Abstract
Background
The cutaneous manifestations of COVID-19 may be useful disease markers and prognostic indicators. Recently, postinfectious telogen effluvium and trichodynia have also been reported.
Objective
To evaluate the presence of trichodynia and telogen effluvium in patients with COVID-19 and describe their characteristics in relation to the other signs and symptoms of the disease.
Methods
Patients with a history of COVID-19 presenting to the clinics of a group of hair experts because of telogen effluvium and/or scalp symptoms were questioned about their hair signs and symptoms in relation to the severity of COVID-19 and associated symptoms.
Results
Data from 128 patients were collected. Telogen effluvium was observed in 66.3% of the patients and trichodynia in 58.4%. Trichodynia was associated with telogen effluvium in 42.4% of the cases and anosmia and ageusia in 66.1% and 44.1% of the cases, respectively. In majority (62.5%) of the patients, the hair signs and symptoms started within the first month after COVID-19 diagnosis, and in 47.8% of the patients, these started after 12 weeks or more.
Limitations
The recruitment of patients in specialized hair clinics, lack of a control group, and lack of recording of patient comorbidities.
Conclusion
The severity of postviral telogen effluvium observed in patients with a history of COVID-19 infection may be influenced by COVID-19 severity. We identified early-onset (<4 weeks) and late-onset (>12 weeks) telogen effluvium.
Key words: COVID-19, hair loss, hair shedding, multicentric study, SARS-CoV-2, scalp allodynia, telogen effluvium, trichodynia
Abbreviations used: OR, odds ratios; TE, telogen effluvium; TR, trichodynia
Capsule Summary.
-
•
Postinfection telogen effluvium observed in patients with a history of COVID-19 infection can develop after 4 weeks (early onset) or 12 weeks (late onset) from the time of infection, with or without associated trichodynia.
-
•
Clinicians should recognize possible hair signs and symptoms in otherwise asymptomatic or minimally symptomatic patients.
Introduction
The COVID-19 pandemic has affected more than 125 million people worldwide and continues to spread rapidly across the globe.1,2 The most common symptoms of this disease are fever, asthenia, headache, muscle or joint pain, anosmia, ageusia, shortness of breath, dry cough, and pneumonia. However, a high number of cutaneous manifestations have also been reported. Acral lesions resembling those of chilblains are the most reported signs in young adults with a pauci or asymptomatic viral course,3 whereas maculo-papular and vesicular rashes are the most common in middle-aged patients.4, 5, 6 It is still debated whether the skin manifestations are a direct consequence of COVID-19 pathogenicity or a consequence of thrombogenic and immune deregulatory responses triggered by SARS-CoV-2 infection.7 Recently, alopecia areata and androgenetic alopecia were shown to be associated with COVID-19; however, acute telogen effluvium (TE) appears to be the most common trichologic disease in patients with COVID-19.8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Trichodynia (TR) is a common accompanying symptom of TE,18,19 but to date, the literature has been silent on its occurrence with respect to COVID-19. For this reason, we decided to collect data, using a multicenter survey, of all patients referred to our hair clinics for TE with or without TR with a history of COVID-19 infection and describe their characteristics.
Methods
An international call addressed to several hair experts, listed as the authors of this paper, was launched in April 2020 by the leading author (Dr Starace) with the aim of collecting epidemiologic, demographic, and clinical data of patients attending their practice for skin or scalp diseases and reporting COVID-19. The authors were asked to complete an online survey 4 times over a 6-month period (from April 2020 to September 2020). At the end of the survey period, the data of patients in whom TE and/or TR developed concomitant with or after COVID-19 were collected and analyzed in detail: sex, age, COVID-19 duration (computed, when available, from the time of the first positive swab to the first negative swab), temporal gap (latency) between TE or TR onset and the first positive swab, other clinical signs and symptoms of COVID-19, treatment received during the infection, possible hospitalization, and/or intensive care unit admission. Patients with a previous history of hair disorder (any kind) were excluded from the study in order to avoid a possible bias. COVID-19–related signs and symptoms were scored as follows: an asymptomatic or a paucisymptomatic course was scored as 1; a symptomatic course with systemic involvement but no hospitalization was scored as 2; and a severe, long-lasting course with hospitalization was scored as 3.
Data processing and statistical analysis
The collected data were error checked and coded before being entered into a computer database. Frequencies were used to summarize qualitative data and median and interquartile range to summarize quantitative data. The association between categorical variables was tested using the χ2 test. The student t test or Mann-Whitney test for independent means was used to compare scale variables according to a parametric or nonparametric distribution, which was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Subsequently, adjusted odds ratios (OR) with their 95% CIs obtained from multivariate logistic regression were computed for variables common to the TE+, TR,+ and TE+/TR+ groups. The backward likelihood ratio method was chosen for variable selection. The assumptions required for logistic regression were checked using the Hosmer and Lemeshow fit test. Heteroschedasticity was assessed using the Breusch-Pagan test. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 23 (IBM Corp).
Results
Survey results
In total, the data of 128 adult patients, 24 men and 104 women, presenting with TE and/or TR were collected and analyzed. To summarize the data obtained from the 4 consecutive surveys, the median of the reported percentages and numerical values was computed.
In decreasing order, morbilliform (33.3%), urticarial (21.4%), erythema polymorphous-like (20%), chickenpox-like (20%), and purpuric (16.7%) rashes were observed in this group of patients. The less common findings were herpes zoster virus reactivation (13.3%) and atopic flares (6.7%). TE was observed in 66.3% (84/128) of the cases and TR in 58.4% (74/128). In 42.4% of the cases, TE was associated with TR. TR was reported as 1 or more of the following manifestations: itching (37.6%), burning (34.7%), pain (26.7%), or paresthesia (4.9%), and it was graded as severe in 32% of the cases. The most commonly reported signs and symptoms of COVID-19 observed in patients with TE or TR were as follows: fever (75.2%), anosmia (57.4), cough (55.8%), dysgeusia (41.6%), asthenia (28.7%), headache (23.8%), myalgia (10.9%), coryza (9.9%), dyspnea (7.9%), and diarrhea (5.9%) (Table I). Relief from TR was achieved in 44% of the cases, in particular by sleeping (20.3%), washing with warm or cold water (13.5%), applying high-potency topical steroids (11.8%), combing (6.7%), massaging (3.3%), and scratching (1.7%). Among patients referred for TE, 47.8% experienced reactive TE, starting from 12 weeks or more after the diagnosis of the infection, whereas 52.2% experienced early-onset TE. Only 69.7% of patients with TE or TR received treatments: high-potency topical steroids (50%), supplements with amino acids and vitamin B complex (27%), topical minoxidil (15%), and pain relief lotions or hair growth promoters (10%).
Table I.
Summary of data collected with the second part∗
| Factors | TR |
TE |
TR + TE |
Total |
|
|---|---|---|---|---|---|
| n = 34 cases | n = 42 cases | n = 25 cases | n = 101 | ||
| F:M ratio | 24:10 | 39:3 | 21:4 | 84:17 | |
| Age (y) | 44 (37.5-54.75) | 45 (28.5-58) | 48 (41.2-61) | 45 (38-58.7) | |
| Latency of symptom onset—positive swab (d) | 3 (0-6.5) | 3 (1.5-4) | 2 (0-5) | 3 (0-5)‡ | |
| Latency of TR (wk) | 2 (1-3) | - | 1† 1.3 (0.9-2) |
1† 1.7 (1-2.6) |
P < .05 |
| Duration of TR (wk) | 5 (3-6.2) | - | 4 (2-6.5) | 5 (3-6)‡ | |
| Latency of TE (wk) | - | 13 (10.5-13.2) | 3 (2-7.5) | 11 [3-13]‡ | P < .05 |
| Duration of TE (wk) | - | 8† 9 (7.2-10) |
2† 8 [7-9] |
10† 8 [7-10] |
|
| Early TE <12 wk (%) | - | 23.8 | 100 | 52.2 | P < .05 |
| Disease severity (%) | - | - | - | P < .05 | |
| 1 | 46.7 | 66.7 | 20 | 48.5 | |
| 2 | 53.3 | 26.2 | 68 | 45.5 | |
| 3 | 0 | 7.1 | 12 | 5.9 | |
| Fever (%) | 76.7 | 71.4 | 80 | 75.2 | |
| Cough (%) | 55.8 | 47.6 | 60 | 53.5 | |
| Ageusia (%) | 32.3 | 26.2 | 80 | 41.6 | P < .05 |
| Anosmia (%) | 67.6 | 38.1 | 72 | 57.4 | P < .05 |
| Diarrhea (%) | 0 | 7.1 | 12 | 5.9 | |
| Myalgia (%) | 5.9 | 11.9 | 16 | 10.9 | |
| Coryza (%) | 11.8 | 11.9 | 4 | 9.9 | |
| Headache (%) | 38.2 | 11.9 | 24 | 23.8 | P < .05 |
| Dyspnea (%) | 5.9 | 4.8 | 16 | 7.9 | |
| Asthenia (%) | 10 | 19 | 72 | 28.7 | P < .05 |
| ICU | 0 | 4.8 | 12 | 5 | |
| COVID duration (d) | 22† 9 (5-15) |
17† 14 (9.7-22.2) |
19† 20.5 (9.7-26.7) |
58† 15 (8.7-23.2) |
P < .05 |
ICU, Intensive care unit; TE, telogen effluvium; TR, trichodynia.
Categorical variables are reported as percentages, whereas quantitative variables are reported as median, followed by the first and third quartile in parentheses. The χ2 test, student t test, and Mann-Whitney test were performed as appropriate. Significant P values (α < 0.05) are set in bold.
Missing cases.
P <.05 at normality tests (Kolmogorov-Smirnov and Shapiro-Wilk).
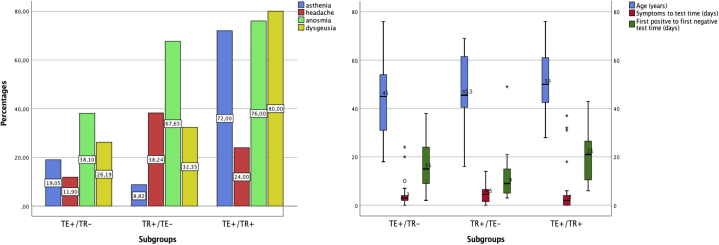
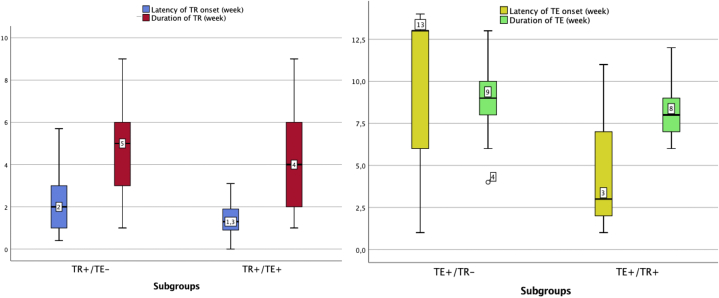
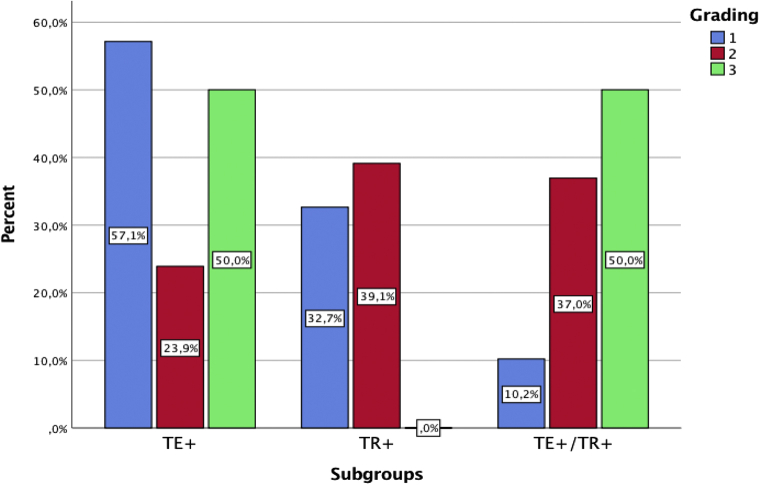
Among the 128 patients, the authors were able to provide detailed data about TR or TE in only 101 and, in particular, in 34 cases of TR alone (TR+/TE−), 42 cases of TE alone (TE+/TR−), and 25 cases of TE associated with TR (TR+/TE+). Upon performing the inferential statistical analysis, these 3 subgroups were found to differ significantly in the following factors: disease severity, ageusia, anosmia, headache, asthenia, and COVID-19 duration (P < .05) (Fig 1). The TR+/TE+ group had significantly shorter COVID-TR latency onset than the TR+/TE− group (1 vs 2 weeks, respectively, P < .05), whereas the 2 groups did not differ significantly in terms of TR duration (4 vs 5 weeks, respectively). The same trend was observed among the TE+/TR− and TE+/TR+ groups with regard to latency time lapse (13 vs 3 weeks, respectively, P < .05) and TE duration (9 vs 8 weeks, respectively) (Fig 2). The TR+/TE+ group was characterized by higher disease severity compared with the TR+/TE− or TE+/TR− group (P < .05) (Fig 3).
Fig 1.
The first bar plot shows the different prevalence of asthenia, headache, anosmia, and dysgeusia among the 3 groups studied. The χ2 test yielded significant P values (P < .05) for all the displayed variables. The second box plot illustrates the distribution of the 3 quantitative variables common to the groups studied. Outliers' values are displayed as stars. The COVID-19 duration was significantly shorter in the TR+ group (P < .05). TE+/TR−, Telogen effluvium without trichodynia; TE+/TR+, telogen effluvium with trichodynia; TR+/TE−, trichodynia without telogen effluvium; TR+/TE+, trichodynia with telogen effluvium.
Fig 2.
The box plot of TR/TE latency and disease duration among the 2 group clusters compared (TE+ vs TE+/TR+ and TR+ vs TR+/TE+) shows the difference in latency (P < .05), whereas disease durations did not differ significantly. TE+/TR−, Telogen effluvium without trichodynia; TE+/TR+, telogen effluvium with trichodynia; TR+/TE−, trichodynia without telogen effluvium; TR+/TE+, trichodynia with telogen effluvium.
Fig 3.
The bar plot shows the distribution of disease severity among the groups studied. TE+, Telogen effluvium alone; TE+/TR+, telogen effluvium with trichodynia; TR+, trichodynia alone.
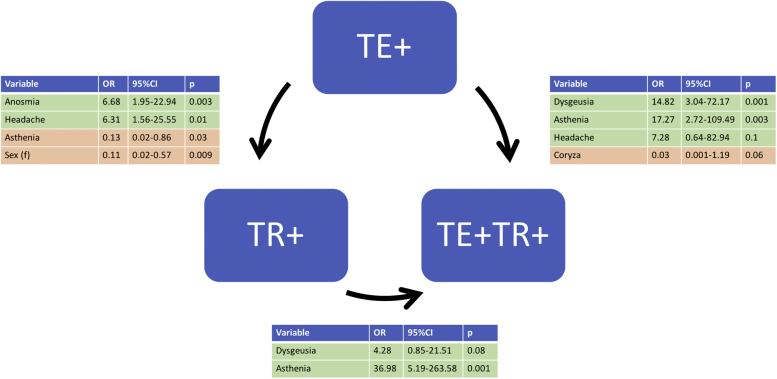
Complete resolution of TE or TR occurred in 91 of 101 patients, whereas 10 cases had persistent active disease at the time of study completion. By setting a cutoff of 12 weeks, 100% of the TE+/TR+ cases occurred within 12 weeks, whereas TE developed within a 3-month time range in only 23.8% of the TE+/TR− patients. Subsequently, multivariate logistic regression was performed to compare the TR+/TE−, TE+/TR−, TR+/TE+ groups in coupled analyses. Anosmia and headache were positively correlated with TR+ (OR: 6.68 and 6.31, respectively), whereas asthenia and female sex had a negative correlation (OR: 0.13 and 0.11, respectively) (Fig 4).
Fig 4.
Graphical representation of the multivariate logistic regression. The arrows indicate the group whose probability is predicted. In a binary response model, the adjusted OR is the estimate of the OR between the 2 events, where the effects of other variables are held constant. OR, Odds ratio; TE+, telogen effluvium alone; TE+/TR+, telogen effluvium with trichodynia; TR+, trichodynia alone.
Dysgeusia and asthenia appeared to be significant risk factors, as determined using the TR+/TE− versus TE+/TR+ analysis (OR: 4.28, 36.98, respectively).
Discussion
TE and TR as complications of COVID-19 were probably still present in the first pandemic wave but were probably overlooked because of other cutaneous features of the disease attracting the most attention. Subsequent reports highlighted the occurrence of TE in patients with a recent history of COVID-19. The evidence of increased postviral TE in patients with a history of COVID-19 has also been reported by 2 web-based studies: the first recorded a 27.9% TE incidence in a sample of 563 surveyed individuals forced to stay at home because of quarantine,17 whereas the other found an increase in searches for the keywords “hair loss” by querying Google Trends.16 Some authors attributed the possible worsening of pre-existing TE to psychosocial stress inflicted by the quarantine imposed on the people by the government.15 However, the presence of emotional stress is not indisputable proof that stress incited the patients' hair loss. This relationship might have also been inversely related.
TE is a frequent cause of hair loss and is characterized by acute or chronic diffuse shedding of hair.20,21 It may be associated with TR and may cause a painful sensation of the scalp upon touching of or brushing through the hair. Acute TE undergoes remission within 3-6 months of onset unless associated with androgenetic alopecia, whereas chronic TE persists beyond 6 months. Different alterations in the hair cycle have been proposed as the pathogenetic mechanism underlying TE. Headington22 proposed 5 functional types of acute TE based on alternations in particular phases of the follicular cycle: immediate or delayed anagen release, short anagen syndrome, and immediate or delayed telogen release. Several triggers, such as drugs, trauma, and emotional and physiologic stress, can lead to the development of acute TE. Chronic TE has been proposed to be due to a persistent reduction in the variance of anagen duration.23 In around 33% of cases, the cause of chronic TE remains unknown.24
In post-COVID-19–infection TE, it has been suggested that the viral insult induces TE through immediate anagen release. Postinfectious hair loss has traditionally been categorized as acute TE; however, it may result from different pathogenetic mechanisms and show different clinical patterns. Evidence exists that the hair follicle might respond to the infection via 2 shedding patterns: dystrophic anagen effluvium and TE according to the type and intensity of the insult. In this way, patients affected by more severe forms of COVID-19 may present with early-onset telogen phase, namely, dystrophic anagen effluvium, suggesting a more profound effect of the levels of proinflammatory cytokines (interleukin 1β, interleukin 6, tumor necrosis factor α, type 1 and type 2 interferon, and metalloproteinases 1 and 3) on the matrix cells of the hair follicle.12
High levels of interleukin 6 have been shown to be associated with asthenia,25 which, in our study, was a strong independent predictor of TE+/TR+ in a bivariate logistic analysis, regardless of whether the comparison group was TR+/TE− (OR: 36.98, 95% CI: 5.19-263.58) or TE+/TR− (OR: 17.27, 95% CI: 2.72-109.49). Additionally, asthenia negatively correlates with TR+ compared with TE+ versus TR+ cohorts (OR: 36.98, 95% CI: 5.19-263.58). Severe cases of COVID-19 were equally distributed among the TE+/TE− and TE+/TR+ groups, whereas less severe cases were more commonly characterized by TR+/TE−.
High levels of interferons have also been shown to be associated with acute TE,13 metalloproteinases 1 and 3 and interleukin 1β with hair follicle growth inhibition,26 and interleukin 6 with the induction of the catagen phase.27
We noticed that in 100% of the patients belonging to the TR+/TE+ group and 23.8% of the patients belonging to the TE+ group, hair loss developed before 12 weeks, with a median latency period of 3 versus 13 weeks, respectively, among the groups. Therefore, we hypothesized that an immune-mediated microthrombotic reaction at the level of the hair follicle vessels, or a direct infection of the hair follicle by the virus, results in inflammation and cell death. However, evidence for this has not yet been provided by a histopathologic examination.
The coagulation cascade, activated in response to COVID-19 infection, is responsible for a decreased concentration of anticoagulant proteins due to reduced production and increased consumption. In addition, these factors can induce microthrombi formation, which may occlude hair follicle blood supply.
Drugs used to treat the infection may also play a role in the pathogenesis of TE, especially in late-onset TE. Watras et al28 highlighted the role of anticoagulants, including enoxaparin, in inducing TE. Moreover, in other studies, the use of hydroxychloroquine, azithromycin, and other medications could not be excluded as potential triggers for the development of TE. The possible limitations of studies on drug-related TE are represented by missing data regarding drug protocols: rapid change of administered drugs and dose regimens as well as the lack of standardized protocols provide data that are too fragmented to be analyzed.
Another important consideration is that a majority of patients with COVID-19 are under psychologic and physiologic stress, both potential triggers for TE and TR.
TR is not specific for TE and has been shown to be associated with androgenetic alopecia and psychologic illnesses, such as depression, obsessive personality disorder, and anxiety.29 In the past, it has been demonstrated that TR does not correlate with the activity of hair loss.30 The only valid information on TR is that it correlates with increased neuropeptide substance P expression.31 Substance P is a stress-related neuronal peptide released by nerve terminals and represents a key factor in neuroinflammation, leading to the release of neuronal mediators implicated in neuropathic pain, as might occur in the scalp with regard to hair loss. Substance P may also play a role in hair cycle modification mediated by stress: stress seems to influence the relationship of substance P with mast cells through neurokinin-1 receptors.
Moreover, substance P has been shown to be associated with sleep deprivation. During the COVID-19 pandemic, people experienced greater anxiety caused by the fear of the disease, worries about the outcomes of the pandemic, family loss, and personal uncertainty about the future, which had a significant impact on sleep. The combination of increased stress, frequent hair wash to avoid virus transmission, and sleep disorders can result in TR more or less being associated with hair loss.32 Interestingly, in our sample, anosmia correlated with TR+/TE− both in the multivariate and univariate analyses, whereas dysgeusia correlated with TE+/TR+ in the multivariate analysis. Overall, both anosmia and dysgeusia largely prevailed in TR+ patients, possibly suggesting its neurogenic role in patients with this condition. Substance P is also a mediator of sterile inflammation of the dura mater, which has been considered as the source of migraine pain. Substance P, together with calcitonin gene-related peptide, leads to a cascade of inflammatory tissue responses, including arteriolar vasodilation, plasma protein extravasation, and degranulation of mast cells in their peripheral target tissue.33 According to our data, headache showed, in fact, a positive correlation with TR+.
Conclusions
Our analysis demonstrated that patients with a recent history of COVID-19 infection might consult a hair specialist for postinfection effluvium of early or late onset, with or without associated TR. In addition to the typical acute TE, which occurs 3-4 months after an infection, we observed early-onset effluvium occurring within less than 2 months after COVID-19 infection. TR, when present, is related to anosmia, ageusia, and headache. We hypothesized that early hair shedding is related to a direct injury caused by the virus and, in particular, due to immune-mediated microthrombotic events within the follicular vasculature and a cytokine storm. Histopathologic evidence is as yet pending.
However, this study had several limitations, including that the survey and the enrollment of the patients were conducted on a select population not representative of the general population. In addition, our data did not include all patients affected by COVID-19 but only those patients who specifically seek a hair expert dermatologist for medical advice on their hair signs and symptoms.
The other limitations of our study were the lack of a control group and the lack of the recording of patient comorbidities that can induce TE or TR.
The aim of the study was, however, not to present data on the prevalence of TE and TR in the general population with COVID-19 but to describe the occurrence of hair findings in patients with a history of COVID-19 presenting for hair evaluation and their correlation with the other known symptoms of COVID-19 as well as to contribute to their understanding. An open question is whether we are dealing with neuropeptide substance P-related TR or scalp allodynia due to associated neuroinflammation. This symptom showed a correlation with other symptoms of neurologic involvement, such as anosmia or dysgeusia and headache. Thus, the question of whether TR is neuro related or caused by microinjuries remains to be determined.
Dermatologists and primary care physicians should be aware of the possible presence of TR, alone or associated with TE, in patients with a history of COVID-19 infection because it might be a presenting sign of the infection in otherwise asymptomatic or minimally symptomatic patients.
Conflicts of interest
Dr Tosti is a consultant for DS Laboratories, Monat Global, Almirall, Tirthy Madison, Eli Lilly, Leo Pharmaceuticals, Bristol Myers Squibb, and P&G. Drs Starace, Lorizzo, Sechi, Alessandrini, Carpanese, Bruni, Vara, Apalla, Asz-Sigall, Barruscotti, Camacho, Doche, Estrada, Dhurat, Gavazzoni, Grimalt, Harries, Ioannidis, McMichael, Melo, Oliveira, Ovcharenko, Pirmez, Ramot, Rudnicka, Shapiro, Silyuk, Sinclair, Vano-Galvan, and Piraccini have no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Reprints not available from the authors.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassi A., Russo T., Argenziano G., et al. Chilblain-like lesions during COVID-19 pandemic: the state of the art. Life (Basel) 2021;11(1):23–27. doi: 10.3390/life11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzano A.V., Genovese G., Fabbrocini G., et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubanov A.A., Deryabin D.G. Skin manifestations in COVID-19 provide a clue for disease's pathophysiology understanding. J Eur Acad Dermatol Venereol. 2020;35(1):e3–e4. doi: 10.1111/jdv.16902. [DOI] [PubMed] [Google Scholar]
- 6.Tan S.W., Tam Y.C., Oh C.C. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119–133. doi: 10.1016/j.jdin.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daneshgaran G., Dubin D.P., Gould D.J. Cutaneous manifestations of COVID-19: an evidence-based review. Am J Clin Dermatol. 2020;21(5):627–639. doi: 10.1007/s40257-020-00558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trüeb R.M., Dutra Rezende H., Gavazzoni Dias M.F. What can the hair tell us about COVID-19? Exp Dermatol. 2021;30(2):288–290. doi: 10.1111/exd.14259. [DOI] [PubMed] [Google Scholar]
- 9.Olds H., Liu J., Luk K., Lim H.W., Ozog D., Rambhatla P.V. Telogen effluvium associated with COVID-19 infection. Dermatol Ther. 2021;34(2):e14761. doi: 10.1111/dth.14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cline A., Kazemi A., Moy J., Safai B., Marmon S. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84(3):773–775. doi: 10.1016/j.jaad.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mieczkowska K., Deutsch A., Borok J., et al. Telogen effluvium: a sequela of COVID-19. Int J Dermatol. 2021;60(1):122–124. doi: 10.1111/ijd.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Arrones O.M., Lobato-Berezo A., Gomez-Zubiaur A., et al. SARS-CoV-2-induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol. 2021;35(3):e181–e183. doi: 10.1111/jdv.17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzetto G., Diotallevi F., Campanati A., et al. Telogen effluvium related to post severe Sars-Cov-2 infection: clinical aspects and our management experience. Dermatol Ther. 2021;34(1):e14547. doi: 10.1111/dth.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domínguez-Santás M., Haya-Martínez L., Fernández-Nieto D., Jiménez-Cauhé J., Suárez-Valle A., Díaz-Guimaraens B. Acute telogen effluvium associated with SARS-CoV-2 infection. Aust J Gen Pract. 2020;49:32. doi: 10.31128/AJGP-COVID-32. [DOI] [PubMed] [Google Scholar]
- 15.Rivetti N., Barruscotti S. Management of telogen effluvium during the COVID-19 emergency: psychological implications. Dermatol Ther. 2020;33(4):e13648. doi: 10.1111/dth.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutlu Ö., Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33(6):e14096. doi: 10.1111/dth.14096. [DOI] [PubMed] [Google Scholar]
- 17.Turkmen D., Altunisik N., Sener S., Colak C. Evaluation of the effects of COVID-19 pandemic on hair diseases through a web-based questionnaire. Dermatol Ther. 2020;33(6):e13923. doi: 10.1111/dth.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trüeb R.M. Telogen effluvium and trichodynia. Dermatology. 1998;196(3):374–375. [PubMed] [Google Scholar]
- 19.Baldari M., Montinari M., Guarrera M., Rebora A. Trichodynia is a distinguishing symptom of telogen effluvium. J Eur Acad Dermatol Venereol. 2009;23(6):733–734. doi: 10.1111/j.1468-3083.2009.03201.x. [DOI] [PubMed] [Google Scholar]
- 20.Kligman A.M. Pathologic dynamics of human hair loss: I. Telogen effluvium. Arch Dermatol. 1961;83(2):175–198. doi: 10.1001/archderm.1961.01580080005001. [DOI] [PubMed] [Google Scholar]
- 21.Trüeb R.M. Telogen effluvium: is there a need for a new classification? Skin Appendage Disord. 2016;2(1-2):39–44. doi: 10.1159/000446119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Headington J.T. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129(3):356–363. doi: 10.1001/archderm.129.3.356. [DOI] [PubMed] [Google Scholar]
- 23.Gilmore S., Sinclair R. Chronic telogen effluvium is due to a reduction in the variance of anagen duration. Australas J Dermatol. 2010;51(3):163–167. doi: 10.1111/j.1440-0960.2010.00654.x. [DOI] [PubMed] [Google Scholar]
- 24.Shrivastava S.B. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venereol Leprol. 2009;75(1):20–27. doi: 10.4103/0378-6323.45215. [DOI] [PubMed] [Google Scholar]
- 25.Grygiel-Górniak B., Puszczewicz M. Fatigue and interleukin-6—a multi-faceted relationship. Reumatologia. 2015;53(4):207–212. doi: 10.5114/reum.2015.53998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong Y., Harmon C.S. Interleukin-1β is differentially expressed by human dermal papilla cells in response to PKC activation and is a potent inhibitor of human hair follicle growth in organ culture. J Interferon Cytokine Res. 1997;17(3):151–157. doi: 10.1089/jir.1997.17.151. [DOI] [PubMed] [Google Scholar]
- 27.Mandt N., Geilen C.C., Wrobel A., et al. Interleukin-4 induces apoptosis in cultured human follicular keratinocytes, but not in dermal papilla cells. Eur J Dermatol. 2002;12(5):432–438. [PubMed] [Google Scholar]
- 28.Watras M.M., Patel J.P., Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? A review of the literature. Drugs Real World Outcomes. 2016;3(1):1–6. doi: 10.1007/s40801-015-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kivanç-Altunay I., Savaş C., Gökdemir G., Köşlü A., Ayaydin E.B. The presence of trichodynia in patients with telogen effluvium and androgenetic alopecia. Int J Dermatol. 2003;42(9):691–693. doi: 10.1046/j.1365-4362.2003.01847.x. [DOI] [PubMed] [Google Scholar]
- 30.Willimann B., Trüeb R.M. Hair pain (trichodynia): frequency and relationship to hair loss and patient gender. Dermatology. 2002;205(4):374–377. doi: 10.1159/000066437. [DOI] [PubMed] [Google Scholar]
- 31.Ericson M., Gabrielson A., Worel S., Lee W.S., Hordinsky M.K. Substance P (SP) in innervated and non-innervated blood vessels in the skin of patients with symptomatic scalp. Exp Dermatol. 1999;8(4):344–345. [PubMed] [Google Scholar]
- 32.Xerfan E.M., Andersen M.L., Facina A.S., Tufik S., Tomimori J. The role of sleep in telogen effluvium and trichodynia: a commentary in the context of the current pandemic. J Cosmet Dermatol. 2021;20(4):1088–1090. doi: 10.1111/jocd.13929. [DOI] [PubMed] [Google Scholar]
- 33.May A., Goadsby P.J. Substance P receptor antagonists in the therapy of migraine. Expert Opin Investig Drugs. 2001;10(4):673–678. doi: 10.1517/13543784.10.4.673. [DOI] [PubMed] [Google Scholar]