Abstract
To improve disease control without increasing the toxicity of a reduced-intensity allogeneic hematopoietic cell transplantation (HCT) in multiple myeloma (MM), a phase 1 trial was performed using an antibody-radionuclide conjugate targeting CD45 (90Y-DOTA-BC8) as conditioning. 90Y-DOTA-BC8 was combined with fludarabine and low-dose TBI followed by allogeneic HCT in patients with MM and ≥1 adverse risk characteristic at diagnosis, relapse after autologous transplant or plasma cell leukemia (PCL). The primary objective was to estimate the maximum tolerated radiation absorbed dose. Fourteen patients were treated (1 with PCL, 9 failed prior autologous HCT, and 9 with ≥1 adverse cytogenetics). Absorbed doses up to 32 Gy to liver were delivered. No dose-limiting toxicities occurred. Nonhematologic toxicities were manageable and included primarily gastrointestinal (43%) and metabolic/electrolyte disturbances (36%). Treatment-related mortality at 100 days was 0%. At a median follow-up of 5 years, the overall survival was 71% (median not reached) and the progression-free survival was 41% (median 40.9 months). The incorporation of CD45-targeted radioimmunotherapy (RIT) into a reduced-intensity allogeneic HCT is well-tolerated and may induce long-term remissions among patients with poor-risk MM, supporting further development of RIT-augmented conditioning regimens for HCT.
Introduction
Recent therapeutic advances have improved survival in patients with multiple myeloma (MM), but patients with certain biological features at diagnosis (elevated β2-microglobulin, lactate dehydrogenase [LDH] or adverse cytogenetics)1 still experience early relapse and shortened survival. Allogeneic hematopoietic cell transplantation (alloHCT) is a potentially curative treatment for MM and may involve an immune-mediated response irrespective of high-risk cytogenetics.2 However, the role of alloHCT in treating patients with MM is controversial, particularly in the setting of novel agents and rapidly evolving immunotherapeutic approaches, including chimeric antigen receptor (CAR) T-cell and bispecific T-cell engager (BiTE) therapies. Transplantation-related mortality (TRM) remains a major limitation to alloHCT and relapse is a major cause of failure despite curative intent. Myeloablative alloHCT resulted in durable remissions for a small subset of patients appearing to be functionally cured, but the approach is associated with unacceptably high rates of TRM ranging between 45–60%.3 Less intensive alloHCT conditioning regimens have reduced TRM to 10–15% but at the cost of increased relapse risk,3, 4 suggesting that the graft-versus-myeloma effect is not sufficiently potent to reliably eliminate disease without additional cytoreduction prior to allografting.
Our study was designed to mitigate the toxicities associated with fully ablative conditioning regimens and simultaneously enhance disease control of a reduced-intensity conditioning (RIC) alloHCT through the use of an antibody radionuclide conjugate to deliver radiation precisely to the bone marrow (BM). Incorporating radiation into conditioning regimens may improve transplant outcomes in MM because myeloma cells are highly sensitive to radiation. 131I-labeled CD45 monoclonal antibody (mAb) has been used as HCT conditioning and demonstrated to be safe and potentially effective in patients with myeloid and lymphoid malignancies.5, 6 In contrast to 131I, yttrium-90 has a shorter half-life (2.5 vs. 8 days) and lacks a gamma component, obviating the need for patient radiation isolation.7 Our study evaluated 90Y conjugated to an IgG1 murine anti-CD45 monoclonal antibody (mAb; BC8) in conjunction with a well-characterized RIC regimen for alloHCT.5 CD45 was selected as the antigen target because it is expressed at high surface density on hematopoietic cells, thus facilitating uniform BM distribution. Despite the fact that CD45 expression on clonal plasma cells is relatively uncommon,8 conjugation of 90Y to anti-CD45 mAb takes advantage of the beta particle’s high emission energy (2.3 Mev maximum) and long traversal pathlengths in tissue (5 mm)7 facilitating a crossfire effect capable of eliminating adjacent radiosensitive CD45-antigen-negative plasma cells.
Methods
Study design
We conducted an open-label, dose-escalation, phase I trial at a single institution. The primary objective of the study was to determine the safety and estimate the maximum tolerated dose (MTD) of radiation delivered via 90Y-DOTA-BC8 mAb combined with fludarabine and 2 Gy total body irradiation (TBI) before an alloHCT for patients with high-risk MM. This study was conducted according to the Declaration of Helsinki and approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board. All study participants provided informed consent. The study was registered at http://www.clinicaltrials.gov as NCT01503242.
Patient and donor selection
Eligible patients were 18 to 65 years-old with least one high-risk characteristic defined by the prevailing definition of high-risk at the time the trial was designed. High-risk included t(4;14), t(14;16), del(17p) by FISH, del(13q) or hypodiploidy by conventional cytogenetics, β2-microglobulin >3.5 μcg/ml, LDH greater than 1.5 times upper limit of normal, primary or secondary plasma cell leukemia (PCL), and/or demonstrating disease persistence/progression after autologous HCT (autoHCT). Patients must have an Eastern Cooperative Oncology Group performance status score of ≤2, measured or estimated creatinine clearance of > 50 ml/minute, BM cellularity ≥50% of age defined normal values by core biopsy, and the ability to provide informed consent.
Eligibility criteria excluded patients with left ventricular ejection fraction <35%, corrected diffusing capacity of the lungs for carbon monoxide <35%, fulminant liver failure, cirrhosis with portal hypertension, chronic viral hepatitis or symptomatic biliary disease. Eligibility criteria also excluded pregnant or breastfeeding females, patients with human anti-mouse antibodies, prior alloHCT, BM plasmacytomas >1 cm, untreated extramedullary plasmacytomas, HIV seropositivity, active central nervous system disease, fertile patients unwilling to use oral contraceptives during and for 12 months post-transplant, prior radiation attaining maximally tolerated levels to any critical organ, or >20 Gy prior radiation to the BM.
Patients were required to have an HLA-matched related or unrelated donor meeting established alloHCT eligibility criteria at the FHCRC. Matching for related and unrelated donors involved high-resolution typing for HLA-A, -B, -C, -DRB1 and -DQB1. Both related and unrelated donors were allowed to have a single allele mismatch without antigen mismatching at any of the HLA-A, -B, or -C loci.
Determination of antibody biodistribution and radiation absorbed dose
Approximately 21 days prior to therapy, patients underwent biodistribution studies to calculate the radiation absorbed dose imparted by 90Y to major organs and whole body. Yttrium-90 lacks discrete gamma emissions; therefore, the gamma emitter, indium-111 (111In) is used as a surrogate radionuclide for biodistribution as previously described.9 Patients received an infusion of 0.5 mg/kg of ideal body weight of BC8 mAb trace labeled with 5‐10 millicuries of 111In. Gamma camera images of the whole body were acquired at four timepoints over the course of 5 days. A BM biopsy obtained approximately 24 hours post-infusion and the activity per unit tissue mass was measured to provide a normalized value for calculating the percent administered activity present in marrow over time. Time-activity curves were generated and integrated for the major organs, and doses were then calculated using methods recommended by the Medical Internal Radiation Dose Committee of the Society of Nuclear Medicine and Medical Imaging (implemented within OLINDA/EXM 1.1, Vanderbilt University, Nashville, Tennessee, USA).10, 11 This information was then used to determine an appropriate therapy infusion activity for 90Y-DOTA-BC8 as previously described.9 The biokinetics of radiolabeled DOTA-BC8 vary considerably from one patient to another; therefore, personalized measurement assessments using 111In-DOTA-BC8 were helpful for assessing predicted biodistributions and dosimetry with subsequent therapy infusions of 90Y-DOTA-BC8 and for ensuring that previously characterized tolerable doses to normal liver would not be exceeded.
Treatment
On approximately day −12, patients received 90Y-BC8-DOTA at an activity calculated from the trace-labeled 111In-DOTA-BC8 biodistribution to deliver the planned radiation dose to the liver (normal critical organ previously demonstrated to receive the highest radiation dose).12 Based on prior experience with 90Y-ibritumomab tiuxetan13 and 131I-anti-CD45 mAb, 12, 14 the starting dose of 90Y was 6 Gy with escalation in increments of 2 Gy for each successive patient. Fludarabine was given at a dose of 30 mg/m2/day on days −4, −3 and −2. Patients received total body irradiation (TBI) at a dose of 2 Gy on day 0 followed by unmanipulated growth factor mobilized donor peripheral blood stem cells. Graft versus host disease (GVHD) prophylaxis consisted of mycophenolate mofetil (MMF) and cyclosporine (CSP). MMF was given at 15 mg/kg orally or intravenously (IV, if not tolerating PO) q12 starting on day 0 to day +27 for patients with related donors, or q8 until day +40 then tapered through day +96 for patients with unrelated donors. CSP was delivered at 3.75 mg/kg orally (or 1.5 mg/kg IV, if not tolerating PO) q12 beginning on day −3 to day +56 and tapered through day +180 for patients with related donors. For patients with unrelated donors, CSP was continued to day +100 and tapered through day +180. Figure 1 illustrates the general treatment schema.
Fig 1. Treatment schema.
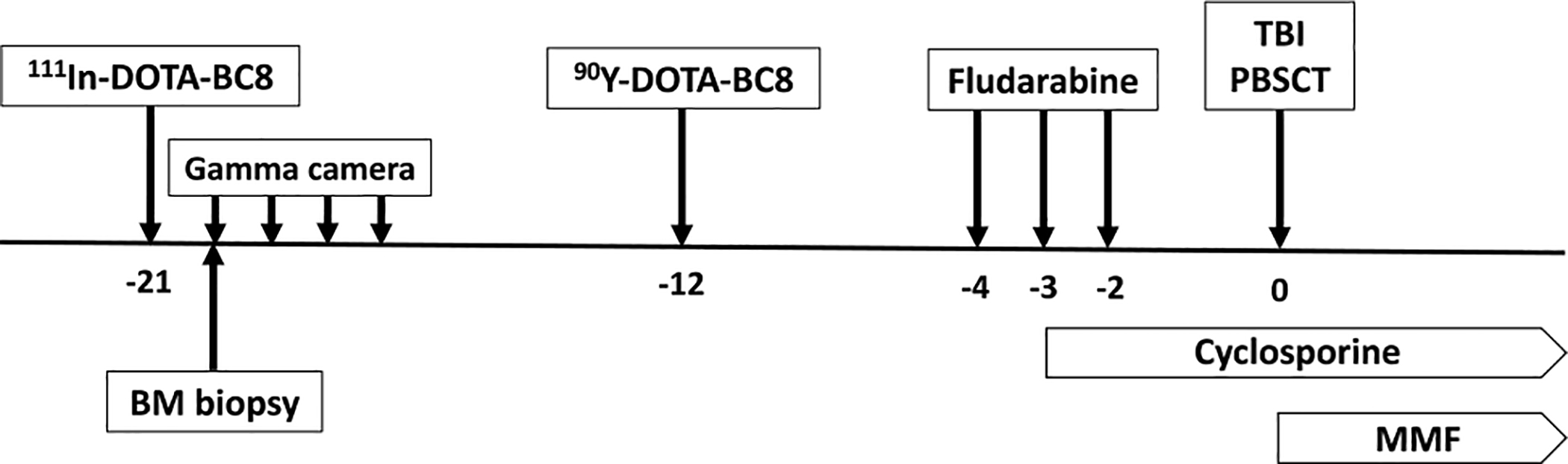
Dosimetry was performed using 111In-DOTA-BC8 infusion followed by gamma camera imaging at 4 timepoints over the course of 5 days and a bone marrow (BM) biopsy was obtained ~24 hours after infusion of 111In-DOTA-BC8. On approximately day −12, 90Y-anti-CD45 antibody was administered followed by fludarabine (30 mg/m2/day) on days −4, −3 and −2, then total body irradiation (TBI; 2 Gy) and allogeneic peripheral blood stem cell transplant (PBSCT) on day 0. Cyclosporine and mycophenolate mofetil (MMF) were started on day −3 and 0, respectively.
Dose-finding algorithm and study endpoints
The primary endpoints were safety and identification of the MTD of radiation delivered via 90Y-BC8-DOTA in combination with fludarabine and low-dose TBI. The MTD was defined as the dose that was associated with a true dose-limiting toxicity (DLT) rate of 25%. A DLT was defined as grade III or IV regimen related toxicity (RRT) occurring within 30 days post-transplant (following Bearman Criteria).15 Dose modification was planned according to Storer’s two-stage approach.16 In the first stage, patients were treated one at a time per dose level until the first DLT is observed. If a DLT had been observed, the second stage would have been initiated at the next lower dose level; and patients would have been treated in cohorts of 4. In this trial, the cohort size was dictated by the prespecified target DLT rate of 25%.
Safety evaluation included adverse event monitoring from the start of 90Y-DOTA-BC8 infusion through day +100 post-transplant or discharge from our institution to the care of the patient’s referring Hematologist/Oncologist. Adverse events (AE) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03.
The secondary endpoint of this study was efficacy assessed by disease response, duration of response, progression-free survival (PFS) and overall survival (OS). Disease response, progression and relapse were evaluated according to the International Myeloma Working Group (IMWG) response criteria between 80–90 days after transplant.
Statistical Analysis
PFS was defined as the time from transplant until disease progression, relapse or death from any cause. OS was defined as the time from transplant to death from any cause. Patients were censored at the date last known to be alive. Probabilities of PFS and OS were calculated using the Kaplan-Meier method.
Results
Patient characteristics
We treated a total of 14 patients from 2012 to 2016, and their characteristics are presented in Table 1. Deletion 17p accounted for 4 (29%), t(4;14) for 4 (29%), and t(14;16) for 1 (7%) of the patients. Five patients had more than one high-risk cytogenetic abnormality [t(4;14), t(14;16), t(14;20), del(17p), gain 1q, del 1p, nonhyperdiploid karyotype, del(13) by karyotyping). Three patients had ≥3 high-risk cytogenetic abnormalities and one patient had primary PCL. Chromosome 1 abnormalities were not included as an independent high-risk feature for determination of study eligibility, but reports have subsequently associated aberrant chromosome 1 features with inferior outcomes.17 Eleven patients (79%) received the allograft as part of a planned tandem auto-allo HCT. The median time from the last autoHCT to alloHCT was 4.4 months (range, 2–15.2 months).
Table 1.
Characteristics per patient.
| Patient No. | Age/Gender | MM subtype | High-risk cytogenetics at diagnosis and/or "high-risk" feature at enrolment | R-ISS at diagnosis | No. of prior regim ens | No. of prior auto-HCT | Disease status prior to allo- HCT | Daysfrom last auto toallo- HCT | Donor type | Disease status at day +80–90 | Current status (days after allo-HCT) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44/M | Kappa light chaln | Progressive disease after auto-HCT | 1 | 2 | 1 | VGPR | 457 | MUD | sCR | Relapsed at day +1 244, alive on other therapy |
| 2 | 53/F | IgG kappa | Persistent disease after auto-HCT and β2M ≥3.5 g/dL | II | 4 | 1 | VGPR | 85 | MUD | VGPR | Relapsed at day +1 169, alive on other therapy |
| 3 | 58/M | IgA lambda | t(4;14), −1p, persistent disease after auto-HCT requiring radiatlon | II | 1 | 1 | CR | 135 | MUD | CR | Alive and disease-free |
| 4 | 55/F | IgG kappa | Primary refractory/perslstent disease after auto-HCT | NE | 4 | 1 | VGPR | 128 | MUD | VGPR | Alive and disease-free |
| 5 | 49/M | IgA lambda | Progressive disease after auto-HCT | NE | 3 | 2 | CR | 155 | MUD | CR | Alive and disease-free |
| 6 | 41/M | IgG kappa | Primary refractory/perslstent disease after auto-HCT | NE | 2 | 1 | VGPR | 237 | MUD | VGPR | Alive and disease-free |
| 7 | 49/M | IgG kappa | t(4;14), −1p, +1q | II | 2 | 1 | VGPR | 127 | MUD | Relapse | Relapsed at day +105, died of relapse at day +185 |
| 8 | 57/F | IgG lambda | t(4;14), Progressive disease after auto- HCT | II | 4 | 2 | Relapse | 424 | MRD | VGPR | Relapsed at day +1 224, alive on other therapy |
| 9 | 61/F | IgA lambda | −1p, +1q, t(14;16), −17p | III | 1 | 1 | sCR | 142 | MUD | sCR | Alive and disease-free |
| 10 | 56/M | IgG lambda | t(4;14) | II | 2 | 1 | sCR | 95 | MUD | Relapse | Relapsed at day +84, died of relapse at day +587 |
| 11 | 55/M | IgA kappa | Persistent disease after auto-HCT, +1q | NE | 1 | 1 | CR | 176 | MRD | sCR | Alive and disease-free |
| 12 | 58/F | IgA kappa | −17p, Progressive disease after auto- HCT | II | 2 | 2 | CR | 75 | MUD | sCR | Died of resplratory fallure In the setting of liver GVHD wlthout relapse at day +262 |
| 13 | 63/F | Prima ry PCL | −17p, −13 by karyotype | II | 2 | 1 | Relapse | 95 | MRD | Relapse | Relapsed at day +63, died of relapse at day +89 |
| 14 | 59/M | IgG kappa | Hypodiploidy (monosomy 7), −13 by karyotype, +1q, −17p | II | 1 | 1 | VGPR | 59 | MUD | VGPR | Alive and disease-free |
MM = multiple myeloma; PCL= plasma celi leukemia; R-ISS = Revised International Staglng System; NE = not evaluable; sCR = stringent complete response; CR = complete response; VGPR = very good partlal response; MUD = matched unrelated donor; MRD = matched related donor
Safety
Common grade ≥3 non-hematologic AEs included gastrointestinal (43%) and metabolic or electrolyte disturbances (36%) (Table 2). No DLTs were observed up to a dose of 32 Gy to the liver (dose level 16); therefore, the MTD could not be estimated. There were no significant liver or kidney function abnormalities observed. There was no occurrence of veno-occlusive disease. TRM, defined as death due to any cause other than disease progression or relapse occurring at any time after transplantation, occurred in 1 patient at day +262 from acute respiratory failure in the setting of severe liver GVHD. The 100-day TRM was 0%.
Table 2.
NCI CTCAE non-hematologic grade 3 and 4 adverse events
| Event | Grade 3 n (%) | Grade 4 n (%) |
|---|---|---|
| Gastrointestinal | ||
| Nausea and vomiting | 3 (21) | 0 |
| Diarrhea | 2 (14) | 0 |
| Oral mucositis | 1 (7) | 0 |
| General disorders | ||
| Fever | 2 (14) | 0 |
| Chills/rigors | 1 (7) | 0 |
| Infections | ||
| Sepsis | 0 | 1 (7) |
| Metabolism and nutritional disorders | ||
| Anorexia | 1 (7) | 0 |
| Hypertriglyceridemia | 1 (7) | 0 |
| Hyperkalemia | 1 (7) | 0 |
| Hypophosphatemia | 1 (7) | 1 (7) |
| Musculoskeletal | ||
| Generalized muscle weakness | 1 (7) | 0 |
| Psychiatric/Neurologic | ||
| Altered mental status | 1 (7) | 0 |
The patterns of hematologic nadir and recovery were relatively consistent with those observed after RIC alloHCT. All patients achieved sustained engraftment for neutrophils (absolute neutrophil count >500/mL for 3 consecutive days) and platelets (>20 000/mL for 7 consecutive days without transfusion support) at a median of 16 days (range, 10–23) and 11 days (range, 9–20), respectively. By day ~28, the median donor-derived CD3 and CD33 chimerisms were 100% (range, 92–100%) and 100% (all patients). In 12 patients with available chimerism data at day ~84, the median donor-derived CD3 and CD33 chimerisms were 100% (94–100%) and 100% (all patients).
Acute GVHD grades ≥2 occurred in 11 (79%) patients, involving the skin (64%), liver (7%) and gut (7%). However, grades 3–4 acute GVHD occurred in only 2 patients (14%). Six patients (43%) developed chronic GVHD classified as either mild or moderate according to the National Institutes of Health consensus criteria.
Efficacy and survival
At day +85±5, patients underwent a comprehensive evaluation to assess disease status according to IMWG criteria. Eleven patients (79%) had a partial response or better: two stringent complete responses (CR), four CR, and five very good partial responses (VGPR). Through day +100, there was one death on day +89 due to progressive disease. At a median follow up of 5 years, six patients relapsed and/or demonstrated disease progression after alloHCT. The 90Y doses administered to these patients were 6, 8, 18, 24 and 30 Gy. Estimates of OS and PFS at 5 years were 71% (95% CI 41–88%) and 41% (95% CI 13–67%), respectively; the median OS and PFS were not reached and 40.9 months, respectively (Fig. 2). At the time of this analysis, 10 patients are alive. The median follow-up among the 10 survivors is 5 years (range, 3–7 years). Four patients have died; three due to progressive MM and one due to respiratory failure (from aspiration) in the setting of liver GVHD.
Fig 2.
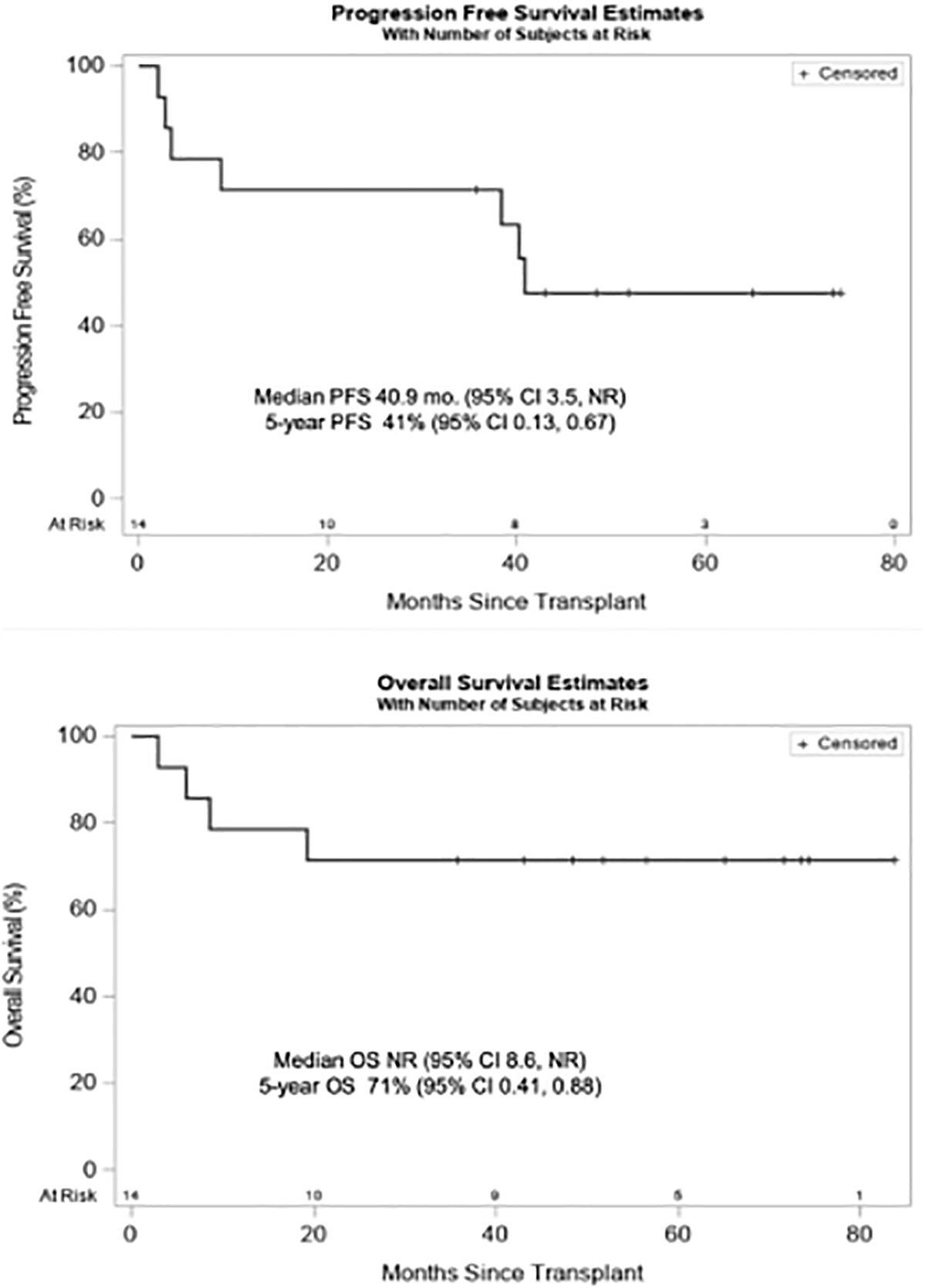
Progression-free and overall survival of patients after allogeneic HCT with 90Y-DOTA-BC8 reduced intensity conditioning.
Discussion
Although therapeutic options for MM have substantially expanded over the past decade, an unmet need remains for patients with high-risk disease. AlloHCT may provide long-term control, yet the high TRM from myeloablative regimens, and increased relapse risk from nonmyeloablative regimens, have represented critical barriers to the success of alloHCT. Our findings demonstrate that the inclusion of 90Y-DOTA-BC8, with a standard RIC regimen before alloHCT for patients with unfavorable risk MM, is both feasible and well-tolerated. This regimen did not result in increased grade ≥3 toxicities beyond those expected with fludarabine and TBI alone.18, 19 Although a maximum dose of 32 Gy was delivered to the liver, no grade III/IV DLTs were observed and no dose related transaminitis was seen, suggesting that patients may tolerate even higher hepatic doses using 90Y-anti-CD45 mAb combined with RIC. At a median follow-up of 5 years, there was no occurrence of myelodysplastic syndrome (MDS), acute myeloid leukemia (AML) or other secondary cancers.
As a dose-finding trial, this study was not powered to determine the efficacy of 90Y-DOTA-BC8 alloHCT. Furthermore, the number of patients enrolled on this trial combined with heterogenous risk factors and prior therapies (including 11 patients who had a tandem auto-alloHCT), do not allow for a formal efficacy determination. Nonetheless, the survival outcomes appear promising in this high-risk cohort. In the context of a RIC alloHCT, the European Group for Blood and Marrow Transplantation reported a 3-year PFS of 21% and a 3-year OS of 41% among patients with MM (without incorporating cytogenetics or other biologic markers).20 A phase III trial comparing tandem autoHCT vs. autoHCT followed by non-myeloablative (TBI 2 Gy) alloHCT reported a 3-year PFS and 3-year OS of 40% and 59%, respectively, among 85 patients with high-risk MM (β2-microglobulin of ≥4 mg/L and del(13) by karyotyping) in the auto-allo HCT group.21 Our group at the FHCRC recently reported long-term outcomes among 244 patients with MM treated with sequential high-dose melphalan and autoHCT followed by nonmyeloablative alloHCT. The study demonstrated inferior outcomes among high-risk and “ultra-high-risk” (≥2 adverse cytogenetic abnormalities) patient subsets and among those with progressive disease after autoHCT. The median PFS was 2.5 and 0.7 years in high- and ultra-high-risk patients, respectively; and only 0.6 years among those who previously failed autoHCT.22
The early relapses observed in three patients (range, days +63–105) on the current study suggest that early integration of maintenance therapy may have been beneficial, because maintenance therapy may eliminate residual disease or facilitate disease control during the critical period of graft maturation. Our study did not require the use of post-allograft maintenance, and the decision to institute maintenance was at the discretion of the primary hematologist/oncologist. Only one patient in the present study received maintenance with lenalidomide beginning day +126 after alloHCT, and that patient remains disease-free without GVHD. Lenalidomide has been associated with GVHD flare when initiated early after alloHCT, but is feasible when instituted later at the time of relapse.23 Bortezomib, on the other hand, is safe and feasible to administer even early after alloHCT.24 Patients treated with novel agents at first relapse after an alloHCT have a prolonged median OS of 7–8 years,22, 25 suggestive of a potential synergy between these agents and a donor-derived immunological milieu. Larger prospective studies incorporating maintenance treatment after alloHCT would help to better define the benefits of such combinations.
Our findings suggest that the radiation crossfire that results from targeting CD45 enables uniform delivery throughout the BM compartment, including target antigen-negative plasma cells. Thirteen patients (93%) on our trial had either low or absent CD45 plasma cell expression at enrollment. Despite this, all patients attained adequate absorbed radiation doses to the BM at an average of 13.4 cGy/mci (4.6–27.6) and 13.5 (4.9–26.5) to the ilium and sacrum, respectively. We are currently exploring targeting a relatively specific MM antigen, CD38, with an antibody conjugated to the alpha-emitter astatine-211 (selected for its favorable properties, including high linear energy transfer [~100 keV/μm] and short path length [50–90 μm] in tissues).26 Our preclinical data has demonstrated that 211At-anti-CD38 mAb has minimal toxicity and can eliminate residual MM cell clones in murine xenograft models designed to replicate minimal residual disease states.26 We will evaluate the addition of 211At-anti-CD38 mAb to HCT conditioning in an anticipated clinical trial at our center.
Given the limitations of alloHCT, and the growing nontransplant therapies for high-risk MM (not limited to, newer generation proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies), we acknowledge a declining enthusiasm for donor transplant in this disease. However, there is mounting evidence indicating that immune surveillance plays a critical role in MM control, and alloHCT offers an important immunotherapeutic platform mediated by alloreactive T-cells. While CAR T-cell therapy trials targeting B cell maturation antigen (BCMA) have shown striking initial anti-myeloma responses, the lack of persistence has thus far precluded long term disease control. In the largest BCMA CAR T-cell trial reported to date, the median PFS was only 11.8 months.27 Early data suggest that antigen loss or reduced antigen density is a key factor for relapse after CAR T-cell therapy.28, 29 This is not an issue with alloHCT because graft-versus-myeloma effect offers a “broad immune surveillance” that is not restricted to a single antigen. Furthermore, the lack of durable functional antimyeloma CAR T-cell responses could be related to T cell exhaustion30 or other CAR T-cell intrinsic factors;31, 32 limitations not shared by alloHCT.
In conclusion, the present study demonstrates that 90Y-anti-CD45 RIT, combined with RIC alloHCT conditioning, represents a safe and feasible therapeutic option. Promising outcomes are observed in a population of MM patients whose disease characteristics portend early death from disease. The potential for enhanced efficacy and reduced toxicity associated with RIT provides a compelling rationale for its integration into alloHCT and the approach should remain an area of active investigation for high-risk MM.
Acknowledgments
This work was supported by grants R21 CA155911, T32 CA009515, R01 CA205248 and P30 CA015704 from the National Institutes of Health, National Cancer Institute and the Conquer Cancer Foundation. The authors thank the patients and family members who enrolled in NCI protocol NCT01503242. We acknowledge the nurses at the University of Washington Medical Center and the Seattle Cancer Care Alliance for clinical care contributions. We also acknowledge Nicholas Brown, Elizabeth Morrigan and Monina Almeda for assistance with data collection, Kelsey Baker for statistical support, and Nancy Press for assistance with the figures.
Sponsor/Funding Source:
NIH/NCI R21 CA155911, NIH/NCI T32 CA009515, NIH/NCI R01 CA205248, NIH/NCI P30 CA015704, Conquer Cancer Foundation
Conflict of Interest Disclosure:
B.M.S. reports consulting for Kiadis Pharma, Actinium Pharmaceuticals, Frazier Healthcare Ventures, and Bristol-Meyers Squibb, equity ownership in AnaptysBio, Oncoresponse, Inipharm, EpiThany, and Blaze Bioscience, employment and equity ownership in Mavupharma, Inc., and research funding from Bellicum. L.A.H reports research funding from Seattle Genetics, Merck, Millennium Pharmaceuticals, Celgene, Sanofi-Aventis; royalties from UpToDate; and consulting for Jazz Pharmaceuticals and the National Comprehensive Cancer Network. J.J.O. receives research funding from Actinium Pharmaceuticals. A.K.G. reports grants and nonfinancial research support from Teva, Bristol-Myers Squibb, Merck, Takeda, Seattle Genetics, Pfizer, Janssen, Takeda, and Effector, and personal fees and nonfinancial support from Seattle Genetics, Pfizer, Janssen, Gilead Sciences, Sanofi-Aventis, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Compliment, Asana Bio, and Incyte. B.G.T. holds patents and receives royalties and research funding from Mustang Bio. J.M.P. reports consulting for Actinium Pharmaceuticals, Astrazeneca Pharmaceuticals, Bayer Healthcare Pharmaceuticals, F. Hoffman-La Roche, Pharmacyclics LLC, Teva Pharmaceuticals, consulting and personal fees from Celgene, Gilead Sciences, Janssen, personal fees from Millennium Pharmaceuticals, and honoraria from Seattle Genetics and Spectrum Pharmaceuticals. W.I.B reports consulting and personal fees from Amgen, Celgene, Janssen and personal fees from Millennium Pharmaceuticals. D.J.G receives research funding from Juno Therapeutics, Celgene, Merck, Seattle Genetics, Sanofi-Aventis, Cellectar Bio and consults for Cellectar Bio, Celgene, Seattle Genetics, and GSK. The remaining authors have disclosed no conflicts of interest.
REFERENCES
- 1.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015; 33(26): 2863–2869. e-pub ahead of print 2015/08/05; doi: 10.1200/jco.2015.61.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roos-Weil D, Moreau P, Avet-Loiseau H, Golmard J-L, Kuentz M, Vigouroux S et al. Impact of genetic abnormalities after allogeneic stem cell transplantation in multiple myeloma: a report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Haematologica 2011; 96(10): 1504–1511. doi: 10.3324/haematol.2011.042713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhakal B, Vesole DH, Hari PN. Allogeneic stem cell transplantation for multiple myeloma: is there a future? Bone Marrow Transplant 2016; 51(4): 492–500. e-pub ahead of print 2016/01/05; doi: 10.1038/bmt.2015.325 [DOI] [PubMed] [Google Scholar]
- 4.Crawley C, Iacobelli S, Bjorkstrand B, Apperley JF, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood 2007; 109(8): 3588–3594. e-pub ahead of print 2006/12/13; doi: 10.1182/blood-2006-07-036848 [DOI] [PubMed] [Google Scholar]
- 5.Pagel JM, Gooley TA, Rajendran J, Fisher DR, Wilson WA, Sandmaier BM et al. Allogeneic hematopoietic cell transplantation after conditioning with 131 I–anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 2009; 114(27): 5444–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassaday RD, Press OW, Pagel JM, Rajendran JG, Gooley TA, Fisher DR et al. Phase I Study of a CD45-Targeted Antibody-Radionuclide Conjugate for High-Risk Lymphoma. Clin Cancer Res 2019. e-pub ahead of print 2019/09/05; doi: 10.1158/1078-0432.Ccr-19-1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheson BD. Radioimmunotherapy of non-Hodgkin lymphomas. Blood 2003; 101(2): 391–398. e-pub ahead of print 2002/10/24; doi: 10.1182/blood-2002-06-1793 [DOI] [PubMed] [Google Scholar]
- 8.Gonsalves WI, Timm MM, Rajkumar SV, Morice WG, Dispenzieri A, Buadi FK et al. The prognostic significance of CD45 expression by clonal bone marrow plasma cells in patients with newly diagnosed multiple myeloma. Leukemia research 2016; 44: 32–39. e-pub ahead of print 2016/03/21; doi: 10.1016/j.leukres.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajendran JG, Fisher DR, Gopal AK, Durack LD, Press OW, Eary JF. High-dose (131)I-tositumomab (anti-CD20) radioimmunotherapy for non-Hodgkin’s lymphoma: adjusting radiation absorbed dose to actual organ volumes. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2004; 45(6): 1059–1064. e-pub ahead of print 2004/06/08; [PubMed] [Google Scholar]
- 10.Loevinger RBT, Watson EE. MIRD primer for absorbed dose calculations, Rev. ed edn Society of Nuclear Medicine, Incorporated: New York, NY, 1991. [Google Scholar]
- 11.Fisher DR. Internal dosimetry for systemic radiation therapy. Seminars in radiation oncology 2000; 10(2): 123–132. e-pub ahead of print 2000/03/23; doi: 10.1053/srao.2000.0123 [DOI] [PubMed] [Google Scholar]
- 12.Pagel JM, Appelbaum FR, Eary JF, Rajendran J, Fisher DR, Gooley T et al. 131I–anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood 2006; 107(5): 2184–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiesa C, Botta F, Coliva A, Maccauro M, Devizzi L, Guidetti A et al. Absorbed dose and biologically effective dose in patients with high-risk non-Hodgkin’s lymphoma treated with high-activity myeloablative 90Y-ibritumomab tiuxetan (Zevalin). Eur J Nucl Med Mol Imaging 2009; 36(11): 1745–1757. e-pub ahead of print 2009/05/21; doi: 10.1007/s00259-009-1141-x [DOI] [PubMed] [Google Scholar]
- 14.Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Bush SA et al. Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131I-labeled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood 1995; 85(4): 1122–1131. e-pub ahead of print 1995/02/15; [PubMed] [Google Scholar]
- 15.Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. Journal of Clinical Oncology 1988; 6(10): 1562–1568. doi: 10.1200/jco.1988.6.10.1562 [DOI] [PubMed] [Google Scholar]
- 16.Storer BE. Small-sample confidence sets for the MTD in a phase I clinical trial. Biometrics 1993; 49(4): 1117–1125. e-pub ahead of print 1993/12/01; [PubMed] [Google Scholar]
- 17.Manier S, Salem KZ, Park J, Landau DA, Getz G, Ghobrial IM. Genomic complexity of multiple myeloma and its clinical implications. Nature reviews. Clinical oncology 2017; 14(2): 100–113. e-pub ahead of print 2016/11/03; doi: 10.1038/nrclinonc.2016.122 [DOI] [PubMed] [Google Scholar]
- 18.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood 2003; 102(9): 3447–3454. doi: 10.1182/blood-2002-09-2955 [DOI] [PubMed] [Google Scholar]
- 19.Rotta M, Storer BE, Sahebi F, Shizuru JA, Bruno B, Lange T et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood 2009; 113(14): 3383–3391. e-pub ahead of print 2008/11/19; doi: 10.1182/blood-2008-07-170746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawley C, Lalancette M, Szydlo R, Gilleece M, Peggs K, Mackinnon S et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood 2005; 105(11): 4532–4539. e-pub ahead of print 2005/02/26; doi: 10.1182/blood-2004-06-2387 [DOI] [PubMed] [Google Scholar]
- 21.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E 3rd et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. The Lancet. Oncology 2011; 12(13): 1195–1203. e-pub ahead of print 2011/10/04; doi: 10.1016/s1470-2045(11)70243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maffini E, Storer BE, Sandmaier BM, Bruno B, Sahebi F, Shizuru JA et al. Long-term follow up of tandem autologous-allogeneic hematopoietic cell transplantation for multiple myeloma. Haematologica 2019; 104(2): 380–391. e-pub ahead of print 2018/09/29; doi: 10.3324/haematol.2018.200253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bensinger WI, Green DJ, Burwick N, Becker PS. A prospective study of lenalidomide monotherapy for relapse after Allo-SCT for multiple myeloma. Bone Marrow Transplant 2014; 49(4): 492–495. e-pub ahead of print 2014/01/15; doi: 10.1038/bmt.2013.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green DJ, Maloney DG, Storer BE, Sandmaier BM, Holmberg LA, Becker PS et al. Tandem autologous/allogeneic hematopoietic cell transplantation with bortezomib maintenance therapy for high-risk myeloma. Blood advances 2017; 1(24): 2247–2256. e-pub ahead of print 2018/01/04; doi: 10.1182/bloodadvances.2017010686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giaccone L, Evangelista A, Patriarca F, Sorasio R, Pini M, Carnevale-Schianca F et al. Impact of New Drugs on the Long-Term Follow-Up of Upfront Tandem Autograft-Allograft in Multiple Myeloma. Biol Blood Marrow Transplant 2018; 24(1): 189–193. e-pub ahead of print 2017/10/11; doi: 10.1016/j.bbmt.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 26.O’Steen S, Comstock ML, Orozco JJ, Hamlin DK, Wilbur DSS, Jones JC et al. The Alpha Emitter Astatine-211 Targeted to CD38 can Eradicate Multiple Myeloma in a Disseminated Disease Model. Blood 2019. e-pub ahead of print 2019/08/10; doi: 10.1182/blood.2019001250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2019; 380(18): 1726–1737. e-pub ahead of print 2019/05/03; doi: 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol 2018; 36(22): 2267–2280. e-pub ahead of print 2018/05/31; doi: 10.1200/jco.2018.77.8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol 2015; 194(3): 911–920. e-pub ahead of print 2014/12/19; doi: 10.4049/jimmunol.1402346 [DOI] [PubMed] [Google Scholar]
- 30.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 2013; 25(2): 214–221. e-pub ahead of print 2013/01/10; doi: 10.1016/j.coi.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLellan AD, Ali Hosseini Rad SM. Chimeric antigen receptor T cell persistence and memory cell formation. Immunol Cell Biol 2019; 97(7): 664–674. e-pub ahead of print 2019/04/23; doi: 10.1111/imcb.12254 [DOI] [PubMed] [Google Scholar]
- 32.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24(5): 563–571. e-pub ahead of print 2018/05/02; doi: 10.1038/s41591-018-0010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


