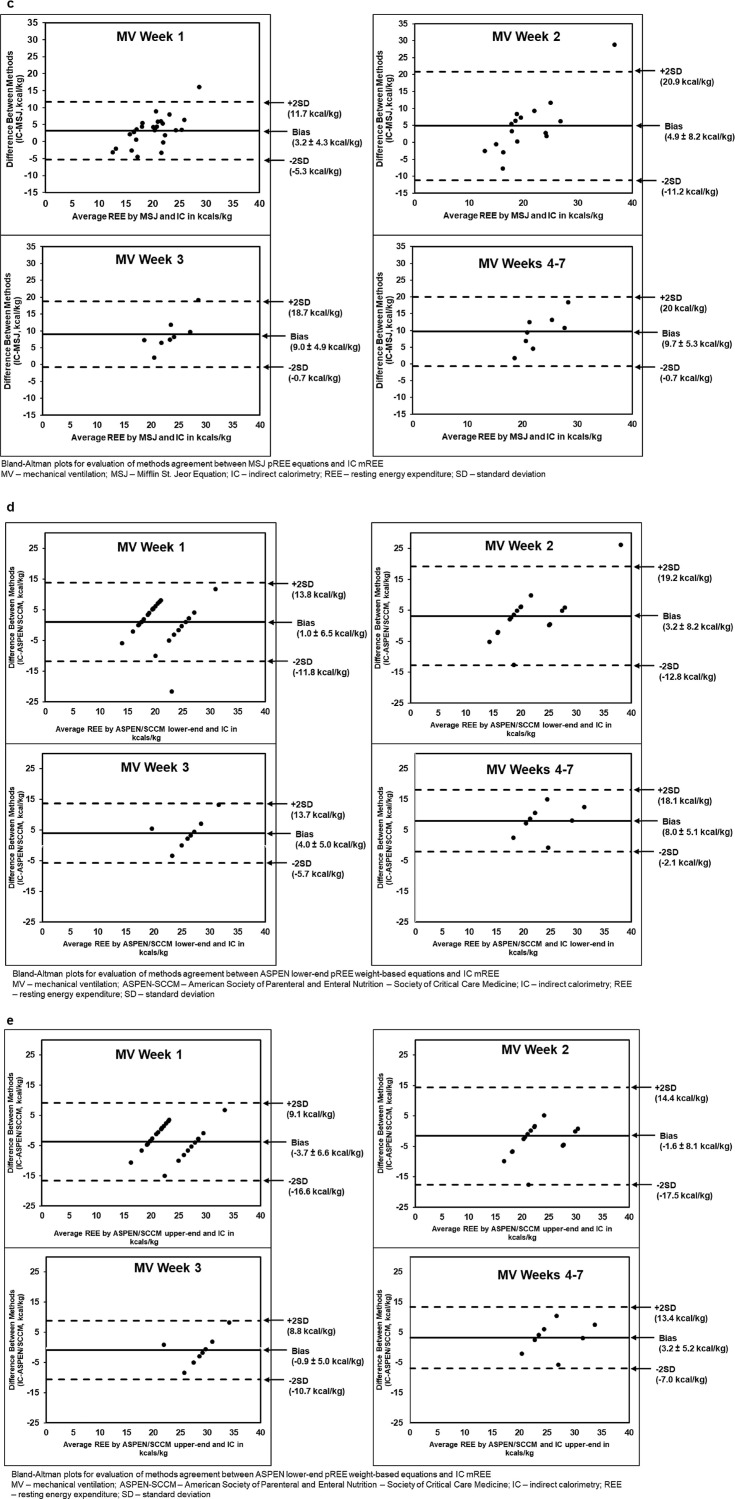
IC mREE was compared to four pREE equations highlighting the static nature of these published pREE equations when comparing MV week 1 to MV weeks 4–7 in addition to the frequent under- and over-prediction of REE (Fig. 2). Throughout ICU LOS, the HB, MSJ, and ASPEN-SCCM lower-end of range most frequently and significantly under-predicted mREE. While the PSU equation showed consistent non-significant differences in all MV weeks, it occasionally over-predicted caloric needs at some time points. ASPEN/SCCM Clinical Guidelines routinely led to under- and over-feeding when used at the lower- and upper-end of the range; respectively, with the exception of MV weeks 4–7 (Table 5 ). When comparing all equations by MV week, ASPEN/SCCM lower-end of range predicted the most accurate REE without over-feeding in MV weeks 1 and 2, followed by PSU equations in MV week 3, and ASPEN/SCCM upper-end of the range in MV weeks 4–7. Without considering the possibility of over-feeding, PSU predicted the most accurate REE in MV weeks 1 and 2 followed by ASPEN/SCCM upper-end of range in MV weeks 3 and 4–7. When comparing mREE to pREE within the BMI groups of obese and non-obese, the MSJ and HB equations consistently under-predicted average REE in both groups in all MV weeks while PSU consistently over-predicted average REE at the same time points. ASPEN/SCCM Clinical Guidelines routinely led to under- and over-predicting when used at the lower- and upper-end of the range; respectively (Table 6 ). Relations between mREE and pREE are also displayed in Bland Altman plots by mechanical ventilation week (Fig. 3 a-e).
Table 5.
IC-derived mREE compared to pREE in COVID-19 ICU patients by mechanical ventilation week.
| pREE Equation (kcal/kg/d) |
IC mREE (kcal/kg/d) |
P Value | |
|---|---|---|---|
| mean ± SEM | mean ± SEM | ||
| MV Week 1 (n = 27) | |||
| MSJ | 18.4 ± 0.6 | 21.6 ± 1.1 | 0.01 |
| PSU | 22.5 ± 1.0 | 21.6 ± 1.1 | 0.51 |
| HB | 19.0 ± 0.5 | 21.6 ± 1.1 | 0.03 |
| ASPEN/SCCM Lower | 20.5 ± 0.9 | 21.6 ± 1.1 | 0.47 |
| ASPEN/SCCM Upper | 25.3 ± 0.9 | 21.6 ± 1.1 | 0.01 |
| MV Week 2 (n=16) | |||
| MSJ | 18.2 ± 0.8 | 23.1 ± 2.4 | 0.07 |
| PSU | 23.6 ± 1.8 | 23.1 ± 2.4 | 0.88 |
| HB | 18.7 ± 0.7 | 23.1 ± 2.4 | 0.09 |
| ASPEN/SCCM Lower | 19.9 ± 1.0 | 23.1 ± 2.4 | 0.23 |
| ASPEN/SCCM Upper | 24.7 ± 1.1 | 23.1 ± 2.4 | 0.54 |
| MV Week 3 (n=8) | |||
| MSJ | 19.0 ± 0.7 | 28.0 ± 1.9 | <0.01 |
| PSU | 25.3 ± 1.8 | 28.0 ± 1.9 | 0.31 |
| HB | 19.6 ± 0.6 | 28.0 ± 1.9 | <0.01 |
| ASPEN/SCCM Lower | 24.0 ± 1.0 | 28.0 ± 1.9 | 0.09 |
| ASPEN/SCCM Upper | 28.9 ± 1.1 | 28.0 ± 1.9 | 0.67 |
| MV Week 4–7 (n=8) | |||
| MSJ | 18.3 ± 0.8 | 27.9 ± 2.1 | <0.01 |
| PSU | 23.5 ± 1.5 | 27.9 ± 2.1 | 0.11 |
| HB | 19.0 ± 0.7 | 27.9 ± 2.1 | <0.01 |
| ASPEN/SCCM Lower | 19.9 ± 1.5 | 27.9 ± 2.1 | 0.01 |
| ASPEN/SCCM Upper | 24.7 ± 1.5 | 27.9 ± 2.1 | 0.24 |
MV – Mechanical Ventilation; MSJ – Mifflin St Jeor; PSU – Penn State University; HB – Harris Benedict; ASPEN – American Society of Parenteral and Enteral Nutrition; SCCM – Society of Critical Care Medicine; IC – Indirect Calorimetry; mREE – Measured Resting Energy Expenditure; pREE – Predicted Resting Energy Expenditure; SEM – Standard Error of Mean.
1 – all mean percentages were derived from individual patient calculations utilizing the following equation (pREE/mREE ∗ 100).
Table 6.
IC-derived mREE compared to pREE in obese and non-obese COVID-19 ICU patients by mechanical ventilation week.
| Non-Obesea (n = 10) |
Obeseb (n = 17) |
|||||
|---|---|---|---|---|---|---|
| pREE (kcal/kg/dc) | IC mREE (kcal/kg/dc) | p value | pREE (kcal/kg/dc) | IC mREE (kcal/kg/dc) | p value | |
| MV Week 1 | ||||||
| MSJ | 21.5 ± 0.5 | 25.1 ± 1.8 | 0.08 | 16.5 ± 0.4 | 19.5 ± 1.0 | 0.01 |
| PSU | 27.5 ± 1.6 | 25.1 ± 1.8 | 0.33 | 19.6 ± 0.7 | 19.5 ± 1.0 | 0.90 |
| HB | 21.5 ± 0.3 | 25.1 ± 1.8 | 0.08 | 17.5 ± 0.5 | 19.5 ± 1.0 | 0.09 |
| ASPEN/SCCM Lower | 25.0 ± 0.0 | 25.1 ± 1.8 | 0.96 | 17.9 ± 1.0 | 19.5 ± 1.0 | 0.28 |
| ASPEN/SCCM Upper |
30.0 ± 0.0 |
25.1 ± 1.8 |
0.03 |
22.5 ± 1.0 |
19.5 ± 1.0 |
0.21 |
|
Non-Obesea(n=10) |
Obeseb(n=10) |
|||||
| MV Weeks 2–3 | ||||||
| MSJ | 20.7 ± 0.7 | 28.0 ± 2.0 | 0.00 | 16.0 ± 0.5 | 19.5 ± 1.5 | 0.04 |
| PSU | 28.1 ± 1.8 | 28.0 ± 2.0 | 0.98 | 19.6 ± 1.3 | 19.5 ± 1.5 | 0.97 |
| HB | 21.2 ± 0.5 | 28.0 ± 2.0 | 0.01 | 17.2 ± 0.6 | 19.5 ± 1.5 | 0.16 |
| ASPEN/SCCM Lower | 25.0 ± 0.0 | 28.0 ± 2.0 | 0.16 | 16.9 ± 0.0 | 19.5 ± 1.5 | 0.10 |
| ASPEN/SCCM Upper | 30.0 ± 0.0 | 28.0 ± 2.0 | 0.35 | 21.5 ± 0.0 | 19.5 ± 1.5 | 0.21 |
Data are means ± SEM.
For ASPEN/SCCM equation in obese patients predicted REE was corrected by divided by 0.65 per guideline specification of equation predicting 65% of what IC-measured REE would yield.
MV – Mechanical Ventilation; MSJ – Mifflin St Jeor; PSU – Penn State University; HB – Harris Benedict; ASPEN – American Society of Parenteral and Enteral Nutrition; SCCM – Society of Critical Care Medicine; IC – Indirect Calorimetry; mREE – Measured Resting Energy Expenditure; SEM – Standard Error of Mean.
BMI <30 kg/m2.
BMI >30 kg/m2.
Kilograms reflects patients' admission weight.
Fig. 3.
a: Bland–Altman Plot of Penn State Predictive Energy Equation vs Indirect Calorimetry. b: Bland–Altman Plot of Harris Benedict Predictive Energy Equation vs Indirect Calorimetry. c: Bland–Altman Plot of Mifflin St. Jeor Predictive Energy Equation vs Indirect Calorimetry. d: Bland–Altman Plot of ASPEN/SCCM Lower End Predictive Energy Equation vs Indirect Calorimetry. e: Bland–Altman Plot of ASPEN/SCCM Upper-End Predictive Energy Equation vs Indirect Calorimetry.
4. Discussion
This prospective observational study demonstrates critically ill, mechanically ventilated COVID-19 patients exhibit unique and progressive hypermetabolism throughout and up to 7-weeks following intubation as measured by IC, the gold-standard for REE estimation. When comparing obese patients to their non-obese counterparts, a persistent hypermetabolic state was observed rather than the progressive increases in mREE observed in their non-obese counterparts. When comparing findings of IC-derived mREE with commonly used pREE equations, the static nature of these published pREE equations was highlighted through their over- and under-prediction of nutritional targets, and inconsistent ability to predict observed progressive hypermetabolism was found. Despite these discrepancies, the PSU and upper-end of the ASPEN-SCCM clinical guidelines appeared to most accurately account for the persistent hypermetabolism and high-metabolic demands within the study population.
Patient demographics within this study appear to be reasonably representative of the demographics of severe COVID-19 ICU patients at other national and international medical centers [1,3,26]. The majority of patients in this analysis were male (61%), Black (47%), non-Hispanic (71%), and obese (58%) with an average BMI of 31.8 ± 1.4 kg/m2. Comparatively, an observational cohort study of critically ill COVID-19 patients admitted to ICUs in the southern region of the US reported the majority of patients as male (55%), Black (70.5%), and obese with a BMI of [median, interquartile range (30, 26–35) kg/m2 [26].
The results of this study validate our preliminary data, in a small cohort of critically ill patients with COVID-19 disease, showing progressive hypermetabolism over a short measurement period in the ICU [2]. A similar hypermetabolic state was found in small a retrospective case series of 7 COVID-19 adults requiring MV when using the CCM Express IC to determine mREE, reporting a median mREE of 4044 kcal/day, or 235.7% ± 51.7% of pREE when using the PSU equation [3]. Since this previous study took single IC measurements during MV at varying times between hospital days 8 and 55, it is not clear how many days’ post-intubation these measurements were taken and, in contrast to our study, cannot accurately describe changes in REE throughout the ICU LOS. The authors did not specify which PSU equation version was used, which would affect results if the age-appropriate modifications were not conducted. A recent observational study of 22 MV COVID-19 patients noted persistent hypermetabolism throughout ICU LOS and higher mREE was higher in the late phase ≥ ICU day 8 for the majority of patients upon intra-individual analysis of mREE [1].
Previous models of the metabolic stress response to injury describe an increase in REE that occurs early, peaking within days following the initial insult before diminishing back toward normometabolism. The traditional Cuthbertson Ebb:Flow ICU model describes an initial short normometabolic Ebb phase (12–24 h) where REE changes very little as counter-regulatory cytokines are generated. This is followed by the flow phase (hypermetabolism) peaking in 3–5 days, and resolving as the patient enters the Flow phase. This latter hypermetabolic phase peaks quickly before resolving completely within 7–10 days (unless late complications of sepsis or multiple organ failure occur and create a subsequent secondary peak) [27]. In a separate model, an initial hypermetabolic response described as the systemic inflammatory response syndrome (SIRS) occurs first peaking early within days, followed by a subsequent opposing hypometabolic compensatory anti-inflammatory response syndrome (CARS), which serves to reverse the changes in REE and hasten the return to normometabolism [28]. In yet a third model, the persistent inflammation catabolism syndrome (PICS), where the SIRS/CARS responses occur simultaneously early after the initial insult, peaking within the first week, but failing to return to baseline or normometabolism by 14 days [28,29].
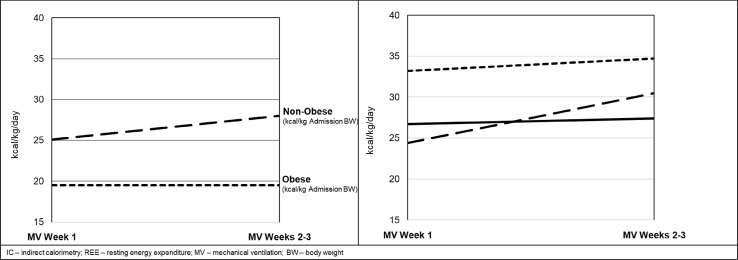
The pattern of REE occurring in response to COVID-19 disease shown in this study is unique and can be easily distinguished from these previously described models and previously published longitudinal IC-measured REE in other forms of critical illness and injury [25]. The rise in REE is slower, but steadily progressive, peaking much later the third week or beyond following admission to the ICU. The response appears to be more exaggerated in non-obese patients, being more truncated and less severe in the obese. This response represents a prolongation of the acute and immediate post-acute phases of critical illness, pushing the transition to the recovery phase (normally expected to occur after 7–10 days) back by 2–3 weeks or even potentially later than 7 weeks in some patients. The reason for the truncated response in obese patients is not clear. Obesity is a major risk factor for contracting COVID-19, is associated with greater disease severity, and has higher mortality [30]. On one hand, the increase in fat mass with obesity represents hypometabolic tissue which might mask the hypermetabolic response being mounted in the lean body mass such that the REE in kcal/kg adjusted BW is lower (Fig. 1). On the other hand, obesity is a pro-inflammatory condition characterized by low-grade SIRS, such that the baseline EE may already be elevated thus reducing the apparent increases over the ensuing 2–3 weeks [31]. Further research is needed to explore and understand this initial finding in COVID-19.
Fig. 1.
IC-derived REE in critically ill obese and non-obese COVID-19 parents by mechanical ventilation week according to kcal/kg of admission, ideal, and adjusted body weight.
Our data further demonstrate the failure of published, commonly-utilized pREE equations to detect the unique prolongation of the hypermetabolic response in critically ill COVID-19. HB, MSJ, and ASPEN-SCCM lower-end of range equations consistently under-predicted REE in both obese and non-obese groups in all MV weeks, confirming their inaccuracies in predicting REE in a critically ill COVID-19 patient population (Table 5, Table 6) This is consistent with previous publications showing poor performance of predictive equations in other ICU populations [16]. Based on our results, it is our recommendation that these pREE equations should be avoided when determining caloric needs for critically ill COVID-19 patients as they will contribute to consistent and prolonged under-feeding which may have deleterious effects on clinical outcomes of this population [7]. In the absence of IC, the PSU equations that were developed specifically for MV patients, can be modified for elderly obese patients, and shown to perform at 67% accuracy when compared to mREE in non-COVID-19 ICU patients, were more comparative to IC-derived mREE in the present study when respective age and BMI status-based PSU equations were used [14]. ASPEN-SCCM upper-end of the range also provided reasonable predictions when estimating 100% of caloric needs within obese patients with fewer instances of over-predicting in the late stages of MV. Although PSU appears to be the best alternative for centers without IC capabilities, like all studied equations, over-predictions in REE depending on MV week are possible (Fig. 2, Table 5, Table 6).
A prominent aspect of this study was the importance of the safety protocol employed to utilize IC in a COVID-19 unit and the features in the design of the Q-NRG calorimeter device which facilitated that process. To combat the high infection risk, strict personal protective equipment (PPE) use was employed for all IC testing. Further thorough cleaning and bleach sterilization of all IC instruments between assessments was strictly employed. Developed with COVID-19 ICU physician and respiratory therapy leadership at DUMC, a system of disconnecting/reconnecting the IC adaptor inside enclosed and plastic-wrapped endotracheal and ventilator tubing to minimize the aerosolization of respiratory virus particles into the room was utilized. Using fully disposable single-patient tubing and connections, the Q-NRG device minimized the number of instruments and equipment requiring sterilization between each measurement.
4.1. Limitations
Despite straightforward PPE, infection safety, and sterilization techniques, the isolation and high infection risk of COVID-19 ICU patients was a limitation to more frequent IC measurements. It was further determined that, to collect longitudinal mREE, IC measurements could most realistically be obtained approximately every 3 days while prioritizing the safety of trained study staff and sufficient time for sterilization. We felt this interval would provide reasonable regular repeated measures in an achievable study design that maximized accuracy, safety, and practical burden on the study and the clinical ICU staff.
Furthermore, fewer patients were able to be tested in successive MV weeks as patients were no longer ventilator dependent or unfortunately were deceased. The subsequent smaller sample sizes in MV weeks 2 and beyond led to the combination of group data to individually analyze obese and non-obese groups, which we feel is a novel and imperative contribution to COVID-19 mREE literature given SARS-CoV-2 infection's significant impact on obese populations [30]. Nevertheless, strengths in the analysis include study sample size and longitudinal analysis with measurements as late as ICU week 7, allowing for sufficiently powered statistical analysis when comparing mREE to pREE determined by four commonly used predictive equations.
5. Conclusions
Indirect calorimetry is essential for the precise and longitudinal assessment of energy needs in critically ill COVID-19 adult patients. Our data demonstrate that IC measures need to be repeated longitudinally and regularly as patients’ REE requirements can differ dramatically over time and according to BMI classification (obese vs. non-obese). To our knowledge, this study shows a previously unreported and unique pattern of a prolonged hypermetabolic stress response to critical illness seen only in patients infected by the SARS-CoV-2 virus and perhaps in some severely burned patients [32]. This prolonged hypermetabolic response appeared more significant in non-obese versus obese patients and was poorly predicted by most all commonly used pREE equations. The use of longitudinal IC measurements is necessary to provide accurate energy targets and delineate the duration of the prolonged hypermetabolic response. In particular, IC measures are vital to prevent early over-feeding and more importantly, significant underfeeding and potential large caloric deficits after the first ICU week, which could easily persist and accrue over prolonged periods of hypermetabolism during ICU stay. If IC is unavailable, the PSU 2003b and 2010 pREE equations (2003b for <60 years of age, and PSU 2010 for >60 years of age) and ASPEN-SCCM upper-end of range weight-based equation may produce the closest REE compared to mREE in mechanically ventilated COVID-19 patients.
Funding statement
This study was funded in part by an investigator-initiated grant from Baxter (Deerfield, IL) to Paul E. Wischmeyer via Duke University. The sponsor (Baxter) did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Only the authors and investigators at Duke University participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authors’ contributions
LN and HM contributed to study conception and design, data analysis and interpretation, and drafting the manuscript for publication. KH contributed to data analysis and interpretation and critical revision of the manuscript. JM contributed to study design, data acquisition and critical revision of the manuscript. JW and DM contributed to study conception and design and data acquisition. PW contributed to study conception and design, data analysis and interpretation and the critical revision and submission of the manuscript. SM contributed to the critical revision and submission of the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
PW: Grant funding from National Institutes of Health, Canadian Institutes of Health Research, Abbott, Baxter, Fresenius, Nutricia, and Takeda. Consultant to Abbott, Fresenius, Baxter, Cardinal Health, Nutricia, and Takeda for research related to this work. Unrestricted gift donations for nutrition research from Musclesound and Cosmed. Honoraria or travel expenses for CME lectures on improving nutrition care from Abbott, Baxter and Danone-Nutricia.
JW: Grant funding from International Anaesthesia Research Society. Royalties or licenses from MuscleSound. Consulting fees from Predicate HPG, Vyaire Medical, Retia Medical. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Retia Medical and EBPOM USA.
SM: Consulting fees from Baxter.
Acknowledgements
We are incredibly grateful and appreciative of the ICU staff who were so bravely committed to the success of this project and volunteered to assist with the study, seeing the essential need to obtain this data to provide insight to ICU providers caring for COVID-19 patients and researchers studying this new pandemic disease. We would also like to acknowledge the LEEP-COVID study team, including Lindsie Boerger, Marat Fudim, Melanie Hollidge, Kathryn Lessig, Jessica Lumbard, Leslie C. Murray, Jhana Parikh, Jacob Ribet, Sue Steves, and Anthony Sung.
References
- 1.Lakenman P.L.M., van der Hoven B., Schuijs J.M., Eveleens R.D., van Bommel J., Olieman J.F. Energy expenditure and feeding practices and tolerance during the acute and late phase of critically ill COVID-19 patients. Clinical Nutrition ESPEN. 2021 Jun;43:383–389. doi: 10.1016/j.clnesp.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittle J., Molinger J., MacLeod D., Haines K., Wischmeyer P.E. Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-03286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu P.J., Cassiere H., DeRosa S., Bocchieri K., Yar S., Hartman A. Hypermetabolism and coronavirus disease 2019. J Parenter Enteral Nutr. 2020;44(7):1234–1236. doi: 10.1002/jpen.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martindale R., Patel J.J., Taylor B., Arabi Y.M., Warren M., McClave S.A. Nutrition therapy in critically ill patients with coronavirus disease 2019. J Parenter Enteral Nutr. 2020;44(7):1174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) JPEN - J Parenter Enter Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 6.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Singer P., Pichard C., De Waele E. Practical guidance for the use of indirect calorimetry during COVID 19 pandemic. Clinical nutrition experimental. 2020;33:18–23. doi: 10.1016/j.yclnex.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Waele E., Spapen H., Honoré P.M., Mattens S., Van Gorp V., Diltoer M. Introducing a new generation indirect calorimeter for estimating energy requirements in adult intensive care unit patients: feasibility, practical considerations, and comparison with a mathematical equation. J Crit Care. 2013;28(5):884. doi: 10.1016/j.jcrc.2013.02.011. e1-. e6. [DOI] [PubMed] [Google Scholar]
- 9.Graf S., Karsegard V.L., Viatte V., Heidegger C.P., Fleury Y., Pichard C. Evaluation of three indirect calorimetry devices in mechanically ventilated patients: which device compares best with the Deltatrac II®? A prospective observational study. Clin Nutr. 2015;34(1):60–65. doi: 10.1016/j.clnu.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Oshima T., Berger M.M., De Waele E., Guttormsen A.B., Heidegger C.-P., Hiesmayr M. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. 2017;36(3):651–662. doi: 10.1016/j.clnu.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Sundström M., Tjäder I., Rooyackers O., Wernerman J. Indirect calorimetry in mechanically ventilated patients. A systematic comparison of three instruments. Clin Nutr. 2013;32(1):118–121. doi: 10.1016/j.clnu.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Garzotto F., Comoretto R.I., Ostermann M., Nalesso F., Gregori D., Bonavina M.G. Preventing infectious diseases in Intensive Care Unit by medical devices remote control: lessons from COVID-19. J Crit Care. 2021;61:119–124. doi: 10.1016/j.jcrc.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thibault R., Coëffier M., Joly F., Bohe J., Schneider S.M., Déchelotte P. How the Covid-19 epidemic is challenging our practice in clinical nutrition—feedback from the field. Eur J Clin Nutr. 2020:1–10. doi: 10.1038/s41430-020-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankenfield D. Validation of an equation for resting metabolic rate in older obese, critically ill patients. J Parenter Enteral Nutr. 2011;35(2):264–269. doi: 10.1177/0148607110377903. [DOI] [PubMed] [Google Scholar]
- 15.Frankenfield D., Smith J.S., Cooney R.N. Validation of 2 approaches to predicting resting metabolic rate in critically ill patients. J Parenter Enteral Nutr. 2004;28(4):259–264. doi: 10.1177/0148607104028004259. [DOI] [PubMed] [Google Scholar]
- 16.Frankenfield D.C., Coleman A., Alam S., Cooney R.N. Analysis of estimation methods for resting metabolic rate in critically ill adults. J Parenter Enteral Nutr. 2009;33(1):27–36. doi: 10.1177/0148607108322399. [DOI] [PubMed] [Google Scholar]
- 17.Mogensen K.M., Andrew B.Y., Corona J.C., Robinson M.K. Validation of the Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition recommendations for caloric provision to critically ill obese patients: a pilot study. J Parenter Enteral Nutr. 2016;40(5):713–721. doi: 10.1177/0148607115584001. [DOI] [PubMed] [Google Scholar]
- 18.Preiser J.-C., van Zanten A.R., Berger M.M., Biolo G., Casaer M.P., Doig G.S. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care. 2015;19(1):1–11. doi: 10.1186/s13054-015-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshima T., Delsoglio M., Dupertuis Y.M., Singer P., De Waele E., Veraar C. The clinical evaluation of the new indirect calorimeter developed by the ICALIC project. Clin Nutr. 2020;39(10):3105–3111. doi: 10.1016/j.clnu.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Baxter Baxter and COSMED announce U.S. FDA 510(k) clearance of Q-NRG+ indirect calorimetry device. 2020. https://www.baxter.com/baxter-newsroom/baxter-and-cosmed-announce-us-fda-510k-clearance-q-nrg-indirect-calorimetry-device [Available from:
- 21.Frankenfield D.C., Rowe W.A., Smith J.S., Cooney R. Validation of several established equations for resting metabolic rate in obese and nonobese people. J Am Diet Assoc. 2003;103(9):1152–1159. doi: 10.1016/s0002-8223(03)00982-9. [DOI] [PubMed] [Google Scholar]
- 22.Harris J.A., Benedict F.G. Carnegie institution of Washington; 1919. A biometric study of basal metabolism in man. [Google Scholar]
- 23.Mifflin M.D., St Jeor S.T., Hill L.A., Scott B.J., Daugherty S.A., Koh Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 24.Bland J.M., Altman D.G. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 25.Uehara M., Plank L.D., Hill G.L. Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Crit Care Med. 1999;27(7):1295–1302. doi: 10.1097/00003246-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Auld S.C., Caridi-Scheible M., Blum J.M., Robichaux C., Kraft C., Jacob J.T. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020 Sep;48(9):e799–e804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuthbertson D. Post-shock metabolic response. Lancet. 1942;239(6189):433–437. [Google Scholar]
- 28.Rosenthal M.D., Moore F.A. Persistent inflammation, immunosuppression, and catabolism: evolution of multiple organ dysfunction. Surg Infect. 2016;17(2):167–172. doi: 10.1089/sur.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moonen H.P.F.X., Beckers K.J.H., Van Zanten A.R.H. Energy expenditure and indirect calorimetry in critical illness and convalescence: current evidence and practical considerations. Journal of Intensive Care. 2021;9(1) doi: 10.1186/s40560-021-00524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefan N., Birkenfeld A.L., Schulze M.B. Global pandemics interconnected—obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021:1–15. doi: 10.1038/s41574-020-00462-1. [DOI] [PubMed] [Google Scholar]
- 31.Karampela I., Chrysanthopoulou E., Christodoulatos G.S., Dalamaga M. Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Current obesity reports. 2020:1–14. doi: 10.1007/s13679-020-00394-x. [DOI] [PubMed] [Google Scholar]
- 32.Clark A., Imran J., Madni T., Wolf S.E. Nutrition and metabolism in burn patients. Burns & trauma. 2017;5 doi: 10.1186/s41038-017-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]