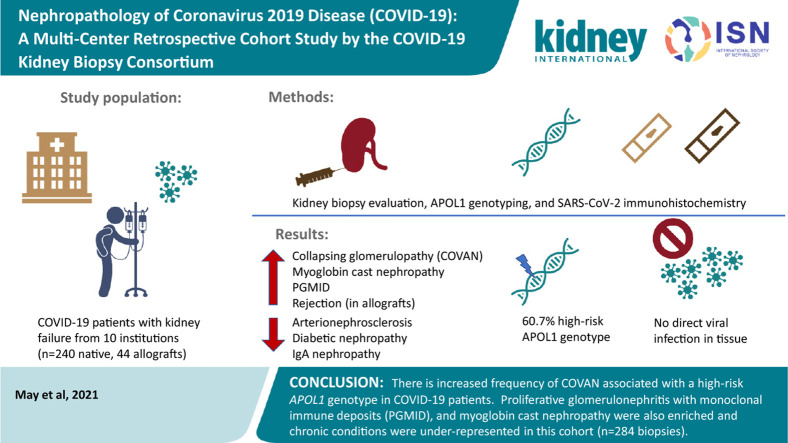
Abstract
Kidney failure is common in patients with Coronavirus Disease-19 (COVID-19), resulting in increased morbidity and mortality. In an international collaboration, 284 kidney biopsies were evaluated to improve understanding of kidney disease in COVID-19. Diagnoses were compared to five years of 63,575 native biopsies prior to the pandemic and 13,955 allograft biopsies to identify diseases that have increased in patients with COVID-19. Genotyping for APOL1 G1 and G2 alleles was performed in 107 African American and Hispanic patients. Immunohistochemistry for SARS-CoV-2 was utilized to assess direct viral infection in 273 cases along with clinical information at the time of biopsy. The leading indication for native biopsy was acute kidney injury (45.4%), followed by proteinuria with or without concurrent acute kidney injury (42.6%). There were more African American patients (44.6%) than patients of other ethnicities. The most common diagnosis in native biopsies was collapsing glomerulopathy (25.8%), which was associated with high-risk APOL1 genotypes in 91.7% of cases. Compared to the five-year biopsy database, the frequency of myoglobin cast nephropathy and proliferative glomerulonephritis with monoclonal IgG deposits was also increased in patients with COVID-19 (3.3% and 1.7%, respectively), while there was a reduced frequency of chronic conditions (including diabetes mellitus, IgA nephropathy, and arterionephrosclerosis) as the primary diagnosis. In transplants, the leading indication was acute kidney injury (86.4%), for which rejection was the predominant diagnosis (61.4%). Direct SARS-CoV-2 viral infection was not identified. Thus, our multi-center large case series identified kidney diseases that disproportionately affect patients with COVID-19 and demonstrated a high frequency of APOL1 high-risk genotypes within this group, with no evidence of direct viral infection within the kidney.
Keywords: acute kidney injury, coronavirus, COVID-19, kidney biopsy, renal pathology, SARS-CoV-2
Graphical abstract
Acute kidney injury (AKI) is a common complication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, impacting 37% of patients hospitalized with coronavirus disease 2019 (COVID-19).1 , 2 Morbidity and mortality levels are significantly higher for COVID-19 patients with AKI3 than for those without kidney disease.4 Up to 40% of patients requiring mechanical ventilation need concurrent kidney replacement therapy, resulting in unprecedented numbers of patients requiring this therapy and a shortage of dialysis units.5 In a study of over 10,000 patients in 920 hospitals, the frequency of in-hospital death was a staggering 73% in patients requiring both mechanical ventilation and dialysis.6
Case reports, small case series, and autopsy studies have shown a wide spectrum of kidney manifestations in COVID-19 patients and have improved our understanding of kidney disease in this population. Reported manifestations in native biopsies include acute tubular injury (ATI),10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 7, 8, 9 myoglobin cast nephropathy,7 thrombotic microangiopathy,7 , 9 , 16 , 20 , 25 , 26 acute interstitial nephritis,14 , 27 noncollapsing focal and segmental glomerulosclerosis,9 , 19 , 20 , 28 collapsing glomerulopathy (also known as COVID-19–associated nephropathy [COVAN]),7, 8, 9 , 15 , 20 , 21 , 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 membranous nephropathy,8 , 20 , 41 minimal change disease,8 , 9 , 21 acute pyelonephritis,13 oxalate nephropathy,14 , 43 immune complex–mediated glomerulonephritis/lupus nephritis,8 infection-associated glomerulonephritis,9 membranoproliferative glomerulonephritis,44 anti-glomerular basement membrane disease,45 , 46 IgA nephropathy,20 , 41 , 47, 48, 49 light chain cast nephropathy,20 arteritis,20 and crescentic glomerulonephritis.20 , 50 , 51 Acute T cell–mediated rejection,8 antibody-mediated rejection,9 calcineurin inhibitor nephrotoxicity,20 atheroemboli,26 collapsing glomerulopathy,42 and cortical necrosis8 , 26 have been reported in COVID-19 kidney allograft biopsies. Chronic conditions have also been reported, including diabetic nephropathy,9 , 11 , 14 , 16 , 26 , 41 obesity-related glomerulopathy,16 , 26 amyloidosis,11 , 20 and arterionephrosclerosis11 , 15 , 24 (Supplementary Tables S1 and S2). These studies have been useful in elucidating the need for kidney biopsy in COVID-19 patients for proper diagnosis and disease management. However, given the large spectrum of diagnoses, it is unclear from small series and case reports which diseases are enriched in COVID-19 patients. Additionally, single-case reports can be subject to publication bias, with those reporting interesting or unusual clinical presentations more likely to be published.
We present the largest kidney biopsy series to date, encompassing 240 native and 44 allograft biopsies in an international collaborative effort to improve our understanding of kidney disease in COVID-19. We sought to determine which kidney diseases are enriched in patients with COVID-19 through comparison of this series to a pre–COVID-19 biopsy cohort.
Methods
Study design
A multi-center clinicopathologic study of kidney pathology in COVID-19 patients was initiated following approval by the Solutions Institutional Review Board, with adherence to the principles of the Declaration of Helsinki. Multiple kidney pathologists and nephrologists were invited to participate and together comprised the COVID-19 Kidney Biopsy Consortium. Biopsies were received from a total of 9 institutions, from the United States, Switzerland, and India. The participating centers included the University of California, Los Angeles (n = 2), the University of California, San Francisco (n = 2), the University of Pennsylvania (n = 2), Medical College of Wisconsin (n = 1), Rush University (n = 2), Ameripath Laboratories (n = 7), the University of Utah (n = 2), the University of Zurich (Switzerland, n = 1), and Manipal Hospital (Bangalore, India, n = 13); the remaining cases were from Arkana Laboratories, which received them from multiple nephrology practices among several states (n = 252). Cases from March 2020 to March 2021 were included. A total of 284 biopsies from COVID-19 patients were evaluated, including 240 native biopsies and 44 allografts. Patients of all ages were included. Inclusion criteria included having a confirmed diagnosis of COVID-19 prior to kidney biopsy, requiring a positive RNA polymerase chain reaction test for SARS-CoV-2 from a nasopharyngeal swab. Cases for which a diagnosis of COVID-19 preceded kidney biopsy by more than 3 months were excluded. Additionally, native kidney biopsy cases in which a prior biopsy with the same diagnosis preceded diagnosis of COVID-19 were excluded. All biopsies with temporal association with COVID-19 were included to avoid selection bias. All cases in the series are novel and are not published elsewhere.
For a control comparison cohort to compare demographics, biopsy indications, and disease frequencies, we utilized a database of kidney biopsies over a 5-year period prior to the pandemic in the United States, from January 1, 2015 to January 1, 2020. This database included 77,530 biopsies overall, comprised of 63,575 native biopsies and 13,955 allograft biopsies. Due to the large number of biopsies in the database, we selected 100 cases a year (500 cases total) through use of a random number generator52 to provide estimates of database characteristics.
Biopsies from patients with COVID-19 were also compared to diagnostic frequencies from 100 HIV-positive patients biopsied prior to the COVID-19 pandemic, as a control for systemic viral infection. The cohort needed to identify these 100 cases of HIV-positive patients spanned a 14-year period (2006–2020). The comparison cohorts included only cases from Arkana Laboratories. Arkana Laboratories receives biopsies from 40 states and is nearly representative of the biopsied population in the entire United States.
Statistics
Categorical variables were compared using the χ2 statistic or Fisher’s exact test, as appropriate. Differences in continuous variables were evaluated using t tests. Kruskal–Wallis testing was utilized for evaluation of group differences. A cutoff of P < 0.01 was considered significant.
Histologic processing of kidney biopsies
Kidney biopsies were prepared by standard light microscopy, immunofluorescence (IF), and electron microscopy techniques, as previously described.53 Formalin-fixed paraffin-embedded (FFPE) tissue sections were stained with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), Masson trichrome, and Jones methenamine silver (JMS). IF testing was performed on frozen tissue sections for IgG, IgA, IgM, C3, C1q, κ light chain, and λ light chains. IF microscopy was available for 283 of 284 cases. Transmission electron microscopy was evaluated for ultrastructural analysis and was available for 198 of 240 native and 34 of 44 allograft biopsies.
For the Arkana Laboratory cases, a Congo red stain was performed on all native biopsies of patients ≥49 years of age. Immunostain tests for C4d and SV-40 were performed on allograft biopsies. Additional immunostain tests were performed within some cases, as indicated for diagnosis. These tests included myoglobin and hemoglobin for cases of ATI with eosinophilic granular or beaded casts, DNAJB9 for fibrillary glomerulopathy, serum amyloid P for confirmation of a diagnosis of membranous-like glomerulopathy with masked IgG kappa deposits (MGMID), IgG subclasses light chain–restricted cases, and for membranous nephropathy subtyping with phospholipase A2 receptor (PLA2R), thrombospondin type 1 domain containing 7A, exostosin-1, and neural epidermal growth factor–like 1 (NELL1).
SARS-CoV-2 immunohistochemistry and in situ hybridization
Immunohistochemistry for SARS-CoV-2 was performed for expression of SARS-CoV-2 within 3-μm formalin-fixed paraffin-embedded tissue sections on the Leica BOND III platform, using a SARS nucleocapsid mouse monoclonal antibody at 1:100 dilution (Thermo Fisher Scientific, catalog #MA1-7404), as described previously.54
For SARS-CoV-2 in situ hybridization, RNAscope was performed on unstained formalin-fixed paraffin-embedded tissue sections on the Leica BOND-III platform using the Leica Bond RNAscope detection kit (catalog #DS9790) according to the standard manufacturer protocol. Commercially available RNA probes targeting nucleotides 21631–23303 of SARS-CoV-2 (ACDBio catalog #848568) were used for in situ hybridization. Positive and negative controls were evaluated with each sample. Peptidylprolyl isomerase B (PP1B), a housekeeping gene, was utilized as a positive RNA integrity control. Diaminopimelate B (DAPB), a bacterial gene, was used as a negative control. The tissue sections were counterstained with periodic acid–Schiff following in situ hybridization.
Histopathologic review
All cases were de-identified, and pathology slides were evaluated at a single center (Arkana Laboratories) and graded by 9 nephropathologists. A subset of glass slides from the referring pathologist or whole slide images were reviewed. Light-microscopic parameters evaluated included the presence or absence of mesangial expansion, mesangial hypercellularity, endocapillary hypercellularity, fibrinoid necrosis, crescents, segmental sclerosis, microangiopathic changes, and presence of capillary loop thrombi. If segmental sclerosis was present, the type according to the Columbia classification55 was specified. Tubulointerstitial parameters (for both native and allograft biopsies) were graded on a 0 to 3 scale according to the Banff classification parameters56 and included interstitial inflammation, edema, tubulitis, tubular injury, and the degree of interstitial fibrosis and tubular atrophy. Vascular parameters included evaluation of arteriosclerosis, arteriolar hyalinosis, endothelialitis, and microangiopathic changes. Arteriosclerosis and arteriolar hyalinosis were scored as mild (10%–25% intimal fibrosis or luminal stenosis), moderate (25%–50%), or severe (>50%). IF staining was graded on a trace to 3+ scale for IgA, IgG, IgM, C3, C1q, C4d, kappa, and lambda light chains, with the character of deposits and compartment specified. For outside cases without IF slides, this information was recorded from the pathology reports. Electron microscopy included the evaluation of electron-dense deposits (subepithelial, intramembranous, subendothelial, or mesangial), and the degree of podocyte foot process effacement (estimated in percentages to the nearest 10% for all cases with segmental sclerosis, and as mild [10%–25%]; moderate [25%–50%], or severe [>50%] for the remainder).
Clinical evaluation
Clinical parameters provided by the nephrologists included patient demographics (age, sex, and ethnicity), medical history for comorbid conditions, and time of biopsy laboratory values. As data were abstracted from clinical records, it is unknown whether patients self-identified with the race provided in the chart. The indication for biopsy (AKI, proteinuria, proteinuria with AKI, hematuria, or AKI on chronic kidney disease [CKD]) was obtained, as well as the time interval between COVID-19 diagnosis and biopsy. Severity of disease was noted, documented as “mild” for outpatients or asymptomatic COVID-19 infection, “moderate” for hospitalized patients, and “severe” in those who required intensive-care unit admission with mechanical ventilation and/or kidney replacement therapy.
AKI was defined according to the Kidney Disease: Improving Global Outcomes Clinical Practice Guideline for Acute Kidney Injury. In patients in whom we did not have a known baseline serum creatinine value, we considered AKI to be present if there was >0.5 mg/dl creatinine above the reference range, with no evidence of chronicity on biopsy, presence of oliguria, requirement for kidney replacement therapy, or a creatinine level >4.0 mg/dl (as this criterion alone meets the Kidney Disease: Improving Global Outcomes criteria for AKI class III). We did not separate AKI into Kidney Disease: Improving Global Outcomes class I, II, or III, as a baseline creatinine value was not available for some patients. CKD was defined as known preexisting CKD in the patient’s medical record or a prior glomerular filtration rate < 60 ml/min.
Demographics included the patient’s age, sex, and ethnicity. Relevant past medical history included presence or absence of comorbidities including preexisting chronic kidney disease, hypertension, diabetes mellitus, and obesity. Laboratory parameters included serum creatinine, quantitative proteinuria, and urinalysis for presence or absence of hematuria (microscopic or macroscopic). Quantitative proteinuria included urine protein-to-creatinine ratio, albumin-to-creatinine ratio, or 24-hour urinary protein measurements. Hematuria was defined as positivity for blood on a dipstick urinalysis, or ≥5 red blood cells/high power field on urine microscopy. Sub-nephrotic proteinuria included 300 mg to <3.5 grams of urinary protein and nephrotic range proteinuria ≥3.5 g/d.
Genetic testing for APOL1 risk alleles
Genetic testing for APOL1 G1 and G2 risk alleles was performed on all African American and Hispanic patient samples with sufficient tissue available for DNA extraction. Polymerase chain reaction was used to amplify the regions of the APOL1 gene carrying 1 of the 2 G1 risk allele pair (rs73885319 / c.1024A>G), the G2 6-base pair insertion/deletion risk allele (rs71785313 / c.1164_1169delATAATT), and wild-type alleles at these 2 loci. The G1 and G2 loci are in near-perfect linkage disequlibrium. Taqman polymerase chain reaction was performed on a ViiA 7 Real-Time polymerase chain reaction system, as previously described.57 Genotyping data were evaluated using ViiA7 sequence detection software, and allelic discrimination plots were used to assess the call results. Genotypes of G0/G0, G0/G1, and G0/G2 were combined into a low-risk status group, and G1/G1, G1/G2, and G2/G2 were grouped as being high risk.
Literature review
All case reports and clinical case series describing manifestations of COVID-19 within kidney biopsies or autopsies were included in a comprehensive review of the literature available in English. Articles were identified through PubMed and Google Scholar, using various search terms, including COVID-19, COVID19, SARS-CoV-2 AND kidney, renal biopsy, kidney biopsy, and autopsy. Conference abstracts from the American Society of Nephrology and from US and Canadian Society of Pathology meetings were also included.
Results
Demographics of the study population
A total of 284 kidney biopsies from patients with COVID-19 were evaluated; these were collected from March 2020 through March 2021 and included 240 native and 44 allograft biopsies. The mean time interval between COVID-19 diagnosis and kidney biopsy was 22.3 ± 26.8 days for native biopsies, and 21.9 ± 23.5 days for allografts. Patients undergoing native biopsy (43.3%) and 40.9% of transplant recipients had a biopsy in the same week that COVID-19 diagnosis was made. There was a slight male dominance in native biopsies (57.1% native biopsies, 50.0% allograft biopsies). African American patients were disproportionately impacted, comprising 44.6% of native COVID-19 kidney biopsies, compared to an estimated 15.4% of patients in the biopsy database (Table 1 ).
Table 1.
Demographics and clinical features of native and allograft COVID-19 kidney biopsies, compared to pre-pandemic native and allograft kidney biopsies
| Parameter | Native |
Pre-pandemic native |
Transplant |
Pre-pandemic transplant |
||||
|---|---|---|---|---|---|---|---|---|
| N | % Total | N | % Total | N | % Total | N | % Total | |
| Number of biopsies | 240 | 429 | 44 | 71 | ||||
| Age, yr, (range) | 53.7 (11–84) | 56.3 (2–94) | 36.6 (12–66) | 52.6 (4–79) | ||||
| Sex | F: 103 M: 137 |
42.9 57.1 |
F: 192 M: 237 |
44.8 55.2 |
F: 22 M: 22 |
50.0 50.0 |
F: 21 M: 50 |
29.6 70.4 |
| Days between diagnosis and biopsy (range) | 22.3 (0–90) | NA | 21.9 (0–85) | NA | ||||
| Severity of infection | NA | NA | ||||||
| Mild | 39 | 16.3 | 10 | 22.7 | ||||
| Moderate | 87 | 36.3 | 20 | 45.5 | ||||
| Severe | 82 | 34.2 | 8 | 18.2 | ||||
| Unknown | 32 | 13.3 | 6 | 13.6 | ||||
| Race | ||||||||
| African American | 107 | 44.6 | 66 | 15.4 | 15 | 34.1 | 14 | 19.7 |
| Caucasian | 45 | 18.8 | 207 | 48.3 | 9 | 20.5 | 33 | 46.5 |
| Native American | 5 | 2.1 | 8 | 1.9 | 1 | 2.3 | 1 | 1.4 |
| Hispanic | 15 | 6.3 | 20 | 4.7 | 8 | 18.2 | 4 | 5.6 |
| Asian/Indian | 11 | 4.6 | 12 | 2.8 | 4 | 9.1 | 0 | 0 |
| Unknown | 57 | 23.8 | 116 | 27.0 | 7 | 15.9 | 19 | 26.8 |
| Comorbidities (≥1) | 205 | 85.4 | 326 | 76.0 | 44 | 100.0 | 71 | 100.0 |
| Obesity | 89 | 37.1 | 110 | 25.6 | 7 | 15.9 | 9 | 12.7 |
| DM | 97 | 40.4 | 127 | 29.6 | 12 | 27.3 | 24 | 33.8 |
| HTN | 174 | 72.5 | 273 | 63.6 | 23 | 52.3 | 33 | 46.5 |
| CKD | 89 | 37.1 | 199 | 46.4 | 44 | 100.0 | 71 | 100.0 |
| Biopsy indication | AKI: 74 AKI on CKD: 35 Proteinuria: 21 AKI and proteinuria: 81 Hematuria and proteinuria: 7 Hematuria: 6 CKD: 12 Unknown: 4 |
30.8 14.6 8.8 33.8 2.9 2.5 5.0 1.7 |
AKI: 81 AKI on CKD: 53 Proteinuria: 113 AKI and proteinuria: 94 Hematuria and proteinuria: 35 Hematuria: 10 CKD: 38 Unknown: 5 |
18.9 12.4 26.3 21.9 8.2 2.3 8.9 1.2 |
Rule out rejection: 38 Delayed graft function: 4 Protocol: 1 Hematuria: 1 |
86.4 9.1 2.3 2.3 |
Rule out rejection: 54 Delayed graft function: 4 Protocol: 7 Proteinuria: 5 Unknown: 1 |
76.1 5.6 9.9 7.0 1.4 |
| Creatinine level, mg/dl (range) | 5.69 (0.32–30.74) Unknown: 9 |
3.24 (104–20.6) Unknown: 83 |
4.21 (0.73–23.0) Unknown: 7 |
2.80 (0.6–11.5) Unknown: 13 |
||||
| Proteinuria (non-nephrotic) | 83 | 34.6 | 155 | 36.1 | 23 | 52.3 | 18 | 25.4 |
| Nephrotic range | 104 | 43.3 | 155 | 36.1 | 3 | 6.8 | 11 | 15.5 |
| No proteinuria | 3 | 1.3 | 22 | 5.1 | 5 | 11.4 | 12 | 16.9 |
| Unknown | 50 | 20.8 | 97 | 22.6 | 13 | 29.5 | 30 | 42.3 |
| Hematuria | 100 | 41.7 | 158 | 36.8 | 14 | 31.8 | 14 | 19.7 |
| None | 17 | 7.1 | 61 | 14.2 | 7 | 15.9 | 16 | 22.5 |
| Unknown | 123 | 51.3 | 210 | 49.0 | 23 | 52.3 | 41 | 57.7 |
| Need for RRT | ||||||||
| Yes | 71 | 29.6 | 32 | 7.5 | 3 | 6.8 | 1 | 1.4 |
| No | 55 | 22.9 | 166 | 38.7 | 18 | 40.9 | 25 | 35.2 |
| Unknown | 114 | 47.5 | 231 | 53.8 | 23 | 52.3 | 45 | 63.4 |
AKI, acute kidney injury; CKD, chronic kidney disease; COVID, coronavirus disease 2019; DM, diabetes mellitus; F, female; HTN, hypertension; M, male; NA, not applicable; RRT, renal replacement therapy.
All demographic and clinical parameters were compared to those from 5 years of biopsies prior to the pandemic (January 1, 2015 to January 1, 2020), comprising 63,575 native and 13,955 allograft biopsies. To provide estimates from the general biopsy database, the data are from a random sampling of 100 cases per year over 5 years (500 cases total, with 429 native and 71 allograft cases) through use of a random-number generator. As a control for systemic viral infection, 100 cases from HIV-positive individuals were also compared.
The mean age of COVID-19 patients undergoing native kidney biopsy was similar to the mean age in the biopsy database (53.7 years vs. 56.3 years), but the HIV comparison cohort was younger (mean age, 43.5 years). Comorbid conditions, including CKD, hypertension, diabetes mellitus, and obesity, were common in COVID-19 patients and in controls, with at least one comorbidity in 85.4% of patients with COVID-19, and in 76.0% from the biopsy database (Table 1). A majority of patients had moderate-to-severe disease at the time of kidney biopsy (70.5% of patients), with moderate disease defined as that requiring hospitalization and/or supplemental oxygen, and severe disease being that involving intensive-care unit admission with mechanical ventilation and/or dialysis (Table 1).
The leading indication for native biopsy was AKI (30.8% patients; mean creatinine = 5.69 mg/dl), followed by AKI on CKD (14.6%), and proteinuria (8.8%). For all patients with COVID-19 with COVAN, all had AKI. For transplant biopsies, the most common indication was a rise in serum creatinine level (86.4%), followed by delayed graft function, hematuria, and protocol surveillance biopsy. Concurrent proteinuria and/or hematuria was seen in a high proportion of patients for both native and transplant biopsies (Table 1).
Kidney biopsy diagnoses in patients with COVID-19
Kidney biopsies from patients with COVID-19 had a wide range of histopathologic diagnoses (Tables 2 and 3). For native biopsies (n = 240), COVAN was the most common (25.8%), with 27 other diagnoses represented (Table 2). There was evidence of ATI within the majority of biopsies (78.3% of native and 88.6% of allografts), although it was the sole or predominant finding within only 13.3% of cases. Within COVAN cases, a higher proportion of cases had concomitant ATI (96.8%). Within allografts, the most common diagnosis was allograft rejection (61.4%), followed by ATI (27.3%; Table 3 ).
Table 2.
Final diagnosis in kidney biopsies from patients with COVID-19 (n = 240), compared with biopsies from HIV-positive patients (n = 64), from 5 years of total biopsies prior to the COVID-19 pandemic (January 1, 2015 to January 1, 2020; n = 63,575)
| Final diagnosis | COVID-19 (n = 240) |
HIV (n = 94) |
Biopsy database (n = 63,575) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | P value | n | % | P value | |
| Collapsing glomerulopathy | 62 | 25.8 | 26 | 27.7 | 0.73 | 1177 | 1.8 | <0.001 |
| Acute tubular injury | 32 | 13.3 | 20 | 21.3 | 0.07 | 7613 | 11.9 | 0.52 |
| Diabetic nephropathy | 29 | 12.1 | 18 | 19.2 | 0.09 | 13,549 | 21.3 | <0.001 |
| Podocytopathies | 18 | 7.5 | 2 | 2.1 | 3877 | 6.1 | ||
| Minimal change disease | 11 | 4.6 | 2 | 2.1 | 0.07 | 1606 | 2.5 | 0.34 |
| Primary, noncollapsing FSGS | 7 | 2.9 | 0 | 0 | 2271 | 3.6 | ||
| Pauci-immune crescentic glomerulonephritis | 11 | 4.6 | 2 | 2.1 | 0.37 | 2782 | 4.3 | 0.87 |
| Membranous nephropathy | 11 | 4.6 | 4 | 4.3 | 1.0 | 3999 | 6.2 | 0.35 |
| Myoglobin cast nephropathy | 8 | 3.3 | 1 | 1.1 | 0.45 | 89 | 0.1 | <0.001 |
| Infection-associated GN | 8 | 3.3 | 10 | 10.6 | 0.01 | 2289 | 3.6 | 1.0 |
| Arterionephrosclerosis | 8 | 3.3 | 5 | 5.3 | 0.53 | 10,441 | 16.4 | <0.001 |
| FSGS, secondary | 8 | 3.3 | 9 | 9.6 | 0.03 | 2144 | 3.3 | 1.0 |
| IgA nephropathy | 7 | 2.9 | 6 | 6.4 | 0.20 | 4729 | 7.4 | 0.004 |
| Lupus nephritis | 6 | 2.5 | 0 | 0 | 0.19 | 3160 | 5.0 | 0.10 |
| Thrombotic microangiopathy | 5 | 2.1 | 3 | 3.2 | 0.69 | 1096 | 1.7 | 0.61 |
| Amyloidosis | 4 | 1.7 | 0 | 0 | 0.58 | 2187 | 3.4 | 0.13 |
| Acute interstitial nephritis | 4 | 1.7 | 10 | 10.6 | <0.001 | 1429 | 2.2 | 0.54 |
| PGMID | 4 | 1.7 | 0 | 0 | 0.12 | 115 | 0.2 | 0.001 |
| Cryoglobulinemic glomerulonephritis | 3 | 1.3 | 0 | 0 | 0.56 | 308 | 0.5 | 0.11 |
| Acute pyelonephritis | 2 | 0.8 | 0 | 0 | 1.0 | 972 | 1.5 | 0.59 |
| Light chain cast nephropathy | 2 | 0.8 | 0 | 0 | 1.0 | 1109 | 1.7 | 0.45 |
| MGMID | 1 | 0.4 | 0 | 0 | 1.0 | 132 | 0.2 | 0.39 |
| Cortical infarct | 1 | 0.4 | 0 | 0 | 1.0 | 107 | 0.2 | 0.33 |
| Anti-glomerular basement membrane antibody disease | 1 | 0.4 | 0 | 0 | 1.0 | 150 | 0.2 | 0.43 |
| Fibrillary glomerulopathy | 1 | 0.4 | 0 | 0 | 1.0 | 507 | 0.8 | 1.0 |
| Light chain deposition disease | 1 | 0.4 | 0 | 0 | 1.0 | 525 | 0.8 | 1.0 |
| Hemoglobin cast nephropathy | 1 | 0.4 | 0 | 0 | 1.0 | 7 | 0.001 | 0.03 |
| Thin glomerular basement membrane disease | 1 | 0.4 | 0 | 0 | 1.0 | 737 | 1.2 | 0.54 |
| Sickle cell nephropathy | 1 | 0.4 | 0 | 0 | 1.0 | 371 | 0.6 | 1.0 |
COVID-19, coronavirus disease 2019; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; MGMID, membranous-like glomerulopathy with monoclonal IgG kappa deposits; PGMID, proliferative glomerulonephritis with monoclonal IgG deposits.
P values represent comparisons to the COVID-19 patient cohort.
Table 3.
Final diagnosis in kidney allograft biopsies from patients with COVID-19 (n = 44), compared to HIV-positive patients (n = 6), from 5 years of total biopsies prior to the COVID-19 pandemic (January 1, 2015 to January 1, 2020; n = 13,955)
| Final diagnosis | COVID (n = 44) |
HIV (n = 6) |
Biopsy database (n = 13,955) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | P value | n | % | P value | |
| Allograft rejection | 27 | 61.4 | 5 | 83.3 | 0.39 | 3788 | 27.1 | <0.001 |
| Antibody-mediated rejection | 17 | 38.6 | 3 | 50 | ||||
| Acute T cell–mediated rejection | 6 | 13.6 | 2 | 33.3 | ||||
| Antibody + T cell–mediated rejection | 4 | 9.1 | 0 | |||||
| Acute tubular injury | 12 | 27.3 | 1 | 16.7 | 1.0 | 2472 | 17.7 | 0.09 |
| Negative for rejection | 2 | 4.5 | 0 | 0 | 1.0 | 5315 | 38.1 | <0.001 |
| Collapsing glomerulopathy | 2 | 4.5 | 0 | 0 | 1.0 | 187 | 1.3 | 0.12 |
| IgA nephropathy | 1 | 2.3 | 0 | 0 | 1.0 | 371 | 2.7 | 1.0 |
COVID, coronavirus; COVID-19, coronavirus disease 2019.
P values represent comparisons to the COVID-19 patient cohort.
Seven children were included in the above analysis—3 had native biopsies, and 4 had allografts. They ranged in age from 11 to 17 years; 5 were girls, and 2 were boys. Two were Caucasian, 2 were Hispanic, 1 was African American, and 2 were of unknown race. Native biopsy diagnoses included membranous nephropathy (NELL1-positive), crescentic IgA nephropathy, and ATI. The transplant diagnoses were antibody-mediated rejection (n = 2), antibody-mediated rejection with concurrent collapsing glomerulopathy, and acute T cell–mediated rejection.
Frequencies of COVID-19 kidney biopsy diagnoses compared to the total population undergoing kidney biopsy
To determine whether there was enrichment in any biopsy diagnosis within patients with COVID-19, compared to the general biopsied populations, the frequencies of each diagnosis were compared to the frequency of diagnosis within the 5-year biopsy database, as well as the cohort of 100 HIV-positive patients (Tables 2 and 3). A comparison of each COVID-19 kidney biopsy diagnosis to disease severity is included in Supplementary Tables S3A and S3B (for native and allograft biopsies, respectively).
Kidney diseases enriched in COVID-19 native biopsies included collapsing glomerulopathy, myoglobin cast nephropathy, and proliferative glomerulonephritis with monoclonal IgG deposits (Figure 1 ). For patients with myoglobin case nephropathy, 4 patients had an elevated creatinine kinase level at the time of biopsy (with the remainder of cases not having data available). A total of 4 patients had proliferative glomerulonephritis with monoclonal IgG deposits (PGMID), of which all contained kappa restricted immune deposits. The patients with PGMID were older adults (mean age, 65.8 years), and 2 had follow-up serum and urine electrophoresis (SPEP/UPEP) and were negative for a monoclonal paraprotein.
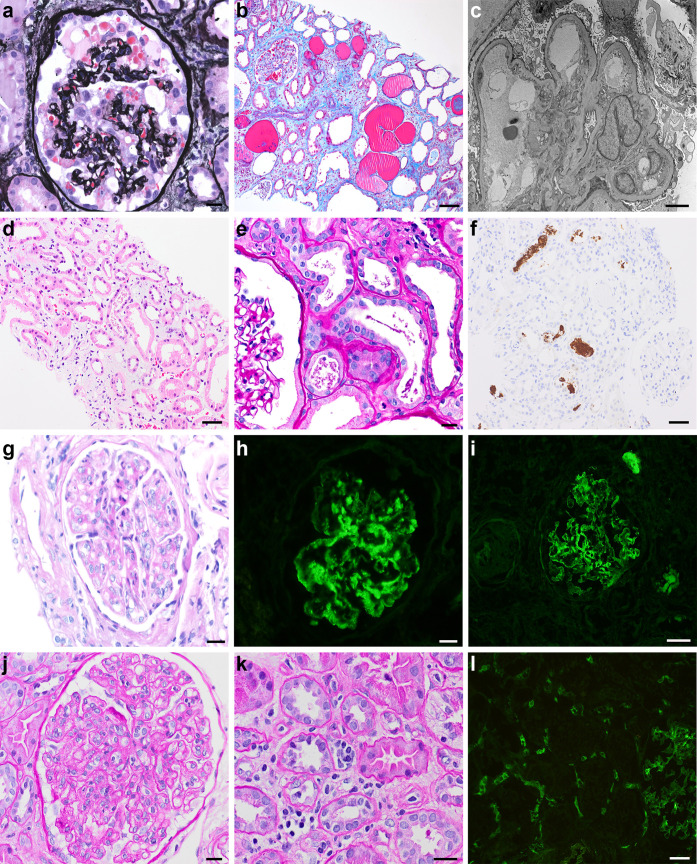
Figure 1.
Representative histopathology of kidney biopsy diagnoses enriched in coronavirus disease 2019 (COVID-19) patients. (a–c) Collapsing glomerulopathy: (a) Jones methenamine silver stain showing glomerular tuft collapse with an overlying epithelial cell proliferation; bar = 20 μm; (b) Masson trichrome stain showing microcystic tubular dilation; bar = 50 μm; (c) electron photomicrograph showing podocyte foot process effacement, original magnification ×1500; (d–f) myoglobin cast nephropathy; (d) hematoxylin and eosin stain showing tubular epithelial simplification and dilation with interstitial edema; bar = 100 μm; (e) periodic acid–Schiff stain showing beaded and granular casts; bar = 20 μm; (f) myoglobin immunohistochemical stain showing positivity within intratubular casts; bar = 100 μm; (g–i): proliferative glomerulonephritis with monoclonal Ig deposits (PGMID); (g) hematoxylin and eosin stain showing mesangial expansion and endocapillary hypercellularity within a glomerulus; bar = 20 μm; (h) granular mesangial and segmental capillary loop staining for IgG; bar = 20 μm; (i) granular mesangial and segmental capillary loop staining for kappa light chain; bar = 20 μm; (j–l) acute antibody-mediated rejection in kidney allograft; (j) periodic acid–Schiff stain showing glomerulitis; bar = 20 μm; (k) periodic acid–Schiff stain showing peritubular capillaritis; bar = 20 μm; and (l) C4d immunofluorescence positive in peritubular capillaries; bar = 100 μm. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Kidney diseases with reduced frequency in the setting of COVID-19 included chronic conditions, such as diabetic nephropathy, arterionephrosclerosis, and IgA nephropathy. Despite reduced diabetic nephropathy and arterionephrosclerosis in COVID-19 kidney biopsies, the frequency of diabetes mellitus and hypertension as clinical comorbidities was increased in patients with COVID-19 (40.4% vs. 29.6% with diabetes; 72.5% vs. 63.6% with hypertension). This finding is likely due to differences in biopsy indication, as there was an increased frequency of patients with COVID-19 presenting with AKI compared to that in the biopsy database (45.4% vs. 31.2%). An additional 21 diagnoses showed no differences from the biopsy database (Table 2).
When comparing diagnostic frequencies with those for HIV-positive patients, there was an increased frequency of both infection-associated glomerulonephritis and acute interstitial nephritis in HIV patients, compared to that for patients with COVID-19 (Table 2). Of note, there was a similar proportion of collapsing glomerulopathy cases (25.8% of patients with COVID-19 and 27.7% in HIV-positive patients), which may suggest similarities between COVAN and HIV-associated nephropathy (HIVAN).
Among allograft biopsies, there was an increased frequency of transplant rejection in patients with COVID-19, compared to that in pre-pandemic biopsies, and there were fewer biopsies with a diagnosis of “negative for rejection.” Other diagnoses were not significantly different between groups (Tables 2 and 3; Figure 1).
Histopathology of COVID-19 kidney biopsies
Histopathologic parameters assessed for all kidney biopsies in patients with COVID-19 included light, IF, and electron microscopic features, as detailed in the Methods section. A majority of the histopathologic findings were as expected for each pathologic diagnosis and were not unique within the setting of COVID-19 (detailed in Supplementary Tables S4–S9), with a few interesting observations.
For biopsies with membranous glomerulopathy, although not increased compared to the biopsy database, there was an enrichment of cases of unknown antigen type. Four of 11 cases were phospholipase A2 receptor–positive, 2 were NELL1-positive, and 5 were negative for phospholipase A2 receptor, NELL1, exostosin-1 or -2, and thrombospondin type 1 domain containing 7A.
All PGMID biopsies were kappa–light chain restricted, with one case being IgG1 kappa, 2 cases IgG3 kappa, and 1 case being kappa–light chain only (light chain–only variant of PGMID). An additional case of membranous-like glomerulopathy with masked IgG kappa deposits was identified. There is one biopsy with a membranous glomerulopathy with IgG1 kappa-restricted immune deposits, yielding a total of 6 patients with an immune complex–mediated glomerulonephritis with kappa light chain–restricted deposits. Other types of paraprotein-associated diseases were not increased in patients with COVID-19. There were 2 cases of light-chain cast nephropathy, and a single case of light-chain deposition disease, all of which were kappa light-chain restricted. AL amyloidosis cases (n = 3) were all lambda light-chain type.
Eleven patients had pauci-immune crescentic glomerulonephritis, including 8 with a known positive antineutrophil cytoplasmic antibody (ANCA) serology at the time of biopsy. The ANCA serologies were MPO-ANCA (n = 4), PR3-ANCA (n = 2), one with a positive ANCA by indirect IF without MPO or PR3 testing, and one with dual ANCA and anti-glomerular basement membrane disease. “Secondary” focal segmental glomerulosclerosis cases (n = 8) also had varied features, with 2 cases with APOL1-risk alleles (APOL1 nephropathy), 2 with concurrent glomerulomegaly (hyperfiltration), 1 with concurrent arterionephrosclerosis (ischemia), 1 with atypical segmental sclerosis with fibrous crescents (sclerosing glomerulopathy), and 2 of unknown etiology. For patients with amyloidosis, 3 had AL amyloidosis, and 1 had leukocyte cell-derived chemotaxin-2 (LECT2) amyloidosis. For lupus nephritis biopsies (n = 6), there were no cases showing activity, with 3 cases of sclerosing lupus nephritis (2 International Society of Nephrology/Renal Pathology Society [ISN/RPS] class IV-C, and 1 advanced sclerosing lupus nephritis ISN/RPS class VI), 2 cases of membranous lupus nephritis (ISN/RPS class V), and 1 case of minimal mesangial lupus nephritis (ISN/RPS class I). In cryoglobulinemic glomerulonephritis biopsies (n = 3), 1 had associated hepatitis C, 1 was favored to be autoimmune in nature (positive antinuclear autoantibodies at 1:1280), and 1 had concurrent vasculitis of unknown etiology.
Histopathologic parameters are documented for podocytopathies (Supplementary Table S4), other glomerular diseases (Supplementary Tables S5A and S5B), tubulointerstitial diseases (Supplementary Table S6), vascular diseases (Supplementary Table S7), CKDs (Supplementary Table S8), and transplants (Supplementary Table S9).
Evaluation of SARS-CoV-2 within biopsy tissue
Immunohistochemistry for SARS-CoV-2 was used to evaluate for direct viral infection within kidney parenchyma. SARS-CoV-2 immunohistochemistry was focally positive within <1% of tubular epithelial cells in 10 of 235 native kidney biopsies and in 1 of 38 allograft biopsies. Most likely, these 3.7% of cases were false positives. However, SARS-CoV-2 in situ hybridization was negative within these positive biopsies. There is equivalent sensitivity and increased specificity of in situ hybridization compared to immunohistochemistry, and therefore, there is no definite evidence of direct viral infection within the samples.
APOL1 genotyping and distribution of low-risk and high-risk genotype to biopsy diagnosis
APOL1 genotyping for G1 and G2 alleles was performed for all patients with collapsing morphology with residual tissue available for testing (n = 48), as well as noncollapsing focal segmental glomerulosclerosis biopsies from all patients of African American or Hispanic descent that had tissue available for testing (n = 59 patients, for a total of 107 patients). Of collapsing glomerulopathy cases, 38 were from African Americans, 2 were from Hispanics, and 8 were from patients of unknown race. Data with additional cases and corresponding genotypes are included in Supplementary Table S10.
The majority of native biopsies from African Americans and Hispanics had a high-risk APOL1 genotype (60.7%). A majority of patients with COVAN had a high-risk genotype (91.7%). Of note, there were only 4 COVAN patients without a high-risk genotype, all of which carried zero APOL1-risk alleles (Table 4 ).
Table 4.
APOL1 TaqMan polymerase chain reaction genotyping results from African American and Hispanic patients with COVID-19 (n = 107): a comparison of biopsy diagnosis to genomic risk allele status—low risk (G0/G0, G1/G0, or G2/G0) or high risk (G1/G1, G1/G2, or G2/G2)
| Diagnosis | Total number | 0 Alleles | 1 Allele | High-risk, 2 alleles | % High risk |
|---|---|---|---|---|---|
| Collapsing | 48 | 4 | 0 | 44 | 91.7 |
| Acute tubular injury | 10 | 3 | 0 | 7 | 70.0 |
| Diabetic nephropathy | 13 | 5 | 4 | 4 | 30.8 |
| Primary podocytopathies | 7 | 1 | 0 | 6 | 85.7 |
| Membranous | 5 | 3 | 2 | 0 | 0 |
| FSGS, favor secondary | 4 | 2 | 0 | 2 | 50 |
| ANCA-associated GN | 2 | 2 | 0 | 0 | 0 |
| Arterionephrosclerosis | 3 | 1 | 1 | 1 | 33.3 |
| Myoglobin casts | 3 | 1 | 2 | 0 | 0 |
| Thrombotic microangiopathy | 1 | 1 | 0 | 0 | 0 |
| Lupus nephritis | 1 | 1 | 0 | 0 | 0 |
| Light chain cast nephropathy | 2 | 1 | 1 | 0 | 0 |
| Amyloidosis | 1 | 1 | 0 | 0 | 0 |
| Cortical infarct | 1 | 1 | 0 | 0 | 0 |
| Acute interstitial nephritis | 1 | 0 | 0 | 1 | 100 |
| Hemoglobin casts | 1 | 1 | 0 | 0 | 0 |
| Cryoglobulinemic GN | 1 | 1 | 0 | 0 | 0 |
| Sickle cell nephropathy | 1 | 0 | 1 | 0 | 0 |
| C3 glomerulonephritis | 1 | 0 | 1 | 0 | 0 |
| Light chain deposition disease | 1 | 1 | 0 | 0 | 0 |
| Total low-risk | 39.3 | ||||
| Total high-risk | 60.7 | ||||
ANCA, antineutrophil cytoplasmic antibody; COVID-19, coronavirus disease 2019; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis.
In native non-COVAN biopsies, 35.6% carried a high-risk APOL1 genotype, with an additional 20.3% with one risk allele. Of non-COVAN cases with segmental sclerosis with or without another concurrent diagnosis (n = 36), 13 had 2 APOL1 risk alleles (36.1%; Supplementary Table S10). Patients with a high-risk APOL1 genotype had a trend toward increased focal segmental glomerulosclerosis lesions, compared to patients with a low-risk genotype (P = 0.03). Additionally, there was an increased degree of podocyte foot process effacement in patients with 2 APOL1 risk alleles (median 90% vs. 30% without risk alleles, P < 0.001).
Review of COVID-19 kidney biopsies reported in the literature
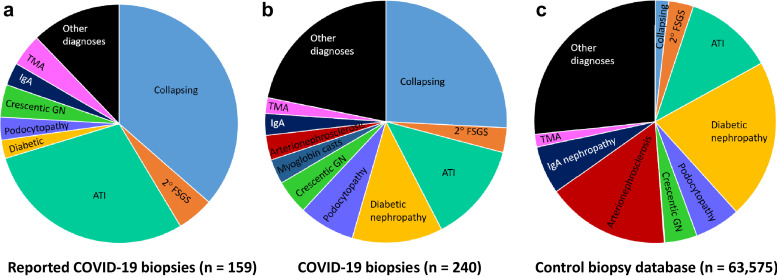
A total of 38 kidney biopsy case studies or case series were reported in the literature, including manuscripts and conference abstracts. Combined, these accounted for 165 cases, of which 158 were native biopsies, and 7 were transplant biopsies. The kidney diseases included COVAN (36.4%), ATI (28.9%), noncollapsing focal segmental glomerulosclerosis (5.0%), thrombotic microangiopathy (4.4%), and crescentic glomerulonephritis (4.4%), with 15 other diagnoses reported within native cases (Table 5 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 7, 8, 9 , 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 and Supplementary Table S1). Seven allograft biopsies were reported, 3 of which showed rejection (Table 5; Supplementary Table S1). Comparison of diagnostic frequencies compared to those among COVID-19 patients in this cohort, as well as to the 5-year biopsy database, is depicted in Figure 2 .
Table 5.
Diagnoses reported within the literature of COVID-19 kidney biopsies (n = 158 native and 7 allograft biopsies)
| Diagnosis | Number of cases | Frequency of cases in literature, % | Published references |
|---|---|---|---|
| Native kidney biopsies (n = 159) | |||
| Collapsing glomerulopathy | 58 | 36.7 | 7, 8, 9,15,20,21,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 |
| Acute tubular injury | 46 | 29.1 | 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 7, 8, 9 |
| FSGS, noncollapsing | 8 | 5.1 | 9,19,20,28 |
| Thrombotic microangiopathy | 7 | 4.4 | 7,9,16,20,25,26 |
| Crescentic GN, pauci-immune | 7 | 4.4 | 20,50,51 |
| IgA nephropathy | 6 | 3.8 | 20,41,47, 48, 49 |
| Minimal change disease | 5 | 3.2 | 8,9,21 |
| Membranous glomerulopathy | 5 | 3.2 | 8,20,41 |
| Diabetic glomerulopathy | 4 | 2.5 | 9,11,14,16,26,41 |
| Oxalate nephropathy | 2 | 1.3 | 14,43 |
| Anti-GBM antibody disease | 2 | 1.3 | 45,46 |
| Granulomatous tubulointerstitial nephritis | 1 | 0.6 | 27 |
| Acute interstitial nephritis | 1 | 0.6 | 14 |
| Lupus nephritis | 1 | 0.6 | 8 |
| MPGN, immune complex type (COVIC) | 1 | 0.6 | 44 |
| Infection-associated glomerulonephritis | 1 | 0.6 | 9 |
| Cortical infarct | 1 | 0.6 | 8 |
| Arteritis | 1 | 0.6 | 20 |
| Amyloidosis | 1 | 0.6 | 11,20 |
| Light chain cast nephropathy | 1 | 0.6 | 20 |
| Transplant kidney biopsies (n = 7) | |||
| Antibody-mediated rejection | 2 | 28.6 | 9 |
| T cell–mediated rejection | 1 | 14.3 | 8 |
| Acute tubular injury | 1 | 14.3 | 22 |
| Calcineurin inhibitor nephrotoxicity | 1 | 14.3 | 20 |
| Collapsing glomerulopathy | 1 | 14.3 | 42 |
| Severe IF/TA | 1 | 14.3 | 20 |
COVIC, COVID-19–associated immune complex disease; COVID-19, coronavirus disease 2019; FSGS, focal segmental glomerulosclerosis; GBM, glomerular basement membrane; GN, glomerulonephritis; IF, interstitial fibrosis; MPGN, membranoproliferative glomerulonephritis; TA, tubular atrophy.
Figure 2.
Frequencies of diagnosis in coronavirus disease 2019 (COVID-19) kidney biopsies compared to the pre-pandemic biopsied population. (a) Frequencies of diagnosis of COVID-19 kidney biopsies reported in the literature (n = 159 patients). (b) Frequencies of diagnosis of COVID-19 kidney biopsies in our multi-institutional cohort (n = 240 patients). (c) Comparison of diagnostic frequencies in the 5-year pre-pandemic biopsy cohort (n = 63,575 patients). ATI, acute tubular injury; FSGS, focal segmental glomerular sclerosis; GN, glomerulonephritis; TMA, thrombotic microangiopathy.
Additionally, 13 autopsy case studies and series were reported in the literature, which together included 176 decedents. ATI was the predominant finding within autopsies (55.1%), with 16 other diseases reported (Supplementary Table S2). Unlike in kidney biopsies, COVAN was only reported in 1.1% of cases, but there was a lower frequency of patients of African descent.
Discussion
We formed a multi-institutional collaboration to compare demographics and determine the frequency of kidney diseases in patients with COVID-19 and compared these frequencies to those in the general population biopsied to find those individuals most at risk and identify diseases that were increased among patients with COVID-19. COVAN, myoglobin cast nephropathy, and proliferative glomerulonephritis with monoclonal Ig deposits are increased in native kidney biopsies in our COVID-19 cohort, and allograft rejection was overrepresented in transplant biopsies.
Within the literature, there was a predominance of COVAN and ATI reported. The high number of COVAN cases is consistent with our cohort, and we did not identify an enrichment in ATI, thrombotic microangiopathy, or crescentic glomerulonephritis in our biopsy series, although several cases are reported in the literature. The reported cases of thrombotic microangiopathy may denote a selection bias in light of coagulopathy described in this population.58 Only 6 transplant biopsies were previously reported, 3 of which had allograft rejection, which is consistent with our study in which 61.3% of transplant biopsies had rejection. There was a lower frequency of diabetic nephropathy, IgA nephropathy, and arterionephrosclerosis as the primary diagnosis in kidney biopsies from patients with COVID-19 in our study and within the literature, likely due to an increased proportion of patients with COVID-19 being biopsied due to AKI rather than CKD.
The most common reported finding in autopsy studies was ATI (55.1%), markedly increased from the frequency in native kidney biopsies (13.3%). A high prevalence of ATI within autopsies could indicate multi-organ system dysfunction in severe illness rather than a primary manifestation and could be an overrepresentation due to postmortem autolysis. Additionally, the demographic distribution of the autopsied population varied from that of COVID-19 kidney biopsies (both reported and in this case series), with only 7.4% of decedents (for which ethnicity data are available) being African American.
We observed a 2.9-fold increase of African Americans biopsied compared to their representation in the general population within the United States (44.6% vs. 15.4%).59 African Americans have a disproportionate burden of COVID-19 infection and development of AKI compared to other ethnic populations, even aside from COVAN, the reasons for which are multifactorial. The incidence of SARS-CoV-2 infection in African Americans is nearly 3 times that in Caucasians.60 Geographic location is a contributing factor, with urban areas and 96% of “hot spots” for SARS-CoV-2 infection having a higher proportion of African Americans.60 African Americans also have a 1.5- to 2.4-fold increased incidence of comorbidities contributing to kidney dysfunction, including diabetes mellitus and obesity.61 Yet, the most important factor contributing to disproportionate AKI is likely related to APOL1 genomic risk alleles.62 Genotyping was selected for African Americans and Hispanics because 14% of all African Americans and 1% of Hispanics carry a high-risk APOL1 genotype.63 , 64 A staggering 60.7% of kidney biopsies from African Americans with COVID-19 were from patients with a high-risk APOL1 genotype, greater than 4 times as many as in the general African American population.
Although the frequency of diagnosis of collapsing glomerulopathy was markedly increased from that in the general population undergoing kidney biopsy, the frequency was similar to that of HIV patients. COVAN and HIVAN are thought to have similar pathophysiology, with a “second hit” to APOL1 risk alleles driven by increased circulating interferon generated as an immune response to viral infection. Supporting this possibility, tubuloreticular inclusions serving as “interferon footprints” have been identified ultrastructurally.35 Although the “second hit” for collapsing glomerulopathy may be short-lived due to viral clearance, it is uncertain whether outcomes in COVAN differ from those with a more persistent trigger, such as HIVAN, systemic lupus erythematosus, and malignancy.
With regard to the increase in myoglobin cast nephropathy within patients with COVID-19, there are reports of rhabdomyolysis associated with COVID-19, supporting this finding. Other viral infections have been reported to cause AKI due to rhabdomyolysis.65 Rhabdomyolysis is often a late complication of SARS-CoV-2 infection, can present prior to AKI,66 and carries a high mortality rate.67 Rhabdomyolysis in the setting of COVID-19 can be due to a necrotizing myopathy, which has been reported.68 , 69 A representative case from our muscle biopsy service is shown in Supplementary Figure S1. Measurement of creatine kinase level on admission could lead to early recognition of rhabdomyolysis, so that hydration therapy can be initiated.
Transient paraproteinemia has been reported in the setting of various infectious and autoimmune diseases.70, 71, 72 Although not previously described, it is possible that SARS-CoV-2 can induce transient paraproteinemia due to hyperactivity of the immune system in response to infection. Transient paraproteinemia has also been associated with kidney dysfunction.70 It is unknown whether this accounts for the increased frequency of proliferative glomerulonephritis with monoclonal IgG deposits in COVID-19 patients.
It has been postulated that SARS-CoV-2 may induce AKI through direct viral infection, as angiotensin 2 (ACE2), a membrane-bound peptidase that acts as receptor for SARS-CoV-2, is expressed within kidney tubular epithelial cells.73 , 74 Although such induction is possible, we were unable to demonstrate SARS-CoV-2 expression within tubular epithelial cells from COVID-19 kidney biopsies. Although there have been reports of viral-like particles seen ultrastructurally, similar ubiquitious intracellular structures were identified within biopsies of non-COVID-19 patients prior to the pandemic.75, 76, 77 Morphologic mimics of virions include secretory vesicles, multivesicular bodies, exosomes, coatomer-coated vesicles, and clathrin-coated vesicles. Therefore, detailed high-power electron microscopy to ultrastructurally evaluate for viral-like particles within podocytes was not performed in this study.19 , 75, 76, 77, 78, 79
Limitations
Our study includes the clinical and histopathologic data from kidney biopsies collected during the COVID-19 pandemic from March 2020 to March 2021, but it is lacking in longitudinal clinical follow-up. Therefore, we are unable to make comparisons of diagnostic data to patient outcomes. In the HIV-positive patient cohort, data on anti-retroviral drug use, CD4+ T cell count, and progression to AIDS are not available. Random sampling was used to estimate demographic data and biopsy indications for a 5-year cohort from the biopsy database, as these data could not be easily queried. Evaluation for direct viral infection included only SARS-CoV-2 immunohistochemistry, with SARS-CoV-2 in situ hybridization performed only as a reflex test for positive cases and not for the entire cohort. Additionally, detailed ultrastructural evaluation was not performed to examine for virions or tubuloreticular inclusions within COVID-19 patient biopsies, although routine electron microscopy was done for most cases.
Conclusion
We present the largest cohort of kidney biopsies from patients with COVID-19 to date, showing an increased frequency of COVAN, PGMID, and myoglobin cast nephropathy. Further studies are required to determine long-term clinical outcomes of patients with kidney disease in the setting of COVID-19, particularly in the setting of COVAN. Pulmonary manifestations have been found to persist long after viral clearance, but whether this is also true for kidney manifestations is unclear and is a subject for further investigation.
Disclosure
All the authors declared no competing interests.
Footnotes
Table S1. Comprehensive overview of published COVID-19 kidney biopsy findings.
Table S2. Review of autopsy findings within kidneys of SARS-CoV-2–infected decedents.
Table S3. Comparison of COVID-19 biopsy diagnosis to COVID-19 disease severity.
Table S4. Histopathologic features of podocyte diseases within native and transplant kidney biopsies from COVID-19 patients.
Table S5. (A) Light microscopic features of glomerular diseases, excluding podocytopathies, in native kidney biopsies from COVID-19 patients; (B) immunofluorescence and ultrastructure of these glomerular diseases.
Table S6. Histopathologic features of tubulointerstitial diseases in COVID-19 native and transplant kidney biopsies.
Table S7. Histopathologic features of vascular diseases in COVID-19 native and transplant kidney biopsies.
Table S8. Histopathologic features of chronic diseases in COVID-19 native and transplant kidney biopsies.
Table S9. Histopathologic features of kidney diseases within transplant biopsies from COVID-19 patients.
Table S10. APOL1 genotypes on individual native biopsy cases to include main diagnosis and presence or absence of focal segmental glomerulosclerosis.
Supplementary References.
Figure S1. Scattered pale staining of acutely necrotic muscle fibers with only minimal associated chronic lymphoid inflammation (necrotizing myopathy) in a 29-year-old African American male with myalgias and clinical nontraumatic rhabdomyolysis characterized by marked CPK elevation (∼180,000), occurring the week of COVID-19 diagnosis, which did not respond to hydration and supportive care, and required steroid treatment (hematoxylin and eosin, original magnification ×100).
Supplementary Material
Table S1. Comprehensive overview of published COVID-19 kidney biopsy findings.
Table S2. Review of autopsy findings within kidneys of SARS-CoV-2–infected decedents.
Table S3. Comparison of COVID-19 biopsy diagnosis to COVID-19 disease severity.
Table S4. Histopathologic features of podocyte diseases within native and transplant kidney biopsies from COVID-19 patients.
Table S5. (A) Light microscopic features of glomerular diseases, excluding podocytopathies, in native kidney biopsies from COVID-19 patients; (B) immunofluorescence and ultrastructure of these glomerular diseases.
Table S6. Histopathologic features of tubulointerstitial diseases in COVID-19 native and transplant kidney biopsies.
Table S7. Histopathologic features of vascular diseases in COVID-19 native and transplant kidney biopsies.
Table S8. Histopathologic features of chronic diseases in COVID-19 native and transplant kidney biopsies.
Table S9. Histopathologic features of kidney diseases within transplant biopsies from COVID-19 patients.
Table S10. APOL1 genotypes on individual native biopsy cases to include main diagnosis and presence/absence of focal segmental glomerulosclerosis.
Supplementary References.
Figure S1. Scattered pale staining of acutely necrotic muscle fibers with only minimal associated chronic lymphoid inflammation (necrotizing myopathy) in a 29-year-old African American male with myalgias and clinical nontraumatic rhabdomyolysis characterized by marked CPK elevation (∼180,000), occurring the week of COVID-19 diagnosis, which did not respond to hydration and supportive care, and required steroid treatment (hematoxylin and eosin, original magnification ×100).
References
- 1.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandhu S., Chand S., Bhatnagar A., et al. Possible association between IgA vasculitis and COVID-19. Dermatol Ther. 2020:e14551. doi: 10.1111/dth.14551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., Luo R., Wang K., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldfarb D.S., Benstein J.A., Zhdanova O., et al. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15:880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karagiannidis C., Mostert C., Hentschker C., et al. Case characteristics, resource use, and outcomes of 10,021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P., Uppal N.N., Wanchoo R., et al. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudose S., Batal I., Santoriello D., et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akilesh S., Nast C.C., Yamashita M., et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. 2021;77:82–93. doi: 10.1053/j.ajkd.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi G.M., Delsante M., Pilato F.P., et al. Kidney biopsy findings in a critically ill COVID-19 patient with dialysis-dependent acute kidney injury: a case against SARS-CoV-2 nephropathy. Kidney Int Rep. 2020;5:1100–1105. doi: 10.1016/j.ekir.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley B.T., Maioli H., Johnston R., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farkash E.A., Wilson A.M., Jentzen J.M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31:1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H., Yang M., Wan C., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golmai P., Larsen C.P., DeVita M.V., et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31:1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoriello D., Khairallah P., Bomback A.S., et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsoukkary S.S., Mostyka M., Dillard A., et al. Autopsy findings in 32 patients with COVID-19: a single-institution experience. Pathobiology. 2021;88:56–68. doi: 10.1159/000511325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diao B., Feng Z., Wang C., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Nat Commun. 2021;12:2506. doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia P., Wen Y., Duan Y., et al. Clinicopathological features and outcomes of acute kidney injury in critically ill COVID-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol. 2020;31:2205–2221. doi: 10.1681/ASN.2020040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadimitriou J.C., Drachenberg C.B., Kleiner D., et al. Tubular epithelial and peritubular capillary endothelial injury in COVID-19 AKI. Kidney Int Rep. 2021;6:518–525. doi: 10.1016/j.ekir.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferlicot S., Jamme M., Gaillard F., et al. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant. 2021 Feb 12:gfab042. doi: 10.1093/ndt/gfab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta R.K., Bhargava R., Shaukat A.-A., et al. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. 2020;21:326. doi: 10.1186/s12882-020-01970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westhoff T.H., Seibert F.S., Bauer F., et al. Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient. Am J Transplant. 2020;20:3216–3220. doi: 10.1111/ajt.16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simms E.L., Chung H., Oberding L., et al. Post-mortem molecular investigations of SARS-CoV-2 in an unexpected death of a recent kidney transplant recipient. Am J Transplant. 2021;21:2590–2595. doi: 10.1111/ajt.16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rapkiewicz A.V., Mai X., Carsons S.E., et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jhaveri K.D., Meir L.R., Flores Chang B.S., et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98:509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvatore S., Borczuk A.C., Seshan S.V. Renal pathology of 34 consecutive COVID autopsies: a single-institution experience. Am Soc Nephrol. 2020;31:299. [Google Scholar]
- 27.Szajek K., Kajdi M.E., Luyckx V.A., et al. Granulomatous interstitial nephritis in a patient with SARS-CoV-2 infection. BMC Nephrol. 2021;22:19. doi: 10.1186/s12882-020-02213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shetty A.A., Tawhari I., Safar-Boueri L., et al. COVID-19-associated glomerular disease. J Am Soc Nephrol. 2021;32:33–40. doi: 10.1681/ASN.2020060804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma Y., Nasr S.H., Larsen C.P., et al. COVID-19-associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020;2:493–497. doi: 10.1016/j.xkme.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H., Larsen C.P., Hernandez-Arroyo C.F., et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol. 2020;31:1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen C.P., Bourne T.D., Wilson J.D., et al. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magoon S., Bichu P., Malhotra V., et al. COVID-19-related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2020;2:488–492. doi: 10.1016/j.xkme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble R., Tan M.Y., McCulloch T., et al. Collapsing glomerulopathy affecting native and transplant kidneys in individuals with COVID-19. Nephron. 2020;144:589–594. doi: 10.1159/000509938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peleg Y., Kudose S., D’Agati V., et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5:940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaillard F., Ismael S., Sannier A., et al. Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int. 2020;98:241. doi: 10.1016/j.kint.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kissling S., Rotman S., Gerber C., et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadosh B.S., Pavone J., Wu M., et al. Collapsing glomerulopathy associated with COVID-19 infection in a heart transplant recipient. J Heart Lung Transplant. 2020;39:855–857. doi: 10.1016/j.healun.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couturier A., Ferlicot S., Chevalier K., et al. Indirect effects of severe acute respiratory syndrome coronavirus 2 on the kidney in coronavirus disease patients. Clin Kidney J. 2020;13:347–353. doi: 10.1093/ckj/sfaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nlandu Y.M., Makulo J.R., Pakasa N.M., et al. First case of COVID-19-associated collapsing glomerulopathy in sub-Saharan Africa. Case Rep Nephrol. 2020;2020:8820713. doi: 10.1155/2020/8820713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izzedine H., Brocheriou I., Arzouk N., et al. COVID-19-associated collapsing glomerulopathy: a report of two cases and literature review. Intern Med J. 2020;50:1551–1558. doi: 10.1111/imj.15041. [DOI] [PubMed] [Google Scholar]
- 41.Nasr S.H., Alexander M.P., Cornell L.D., et al. Kidney biopsy findings in patients with COVID-19, kidney injury, and proteinuria. Am J Kidney Dis. 2021;77:465–468. doi: 10.1053/j.ajkd.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazareth H., Péré H., Binois Y., et al. COVID-19-related collapsing glomerulopathy in a kidney transplant recipient. Am J Kidney Dis. 2020;76:590–594. doi: 10.1053/j.ajkd.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontana F., Cazzato S., Giovanella S., et al. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Rep. 2020;5:1815–1822. doi: 10.1016/j.ekir.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sethi S., D’Costa M.R., Hermann S.M., et al. Immune-complex glomerulonephritis following COVID-19 infection. Kidney Int Rep. 2021;6:1170–1173. doi: 10.1016/j.ekir.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brix S.R., Jones R.B., Jayne D.R.W. Glomerular basement membrane nephritis—crescentic renal inflammation and immunosuppressive intervention in the time of the severe acute respiratory syndrome coronavirus 2 pandemic. Kidney Int. 2021;99:1234–1235. doi: 10.1016/j.kint.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koc N.S., Yildirim T., Saglam A., et al. A patient with COVID-19 and anti-glomerular basement membrane disease. Nefrologia. 2021;41:471–473. doi: 10.1016/j.nefroe.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y., Li X.J., Li Y.Q., et al. Clinical and pathological findings of SARS-CoV-2 infection and concurrent IgA nephropathy: a case report. BMC Nephrol. 2020;21:504. doi: 10.1186/s12882-020-02163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suso A.S., Mon C., Oñate Alonso I., et al. IgA vasculitis with nephritis (Henoch-Schönlein Purpura) in a COVID-19 patient. Kidney Int Rep. 2020;5:2074–2078. doi: 10.1016/j.ekir.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N.L., Papini A.B., Shao T., et al. Immunoglobulin-A vasculitis with renal involvement in a patient with COVID-19: a case report and review of acute kidney injury related to SARS-CoV-2. Can J Kidney Health Dis. 2021;8 doi: 10.1177/2054358121991684. 2054358121991684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moeinzadeh F., Dezfouli M., Naimi A., et al. Newly diagnosed glomerulonephritis during COVID-19 infection undergoing immunosuppression therapy, a case report. Iran J Kidney Dis. 2020;14:239–242. [PubMed] [Google Scholar]
- 51.Uppal N.N., Kello N., Shah H.H., et al. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5:2079–2083. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.RANDOM.ORG Random number generator. https://www.random.org/integer-sets Available at:
- 53.Walker P.D., Cavallo T., Bonsib S.M. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555–1563. doi: 10.1038/modpathol.3800239. [DOI] [PubMed] [Google Scholar]
- 54.Best Rocha A., Stroberg E., Barton L.M., et al. Detection of SARS-CoV-2 in formalin-fixed paraffin-embedded tissue sections using commercially available reagents. Lab Invest. 2020;100:1485–1489. doi: 10.1038/s41374-020-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Agati V.D., Alster J.M., Jennette C., et al. Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol. 2013;8:399–406. doi: 10.2215/CJN.06100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roufosse C., Simmonds N., Clahsen-van Groningen M., et al. 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larsen C.P., Beggs M.L., Saeed M., et al. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol. 2013;24:722–725. doi: 10.1681/ASN.2012121180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goswami J., MacArthur T.A., Sridharan M., et al. A review of pathophysiology, clinical features, and management options of COVID-19 associated coagulopathy. Shock. 2021;55:700–716. doi: 10.1097/SHK.0000000000001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.US Census Bureau. US Census Bureau Quick Facts United States (vol. 2020). Available at: https://www.census.gov/quickfacts/fact/table/US/PST045219. Accessed January 1, 2021.
- 60.Moore J.T., Ricaldi J.N., Rose C.E., et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5-18, 2020—22 states, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brancati F.L., Kao W.H.L., Folsom A.R., et al. Incident type 2 diabetes mellitus in African American and White adults: The Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 62.Genovese G., Friedman D.J., Ross M.D., et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Limou S., Nelson G.W., Kopp J.B., Winkler C.A. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21:426–433. doi: 10.1053/j.ackd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kramer H.J., Stilp A.M., Laurie C.C., et al. African ancestry-specific alleles and kidney disease risk in Hispanics/Latinos. J Am Soc Nephrol. 2017;28:915–922. doi: 10.1681/ASN.2016030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munir I., Mehmood T., Mohiuddin A.F., et al. A rare complication of seasonal influenza: case report and a brief review of the literature. Am J Med Case Rep. 2020;8:293–298. [Google Scholar]
- 66.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26:1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh B., Kaur P., Reid R.R. Case reports: rhabdomyolysis associated with COVID-19. Am Fam Phys. 2020;102:645–648. [PubMed] [Google Scholar]
- 68.Román G.C., Spencer P.S., Reis J., et al. The neurology of COVID-19 revisited: a proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J Neurol Sci. 2020;414:116884. doi: 10.1016/j.jns.2020.116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basiratnia M., Derakhshan D., Yeganeh B.S., et al. Acute necrotizing glomerulonephritis associated with COVID-19 infection: report of two pediatric cases. Pediatr Nephrol. 2021;36:1019–1023. doi: 10.1007/s00467-021-04944-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vodopick H., Chaskes S.J., Solomon A., et al. Transient monoclonal gammopathy associated with cytomegalovirus infection. Blood. 1974;44:189–195. [PubMed] [Google Scholar]
- 71.Stoimenis D., Spyridonidou C., Papaioannou N. Transient monoclonal gammopathy induced by disseminated Staphylococcus aureus infection. Case Rep Med. 2012;2012:607104. doi: 10.1155/2012/607104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strobel S.L. Transient paraproteinemia: an intriguing immunological anomaly. Ann Clin Lab Sci. 2003;33:265–270. [PubMed] [Google Scholar]
- 73.Li N., Zimpelmann J., Cheng K., et al. The role of angiotensin converting enzyme 2 in the generation of angiotensin 1-7 by rat proximal tubules. Am J Physiol Renal Physiol. 2005;288:F353–F362. doi: 10.1152/ajprenal.00144.2004. [DOI] [PubMed] [Google Scholar]
- 74.Koitka A., Cooper M.E., Thomas M.C., et al. Angiotensin converting enzyme 2 in the kidney. Clin Exp Pharmacol Physiol. 2008;35:420–425. doi: 10.1111/j.1440-1681.2008.04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cassol C.A., Gokden N., Larsen C.P., et al. Appearances can be deceiving—viral-like inclusions in COVID-19 negative renal biopsies by electron microscopy. Kidney360. 2020;1:824–828. doi: 10.34067/KID.0002692020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calomeni E., Satoskar A., Ayoub I., et al. Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int. 2020;98:233–234. doi: 10.1016/j.kint.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roufosse C., Curtis E., Moran L., et al. Electron microscopic investigations in COVID-19: not all crowns are coronas. Kidney Int. 2020;98:505–506. doi: 10.1016/j.kint.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frelih M., Erman A., Wechtersbach K., et al. SARS-CoV-2 virions or ubiquitous cell structures? Actual dilemma in COVID-19 era. Kidney Int Rep. 2020;5:1608–1610. doi: 10.1016/j.ekir.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akilesh S., Nicosia R.F., Alpers C.E., et al. Characterizing viral infection by electron microscopy: lessons from the Coronavirus disease 2019 pandemic. Am J Pathol. 2021;191:222–227. doi: 10.1016/j.ajpath.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.