Highlights
-
•
Compared to heathy controls, post-COVID-19 patients had raised T2 mapping values.
-
•
Lower FT-CS and FT-RS values for the shorter contact-to-CMR interval (<8 weeks).
Keywords: Cardiac magnetic resonance imaging, Feature tracking, Mapping, COVID-19
Abbreviations: CMR, Cardiac-magnetic-resonance-imaging; COVID-19, coronavirus disease-2019; CRP, C-reactive-protein; CS, circumferential-strain; ECG, electrocardiogram; ECV, extracellular-volume; EGE, early-gadolinium-enhancement; FT, feature-tracking; ICU, intensive-care-unit; IQR, interquartile-range; IR, inversion-recovery; LAX, long-axis-view; LGE, late-gadolinium-enhancement; LS, longitudinal-strain; LV, left-ventricle; LVEDV, LV end-diastolic-volume; LVEF, LV-Ejection-fraction; LVESV, LV-end-systolic-volume; MERS-CoV, Middle-East-respiratory-syndrome-coronavirus; PCR, polymerase-chain-reaction; ROI, region-of-interest; RS, radial-strain; RV, right-ventricle; RVEDV, end-diastolic-volume; RVEF, RV-ejection-fraction; RVESV, RV-end-systolic-volume; SARS-CoV-2, severe-acute-respiratory-syndrome-coronavirus-2; SAX, short-axis-view; SCLS, systemic-capillary-leak-syndrome; SD, standard-deviation; SIRS, systemic-inflammatory-response-syndrome; SSFP, steady-state-free-precession; STIR, short-tau-inversion-recovery; TTE, transthoracic-echocardiography; TR, repetition-time
Abstract
Background
Post-COVID-19 patients may incur myocardial involvement secondary to systemic inflammation. Our aim was to detect possible oedema/diffuse fibrosis using cardiac magnetic resonance imaging (CMR) mapping and to study myocardial deformation of the left ventricle (LV) using feature tracking (FT).
Methods
Prospective analysis of consecutively recruited post-COVID-19 patients undergoing CMR. T1 and T2 mapping sequences were acquired and FT analysis was performed using 2D steady-state free precession cine sequences. Statistical significance was set to p < 0.05.
Results
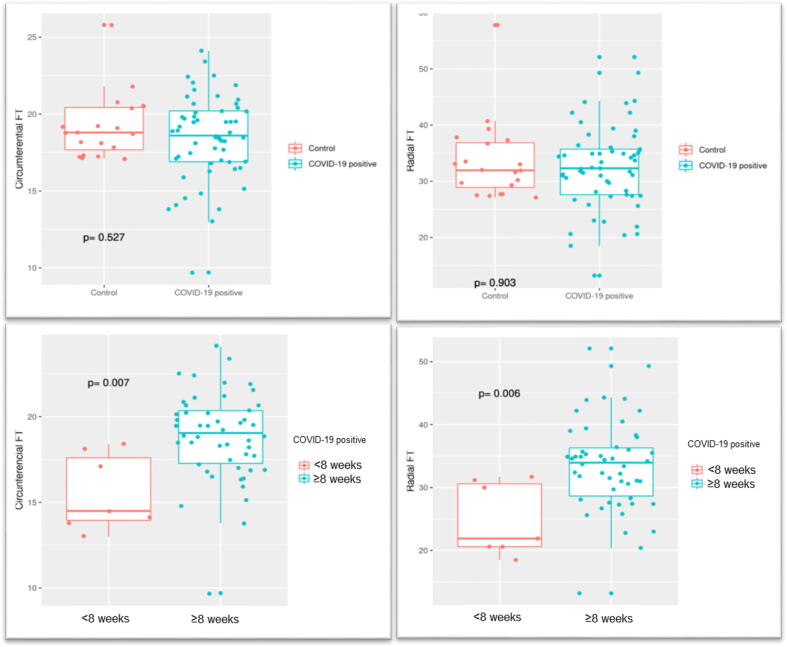
Included were 57 post-COVID-19 patients and 20 healthy controls, mean age 59 ± 15 years, men 80.7%. The most frequent risk factors were hypertension (33.3%) and dyslipidaemia (36.8%). The contact-to-CMR interval was 81 ± 27 days. LV ejection fraction (LVEF) was 61 ± 10%. Late gadolinium enhancement (LGE) was evident in 26.3% of patients (19.3%, non-ischaemic). T2 mapping values (suggestive of oedema) were higher in the study patients than in the controls (50.9 ± 4.3 ms vs 48 ± 1.9 ms, p < 0.01). No between-group differences were observed for native T1 nor for circumferential strain (CS) or radial strain (RS) values (18.6 ± 3.3% vs 19.2 ± 2.1% (p = 0.52) and 32.3 ± 8.1% vs 33.6 ± 7.1% (p = 0.9), respectively). A sub-group analysis for the contact-to-CMR interval (<8 weeks vs ≥ 8 weeks) showed that FT-CS (15.6 ± 2.2% vs 18.9 ± 2.6%, p < 0.01) and FT-RS (24.9 ± 5.8 vs 33.5 ± 7.2%, p < 0.01) values were lower for the shorter interval.
Conclusions
Post-COVID-19 patients compared to heathy controls had raised T2 values (related to oedema), but similar native T1, FT-CS and FT-RS values. FT-CS and FT-RS values were lower in post-COVID-19 patients undergoing CMR after < 8 weeks compared to ≥ 8 weeks.
1. Introduction
The World Health Organization confirmed, as of 25 May 2021, 166,352,007 cases and 3,449,189 deaths worldwide from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen responsible for coronavirus disease 2019 (COVID-19) [1]. People at cardiovascular risk are estimated to have around 15% greater probability of a fatal outcome [2], [3], [4], while mortality in patients with COVID-19 and cardiovascular disease is five times higher than in patients without heart disease [5].
Myocardial injury in the COVID-19 context has been defined as an increase in troponin above the 99th percentile of the upper reference limit [2]. Although elevated cardiac biomarkers are related to higher in-hospital mortality of patients with COVID-19 [2], [4], [6], they may simply reflect disease severity (hypoxia, hypotension, etc) rather than any specific pathology [7], [8], [9]. Biomarker determination and interpretation nonetheless represents a challenge for clinical management of these patients, as they may originate in numerous conditions, including myocarditis, acute coronary syndrome, arrhythmias, heart failure, cytokine storm, microangiopathy, chronic kidney disease, or pulmonary thromboembolism [8], [9], [10], [11]. While cardiac imaging techniques such as echocardiography are undoubtedly useful in clarifying diagnosis and prognosis, cardiac imaging society pandemic-related recommendations are that imaging be performed in the acute disease phase when a substantial change in clinical management may determine morbidity-mortality [12], [13].
Limitations on defining myocardial involvement during the critical COVID-19 phase may modify medium- and long-term prognosis for this population, thereby posing a new post-discharge COVID-19 challenge for cardiologists: to detect myocardial sequelae that may influence clinical evolution. It is therefore an opportune moment to define and characterize tissue and myocardial deformation using more advanced techniques, in particular, cardiac magnetic resonance imaging (CMR).
CMR can detect chronic myocardial oedema. While black-blood sequences are generally used, these are susceptible to artifacts [14] that may mislead interpretations regarding water/myocardial oedema. T2 mapping, a highly reproducible technique [14], has been successfully used to quantify oedema, given the higher signal-to-noise ratio, shorter breath-holds, and fewer breathing motion artifacts [15].
Myocardial deformation has also been evaluated using feature tracking (FT) (analogous to speckle tracking in echocardiography), which allows strain to be evaluated from conventional cine sequences [16]. It is as yet uncertain whether studying myocardial deformation could determine the presence and severity of myocardial involvement in patients recovering from COVID-19.
Our aim was to detect possible oedema or diffuse fibrosis reflecting an inflammatory pathology using CMR mapping sequences, and to study myocardial deformation of the left ventricle (LV) using FT to complement myocardial studies.
2. Materials and Methods
The study population was patients who had been hospitalized for polymerase chain reaction (PCR)-confirmed COVID-19 in our hospital. Discharged patients were prospectively recruited and were evaluated in individual face-to-face cardiology visits. Referred for CMR, provided they tested PCR-negative, were patients with dyspnoea or chest pain and/or transthoracic echocardiogram (TTE) (LV dysfunction/alterations in segmental contractility, suspected right ventricle (RV) dilatation/dysfunction) or ECG alterations (Table-1).
Given that myocardial involvement is reported to occur 8–14 days after COVID-19 symptom onset [17], to calculate the contact-to-CMR interval, we took hospital admission/emergency department visit as the first day of a possible diagnosis. First hospital contacts were between March-May 2020. CMR studies were conducted between April-July 2020.
Patients with a known history of ischaemic heart disease, previous cardiomyopathy, or previous myocarditis episodes were excluded; in addition, conventional exclusion criteria for magnetic resonance (MR) were applied. The study group included 57 patients, while 20 healthy individuals were included in a control group. Sex- and age-matched healthy controls with normal ECG, and without any prior known cardiac or inflammatory disease who previously underwent CMR in our hospital were selected. Baseline characteristics and hospital treatment details were obtained from patients’ medical histories.
The study was approved by the ethics committee of our hospital. All subjects gave their written informed consent prior to CMR and to use of imaging data for educational or scientific purposes. No external funding was used.
CMR studies were carried out with a 1.5 Tesla device (Optima MR450w-GE; GE Healthcare) using a 32-channel multi-element surface antenna and ECG synchronization. The cine images, taken in end-expiration with a retrospective ECG protocol, were captured using conventional steady-state free-precession (SSFP) sequences in longitudinal axes with 2-, 3- and 4-chamber views and in 10–15 contiguous short-axis (SAX) slices covering both ventricles base to apex. Non-contrast CMR exams in control group. In study group, approximately 8–10 min after intravenous infusion of 0.15 mmol/kg of gadobutrol (Gadovist® 1 mmol/mL), late gadolinium enhancement (LGE) images were acquired, in the same views as for the cine images, employing a T1-weighted gradient echo inversion-recovery (IR) sequence.
To evaluate oedema, before gadolinium administration, T2-weighted short tau inversion recovery (STIR) sequences were acquired in SAX slices from base to apex, and T2 maps were acquired in end diastole in basal, mid, and apical SAX and one long-axis (LAX) plane using a T2-prepared turbo spin echo (TSE) sequence. Acquired were four T2-prepared TSE images with different T2-prepared times (11, 37, 63, and 90 ms; repetition time (TR) = 1 × R-R). All images were acquired with attempted breath-hold. T1 mapping was performed with a modified Look-Locker IR sequence using a 3(3)5 scheme, immediately before and 15 min after contrast application, in order to quantify extracellular volume (ECV), in the same four planes as the T2 mapping sequence. The updated Lake Louise criteria were used for the diagnosis of myocarditis [15]
All CMR studies were analysed by a cardiologist (**) and a radiologist (**) with extensive CMR experience (>4 and > 8 years, respectively). Cardiac functional analysis was performed offline using CVI42 dedicated software (v.5.11; Circle Cardiovascular Imaging). LV and RV ejection fractions, LV and RV end-diastolic/end-systolic volumes were obtained from the cine sequences using the disc summation method. The presence of focal myocardial oedema in T2-STIR was visually evaluated and agreed by the two experts, while the LGE of each myocardial segment was rated visually as subepicardial, midwall, subendocardial, transmural, or zero.
For the mapping analysis, for diffuse disease and global evaluations, single regions of interest (ROIs) were drawn in the septum in each mid-cavity SAX map to avoid lung, liver, and veins as possible sources of susceptibility artifacts [18]. If artifacts or non-conclusive results resulted, basal ROIs were used for validation [18].
FT was evaluated (using the CVI42 software) by analysing peak circumferential strain (CS) and radial strain (RS) for each segment, including basal, mid, and apical SAX breath-held SSFP cines. Contours were drawn in the end-diastolic phase along LV epicardial and endocardial borders in all short axis slices; except the first two–three most basal slices with<50% myocardium, and in a reference cut of the 2-chamber, 4-chamber and 3-chamber cines. Subsequently automatically propagated through all frames (if propagation was faulty, editing was performed in end-diastolic phase on the respective slice and again automatically propagated). The LV was divided according to the AHA 16 segment model. FT-CS and FT-RS values were obtained by tracking features within each voxel throughout the cardiac cycle, resulting in global circumferential and radial strain values.
FT measurements obtained independently by the cardiologist and radiologist were compared to determine intra- and inter-observer variability.
Statistical analyses were performed using R (www.r-project.org). Continuous variables were Shapiro-Wilk-tested for normality and reported as mean ± standard deviation (SD) if normality could be assumed, or median ± interquartile range (IQR) otherwise. Qualitative values were reported as percentages. For normally distributed data, the unpaired T-test was used under the non-equal variances condition, and the Wilcoxon test otherwise. Qualitative variables were compared using the chi-square test. Absolute intraclass coefficient correlation (ICC) values were calculated to determine intra- and inter-observer reliability. For all tests, significance was set to p < 0.05.
3. Results
Of 377 patients evaluated in post-COVID-19 cardiology visits, 62 were recruited for CMR studies, of whom 57 met the inclusion criteria. Most patients were referred due to control TTE alterations (71.9%), and most were asymptomatic (82.5%) according to the post-discharge cardiology evaluation. The contact-to-CMR interval was 81 ± 27 days.
Table 1 describes baseline characteristics, and CMR results in Table 2. LVEF by the disc summation method was 61%±10%. No visual segmental alterations in contractility were observed in 80.7% of patients, while contractile abnormality was mainly driven by mild global hypokinesia (8.7%). Most patients (73.7%) had no pathological findings for focal fibrosis by LGE, 19.3% showed non-ischaemic LGE, and 3.5% each showed ischaemic and pericardial LGE.
Table 1.
Patient baseline characteristics (n (%), unless otherwise indicated).
| Baseline characteristics (n = 57) | |
|---|---|
| Age, years | 59 ± 15 |
| Male | 46 (80.7%) |
| Hypertension | 19 (33.3%) |
| Diabetes | 8 (14%) |
| Hypercholesterolaemia | 21 (36.8%) |
| Smoking | 2 (3.5%) |
|
Immunomodulator treatment Ciclosporin Tocilizumab Ciclosporin + tocilizumab |
29 (50.9%) 17 (29.8%) 1 (1.8%) 11 (19.3%) |
| Corticosteroid treatment | 14 (24.6%) |
| ICU admission | 4 (7%) |
| TTE LVEF, % | 60 ± 9 |
| CRP levels (in-hospital) | 12.7 ± 9.8 |
| D-dimer levels (in-hospital) | 831.9 ± 1240.7 |
| Ferritin levels (in-hospital) | 1436.3 ± 1068.8 |
|
ECG findings RBBB Atrial fibrillation Ventricular extrasystole Negative precordial T wave Pathological Q wave |
24 (42%) 9 (15.8%) 3 (5.3%) 1 (1.8%) 7 (12.3%) 4 (7%) |
|
CMR referral Symptoms No symptoms TTE findings ECG findings TTE + ECG findings |
10 (17.5%) 47 (82.5%) 41 (71.9%) 4 (7%) 2 (3.5%) |
Abbreviations: CRP, C-reactive protein; ECG, electrocardiogram; ICU, intensive care unit; LVEF, LV ejection fraction; RBBB, right bundle branch block; CMR, cardiac magnetic resonance; TTE, transthoracic echocardiography.
Table 2.
Cardiac magnetic resonance imaging (CMR) findings (n (%), unless otherwise indicated).
| CMR findings | |||
|---|---|---|---|
| Post-COVID-19 (n = 57) | Controls (n = 20) | p | |
| LVEF, % | 61 ± 10 | 63 ± 4 | 0.68 |
| LVEDV (index), mL/m2 | 76 ± 17 | 74 ± 12 | 0.75 |
| LVESV (index), mL/m2 | 30 ± 9 | 27 ± 6 | 0.83 |
| RVEF, % | 60 ± 9 | 62 ± 6 | 0.84 |
| RVEDV (index), mL/m2 | 74 ± 18 | 75 ± 14 | 0.67 |
| RVESV (index), mL/m2 | 30 ± 9 | 29 ± 8 | 0.83 |
|
Abnormal STIR Myocardial Pericardial |
6 (10.5%) 4 (7%) 2 (3.5%) |
||
|
Native T1, ms <8 weeks (n = 7) ≥8 weeks (n = 50) |
996.4 ± 43.9 995 ± 88.3 996.5 ± 38.4 |
981.5 ± 21.2 | 0.34 |
|
ECV, % Abnormal, % <8 weeks, % (n = 7) ≥8 weeks, % (n = 50) |
26.6 ± 3.1 11 (19.3%) 28.3 ± 6 26.5 ± 2.75 |
– | |
|
T2 mapping, ms <8 weeks (n = 7) ≥8 weeks (n = 50) |
50.9 ± 4.3 55 ± 12.6 50.51 ± 2.8 |
48 ± 1.9 | <0.01 |
|
LGE Non-ischaemic Ischaemic Pericardial |
15 (26.3%) 11 (19.3%) 2 (3.5%) 2 (3.5%) |
||
|
FT-CS <8 weeks (n = 7) ≥8 weeks (n = 50) |
18.6 ± 3.3% 15.6 ± 2.2% 18.9 ± 2.6%. |
19.2 ± 2.1% | 0.52 |
|
FT-RS <8 weeks (n = 7) ≥8 weeks (n = 50) |
32.3 ± 8.1% 24.9 ± 5.8% 33.5 ± 7.2% |
33.6 ± 7.1% | 0.90 |
Abbreviations: ECV, extracellular volume; FT, feature tracking; FT-CS, FT circumferential strain; FT-RS, FT radial strain; LGE, late gadolinium enhancement; LV, left ventricle; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume; RV, right ventricle; RVEDV, end-diastolic volume; RVEF, RV ejection fraction; RVESV, RV end-systolic volume; STIR, short tau inversion recovery.
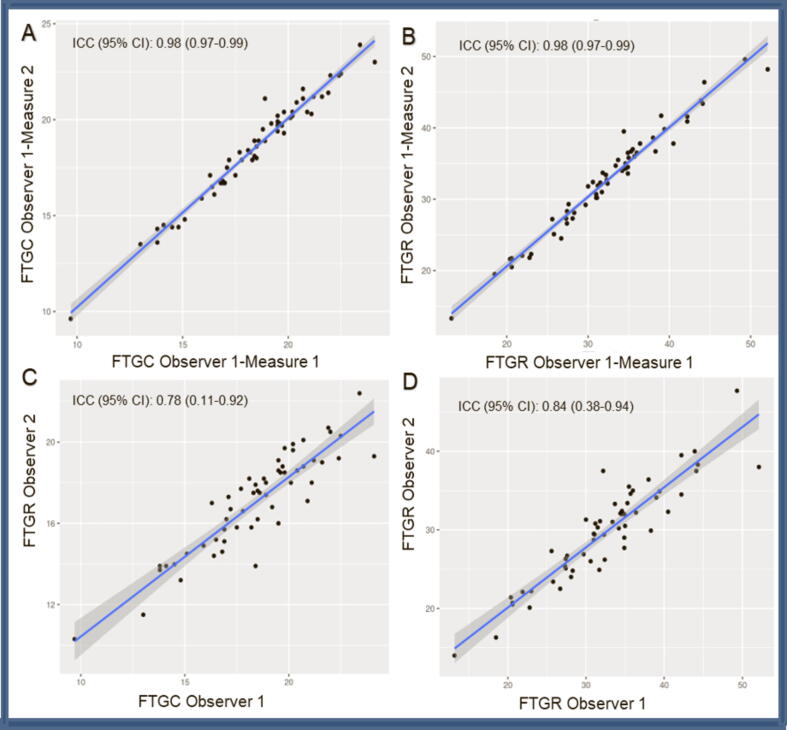
In relation to FT-measured LV deformation parameters, intra- and inter-observer reliability was good to excellent (Figure 1) (ICC 0.98 and 0.78 for FT-CS, and 0.98 and 0.84 for FT-RS). While FT-CS differences between the study group vs the control group were statistically non-significant (18.6 ± 3.3% vs 19.2 ± 2.1%, p = 0.52), on differentiating between study group patients according to contact interval (<8 weeks, n = 7 vs ≥ 8 weeks, n = 50, i.e., up to 49 days and after 50 days), FT-CS values for the former were found to be significantly lower (15.6 ± 2.2% vs 18.9 ± 2.6%, p < 0.01). FT-RS differences between the study group vs the control group were statistically non-significant (32.3 ± 8.1% vs 33.6 ± 7.1%, p = 0.9), but were significantly reduced for the shorter contact interval (<8 weeks 24.9 ± 5.8 vs ≥ 8 weeks 33.5 ± 7.2%, p < 0.01) (Figure 2).
Fig. 1.
Cardiac-magnetic-resonance-imaging (CMR). Feature-tracking (FT) radial-strain (RS) and circumferential-strain (CS). Intra-(A-B) and inter-observer(A-B) variability. ICC: intraclass correlation coefficient.
Fig. 2.
Feature-tracking (FT) radial-strain (RS) and circumferential-strain (CS). Post-COVID-19 patients vs controls (A-B). CMR < 8 weeks vs CMR ≥ 8 weeks (C-D).
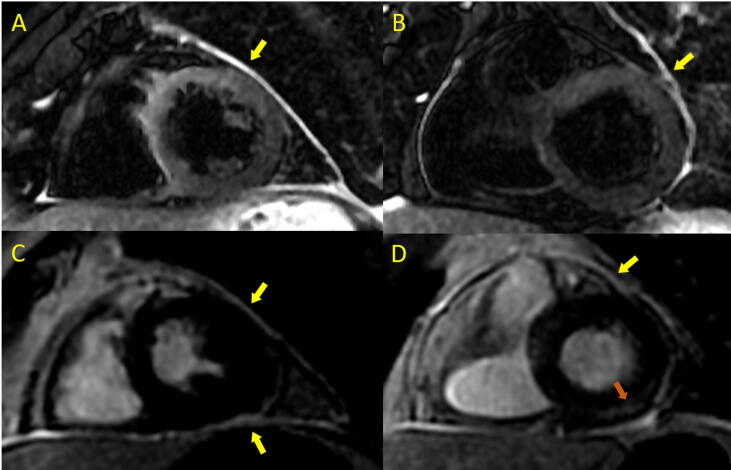
For myocardial mapping, we observed a statistically significant difference between the study and control groups for oedema detection in T2 mapping, with high values suggestive of myocardial oedema in post-COVID-19 patients (50.9 ± 4.3 ms vs 48 ± 1.9 ms for the study group vs the controls, p < 0.01). STIR sequence sensitivity to oedema evaluation was pathological in only 10.5% of cases; in two cases (3.5%), however, signal hyperintensity was observed at the pericardial level which, in view of LGE in IR sequences, resulted in diagnoses of pericarditis or myopericarditis (Figure 3). We found no differences with respect to the following: native myocardial T1 for the study group vs the controls (996.4 ± 43.9 ms vs 981.5 ± 21.2 ms, p = 0.34), contact-to-CMR interval (<8 weeks vs ≥ 8 weeks), native myocardial T1, or ECV.
Fig. 3.
Post-COVID-19 patient with myopericarditis. A-B: Short-tau-inversion-recovery (STIR) sequences showing signal hyperintensity at the pericardial level consistent with pericardial oedema (yellow arrow). C-D: 2D-phase-sensitive-inversion-recovery (2D-PSIR) sequence showing pericardial gadolinium capture (yellow arrow) and myocardial focal fibrosis in the basal inferolateral segment (orange arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Patients recovering from COVID-19, with a contact-to-CMR interval of 81 ± 27 days, had higher T2 mapping values, suggestive of residual myocardial oedema, than controls. Only T2 mapping sequences detected myocardial involvement. No differences were found for native T1, FT-CS, or FT-RS between the study and control groups. However, when patients were analysed according to contact-to-CMR interval (<8 weeks and ≥ 8 weeks), we found significantly lower FT-CS and FT-RS values for the shorter interval.
Myocardial involvement is associated with greater mortality in critically ill patients with COVID-19 [19], [20]. While the pathophysiological mechanisms are not fully understood, several theories have been proposed. One theory is that direct virus-induced damage at the cardiomyocyte level is managed through interaction with angiotensin-converting-enzyme-2 receptors [7], although autopsies have frequently reported an inflammatory process in the absence of the virus [21], [22]. The main route of action may therefore be indirect: a disproportionate systemic inflammatory response that launches a pathological immune response, leading, in turn, to reduced coronary blood flow and myocardial oxygen supply, coronary plaque destabilization, and microthrombogenesis [23]. Cardiac manifestations in COVID-19 potentially encompass arrhythmias, heart failure, ischaemic heart disease, myocardial injury, and myocardial infarction with nonobstructive coronary arteries that includes myocarditis and cardioembolic events [7], [9], [17].
Although myocarditis needs to be considered in the differential diagnosis of myocardial injury in patients with COVID-19, few cases have been histologically confirmed. Myocardial biopsies in some cases have returned findings of SARS-CoV-2 [24] or of an inflammatory infiltrate without virus [22]; in other cases, no biopsy was performed to demonstrate viral presence [25]. A study of 22 biopsies for patients who died after COVID-19 did not report the typical finding of lymphocytic infiltration associated with myocarditis, but did report that hearts showed necrosis/apoptosis patterns unrelated to lymphocyte infiltration [26].
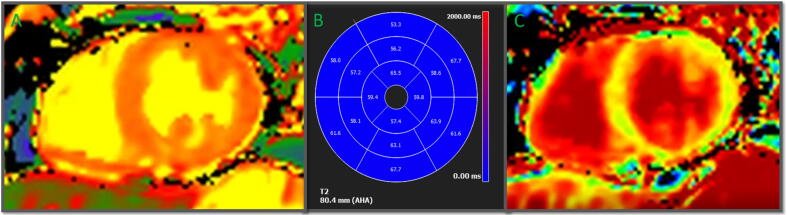
We therefore support the idea that, while a myocarditis process is certainly possible [27], myocardial injury is more likely to be the result of a systemic inflammatory response syndrome (SIRS) that includes cytokine storm, dysregulated immunocytes, and uncontrolled inflammation [8], [9], [17] – and, as in our post-COVID-19 patients, also involving the myocardium. Infection-associated fever and tachycardia increase myocardial oxygen demand, while hypoxemia secondary to pneumonitis reduces myocardial oxygen supply, leading to a supply–demand imbalance that causes myocardial injury [28]. Also described in the literature is systemic capillary leak syndrome (SCLS), defined as a paroxysmal permeability disorder of the capillary system that causes abrupt and massive intravascular space-to-interstitial space passage of fluids and proteins. SCLS may explain raised T2 mapping values, native myocardial T1, and ECV in the absence of focal fibrosis in some patients with COVID-19 [29] (Figure 4). We suggest that myocardial involvement in most cases is secondary to a major systemic inflammatory process, rather than a primary lesion [8], [28].
Fig. 4.
T1-T2-mapping in a post-COVID-19 patient. A: Raised T1-mapping (1067 ms) suggestive of diffuse fibrosis. B: Globally raised T2-mapping (59 ms) suggestive of residual oedema. C: Slightly raised (29%) extracellular volume (ECV) suggestive of diffuse fibrosis.
In CMR studies, increased oedema causes an increase in T1 and especially in T2 relaxation times in the myocardium [15], reflected, in turn, in signal hyperintensity in T2-weighted images and in T1-T2 mapping prolongation at the myocardial level. Higher T1, T2, and ECV values for an inflamed myocardium can be quantified directly by sequence mapping, which has none of the limitations of qualitative and semiquantitative tissue characterization techniques. High T1 and T2 values in the early acute inflammation stages [30] decrease as the inflammation and oedema resolve. Luetkens et al. [30] found that only native myocardial T1 and T2 mapping can detect residual inflammation in patients with myocarditis at 4–8 weeks of follow-up, reporting normal values for other myocardial oedema evaluation parameters, such as early gadolinium enhancement (EGE) or T2-STIR ratios (i.e., skeletal muscle/myocardial signal intensity in the T2-black blood sequence); those authors further reported 35% (reduced from 88%) visible myocardial oedema at 4–8 weeks of follow-up (only 4% at longer follow-up), indicating that only the T2 map remained altered for the study group at 8 weeks of follow-up (this was the reason why we took 8 weeks as our cut-off). Lurz et al. [31] also concluded that only T2 mapping yields an acceptable diagnosis 14 days after an inflammatory cardiac process with chronic symptoms – a conclusion of direct relevance to our results, as most of our CMR studies were performed ≥ 8 weeks (81 ± 27 days) after first contact.
For 100 post-COVID-19 patients (33% hospitalized, 8% treated with corticosteroids) studied using CMR (median (IQR) interval between diagnosis and CMR of 71 (64–92) days), Puntmann et al. [32] reported abnormal CMR findings for 78% of their patients, including LGE (32%) or pericardial enhancement (22%), raised native myocardial T1 values (73%), and raised T2 mapping values (60%), concluding, furthermore, that mapping techniques have the greatest discriminatory power in detecting cardiac involvement in the COVID-19 context. We likewise observed altered T2 mapping values in our patients, but no difference in native T1 values, a divergence probably explained by the contact-to-CMR interval (81 ± 27 days).
It is striking that, in four of our patients with abnormal myocardial STIR (7%), the oedema was predominantly located at the anteroseptal segment level, corroborating findings by Huang et al. [33]. While we could not definitively confirm this by segmental evaluation of T2 mapping sequences where involvement was generally global, the LGE percentage in our study (26.3%) was similar to that reported (31%) by those authors.
The question of whether we are dealing with a classic myocarditis process is, therefore, at the very least debatable. While different myocardial segments may be involved in myocarditis, the inferolateral segments are a typical location for viruses similar to SARS-CoV-2, e.g., Middle East respiratory syndrome coronavirus (MERS-CoV) [34], [35], for which non-ischaemic (subepicardial/intramyocardial) LGE tends to be much greater [30]. Recently, however, Li et al. [36] observed a different LGE pattern for patients with acute fulminant compared to acute non-fulminant myocarditis, predominantly at the septal level, with higher T1, T2, and ECV values, and lower FT-CS, FT-RS, and FT longitudinal strain (FT-LS) values, further reporting significant positive correlation between quantitative myocardial oedema/inflammation parameters and FT-CS. We observed no relationship between myocardial oedema parameters and FT-CS/FT-RS in our patients, probably because of their preserved systolic function.
Another factor in myocardial inflammatory processes is segmental contractility alterations, representing a support criterion for the updated Lake Louise criteria [15]. Thavendiranathan et al. [35] found in patients with acute myocarditis, FT values reductions in study group in comparison with controls for both FT-CS (-13.7 ± 6.3% vs –23.3 ± 5.8%) and FT-RS (16.8 ± 7.6% vs 38.5 ± 6.7%), suggesting greater FT sensitivity in detecting abnormal wall motion of segments with altered T2. Similarly, Baeßler et al. [37] studied the additive role of T2 mapping and FT in patients with myocarditis and a preserved LVEF (>50%), reporting greater diagnostic precision and greater reproducibility for FT-CS compared to FT-LS, and suggesting, furthermore, that T2 mapping, FT-CS, and LGE evaluation combined was diagnostically more sensitive than separate imaging parameter analyses or Lake Louise criteria. Gatti et al. [38] found no differences between 2D FT-CS, FT-RS, or FT-LS for patients with myocarditis and LVEF > 55% compared with healthy controls. In view of the divergent results and opinions, therefore, the debate on FT for patients with inflammatory myocardial processes remains open.
In our study of a systemic inflammatory process in which many patients did not have focal fibrosis, results pointed to broadly preserved LV contractility, corroborating Huang et al. [30]. We found no FT-CS and FT-RS differences, however, between the study and control groups, possibly explained by preserved ventricular function, less critical disease phase (only 7% ICU admissions), and a lengthy contact interval (2–3 months) that probably meant recovery in the evaluation phase – as evidenced by the sub-analyses of FT-CS (<8 weeks 15.6 ± 2.2% vs ≥ 8 weeks 18.9 ± 2.6%, p < 0.01) and of FT-RS (<8 weeks 4.9 ± 5.8 vs ≥ 8 weeks 33.5 ± 7.2%, p < 0.01) (Figure 5; Video 1), as Chen et al [39] have recently proved with global longitudinal
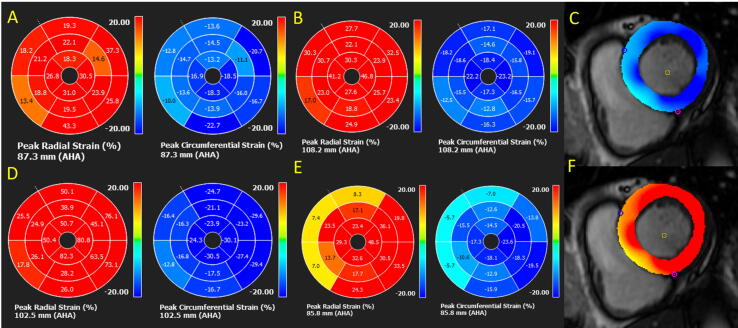
Fig. 5.
CMR FT-CS and FT-RS (blue and red bullseyes, respectively) in post-COVID-19 patients. A: LVEF = 54%. Reduced FT-CS (14.1%) and FT-RS (20.6%), with regional analysis showing predominantly septal segment involvement. B: Preserved LVEF = 59%. Global FT-CS (16.5%) and FT-RS (25.8%) involvement. C: FT-CS showing segments with preserved contractility (dark blue) and altered contractility (light blue). D: Preserved FT-CS (21.1%) and FT-RS (40.5%) with regional septal involvement. E: Reduced FT-CS (13.8%) and FT-RS (20.6%) with extensive regional involvement. F: FT-RS, showing segments with preserved contractility (dark red) and altered contractility (yellow/orange). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. Limitations
Following recommendations, our CMR studies were performed after recovery from COVID-19 and following individual consultations. An obvious limitation in evaluating the impact of our findings is the contact-to-CMR interval, even though T2 mapping alterations were striking. Our results should be viewed in relation to the observation by Zhou et al. [40] that myocardial injury can occur in different phases of COVID-19, including late phases.
Another limitation was that we did not have cardiac biomarker evaluations for some of our patients (due to early-pandemic hospital protocol). Furthermore, selection bias cannot be ruled out as all CMR studies were performed after clinical and echocardiographic indication.
Other limitations are technical ones inherent to sequence mapping and FT. Results for mapping depend on multiple factors, including hardware, software, measurements, image quality, user experience, and protocol adherence [15]; furthermore, sensitivity/specificity values drop for studies performed > 14 days after symptom onset [31]. We did not consider FT-LS because of intra- and inter-observer variability regarding our sample; however, FT-CS is reported to have greater validity in CMR for myocarditis-type inflammatory processes [37], including COVID-19 scenario [41]. Since intra- and inter-observer reliability in our sample was highly variable depending on the segment evaluated (ICC 0.03–0.89), we did not take quantitative segmental results into account, considering the global FT value to be more robust.
6. Conclusions
In our study of myocardial involvement in post-COVID-19 patients compared to healthy controls, we found raised T2 mapping values (related to oedema) but no differences in native T1 (mainly associated with diffuse fibrosis), or in myocardial deformation parameters; FT-CS and FT-RS values. When study group patients were sub-grouped according to contact-to-CMR interval (<8 weeks and ≥8 weeks), we found lower FT-CS and FT-RS values for the shorter interval.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Our thanks to Ailish Maher for English language assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100854.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19). Weekly Operational Update.
- 2.Cosyns B., Lochy S., Luchian M.L. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc. Imaging. 2020 doi: 10.1093/ehjci/jeaa136. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 4.Chapman A.R., Bularga A., Mills N.L. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648 [Epub ahead of print] [DOI] [PubMed]
- 6.Shi S., Qin M., Cai Y. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa408. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik T., Mohiddin S., Dimarco A. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imazio M., Klingel K., Kindermann I. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020 doi: 10.1136/heartjnl-2020-317186. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Bavishi C., Bonow R., Trivedi V. Acute Myocardial Injury in Patients Hospitalized With COVID-19 Infection: A Review. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.05.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long B., Brady W.J., Koyfman A. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.048. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tersalvi G., Vicenzi M., Calabretta D. Elevated troponin in patients with Coronavirus Disease 2019 (COVID-19): possible mechanisms. J Card Fail. 2020 doi: 10.1016/j.cardfail.2020.04.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skulstad H., Cosyns B., Popescu B.A. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Hear J Cardiovasc Imaging. 2020 doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y., Chen T., Bryant J. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson. 2020;22:26. doi: 10.1186/s12968-020-00628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesmueller M., Wuest W., Heiss R. Cardiac T2 mapping: robustness and homogeneity of standardized in-line analysis. J Cardiovasc Magn Reson. 2020;22(1):39. doi: 10.1186/s12968-020-00619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira V., Schulz-Menger J., Holmvang G. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 16.Claus P., Omar A.M.S., Pedrizzetti G., Sengupta P.P., Nagel E. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc Imaging. 2015;8:1444–1460. doi: 10.1016/j.jcmg.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Atri D., Siddiqi H., Lang J. COVID-19 for the cardiologist: A current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020 Apr 10;5(5):518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messroghli D., Moon J., Ferreira V. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017;19(1):75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santoso A, Pranata R, Wibowo A et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020. doi: 10.1016/j.ajem.2020.04.052. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 20.Wentao N., Xiuwen Y., Jie L. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sala S, Peretto G, Gramegna M et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:doi: 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed]
- 23.Guo T., Fan Y., Ming C. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020 doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inciardi R., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox S., Li G., Akmatbekov A. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.049465. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Salamanca J., Díez-Villanueva P., Martínez P. Covid-19 “Fulminant myocarditis” Successfully treated with temporary mechanical circulatory support. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.05.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libby P. The heart in COVID19: Primary target or secondary bystander? JACC Basic Transl Sci. 2020 doi: 10.1016/j.jacbts.2020.04.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luetkens J.A., Homsi R., Dabir D. Comprehensive cardiac magnetic resonance for short-term follow-up in acute myocarditis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lurz P., Luecke C., Eitel I. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis. J Am Coll Cardiol. 2016;67:1800–1811. doi: 10.1016/j.jacc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Puntmann V, Carerj L, Wieters I et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. doi:10.1001/jamacardio.2020.3557 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 33.Huang L., Zhao P., Tang D. Cardiac involvement in recovered COVID-19 patients identified by magnetic resonance imaging. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.05.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alhogbani T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann Saudi Med. 2016;36(1):78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thavendiranathan P., Walls M., Giri S. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging. 2012;5(1):102–110. doi: 10.1161/CIRCIMAGING.111.967836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H., Zhu H., Yang Z. Tissue characterization by mapping and strain cardiac MRI to evaluate myocardial inflammation in fulminant myocarditis. J Magn Reson Imaging. 2020 doi: 10.1002/jmri.27094. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Baeßler B., Treutlein M., Schaarschmidt F. A novel multiparametric imaging approach to acute myocarditis using T2-mapping and CMR feature tracking. J Cardiovasc Magn Reson. 2017;19(1):71. doi: 10.1186/s12968-017-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatti M., Palmisano A., Faletti R. Two–dimensional and three–dimensional cardiac magnetic resonance feature–tracking myocardial strain analysis in acute myocarditis patients with preserved ejection fraction. Int J Cardiovasc Imaging. 2019;35(6):1101–1109. doi: 10.1007/s10554-019-01588-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen BH, Shi NN, Wu CW et al. Early cardiac involvement in patients with acute COVID-19 infection identified by multiparametric cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2021. doi: 10.1093/ehjci/jeab042. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 40.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., Zhou Z., Jiang H. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23(1):14. doi: 10.1186/s12968-021-00710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.