Abstract
Erjing prescription (EJP) was an ancient formula that was recorded in the General Medical Collection of Royal Benevolence of the Song Dynasty. It has been frequently used to treat type 2 diabetes mellitus (T2DM) in the long history of China. The formula consists of Lycium barbarum L. and Polygonatum sibiricum F. Delaroche with a ratio of 1 : 1. This study aimed to identify the potential effects and mechanisms of EJP treatment T2DM. The target proteins and possible pathways of EJP in T2DM treatment were investigated by the approach of network pharmacology and real-time PCR (RT-PCR). 99 diabetes-related proteins were regulated by 56 bioactive constituents in EJP in 26 signal pathways by Cytoscape determination. According to GO analysis, 606 genes entries have been enriched. The PPI network suggested that AKT1, EGF, EGFR, MAPK1, and GSK3β proteins were core genes. Among the 26 signal pathways, the PI3K-AKT signal pathway was tested by the RT-PCR. The expression level of PI3K p85, AKT1, GSK3β, and Myc mRNA of this pathway was regulated by EJP. The study based on network pharmacology and RT-PCR analysis revealed that the blood sugar level was regulated by EJP via regulating the PI3K-AKT signal pathway. Plenty of new treatment methods for T2DM using EJP were provided by network pharmacology analysis.
1. Introduction
Type 2 diabetes (T2DM, Chinese name: Xiaoke) is a common chronic metabolic disease with a high prevalence. In 2019, over 463 million people suffered from diabetes. It is estimated that there will be 578 and 700 million diabetic patients in 2030 and 2045, respectively [1, 2], which would bring up a huge economic burden on society. In general, many cases may be undiagnosed in a long predetection period [3]. A lot of complications, such as heart failure, cardiovascular complications, and diabetic nephropathy, are always accompanied with the occurrence of diabetes [4–7]. Low immunity is a common feature for almost all forms of diabetes [8]. Therefore, the researcher devotes to exploring effective drugs to prevent and treat T2DM. Nowadays, traditional Chinese medicine (TCM) has been increasingly popular for the T2DM treatment [9]. For example, Liuwei Dihuang pills and Huanglian decoction have been widely researched for treating T2DM [10–12]. Alizarin, isolated from TCM (Rubia cordifolia), reduced blood sugar levels and alleviated insulin resistance through the PI3K/Akt pathway [13].
EJP (also known as Erjingwan) is a formula consisting of Lycium barbarum L. and Polygonatum sibiricum F. Delaroche, which is recorded in General Medical Collection of Royal Benevolence, early traced back to the Song Dynasty. Erjingwan, a classic formula of nourishing, showed antiaging effects in the skin through activation of Nrf2 and inhibition of NF-κB [14]. These two medicines possessed a certain hypoglycemic effect and mainly contained polysaccharides and flavonoids [15]. The extract of EJP exhibited hypoglycemic and hypolipidemic activities on the model mouse of T2DM [16]. L. barbarum had a substantial value as a homology of medicine and food [17]. Rui Zhao reported L. barbarum polysaccharides improved the insulin resistance in NIDDM rats [18]. Galactomannan extracted from Iraqi L. barbarum fruit treatment improved the bodyweight and alleviated diabetic complications associated with lipid peroxidation in the alloxan-induced diabetes in rats [19]. Besides, phenolic amides from L. barbarum also had an immunomodulatory activity [20]. It was recorded that P. sibiricum had a rich pharmacological function, such as strengthen immunity, cardioprotection, antibacterial, and antidiabetes [21], in modern and ancient pharmacology literatures. P. sibiricum polysaccharide improves inflammatory cytokines and promotes glucose uptake in high glucose and high insulin-induced 3T3-L1 adipocytes by promoting Nrf2 expression [22]. Although EJP had been applied to treat T2DM, the mechanism was not completely elucidated.
In this study, network pharmacology can be used to elucidate the correlation between components (except for the L. barbarum polysaccharide) and pathways by constructing a network model. First, effective components from EJP, certain targets of these components, and the gene of T2DM were excavated through the TCMSP database. Second, the interaction of compound and proteins were elucidated through the network models of compound-target and key proteins. The network of the component-target-pathway was also built to analyze the mechanism of T2DM treated by EJP. Third, the PI3K p85, AKT1, GSK3β, and Myc mRNA of PI3K-AKT signal pathways were validated at the RT-PCR analysis. In total, the research will be expected to provide new strategy and thinking on the study for EJP.
2. Materials and Methods
2.1. The Preparation of Aqueous Extract from EJP
The Lycium barbarum L. were purchased from Ningxia, PR China (batch no. 070101, 104.17°–107.39°E, 35.14°–39.23°N), and Polygonatum sibiricum F. Delaroche were purchased from Sichuan, PR China (batch no. 070101, 97.21°–108.31°E, 26.03°–34.19°N). L. barbarum and P. sibiricum were dried severally and extracted (1 h × 3) with water at 1 : 1 ratio. Then, the aqueous extract was concentrated and dried. After that, the extract with a concentration of 1 mg/mL was prepared to treat IR-HepG2.
2.2. Ingredients Database Construction
All potential compounds of EJP were obtained from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database analysis platform database (http://lsp.nwu.edu.cn/tcmsp.php/) [23]. In the TCMSP database, 500 Chinese herbal medicines and 30,069 constituents were registered from the Chinese Pharmacopoeia (2010 edition). A total of 56 compounds were gained from EJP, including 45 in L. barbarum and 12 in P. sibiricum. The details of each class of compounds are summarized in Table 1.
Table 1.
Compounds database of two herbs in Erjing prescription.
| MOL ID | Molecule | MW | OB (%) | DL | Medicine |
|---|---|---|---|---|---|
| MOL001323 | Sitosterol α1 | 426.8 | 43.28 | 0.78 | L. barbarum |
| MOL003578 | Cycloartenol | 426.8 | 38.69 | 0.78 | L. barbarum |
| MOL001494 | Mandenol | 308.56 | 42 | 0.19 | L. barbarum |
| MOL001495 | Ethyl linolenate | 306.54 | 46.1 | 0.2 | L. barbarum |
| MOL001979 | LAN | 426.8 | 42.12 | 0.75 | L. barbarum |
| MOL000449 | Stigmasterol | 412.77 | 43.83 | 0.76 | L. barbarum |
| MOL005406 | Atropine | 289.41 | 45.97 | 0.19 | L. barbarum |
| MOL005438 | Campesterol | 400.76 | 37.58 | 0.71 | L. barbarum |
| MOL006209 | Cyanin | 411.66 | 47.42 | 0.76 | L. barbarum |
| MOL007449 | 24-Methylidenelophenol | 412.77 | 44.19 | 0.75 | L. barbarum |
| MOL008173 | Daucosterol_qt | 414.79 | 36.91 | 0.75 | L. barbarum |
| MOL008400 | Glycitein | 284.28 | 50.48 | 0.24 | L. barbarum |
| MOL010234 | δ-Carotene | 536.96 | 31.8 | 0.55 | L. barbarum |
| MOL000953 | CLR | 386.73 | 37.87 | 0.68 | L. barbarum |
| MOL009604 | 14b-Pregnane | 288.57 | 34.78 | 0.34 | L. barbarum |
| MOL009612 | (24R)-4α-Methyl-24-ethylcholesta-7, 25-dien-3β-yl acetate | 482.87 | 46.36 | 0.84 | L. barbarum |
| MOL009615 | 24-Methylenecycloartan-3β, 21-diol | 456.83 | 37.32 | 0.8 | L. barbarum |
| MOL009617 | 24-Ethylcholest-22-enol | 414.79 | 37.09 | 0.75 | L. barbarum |
| MOL009618 | 24-Ethylcholesta-5, 22-dienol | 412.77 | 43.83 | 0.76 | L. barbarum |
| MOL009620 | 24-Methyl-31-norlanost-9 (11)-enol | 428.82 | 38 | 0.75 | L. barbarum |
| MOL009621 | 24-Methylenelanost-8-enol | 440.83 | 42.37 | 0.77 | L. barbarum |
| MOL009622 | Fucosterol | 412.77 | 43.78 | 0.76 | L. barbarum |
| MOL009631 | 31-Norcyclolaudenol | 440.83 | 38.68 | 0.81 | L. barbarum |
| MOL009633 | 31-Norlanost-9 (11)-enol | 414.79 | 38.35 | 0.72 | L. barbarum |
| MOL009634 | 31-Norlanosterol | 412.77 | 42.2 | 0.73 | L. barbarum |
| MOL009635 | 4, 24-Methyllophenol | 414.79 | 37.83 | 0.75 | L. barbarum |
| MOL009639 | Lophenol | 400.76 | 38.13 | 0.71 | L. barbarum |
| MOL009640 | 4α, 14α, 24-Trimethylcholesta-8, 24-dienol | 426.8 | 38.91 | 0.76 | L. barbarum |
| MOL009641 | 4α, 24-Dimethylcholesta-7, 24-dienol | 412.77 | 42.65 | 0.75 | L. barbarum |
| MOL009642 | 4α-Methyl-24-ethylcholesta-7, 24-dienol | 426.8 | 42.3 | 0.78 | L. barbarum |
| MOL009644 | 6-Fluoroindole-7-dehydrocholesterol | 402.7 | 43.73 | 0.72 | L. barbarum |
| MOL009646 | 7-O-Methylluteolin-6-C-β-glucoside | 318.3 | 40.77 | 0.3 | L. barbarum |
| MOL009650 | Atropine | 289.41 | 42.16 | 0.19 | L. barbarum |
| MOL009651 | Cryptoxanthin monoepoxide | 568.96 | 46.95 | 0.56 | L. barbarum |
| MOL009653 | Cycloeucalenol | 426.8 | 39.73 | 0.79 | L. barbarum |
| MOL009656 | (E, E)-1-Ethyl octadeca-3, 13-dienoate | 308.56 | 42 | 0.19 | L. barbarum |
| MOL009660 | Methyl (1R, 4aS, 7R, 7aS)-4a, 7-dihydroxy-7-methyl-1-[(2S, 3R, 4S, 5S, 6R)-3, 4, 5-trihydroxy-6-(hydroxymethyl) oxan-2-yl] oxy-1, 5, 6, 7a-tetrahydrocyclopentapyran-4-carboxylate | 406.43 | 39.43 | 0.47 | L. barbarum |
| MOL009662 | Lantadene A | 552.87 | 38.68 | 0.57 | L. barbarum |
| MOL009664 | Physalin A | 526.58 | 91.71 | 0.27 | L. barbarum |
| MOL009665 | Physcion-8-O-β-D-gentiobioside | 608.6 | 43.9 | 0.62 | L. barbarum |
| MOL009677 | Lanost-8-en-3β-ol | 428.82 | 34.23 | 0.74 | L. barbarum |
| MOL009678 | Lanost-8-enol | 428.82 | 34.23 | 0.74 | L. barbarum |
| MOL009681 | Obtusifoliol | 426.8 | 42.55 | 0.76 | L. barbarum |
| MOL000098 | Quercetin | 302.25 | 46.43 | 0.28 | L. barbarum |
| MOL001792 | DFV | 256.27 | 32.76 | 0.18 | L. barbarum |
| MOL002714 | Baicalein | 270.25 | 33.52 | 0.21 | L. barbarum |
| MOL002959 | 3′-Methoxydaidzein | 284.28 | 48.57 | 0.24 | L. barbarum |
| MOL000358 | β-Sitosterol | 414.79 | 36.91 | 0.75 | L. barbarum/P. sibiricum |
| MOL000359 | Sitosterol | 414.79 | 36.91 | 0.75 | P. sibiricum |
| MOL003889 | Methylprotodioscin_qt | 446.74 | 35.12 | 0.86 | P. sibiricum |
| MOL004941 | (2R)-7-Hydroxy-2-(4 hydroxyphenyl) chroman-4-one | 256.27 | 71.12 | 0.18 | P. sibiricum |
| MOL000546 | Diosgenin | 414.69 | 80.88 | 0.81 | P. sibiricum |
| MOL006331 | 4′, 5-Dihydroxyflavone | 254.25 | 48.55 | 0.19 | P. sibiricum |
| MOL009760 | Sibiricoside A | 432.71 | 35.26 | 0.86 | P. sibiricum |
| MOL009763 | (+)-Syringaresinol-O-β-D-glucoside | 580.64 | 43.35 | 0.77 | P. sibiricum |
| MOL009766 | Zhonghualiaoine 1 | 458.75 | 34.72 | 0.78 | P. sibiricum |
First, effective constituents that contributed to its efficacy were selected by absorption, distribution, metabolism, and excretion parameters (ADME), meanwhile those are ineffective or even toxic were removed [24]. Second, higher oral absorption, bioavailability, and biological properties were essential for candidate constituents. Therefore, these compounds needed to satisfy the 30% in oral bioavailability (OB) and 0.18 in drug-likeness (DL).
The related proteins of active components in EJP were acquired from TCMSP databases. Genes related to proteins were retrieved from the UniProt Knowledgebase (UniProtKB) (http://www.uniprot.org), which was a protein database containing 54,247,468 sequence entries [25].
2.3. Predicting Targets of T2DM
The target proteins of T2DM were obtained from four sources: (1) 15,500 gene entries were included in OMIM database (http://www.omim.org/), which was focused on illustrating gene and genetic disorders [26]. (2) GeneCards (https://www.genecards.org/) is a comprehensive database that provided human genes of annotation and prediction. It could effectively establish the linkages of gene disease [27]. (3) Dis-GeNET (http://www.disgenet.org/) database, the current release contains more than 24,000 diseases and traits, 17,000 genes, and 117,000 genomic variants [28]. (4) Therapeutic target database (TTD) contained information from three aspects: (i) the microRNAs and transcription factors of target regulation, (ii) the proteins of target interaction, and (iii) targeting agents and targets, which can be easily retrieved and further enriched by the mechanisms of regulation or biochemical classes [29]. All targets linked to T2DM were only limited to Homo sapiens. The 1,260 genes totally were gained. The 99 same target proteins of compound and disease were selected as the main target of EJP in the treatment of T2DM (Table 2).
Table 2.
99 genes related to compounds of EJP and T2DM.
| Symbol name | Gene name | Symbol name | Gene name | Symbol name | Gene name |
|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 | PTGS2 | Acetylcholinesterase | ACHE | Mitogen-activated protein kinase 14 | MAPK1 |
| Prostaglandin G/H synthase 1 | PTGS1 | Epidermal growth factor receptor | EGFR | Interstitial collagenase | MMP1 |
| Mineralocorticoid receptor | NR3C2 | RAC-α serine/threonine-protein kinase | AKT1 | Hypoxia-inducible factor 1-α | HIF1A |
| β-2 Adrenergic receptor | ADRB2 | Vascular endothelial growth factor A | VEGFA | Signal transducer and activator of transcription 1-α/β | STAT1 |
| Urokinase-type plasminogen activator | PLAU | Apoptosis regulator Bcl-2 | BCL2 | Receptor tyrosine-protein kinase erbB-2 | ERBB2 |
| Sodium channel protein type 5 subunit α | SCN5A | Bcl-2-like protein 1 | BCL2L1 | Peroxisome proliferator-activated receptor gamma | PPARG |
| 5-Hydroxytryptamine 2A receptor | HTR2A | Proto-oncogene c-Fos | FOS | Heme oxygenase 1 | HMOX1 |
| Sodium-dependent serotonin transporter | SLC6A4 | Cyclin-dependent kinase inhibitor 1 | CDKN1A | Cytochrome P450 3A4 | CYP3A4 |
| D (2) dopamine receptor | DRD2 | Apoptosis regulator BAX | BAX | Cytochrome P450 1A2 | CYP1A2 |
| Estrogen receptor | ESR1 | 72 kDa type IV collagenase | MMP2 | Caveolin-1 | CAV1 |
| Androgen receptor | AR | Interleukin-10 | IL10 | Myc proto-oncogene protein | MYC |
| Peroxisome proliferator-activated receptor-γ | KCNH2 | Proepidermal growth factor | EGF | Tissue factor | F3 |
| Estrogen receptor β | ESR2 | Retinoblastoma-associated protein | RB1 | Gap junction α-1 protein | GJA1 |
| Mitogen-activated protein kinase 14 | MAPK14 | Tumor necrosis factor | TNF | Intercellular adhesion molecule 1 | ICAM1 |
| Glycogen synthase kinase-3 β | GSK3β | Transcription factor AP-1 | JUN | C-C motif chemokine 2 | CCL2 |
| Cell division protein kinase 2 | CDK2 | Caspase-3 | CASP3 | E-selectin | SELE |
| Nitric oxide synthase, inducible | NOS2 | Cellular tumor antigen p53 | TP53 | Vascular cell adhesion protein 1 | VCAM1 |
| Collagenase 3 | MMP13 | Ornithine decarboxylase | ODC1 | Interleukin-8 | CXCL8 |
| Phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit, γ isoform | PIK3CG | Xanthine dehydrogenase/oxidase | XDH | Protein kinase C β type | PRKCB |
| Dipeptidyl peptidase IV | DPP4 | Caspase-8 | CASP8 | Heat shock protein β-1 | HSPB1 |
| Stromelysin-1 | MMP3 | RAF proto-oncogene serine/threonine-protein kinase | RAF1 | Transforming growth factor β-1 | TGFB1 |
| Coagulation factor VII | F7 | Superoxide dismutase (Cu-Zn) | SOD1 | Maltase-glucoamylase, intestinal | MGAM |
| Nitric oxide synthase, endothelial | NOS3 | Protein kinase C alpha type | PRKCA | Interleukin-2 | IL2 |
| Tissue-type plasminogen activator | PLAT | Interferon gamma | IFNG | Poly (ADP-ribose) polymerase 1 | PARP1 |
| Thrombomodulin | THBD | Interleukin-1α | IL1A | Solute carrier family 2, facilitated glucose transporter member 4 | SLC2A4 |
| Plasminogen activator inhibitor 1 | SERPINE1 | Myeloperoxidase | MPO | Collagen α-1 (III) chain | COL3A1 |
| Collagen α-1 (I) chain | COL1A1 | Nuclear factor erythroid 2-related factor 2 | NFE2L2 | Serine/threonine-protein kinase Chk2 | CHEK2 |
| C-reactive protein | CRP | C-X-C motif chemokine 10 | CXCL10 | Osteopontin | SPP1 |
| Runt-related transcription factor 2 | RUNX2 | Cathepsin D | CTSD | Insulin-like growth factor-binding protein 3 | IGFBP3 |
| Insulin-like growth factor II | IGF2 | Serum paraoxonase/arylesterase 1 | PON1 | Cytosolic phospholipase A2 | PLA2G4A |
| CD40 ligand | CD40LG | Type I iodothyronine deiodinase | DIO1 | Canalicular multispecific organic anion transporter 1 | ABCC2 |
| Receptor tyrosine-protein kinase erbB-3 | ERBB3 | Catalase | CAT | Serine/threonine-protein kinase mTOR | MTOR |
| Insulin receptor | INSR | Peroxisome proliferator-activated receptor-α | PPARA | Peroxisome proliferator-activated receptor δ | PPARD |
2.4. Network Construction
2.4.1. Compound-Target Network
The pharmacological mechanisms of action were explored by the compound-target network, which was founded for the 57 compounds and relevant protein targets in T2DM utilizing Cytoscape 3.7.2. [30].
2.4.2. GO and KEGG Analyses
Gene ontology (GO) is to annotate genes and their expression products. It is mainly divided into 3 parts: cell component (CC), biological process (BP), and molecular function (MF) [31]. Kyoto Encyclopedia of Genes and Genomes (KEGG) can analyze the signal pathways of drug targets in order to search the disease signal pathways that have maximum correlation, which is significant for discovering the possible mechanism of EJP in the treatment of T2DM [32]. In this study, the enrichment analysis of GO and KEGG were performed through DAVID bioinformatics resources (https://david.ncifcrf.gov) [33] in order to explore the related CC, BP, MF, and pathways. It illustrated the connection of genes and target proteins in diabetes.
2.4.3. PPI Network
The effective components of drugs effected on the human body not only by directly acting on one target but also by indirectly acting on other targets. The prevention and control disease was regulated by multifarious intricate signal pathways, which are interactions and transductions between upstream and downstream targets. The complex relationship between targets and targets can be clearly displayed by constructing PPI. Target proteins related to diabetes were analyzed by online STRING 11.0 (https://string-db.org/cgi/input?sessionId=biiGmvCwYzjy&input_page_show_search=on) to construct the PPI network [34, 35].
2.4.4. Component-Target-Pathway Network
The relationships of component, target, and pathway were clarified utilizing Cytoscape 3.7.2.
2.5. Cell Culture
HepG2 cells were grown in Dulbecco's modified eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin at 37°C, 5% CO2 having 95% relative humidity atmosphere. The cells were seeded at a density of 1.0 × 104 cells/well in a 96-well plate for 24 h, and then, the cells were treated with high concentrations of insulin (1 × 10−5) for 48 h. After the period, the cells were divided into four groups (control, model, DMBG, and HG group). The control and model group were treated DMEM and DMBG, and the HG group was treated metformin (100 μg/mL) and EJP (100 μg/mL). After 24 h, the glucose content was measured using a spectrophotometric microtiter plate reader at 520 nm, and IR-HepG2 cells were prepared.
2.6. RT-PCR Analysis
The IR-HepG2 cells were extracted for RT-PCR analysis. First, the total RNA of IR-HepG2 was extracted with RNA rapid extraction solution, after treated as extracts of concentrations ranging from 100 to 500 ng/L. Second, the RNA was reverse-transcribed through a PCR instrument. Third, RT-PCR was performed using the PCR amplification instrument. The primer sequences were as follows: H-ACTIN-S: CACCCAGCACAATGAAGATCAAGAT; H-ACTIN-A: CCAGTTTTTAAATCCTGAGTCAAGC; H-PIK3r1-S: GGAAGCAGCAACCGAAACAA; H-PIK3r1-A: TCGCCGTCCACCACTACAGA; H-AKT (1)-S: GCTCAGCCCACCCTTCAAG; H-AKT (1)-A: GCTGTCATCTTGGTCAGGTGGT; H-GSK3β-S: GTTAGCAGAGACAAGGACGGCA; H-GSK3β-A: GCAATACTTTCTTGATGGCGAC; H-MYC-S: CTCGACTACGACTCGGTGCA; H-MYC-A: CGGGTCGCAGATGAAACTCT.
3. Results
3.1. Compound-Target Network
Ninety-nine (99) kinds of candidate genes relevant to diabetes were excavated from OMIM, GeneCards, Dis-GeNET, and TTD databases. As shown in Figure 1, 130 nodes (56 compound nodes and 99 target nodes) and 288 edges were seen in the compound-target network. The yellow and blue nodes represented the compounds and targets, respectively. Furthermore, each edge represented the correlation of compound and target. In the network, larger degree represents a stronger interaction. Furthermore, the larger degree nodes may represent the key compound or target in the network. Quercetin, β-sitosterol, diosgenin, baicalein, and 3′-methoxydaidzein were found as the potential composition of treating diabetes in EJP. Quercetin was connected to 85 proteins, β-sitosterol was associated with 15 proteins, diosgenin was related to 12 proteins, baicalein was relevant in 16 proteins, and 3′-methoxydaidzein was connected to 10 proteins.
Figure 1.

Components-target network was structured. The yellow 56 nodes represent the compounds; the blue 99 nodes represent the compound targets.
3.2. GO and KEGG Analyses
The DAVID 6.8 database (https://david.ncifcrf.gov) was utilized to elucidate the CC, BP, and MF annotations of the selected 99 proteins. There were 606 genes entries (FDR, Figure 2 shows the top 8 according to FDR <0.05), out of which 46 genes entries were relevant to CC including the extracellular region, plasma membrane, and cytosol. 477 items were related to BP, including the hypoxia and drug responsion, positive regulation of gene expression and transcription, DNA-templated, angiogenesis, inflammatory response, and positive regulation of cell proliferation. 83 items were related to MF, including protein binding, identical protein binding, and the homodimerization activity of protein. Therefore, the positive regulation of transcription with RNA polymerase II promoter, DNA-templated, and responsion of hypoxia about the action of EJP were regulated through the binding of protein, identical protein, and enzyme, as well as plasma membrane, cytosol, and the homodimerization activity of protein. A target-pathway network was constructed from the data screened of DAVID for determining the relationship between T2DM proteins and related pathways. Furthermore, the key signal pathways (HIF-1, PI3K-AKT, and MAPK) were based on the KEGG analysis (FDR <0.05) and literature analysis (Figures 3 and 4).
Figure 2.

GO analysis, the distribution of GO entries in CC, BP, and MF (top 8 according to FDR <0.05) are shown.
Figure 3.

Target-pathway network constructed based on KEGG analysis from the DAVID database. Blue represents 26 pathways and green represents 66 protein targets.
Figure 4.
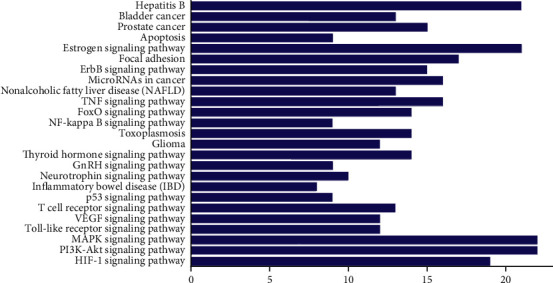
KEGG enrichment analysis of predicted pathways.
3.3. PPI Network Analysis
The network of protein and protein was constructed for exploring the interaction of 99 antidiabetic protein. As shown in Figure 5, AKT1, EGF, EGFR, MAPK1, and GSK3β proteins were located at the core position. These five proteins mainly involved PI3K/AKT, MAPK, and VEGF signaling pathways. AKT1 regulated many processes including metabolism, proliferation, cell survival, growth, and angiogenesis. AKT1 was indirectly activated by insulin and other growth factors [36]. Inhibition of EGFR or HB-EGF intercepted the proliferative response to HB-EGF and glucose in rat islets [37]. MiR-133 may be an effective target for the treatment of diabetic nephropathy via the MAPK/ERK pathway [38]. The antidiabetic effect of SJE might be dependent on the AMPK pathway, which was indicated through the inhibition of gene expression in INS1 and GSK3β, and upregulating the hepatic phosphorylation of AMPKα in liver of mice [39].
Figure 5.

The network of protein-protein interaction.
3.4. Component-Target Pathway Network
To further clarify the mechanism of action of EJP in the treatment of T2DM, the component-target-pathway network was established. As shown in Figure 6, the network was composed of chemical components, protein targets, and pathways, including 156 nodes and 643 edges. 56 components interacted with 99 target proteins and were associated with 26 pathways. Only 66 proteins were related to 26 pathways, so these 66 proteins were potential key proteins of EJP for T2DM. This prediction provided a scientific evidence for further research into the mechanism of EJP in the T2DM treatment.
Figure 6.

Component-target-pathway network was structured. From left to right, the components of EJP, target, pathways, and component represent the green, red, and blue, severally. The edges show the relationship of the component-target-pathway.
3.5. RT-PCR Analysis
Based on the prediction of network pharmacology, PI3K p85, AKT1, GSK3β, and Myc proteins in the PI3K-AKT signal pathway were selected and used to verify the mechanism of action of EJP in treating T2DM at the mRNA level by RT-PCR. As shown in Figure 7, compared to the control group, the expression level of the model group of PI3K p85 and AKT1 mRNA reduced while GSK3β and Myc mRNA increased. The expression level of DMBG and HG groups of PI3K p85 and AKT1 mRNA increased while GSK3β and Myc mRNA reduced compared with the model group. Thus, EJP controlled the blood glucose levels of T2DM mice via upregulating mRNA of PI3K p85 and AKT1 and downregulating mRNA of GSK3β and Myc in PI3K-AKT signal pathways.
Figure 7.

The RT-PCR analysis of the PI3K-AKT signal pathway. The expression level of PI3K p85, AKT1, GSK3β, and Myc mRNA. ∗P < 0.05. ∗∗P < 0.01.
4. Discussion
The prevalence rate of T2DM is gradually increasing due to the aging of the population and changes in lifestyle [40]. Long-term medication is inevitable for the treatment of such chronic metabolic disease. Due to TCM featured multitarget, multicomponent, and low toxicity became more and more popular in the treatment of diabetes. So, it is vital to elucidate the pharmacological mechanism of TCM formula. The active ingredient and targets of TCM could be preferentially predicted by network pharmacology [41]. In this study, the bioactive component and the action mechanism of EJP for diabetes treatment was predicted via the network pharmacology method, and some proteins were verified through RT-PCR monitoring.
The compound-target network clarified that the treatment efficacy of EJP against T2DM was exactly related 56 compounds. Quercetin, β-sitosterol, diosgenin, baicalein, and 3′-methoxydaidzein were found as the potential composition of treating diabetes in EJP. The hyperglycemia was modulated through regulating the enzymes activities as for metabolism in glucose and improving the antioxidant status of pancreatic in rats of T2DM model from the results of Oyedemi' studies [42]. Quercetin can be used for the treatment of T2DM by lowering the pancreatic iron deposition and PBC ferroptosis [43]. β-Sitosterol attenuates insulin resistance and high fat diet-induced detrimental changes via mediating IRS-1/AKT signaling for the management of T2DM [44]. Diosgenin ameliorates cognitive deficits in T2DM, owing to its amelioration of astrogliosis, inflammation, and oxidative stress [45]. Baicalein (10−6 and 10−5 mol/L) regulated glucose uptake, glycolysis, and gluconeogenesis of hepatocytes to treat T2DM [46]. 3′-Methoxydaidzein has not been reported about the antidiabetic effect and may be as a potential T2DM drug. Therefore, T2DM treated by EJP was closely related to key target compound including quercetin, β-sitosterol, diosgenin, baicalein, and 3′-methoxydaidzein. The network pharmacology prediction of EJP for the T2DM treatment has been proved to be appropriate, and the screening of new compounds for the T2DM treatment provides ideas for the development of new drugs.
The component-target-pathway network depicted that the therapeutic effect of EJP on T2DM directly interacted with 99 genes. The results of KEGG pathway enrichment analysis of 66 proteins indicated that 26 pathways were exactly connected to the occurrence and progression of T2DM, suggesting that these pathways might be the molecular mechanism of EJP against T2DM. The relationships of some pathways with T2DM were succinctly discussed as follows. Hepatitis B: A study showed hepatitis B virus (HBV) coinfection was significantly related to blood glucose levels. The participants of 28% with HBV coinfection developed T2DM. It was an increasing evidence that infection of HBV is strongly associated with the development of T2DM [47]. Bladder and prostate cancer pathway: T2DM are becoming increasingly prevalent worldwide and is associated with the increased incidence of bladder cancer [48]. However, a personal history of T2DM is connected with a lower incidence of prostate cancer [49]. Apoptosis pathway: apoptosis plays important roles in the pathophysiology of T2DM. The prevention and revert of β-cell apoptosis by regulating the balance of Bcl family, and apoptotic genes against apoptosis might be a new path for prevention and therapeutic application on T2DM [50]. FoxO signaling pathway: forkhead box protein O1 (FOXO1) played important roles in β-cell growth and function. FOXO1 mRNA levels were increased in the islets of patients with T2DM [51]. TNF signaling pathway: there was a growing evidence that tumor necrosis factor-α (TNF-α) involved in insulin resistance, and it is associated with the development of T2DM [52]. Glioma pathway: it was inverse relations between diabetes and glioma risk [53]. Thyroid hormone signaling pathway: the downregulation of FT3 was significantly related to the prevalence of DR in T2DM with normal thyroid function [54, 55]. Neurotrophin signaling pathway: the changes in the serum neurotrophic factor levels were associated with metabolic syndrome components in T2DM [56, 57]. T cell signaling pathway: in a study, it showed that the presence of senescent T cells exerted a detrimental influence on immune function during T2DM [58]. VEGF signaling pathway: the redox environment influences vascular endothelial growth factor (VEGF) production in response to proinflammatory stimuli in T2DM [59]. Toll-like receptor signaling pathway: toll-like receptor, the central of innate immunity, was significantly involved in progression of T2DM [60]. MAPK and PI3K-AKT signaling pathways: MAPK and PI3K-AKT are essential for glucose homeostasis. The PI3K-AKT signaling pathway is the major effector of metabolic insulin action [61, 62]. HIF-1 signaling pathway: hypoxia inducible factors (HIFs) play a value in T2DM. Furthermore, the HIF pathway is disordered in major metabolic tissues involved in the pathogenesis of diabetes [63]. According to the PPI network and RT-PCR analysis, PI3K-AKT, MAPK, and VEGF signaling pathways might play significant roles on T2DM.
5. Conclusion
99 targets and 58 signal pathways were screened via network pharmacology. Only 66 targets and 26 pathways were directly related to diabetes with literature analysis. In the compound-target network, quercetin, β-sitosterol, diosgenin, baicalein, and 3′-methoxydaidzein were core compounds. Furthermore, PI3K-AKT, MARK, and estrogen signal pathways might be significant. The expression of PI3K p85 and AKT1 p85 mRNA were upregulated and GSK3β and Myc mRNA were downregulated in the analysis of RT-PCR. It implied that the hyperglycemia of T2DM model rats were alleviated via the PI3K-AKT pathway. In conclusion, EJP was proved to treat T2DM. It is helpful for researchers to further design pharmacodynamics experiments on these components in the next protocol. Meanwhile, it provided a reference to study the effective constituents of EJP and mechanism of treating T2DM. These specific targets were also worth researching deeply in future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81760839).
Abbreviations
- EJP:
Erjing prescription
- T2DM:
Type 2 diabetes mellitus
- RT-PCR:
Real-time PCR
- CC:
Cell component
- BP:
Biological process
- MF:
Molecular function
- PPI:
Protein-protein interactions
- TCM:
Traditional Chinese medicine
- TCMSP:
Traditional Chinese medicine systems pharmacology
- OB:
Oral bioavailability
- DL:
Drug-likeness
- GO:
Gene Ontology
- TTD:
Therapeutic target database
- KEGG:
Kyoto Encyclopedia of Genes and Genomes.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All authors participated in this study. Liming Zhang designed this manuscript. Jiexin Wang and Haiqing Chu conducted the experiments, the literature survey, and drafted the manuscript. Hangying Li revised the manuscript. Wenqian Yang, Yu Zhao, and Tong Shen processed the data and checked the manuscript. John Cary checked the grammar of the manuscript and revised it. Jiexin Wang and Haiqing Chu contributed equally.
Supplementary Materials
The graphical abstract is the overall flowchart of this study and may be found online in the supporting materials section at the end of the article.
References
- 1.Forouhi N. G., Wareham N. J. Epidemiology of diabetes. Medicine. 2014;42(12):698–702. doi: 10.1016/j.mpmed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Reserch and Clinical Practice. 2019;157 doi: 10.1016/j.diabres.2019.107843.107843 [DOI] [PubMed] [Google Scholar]
- 3.Bantie G. M., Wondaye A. A., Arike E. B., et al. Prevalence of undiagnosed diabetes mellitus and associated factors among adult residents of Bahir Dar city, northwest Ethiopia: a community-based cross-sectional study. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2019-030158.e030158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dal Canto E., Ceriello A., Rydén L., et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. European Journal of Preventive Cardiology. 2019;26(2):25–32. doi: 10.1177/2047487319878371. [DOI] [PubMed] [Google Scholar]
- 5.Ferrini M., Johansson I., Aboyans V. Heart failure and its complications in patients with diabetes: mounting evidence for a growing burden. European Journal of Preventive Cardiology. 2019;26(2):106–113. doi: 10.1177/2047487319885461. [DOI] [PubMed] [Google Scholar]
- 6.Gan Q., Wang J., Hu J., et al. The role of diosgenin in diabetes and diabetic complications. The Journal of Steroid Biochemistry and Molecular Biology. 2020;198 doi: 10.1016/j.jsbmb.2019.105575.105575 [DOI] [PubMed] [Google Scholar]
- 7.Jebur A. B., El-Demerdash F. M., Kang W. Bromelain from ananas comosus stem attenuates oxidative toxicity and testicular dysfunction caused by aluminum in rats. Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements (GMS) 2020;62 doi: 10.1016/j.jtemb.2020.126631.126631 [DOI] [PubMed] [Google Scholar]
- 8.Moffa S., Mezza T., Cefalo C., et al. The interplay between immune system and microbiota in diabetes. Mediators of Inflammation. 2019;2019:10. doi: 10.1155/2019/9367404.9367404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saadeldeen F. S. A., Niu Y., Wang H., et al. Natural products: regulating glucose metabolism and improving insulin resistance. Food Science and Human Wellness. 2020;9(3):214–228. doi: 10.1016/j.fshw.2020.04.005. [DOI] [Google Scholar]
- 10.Pan L., Li Z., Wang Y., Zhang B., Liu G., Liu J. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian decoction in rats with type 2 diabetes mellitus. Journal of Ethnopharmacology. 2020;258 doi: 10.1016/j.jep.2020.112842.112842 [DOI] [PubMed] [Google Scholar]
- 11.Qiu Z., Dong J., Xue C., et al. Liuwei dihuang pills alleviate the polycystic ovary syndrome with improved insulin sensitivity through PI3K/Akt signaling pathway. Journal of Ethnopharmacology. 2020;250 doi: 10.1016/j.jep.2019.111965.111965 [DOI] [PubMed] [Google Scholar]
- 12.Zheng W., Wang G., Zhang Z., Wang Z., Ma K. Research progress on classical traditional Chinese medicine formula Liuwei Dihuang pills in the treatment of type 2 diabetes. Biomedicine & Phareacotherapy. 2020;121 doi: 10.1016/j.biopha.2019.109564.109564 [DOI] [PubMed] [Google Scholar]
- 13.Xu L., Xing M., Xu X., et al. Alizarin increase glucose uptake through PI3K/Akt signaling and improve alloxan-induced diabetic mice. Future Medicinal Chemistry. 2019;11(5):395–406. doi: 10.4155/fmc-2018-0515. [DOI] [PubMed] [Google Scholar]
- 14.Zhong H., Hong C., Han Z., et al. Erjingwan extracts exert antiaging effects of skin through activating Nrf2 and inhibiting NF-κB. Evidence-Based Complementary and Alternative Medicine. 2019;2019:15. doi: 10.1155/2019/5976749.5976749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S., Yang M., Li Y., et al. Variance analysis on polysaccharide, total flavonoids and total phenols of Lycium barbarum leaves from different production areas. Zhongguo Zhong Yao Za Zhi. 2019;44(9):1774–1780. doi: 10.19540/j.cnki.cjcmm.20190325.104. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Guan E., Zhang L. Effects of erjingfang polysaccharide extracts on blood glucose and lipids in mice with 2 diabetes. Chinese Journal of Hospital Pharmacy. 2015;35(5):405–409. [Google Scholar]
- 17.Zhao R., Gao X., Zhang T., Li X. Effects of Lycium barbarum. polysaccharide on type 2 diabetes mellitus rats by regulating biological rhythms. Iranian Journal of Basic Medical Sciences. 2016;19(9):1024–1030. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao R., Li Q., Xiao B. Effect of Lycium barbarum polysaccharide on the improvement of insulin resistance in NIDDM rats. Yakugaku Zasshi. 2005;125(12):981–988. doi: 10.1248/yakushi.125.981. [DOI] [PubMed] [Google Scholar]
- 19.Al-Fartosy A. J. M. Protective effect of galactomannan extracted from Iraqi Lycium barbarum L. fruits against alloxan-induced diabetes in rats. American Journal of Biochemistry and Biotechnology. 2015;11(2):73–83. doi: 10.3844/ajbbsp.2015.73.83. [DOI] [Google Scholar]
- 20.Zhu P.-F., Zhao Y.-L., Dai Z., et al. Phenolic amides with immunomodulatory activity from the nonpolysaccharide fraction of Lycium barbarum fruits. Journal of Agricultural and Food Chemistry. 2020;68(10):3079–3087. doi: 10.1021/acs.jafc.9b07499. [DOI] [PubMed] [Google Scholar]
- 21.Zhao P., Zhao C., Li X., et al. The genus polygonatum: a review of ethnopharmacology, phytochemistry and pharmacology. Journal of Ethnopharmacology. 2018;214:274–291. doi: 10.1016/j.jep.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Cai J., Zhu Y., Zuo Y., et al. Polygonatum sibiricum polysaccharide alleviates inflammatory cytokines and promotes glucose uptake in high-glucose-and high-insulin-induced 3T3-L1 adipocytes by promoting Nrf2 expression. Molecular Medicine Reports. 2019;20(4):3951–3958. doi: 10.3892/mmr.2019.10626. [DOI] [PubMed] [Google Scholar]
- 23.Ru J., Li P., Wang J., et al. A database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6 doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi X.-Q., Yue S.-J., Tang Y.-P., et al. A network pharmacology approach to investigate the blood enriching mechanism of Danggui Buxue decoction. Journal of Ethnopharmacology. 2019;235:227–242. doi: 10.1016/j.jep.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Breuza L., Poux S., Estreicher A., et al. The UniProtKB guide to the human proteome. Database (Oxford) 2016;2016 doi: 10.1093/database/bav120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamosh A., Scott A., Amberger J., Bocchini C., McKusick V. Online mendelian inheritance in man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Research. 2005;33:D514–D517. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stelzer G., Rosen N., Plaschkes I., et al. The genecards suite: from gene data mining to disease genome sequence analyses. Current Protocols in Bioinformatics. 2016;54:1–33. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 28.Piñero J., Ramírez-Anguita J. M., Saüch-Pitarch J., et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Research. 2020;48(D1):p. D845. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Zhang S., Li F., et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Research. 2020;48(D1):p. D1031. doi: 10.1093/nar/gkz981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu B. F., Liu R., Huang C. X., et al. Identification of key genes in ruptured atherosclerotic plaques by weighted gene correlation network analysis. Scientific Reports. 2020;10(1):p. 10847. doi: 10.1038/s41598-020-67114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren L., Zheng X., Liu J., et al. Network pharmacology study of traditional Chinese medicines for stroke treatment and effective constituents screening. Journal of Ethnopharmacology. 2019;242 doi: 10.1016/j.jep.2019.112044.112044 [DOI] [PubMed] [Google Scholar]
- 32.Pan Q., Zhou R., Su M., Li R. The effects of plumbagin on pancreatic cancer: a mechanistic network pharmacology approach. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2019;25:4648–4654. doi: 10.12659/MSM.917240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang D. W., Sherman B. T., Tan Q., et al. DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Research. 2007;35:W169–W175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szklarczyk D., Gable A. L., Lyon D., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research. 2019;47(D1):p. D607. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vella D., Marini S., Vitali F., et al. PPI network analysis via topological and functional module identification. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-23672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Y., Zhang X., Ma F., et al. The role of Akt2 in the protective effect of fenofibrate against diabetic nephropathy. International Journal of Biological Sciences. 2020;16(4):553–567. doi: 10.7150/ijbs.40643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maachi H., Fergusson G., Ethier M., et al. HB-EGF signaling is required for glucose-induced pancreatic β-cell proliferation in rats. Diabetes. 2020;69(3):369–380. doi: 10.2337/db19-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao X., Kong W. X., Li Y. T. MiR-133 inhibits kidney injury in rats with diabetic nephropathy via MAPK/ERK pathway. European Review for Medical and Pharmacological Sciences. 2019;23(24):10957–10963. doi: 10.26355/eurrev_201912_19799. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Zhang X., Zhan L., et al. LC-Q-TOF-MS characterization of polyphenols from white bayberry fruit and its antidiabetic effect in KK-ay mice. ACS Omega. 2020;5(28):17839–17849. doi: 10.1021/acsomega.0c02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sattar N., Rawshani A., Franzén S., et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139(19):2228–2237. doi: 10.1161/circulationaha.118.037885. [DOI] [PubMed] [Google Scholar]
- 41.Wang K. X., Gao Y., Lu C., et al. Uncovering the complexity mechanism of different formulas treatment for rheumatoid arthritis based on a novel network pharmacology model. Frontiers in Pharmacology. 2020;11:p. 1035. doi: 10.3389/fphar.2020.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oyedemi S. O., Nwaogu G., Chukwuma C. I., et al. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: in silico studies of molecular interaction of quercetin with hexokinase and catalase. Journal of Food Biochemistry. 2020;44(2) doi: 10.1111/jfbc.13127.e13127 [DOI] [PubMed] [Google Scholar]
- 43.Li D., Jiang C., Mei G., et al. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients. 2020;12(10) doi: 10.3390/nu12102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z., Huang W., Zhang J., Xie M., Wang X. Baicalein improves glucose metabolism in insulin resistant HepG2 cells. European Journal of Pharmacology. 2019;854:187–193. doi: 10.1016/j.ejphar.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Mahmoudi N., Kiasalari Z., Rahmani T., et al. Diosgenin attenuates cognitive impairment in streptozotocin-induced diabetic rats: underlying mechanisms. Neuropsychobiology. 2021;80(1):25–35. doi: 10.1159/000507398. [DOI] [PubMed] [Google Scholar]
- 46.Babu S., Krishnan M., Rajagopal P., et al. Beta-sitosterol attenuates insulin resistance in adipose tissue via IRS-1/Akt mediated insulin signaling in high fat diet and sucrose induced type-2 diabetic rats. European Journal of Pharmacology. 2020;873 doi: 10.1016/j.ejphar.2020.173004.173004 [DOI] [PubMed] [Google Scholar]
- 47.Lin P. Y., Chen S. C., Lo T. C., Kuo H. W. Dual infection with hepatitis B virus and hepatitis C virus correlated with type 2 diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes: Official Journal, German Society of Endocrinology [and] German Diabetes Association. 2020;128(1):38–42. doi: 10.1055/a-0794-6135. [DOI] [PubMed] [Google Scholar]
- 48.Pan Y., Lee C. Y., Lee L. M., et al. Incidence of bladder cancer in type 2 diabetes mellitus patients: a population-based cohort study. Medicina (Kaunas, Lithuania) 2020;56(9) doi: 10.3390/medicina56090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji J., Sundquist J., Sundquist K. Association of family history of type 2 diabetes with prostate cancer: a national cohort study. Frontiers in Oncology. 2016;6:p. 194. doi: 10.3389/fonc.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomita T. Apoptosis in pancreatic β-islet cells in type 2 diabetes. Bosnian Journal of Basic Medical Sciences. 2016;16(3):162–179. doi: 10.17305/bjbms.2016.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., Wan T., Li Y. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nature Reviews Endocrinology. 2013;9(10):615–623. doi: 10.1038/nrendo.2013.119. [DOI] [PubMed] [Google Scholar]
- 52.Liu C., Feng X., Li Q., Wang Y., Li Q., Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100–109. doi: 10.1016/j.cyto.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 53.Seliger C., Ricci C., Meier C. R., et al. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro-Oncology. 2016;18(3):340–349. doi: 10.1093/neuonc/nov100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bril F., Kadiyala S., Portillo Sanchez P., et al. Plasma thyroid hormone concentration is associated with hepatic triglyceride content in patients with type 2 diabetes. Journal of Investigative Medicine. 2016;64(1):63–68. doi: 10.1136/jim-2015-000019. [DOI] [PubMed] [Google Scholar]
- 55.Zou J., Li Z., Tian F., et al. Association between normal thyroid hormones and diabetic retinopathy in patients with type 2 diabetes. Biomed Research International. 2020;2020:7. doi: 10.1155/2020/8161797.8161797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujinami A., Ohta K., Obayashi H., et al. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: relationship to glucose metabolism and biomarkers of insulin resistance. Clinical Biochemistry. 2008;41(10-11):812–817. doi: 10.1016/j.clinbiochem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Civelek S., Konukoglu D., Erdenen F., Uzun H. Serum neurotrophic factor levels in patients with type 2 diabetes mellitus: relationship to metabolic syndrome components. Clinical Laboratory. 2013;59(3-4):369–374. doi: 10.7754/clin.lab.2012.120404. [DOI] [PubMed] [Google Scholar]
- 58.Lau E. Y. M., Carroll E. C., Callender L. A., et al. Type 2 diabetes is associated with the accumulation of senescent T cells. Clinical & Experimental Immunology. 2019;197(2):205–213. doi: 10.1111/cei.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Earle K. A., Zitouni K., Nourooz-Zadeh J. Lipopolysaccharide-induced VEGF production and ambient oxidative stress in type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2019;104(1):1–6. doi: 10.1210/jc.2018-00836. [DOI] [PubMed] [Google Scholar]
- 60.Sepehri Z., Kiani Z., Nasiri A., Kohan F. Toll-like receptor 2 and type 2 diabetes. Cellular & Molecular Biology Letters. 2016;21 doi: 10.1186/s11658-016-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho H., Mu J., Kim J., et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBbeta) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 62.Schultze S. M., Hemmings B. A., Niessen M., Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Reviews in Molecular Medicine. 2012;14:p. e1. doi: 10.1017/S1462399411002109. [DOI] [PubMed] [Google Scholar]
- 63.Girgis C. M., Cheng K., Scott C. H., Gunton J. E. Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends in Endocrinology & Metabolism. 2012;23(8):372–380. doi: 10.1016/j.tem.2012.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The graphical abstract is the overall flowchart of this study and may be found online in the supporting materials section at the end of the article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


