Abstract
The trace fear conditioning protocol is designed to measure hippocampal function in mice. The protocol includes a neutral conditioned stimulus (tone) and an aversive unconditioned stimulus (shock), separated in time by a trace interval. The trace interval between the tone and the shock critically involves the hippocampus and could be used to evaluate hippocampal-dependent learning and memory. In this protocol, we presented mice with five pairings of tone and shock separated by a 20 sec trace interval. Freezing was measured 24 h after conditioning to evaluate contextual memory by placing mice in the conditioned chamber. In addition, 48 h after conditioning, freezing was measured in a dark chamber, which served as a different context. This method enables precise detection of hippocampal-dependent learning and memory following pharmacological and genetic manipulations that impair or enhance hippocampal function.
Keywords: Trace fear conditioning (TFC), Contextual memory, Hippocampus function, Memory enhancement, Learning and memory deficits
Background
The trace fear conditioning (TFC) paradigm differs from standard fear conditioning paradigms ( Heise et al., 2017 ; Segev et al., 2013 and 2015) by the simple insertion of a trace interval between a conditioned stimulus (CS, e.g., tone) and an unconditioned stimulus (US, e.g., electric foot shock), and repeated application of their combination at fixed intervals. The TFC paradigm involves the formation of temporally non-contiguous associations in both natural and pathological conditions, and is considered a complex, hippocampal-dependent paradigm, in contrast to simple cortical-dependent learning paradigms such as taste learning ( Stern et al., 2013 ; Ounallah- Saad et al., 2014 ; Rappaport et al., 2015 ; Levitan et al., 2016 ; Sharma et al., 2018 ). A remarkable aspect of trace fear conditioning is that it provides a reliable model of attention-dependent associative learning that reflects the complex processing of the hippocampus and alters the circuitry recruited for learning. Several studies have shown that hippocampal lesions before and after training impair the ability of the animal to associate the CS and US stimuli when they are separated by the trace interval ( Bangasser et al., 2006 ; Esclassan et al., 2009 ). However, animals with hippocampal lesions could associate the CS and US in a delay situation, where no trace interval separates them, but the CS and US co-terminate ( McEchron et al., 1998 ; McEchron et al., 2000 ; Quinn et al., 2002 ). Although other brain regions such as the medial prefrontal cortex ( Peters et al., 2009 ; Beeman et al., 2013 ), the entorhinal and perirhinal cortices ( Esclassan et al., 2009 ; Kent and Brown, 2012), and the amygdala (Pape and Pare, 2010; Gilmartin et al., 2012 ) are involved in relaying stimulus inputs and response outputs, the hippocampus is selectively involved in trace conditioning rather than general fear learning or expression. Moreover, the acquisition of trace fear conditioning increases intrinsic excitability and facilitates LTP in pyramidal neurons of the hippocampus ( Song et al., 2012 ), which makes trace fear conditioning an ideal paradigm to test hippocampal function in young and aged mice ( Sharma et al., 2018 ).
Materials and Reagents
-
Animals
Male C57BL/6 mice (Envigo, Jerusalem) weighing 20-25 g and approximately 12 weeks old were used in this study. This protocol can also be used to study the function of the hippocampus in other strains and different age groups of mice ( Shoji et al., 2016 ; Sharma et al., 2018 ). The mice were housed individually, on a 12/12 h light/dark cycle, and provided with water and standard rodent chow ad libitum.
70% ethanol (Fisher Scientific, catalog number: BP82011)
Equipment
-
TFC chambers (Coulbourn Instruments, model: H10-11M-TC)
Place TFC chambers measuring 25 x 25 x 25 cm internally inside a larger, insulated plastic cabinet that excludes external light and noise (Panlab, Harvard Apparatus, model: LE116 76-0280).
Visual (CCD) and infrared camera (Sensor Technologies America, model: STC-CMB4MPOE) along with an infrared illuminator (Bosch, model: EX12LED-3BD-8W).
Software
-
FreezeFrame 3.0 and FreezeView software (Coulbourn Instruments)
Note: Both software components can be downloaded from the Actimetrics website.
-
FreezeView manual
Note: The manual can be downloaded from the Coulbourn webpage.
Procedure
See Video 1 for the procedure to perform the experiment described in this protocol.
Video 1. Trace Fear Conditioning Protocol.
This video describes the trace fear conditioning protocol to assess hippocampal function in mice (Animals were handled according to approved protocols and animal welfare regulations of the University of Haifa Institutional Ethics Committee).
-
Acquisition of TFC
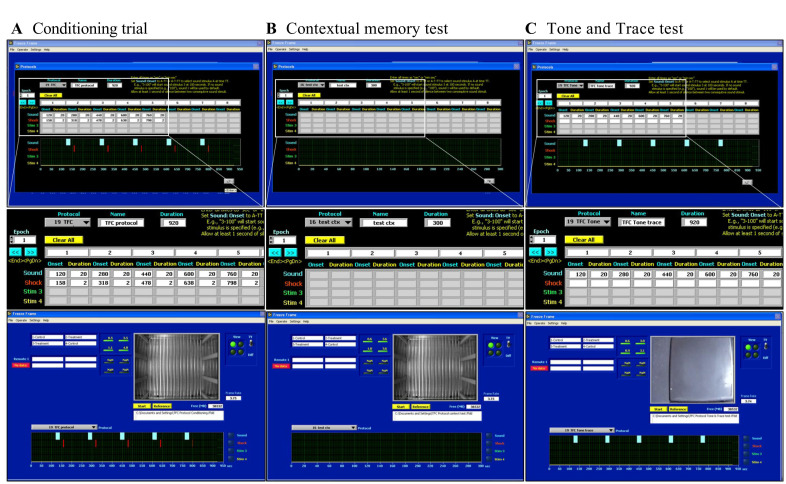
Start the FreezeFrame 3.0 software (Coulbourn Instruments) and select the protocol for TFC See Figure 1 for protocol settings in FreezeFrame 3.0.
Turn on the light and fan of the conditioning box and calibrate the shock levels, light levels, and sound intensity levels for the testing chamber.
For the TFC protocol, place mice in a chamber (with a 20 W bulb and a 16-bar metal grid floor) for 120 sec. Apply a 2.9 kHz tone for 20 sec at 80 dB (conditioned stimulus, CS) and a 0.5 mA foot shock for 2 sec (unconditioned stimulus, US) at the end of the 20 sec trace interval.
Repeat the previous step four times and separate each trial by a 120 sec inter trial interval (ITI).
After administration of the last shock, keep the animals in the chamber for 120 sec before taking them back to the home cage in order to maintain a constant ITI during the conditioning procedure. Do not house the mice in the same room as the testing room.
Clean the chambers with 10% ethanol between successive sets of mice.
-
Contextual memory testing
Place mice in the conditioning chamber 24 h after conditioning and record freezing for 300 sec (without tone or foot shock) using a visual camera. Analyze the data with FreezeView software (Coulbourn Instruments).
Clean chambers with 10% ethanol between successive sets of mice.
-
Tone and Trace memory testing
For the tone-trace test, place animals in the dark chambers 48 h after conditioning, but hide the grid floor with black plastic to create another context.
Present the animals with the TFC protocol as on the conditioning day, but without shock.
Record animal behavior using an infrared camera.
Clean the chambers with water between successive sets of mice.
-
Extinction of fear memory
Extinction of TFC refers to a reduction in the freezing response after the repeated presentation of a CS in the absence of the previously paired US.
Measure extinction of tone-trace fear response 24 h and 48 h after Tone and Trace testing.
Place mice in the dark chambers and hide the grid floor with black plastic to create another context.
Present the animals with the TFC protocol as on the conditioning day, but without shock.
Record animal behavior using an infrared camera.
Clean the chambers with water between successive mice. Do not use 10% ethanol, since the mice may associate the smell with the conditioned context. In this case, water constitutes a different context, allowing to test auditory fear response.
Figure 1. The screenshots from FreezeFrame 3.0 software showing settings used in the TFC protocol.
A. Conditioning trial; B. Contextual test; C. Tone and trace test.
Data analysis
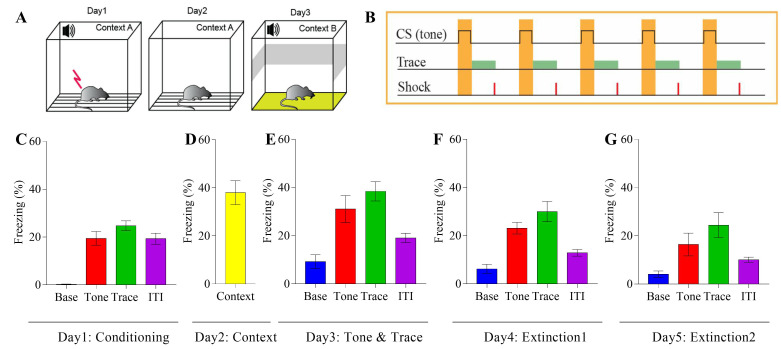
The indication for fear memory is the percentage of time spent freezing during the context, tone, and trace event. See Figure 2 for an overview of the experiment and representative results.
The software reports freezing behavior to the tone and trace interval as percent freezing across all five trials.
Shapiro–Wilk test was used as a numerical means of assessing normality. Independent-samples t-test was used as a parametric test and the Mann-Whitney U test was used for the nonparametric equivalent.
Figure 2. Mice tested in the TFC protocol show evidence of contextual fear and freezing during tone and trace interval.
A. Experimental design; B. TFC protocol used for the conditioning; C. Freezing response of mice during the conditioning, Base (% freezing during first 120 sec), Tone (average % freezing during the tones), Trace (average % freezing during the traces) and ITI (average % freezing during the ITI); D. Contextual memory test (% freezing during 300 sec in context); E. Tone and trace fear response, Base (% freezing during first 120 sec), Tone (average % freezing during the tones), Trace (average % freezing during the traces) and ITI (average % freezing during the ITI); F-G. The mice show normal extinction of fear response during tone and trace interval.
Notes
To establish a contextual memory, it is important that the mice be kept in the chamber for 120 sec prior to the stimuli. In addition, a 20 sec trace interval ensures a proper association between the conditional and unconditional stimuli. Five repetitions of this pairing induces a strong response to the trace interval.
Acknowledgments
Our laboratory used this protocol to assess TFC in mice in a recent publication ( Sharma et al., 2018 ). That work was supported by the Israeli Ministry of Science, Technology, and Space (MOST 3-12080 to K.R.); the Israel Science Foundation (ISF 1003/12, ISF-IDRC 2395/2015 to K.R.); the Wolfson Charitable Trust (K.R.); and the Israeli Planning and Budgeting Committee Program Fellowships for Outstanding Post-Doctoral Fellows from China and India (V.S.) and the Ministry of Science and Technology (Eshkol post-doctoral fellowship) and the Tauber foundation fellowship to HO-S. We have modified the protocol in an attempt to make it more efficient to evaluate hippocampus function ( Lugo et al., 2014 ).
Competing interests
The authors declare that there are no conflicting and/or competing interests.
Ethics
Animals were handled according to approved protocols and animal welfare regulations of the University of Haifa Institutional Ethics Committee.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Bangasser D. A., Waxler D. E., Santollo J. and Shors T. J.(2006). Trace conditioning and the hippocampus: the importance of contiguity. J Neurosci 26(34): 8702-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beeman C. L., Bauer P. S., Pierson J. L. and Quinn J. J.(2013). Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learn Mem 20(6): 336-343. [DOI] [PubMed] [Google Scholar]
- 3. Esclassan F., Coutureau E., Di Scala G. and Marchand A. R.(2009). A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning. J Neurosci 29(25): 8087-8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gilmartin M. R., Kwapis J. L. and Helmstetter F. J.(2012). Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiol Learn Mem 97(4): 452-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heise C., Taha E., Murru L., Ponzoni L., Cattaneo A., Guarnieri F. C., Montani C., Mossa A., Vezzoli E., Ippolito G., Zapata J., Barrera I., Ryazanov A. G., Cook J., Poe M., Stephen M. R., Kopanitsa M., Benfante R., Rusconi F., Braida D., Francolini M., Proud C. G., Valtorta F., Passafaro M., Sala M., Bachi A., Verpelli C., Rosenblum K. and Sala C.(2017). eEF2K/eEF2 pathway controls the excitation/inhibition balance and susceptibility to epileptic seizures. Cereb Cortex 27(3): 2226-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kent B. A. and Brown T. H.(2012). Dual functions of perirhinal cortex in fear conditioning. Hippocampus 22(10): 2068-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levitan D., S. Gal-Ben-Ari, Heise C., Rosenberg T., Elkobi A., Inberg S., Sala C. and Rosenblum K.(2016). The differential role of cortical protein synthesis in taste memory formation and persistence. NPJ Sci Learn 1: 16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McEchron M. D., Bouwmeester H., Tseng W., Weiss C. and Disterhoft J. F.(1998). Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 8(6): 638-646. [DOI] [PubMed] [Google Scholar]
- 9. McEchron M. D., Tseng W. and Disterhoft J. F.(2000). Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate(fear) conditioning in rabbits. Hippocampus 10(6): 739-751. [DOI] [PubMed] [Google Scholar]
- 10. Ounallah-Saad H., Sharma V., Edry E. and Rosenblum K.(2014). Genetic or pharmacological reduction of PERK enhances cortical-dependent taste learning. J Neurosci 34(44): 14624-14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pape H. C. and Pare D.(2010). Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90(2): 419-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters J., Kalivas P. W. and Quirk G. J.(2009). Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16(5): 279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quinn J. J., Oommen S. S., Morrison G. E. and Fanselow M. S.(2002). Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus 12(4): 495-504. [DOI] [PubMed] [Google Scholar]
- 14. Rappaport A. N., Jacob E., Sharma V., Inberg S., Elkobi A., Ounallah-Saad H., Pasmanik-Chor M., Edry E. and Rosenblum K.(2015). Expression of quinone reductase-2 in the cortex is a muscarinic acetylcholine receptor-dependent memory consolidation constraint. J Neurosci 35(47): 15568-15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Segev Y., Barrera I., Ounallah-Saad H., Wibrand K., Sporild I., Livne A., Rosenberg T., David O., Mints M., Bramham C. R. and Rosenblum K.(2015). PKR inhibition rescues memory deficit and ATF4 overexpression in ApoE epsilon4 human replacement mice. J Neurosci 35(38): 12986-12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Segev Y., Michaelson D. M. and Rosenblum K.(2013). ApoE epsilon4 is associated with eIF2alpha phosphorylation and impaired learning in young mice. Neurobiol Aging 34(3): 863-872. [DOI] [PubMed] [Google Scholar]
- 17. Sharma V., Ounallah-Saad H., Chakraborty D., Hleihil M., Sood R., Barrera I., Edry E., Kolatt Chandran S., S. Ben Tabou de Leon, Kaphzan H. and Rosenblum K.(2018). Local inhibition of PERK enhances memory and reverses age-related deterioration of cognitive and neuronal properties. J Neurosci 38(3): 648-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shoji H., Takao K., Hattori S. and Miyakawa T.(2016). Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song C., Detert J. A., Sehgal M. and Moyer J. R., Jr (2012). Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. J Neurophysiol 107(12): 3397-3408. [DOI] [PubMed] [Google Scholar]
- 20. Stern E., Chinnakkaruppan A., David O., Sonenberg N. and Rosenblum K.(2013). Blocking the eIF2alpha kinase(PKR) enhances positive and negative forms of cortex-dependent taste memory. J Neurosci 33(6): 2517-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]