Abstract
The mammary gland undergoes extensive remodeling during pregnancy and is also subject to neoplastic processes both of which result in histological changes of the gland epithelial structure. Since the mammary tree is a complex three-dimensional structure a method is needed that provides an overview of the entire gland. Whole mounts provide this information, are inexpensive and do not require specialized equipment. This protocol describes mammary gland isolation, whole mount preparation and analysis. Mammary gland tissue, which is removed postmortem, is stained with Carmine Alum, a nuclear stain, allowing detection of epithelial structures embedded in the adipose tissue of the mammary fat pad. Stained mammary glands are imaged by light microscopy or embedded and sectioned for histological examination. Image analysis software such as Image J can be used to quantify extensity of branching complexity, epithelial structure remodeling or hyperplastic changes.
Keywords: Mammary gland, Whole mount, Branching morphogenesis, Mammary epithelial cell, Mammary tree
Background
Although development of the mammary gland begins during embryonic development and a rudimentary epithelial structure is present at birth, the epithelial mammary tree undergoes extensive expansion postnatally. In response to hormonal changes, mammary epithelial cells proliferate and invade the mammary fat pad. During pregnancy, the mammary gland epithelium undergoes further differentiation and remodeling to prepare for milk production. Subsequently, these epithelial structures involute in response to weaning. These remodeling processes are driven by hormones, growth factors, cytokines and the extracellular matrix. In addition to remodeling in response to physiological processes, the mammary gland is subject to pathological processes such as neoplastic transformation. This complex biology together with the relatively ease of isolation make the mammary gland a useful experimental model. Experimental studies analyzing mammary gland biology or neoplastic transformation often employ mouse models to quantify the effect of gene deletion or overexpression on mammary gland development, remodeling and neoplastic transformation. Mammary gland whole mounts allow routine examination of these normal and disease processes on the entire 3D epithelial structure of the mammary gland ( Plante et al., 2011 ; Inman et al., 2015 ; Tucker et al., 2016 and 2017; Kolla et al., 2017 ; Tolg et al., 2017 ). Furthermore, injection of mice with potential therapeutic compounds combined with whole mount analysis allows in vivo testing of future cancer treatment strategies. This protocol provides details of the procedure starting with removal of the mammary gland and ending with image analysis. A video of mammary gland isolation, photos, figures and referenced literature make it more complete compared to existing whole mount protocol.
Materials and Reagents
Glass slide (Micro Slides Superfrost Plus) (VWR, catalog number: 48311-703)
Micro cover glass (VWR, catalog number: 48393-081)
Glass Pasteur pipette (VWR, catalog number: 14672-380)
Glass Slide Staining Dish (DWK Life Sciences, WheatonTM, catalog number: 900200)
Coplin Staining Dish (Variety Glass, catalog number: 674EMD)
50 ml Crew Cap tubes (SARSTEDT, catalog number: 62.547.254)
Mice
100% ethanol (Greenfield Global, Commercial Alcohols, catalog number: P016EAAN)
Glacial acetic acid (Merck, catalog number: AX0073-9)
Carmine dye: Carmin Alum (Fisher Scientific, catalog number: C579-25)
Aluminum potassium dodecahydrate (Merck, catalog number: 101042)
Hydrochloric acid (HCl, Sigma-Aldrich, catalog number: H1758)
Xylene (LabChem, catalog number: LC269704)
Permount SP15 (Fisher Scientific, catalog number: SP15-100)
Carnoy’s solution (see Recipes)
Carmine alum solution (see Recipes)
Destaining solution (see Recipes)
Equipment
Small scissors (Delicate dissecting scissors, Fisher Scientific, FisherbrandTM, catalog number: 08-951-5; Sharp-pointed dissecting scissors, Fisher Scientific, FisherbrandTM, catalog number: 08-940)
Forceps (Fisherbrand curved medium point general purpose forceps)
Chemical hood (Fisher Scientific)
Dissecting microscope with digital camera (Q color 3 camera, OLYMPUS, model: SZX 12)
Stirring Hotplate (Fisher Scientific)
Weighing scale (Denver Instrument, model: XS-310D, catalog number: 8531.1)
pH meter (Fisher Scientific, Accumet Research, model: AR15, catalog number: 13-636-AR15)
Software
ImageJ: free download available at https://imagej.nih.gov/ij
Procedure
-
Preparation of female mice that are older than 2 weeks for mammary gland whole mounts
Notes:
Any mouse strain can be used. We routinely use C57Bl/6 and FVB mice that we breed in our animal facility. These mice are also available from commercial breeders (TACONIC model # B6-F and FVB).
Rat mammary glands can likely also be used but fixation, staining, de-staining and de-hydration times need to be increased.
To study mouse mammary gland branching during gestation, the beginning of pregnancy has to be known. To obtain timed pregnancies, breed one adult male with two 6-8 weeks old females. Add the females to the male cage at the end of the day and inspect females for copulation plugs the following morning. Remove both females from the male cage. Mice that show a copulation plug are potential pregnant and should be housed separately. The day the copulation plug is observed is day 1 of the pregnancy. Images of copulation plugs are available online e.g., Reproductive Biology of Breeding Mice (Soper, The Jackson Laboratory).
To isolate the mammary gland, sacrifice the mouse at the desired day of gestation using CO2 inhalation. Using small scissors, make one skin incision across the abdomen and a second incision down the midline. The skin can now be peeled back and the mammary gland becomes visible underneath the skin.
Use small scissors to remove the mammary gland from the skin. Please see provided video (Video 1) for details.
Transfer the gland to an electrostatically adherent microscopy slide and use blunt end forceps to spread out the gland over the glass slide as much as possible without tearing apart the tissue.
Lay slide with the tissue side facing up on a flat surface for 5 min to allow the tissue to partially dry and stick to the glass surface.
Submerge the slides in sufficient amount of Carnoy’s solution to cover the entire mammary gland and store inside a chemical hood for 18-24 h at room temperature.
Tissue staining, de-staining, dehydration, and mounting has to be performed inside a chemical hood.
Transfer the slides into Carmine alum solution and store in the solution 24 h-3 days, depending on the thickness of the mammary gland, at room temperature. Staining is completed when red dye penetrated entire tissue and unstained areas are visible. The Carmine alum solution can be used up to three times.
The Carmine alum will stain the epithelial structures of the gland. To remove excess dye submerge the slides in de-staining solution at room temperature. Change the solution every 30 min until solution remains clear. The time required for de-staining varies and depends on the thickness of the mammary gland but 3 h is sufficient for most samples.
Dehydrate mammary glands by submerging slides for 5 min each in 70, 80, 95 and 100% ethanol, then submerge slides in xylene overnight at room temperature.
Slides can be stored submerged in xylene in glass containers or be mounted with Permount SP15. Glass containers with slides and xylene need to be stored inside a chemical hood. Evaporated Xylene needs to be replaced from time to time to prevent samples from drying out. Therefore, mounting slides with Permount is a better alternative for long-term storage.
To mount samples: lay slides tissue side up on a flat surface inside a chemical hood. Cover the sample with sufficient Permount SP15 to cover the mammary gland tissue. Slowly lower a cover glass on top of the mammary tissue and apply light pressure.
Lay slides tissue side up on a flat surface inside a chemical hood. Let slides dry for 24 h. Permount will shrink while drying, exposing outer edges of the mammary gland tissue and making it necessary to add more Permount. Add Permount underneath the coverslip edges using a glass Pasteur pipette with a rubber ball. Lay slides tissue side up on a flat surface inside a chemical hood and inspect slides again after 24 h. Continue adding Permount for 3-7 days or until mammary gland tissue remains covered with Permount. Store mounted slides laying flat inside a chemical hood for one week. Slides can be stored inside a plastic slide box.
Images of the mammary gland can be taken with a digital camera attached to a dissecting microscope.
Video 1. Skin incision, removal of the mammary gland and spreading of the tissue onto glass slides.
Data analysis
Branching of the mammary epithelial tree can be analyzed by image analysis. The 4th mammary gland contains a lymph node which will be clearly visible in whole mounts due to its high cell density and is often used as reference point when measuring branch length or counting branch points (Figures 1 and 2). Image analysis programs such as ImageJ are useful in measuring the length of primary and secondary branches.
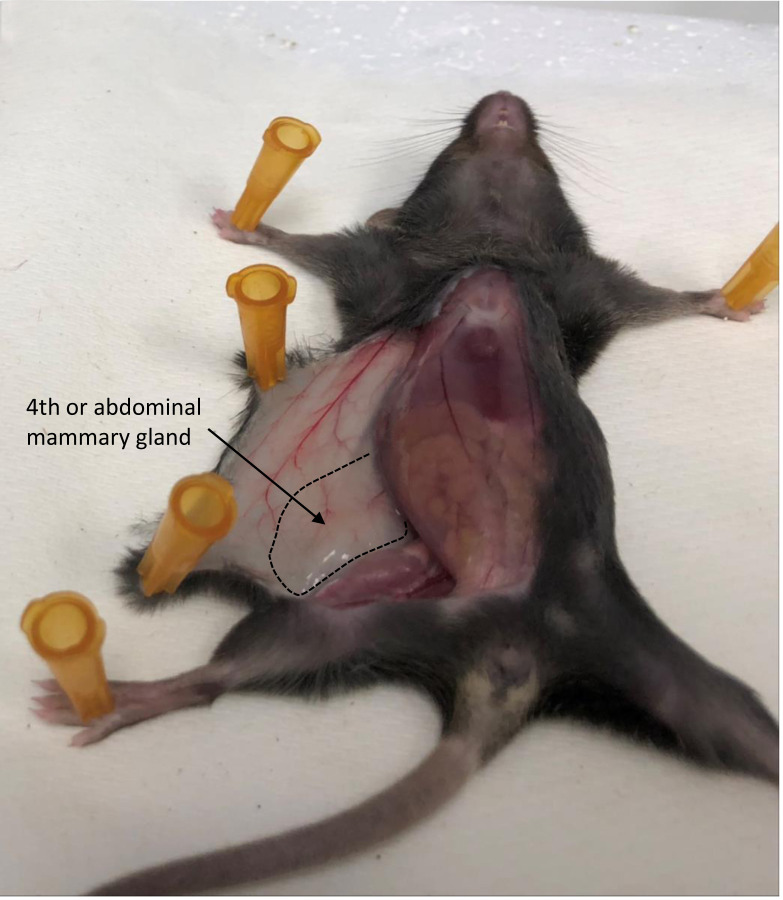
Figure 1. 4th mammary gland location in the mouse.
Area of the 4th mammary gland is outlined and indicated.
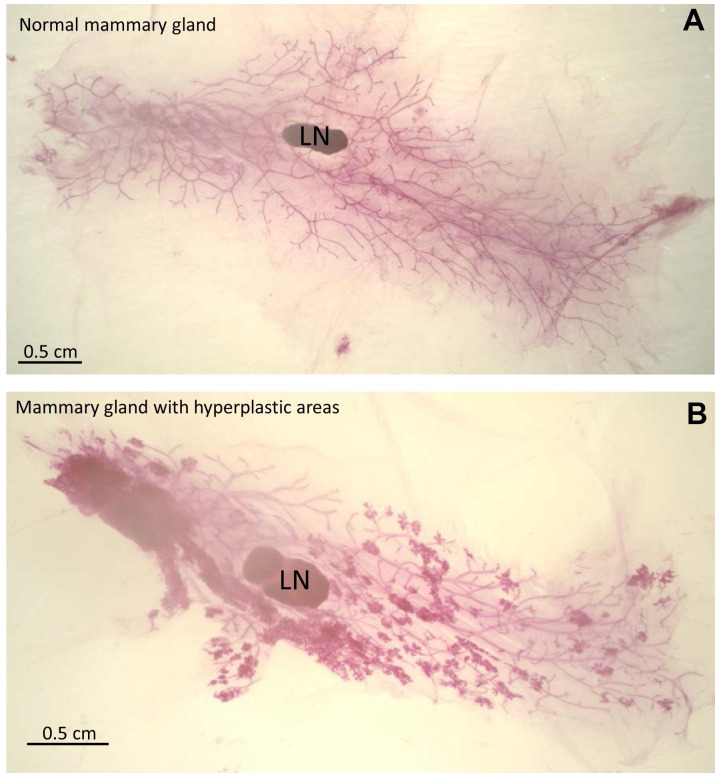
Figure 2. Examples of mammary gland whole mounts.
Stained mammary gland whole mounts were mounted using Permount and images were taken with a dissecting microscope. Lymph nodes are labeled LN. A. Image of a normal mammary gland. B. Image of mammary gland containing hyperplastic areas.
Open the image in ImageJ. Choose the freehand option of the “Line” tool from the main menu and follow the mammary gland branch on the screen (Figure 3A). Then click “measure” from the main menu of ImageJ. The measurement will be added to a result table which can be copied into “excel”. Alternatively, the number of branch points/area can be counted to describe branching complexity without the use of any special software or using the “multi point” option in the main menu of ImageJ (Figure 3A). Staining intensity is increased in areas of mammary gland hyperplasia. The thresholding function of ImageJ allows quantification of these areas (Figure 3B).
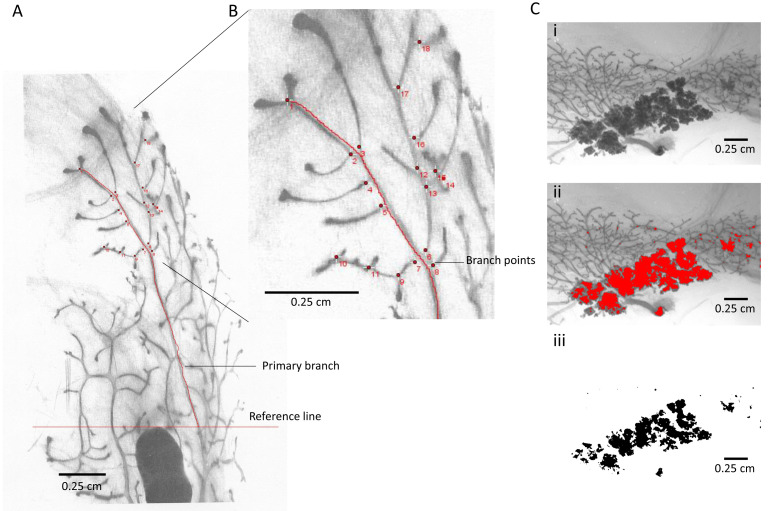
Figure 3. Examples of image analysis.
A. Black and white image of mounted mammary gland whole mount. The image was taken using a digital camera attached to a dissecting microscope. The image was saved as .tiff and re-opened in ImageJ. Reference line (red) was drawn using the outer edge of the lymph note as reference point. One primary branch was outlined in red starting at the reference line. B. Magnified area of (A): Branch points (red dots) were counted using the multi-point function of ImageJ. C. Example of image analysis using the thresholding function of ImageJ. i. Black and white image of mammary gland area containing hyperplasia. ii. Hyperplastic area was selected using the thresholding function of Image J and highlighted in red. iii. The selected hyperplastic area is shown in black on a white background. Scale bars = 0.25 cm.
Open the image in ImageJ, then go to “Image” and choose “Adjust”, then choose “Threshold”. Adjust the threshold until the area you want to measure is highlighted then click “apply”. Go to “set measurement” in the main menu, make sure “area” and “limited to threshold” are clicked. Then click “measure” in the main menu. The measurement will be added to a result table which can be copied into “excel”.
Recipes
-
Carnoy’s solution
For 1 L Carnoy’s buffer:
Mix 750 ml 100% ethanol with 250 ml glacial acetic acid under a chemical hood
Buffer can be stored at room temperature in a glass bottle
-
Carmine alum solution
For 100 ml Carmin alum:
Add 200 mg Carmine dye and 500 mg aluminum potassium to 100 ml demineralized H2O
Boil the mixture for 5 sec, then let it cool down to room temperature before use
Carmin alum can be reused multiple times and can be stored at room temperature in a glass bottle
-
Destaining solution
For 1 L de-staining solution:
Mix 700 ml 100% ethanol with 298 ml demineralized H2O and add 2 ml 12 N HCl or add 2 ml 12 N HCl to 70% ethanol
Acknowledgments
This work was supported by a CBCF grant, and Breast Cancer Society of Canada to ET, The Endre A. Balazs Foundation to MC. This protocol was adapted from Sympson et al., 1994 . The authors do not have any conflict of interest to declare.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Inman J. L., Robertson C., Mott J. D. and Bissell M. J.(2015). Mammary gland development: cell fate specification, stem cells and the microenvironment. Development 142(6): 1028-1042. [DOI] [PubMed] [Google Scholar]
- 2. Kolla S., Pokharel A. and Vandenberg L. N.(2017). The mouse mammary gland as a sentinel organ: distinguishing'control' populations with diverse environmental histories. Environ Health 16(1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Plante I., Stewart M. K. and Laird D. W.(2011). Evaluation of mammary gland development and function in mouse models. J Vis Exp (53). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tolg C., Yuan H., Flynn S. M., Basu K., Ma J., Tse K. C. K., Kowalska B., Vulkanesku D., Cowman M. K., McCarthy J. B. and Turley E. A.(2017). Hyaluronan modulates growth factor induced mammary gland branching in a size dependent manner. Matrix Biol 63: 117-132. [DOI] [PubMed] [Google Scholar]
- 5. Tucker D. K., Foley J. F., Bouknight S. A. and Fenton S. E.(2017). Sectioning mammary gland whole mounts for lesion identification. J Vis Exp (125). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tucker D. K., Foley J. F., Hayes-Bouknight S. A. and Fenton S. E.(2016). Preparation of high-quality hematoxylin and eosin-stained sections from rodent mammary gland whole mounts for histopathologic review. Toxicol Pathol 44(7): 1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soper, B. W. Reproductive Biology of Breeding Mice.
- 8. Sympson C. J., Talhouk R. S., Alexander C. M., Chin J. R., Clift S. M., Bissell M. J. and Werb Z.(1994). Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol 125(3): 681-693. [DOI] [PMC free article] [PubMed] [Google Scholar]