Abstract
Neurotrophins are a family of proteins that support neuronal proliferation, survival, and differentiation in the central and peripheral nervous systems, and are regulators of neuronal plasticity. Nerve growth factor is one of the best-described neurotrophins and has advanced to clinical trials for treatment of ocular and brain diseases due to its trophic and regenerative properties. Prior trials over the past few decades have produced conflicting results, which have principally been ascribed to adverse effects of systemic nerve growth factor administration, together with poor penetrance of the blood-brain barrier that impairs drug delivery. Contrastingly, recent studies have revealed that topical ocular and intranasal nerve growth factor administration are safe and effective, suggesting that topical nerve growth factor delivery is a potential alternative to both systemic and invasive intracerebral delivery. The therapeutic effects of local nerve growth factor delivery have been extensively investigated for different ophthalmic diseases, including neurotrophic keratitis, glaucoma, retinitis pigmentosa, and dry eye disease. Further, promising pharmacologic effects were reported in an optic glioma model, which indicated that topically administered nerve growth factor diffused far beyond where it was topically applied. These findings support the therapeutic potential of delivering topical nerve growth factor preparations intranasally for acquired and degenerative brain disorders. Preliminary clinical findings in both traumatic and non-traumatic acquired brain injuries are encouraging, especially in pediatric patients, and clinical trials are ongoing. The present review will focus on the therapeutic effects of both ocular and intranasal nerve growth factor delivery for diseases of the brain and eye.
Keywords: Alzheimer's disease, eye drops, group B streptococcus meningitis, glioma, intranasal delivery, neurotrophic keratitis, nerve growth factor, proNGF, stroke, traumatic brain injury
Introduction
World Health Organization (WHO) projections predict that over the next decade, neurological disorders will cause more than 1.5 billion annual Disability-Adjusted Life Years, which occur due to ill health, disability, or early death (WHO report, 2006). No currently available therapeutics restore neuronal loss or significantly improve clinical outcomes in these diseases. Consequently, novel therapeutic approaches should be developed for the treatment of degenerative and acquired brain injuries.
The pro-survival and regenerative properties of neurotrophic factors suggest that these molecules have therapeutic potential in a wide range of neurodegenerative diseases (Aloe et al., 2012; Rocco et al., 2018). Nerve growth factor (NGF) in particular has emerged as a promising potential therapeutic for treatment of neurodegenerative diseases (Aloe et al., 2012; Manni et al., 2013). NGF is the founding and best-characterized member of the neurotrophins, a family of four neuronal growth factors including NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3, and neurotrophin-4/5 (Aloe et al., 2012; Skaper, 2012, 2017; Bothwell, 2014, 2016; Rocco et al., 2018).
As all other neurotrophins, NGF is synthetized as a 13 kDa monomer, usually found in biological tissues as a non-covalently linked 26 kDa protein dimer, from a 26–32 kDa precursor molecule, nerve growth factor precursor (proNGF), which undergoes intracellular processing to generate mature NGF (mNGF) (Figure 1) (Hempstead, 2014). After its release in the extracellular space, NGF exerts its biological action by challenging the specific receptor tropomyosin kinase receptor A (TrkA), which is a typical tyrosine kinase receptor (Huang and Reichardt, 2003). The major cytosolic/endosomal pathways activated by the TrkA are Ras-mitogen activated protein kinase, extracellular signal-regulated kinase, phosphatidylinositol 3-kinase-Akt, and phospholipase C-γ (Klesse and Parada, 1999; Chao et al., 2006; Reichardt, 2006). NGF also binds to and activates the low-affinity, non-selective p75 neurotrophin receptor (p75NTR). This receptor is a transmembrane glycoprotein that regulates signaling through TrkA (Friedman and Greene, 1999; Schor, 2005; Reichardt, 2006); binding of NGF to p75NTR activates additional signaling pathways, namely Jun kinase signaling cascade, NF-κB and ceramide generation that, in the absence of co-expressed TrkA, may signal a cell to die via apoptosis (Friedman and Greene, 1999; Miller and Kaplan, 2001; Schor, 2005).
Figure 1.
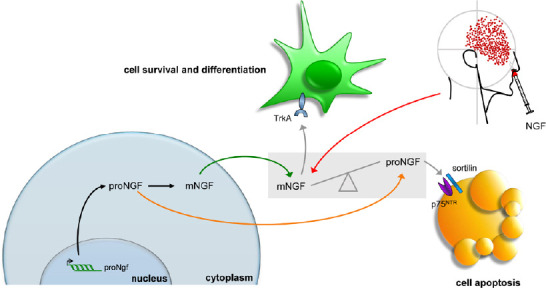
Schematic representation of mNGF metabolism.
The synthesis of nerve growth factor (NGF) as a precursor molecule (proNGF) is followed by the processing of this latter in a mature peptide (mNGF) that, after its release, preferentially challenges the neurotrophic TrKA receptor. In neurodegenerative and traumatic conditions, accumulation of proNGF has been reported, with consequent activation of the apoptotic p75 neurotrophin receptor (p75NTR) signaling. After NGF delivery, the balance between proNGF and mNGF, altered by disease development, could be shifted toward the latter.
At first, it has generally been assumed that proNGF was largely processed into mNGF and that the mature form accounted for the biological activity in most tissues. Instead, in 2001, Lee et al. shattered the traditional view that the precursor of NGF was functionally inactive, by showing that proNGF has its own function and that secreted proNGF promotes cell death (Lee et al., 2001) by activating the p75NTR /sortilin receptor complex and the Jun kinase signaling cascade (Nykjaer et al., 2004). Moreover, Fahnestock et al. (2001) showed that proNGF is the predominant form of NGF in mouse, rat, and human brain tissue, whereas little or no mature NGF is detected in the same tissues. It was later discovered that proNGF is increased in Alzheimer’s disese (AD) and its accumulation may be related to AD peculiar cholinergic degeneration and memory impairment (Fahnestock et al., 2001). proNGF accumulation was also observed after spinal cord injury, leading to apoptosis of oligodendrocytes and lesion extension along fiber tracts undergoing Wallerian degeneration (Beattie et al., 2002; Harrington et al., 2004). In addition to the unbalance between proNGF and mNGF, it was also shown that in AD the expression of the NGF-receptor TrkA is decreased (Mufson et al., 1997) and p75NTR expression is often increased in dying neurons following seizure (Roux et al., 1999), ischemia (Park et al., 2000) and brain injury (Harrington et al., 2004). Taken together, these evidences have suggested an unbalance in proNGF and its receptors relative abundance, which may favor proNGF binding to the p75NTR /sortilin receptor complex with consequent triggering of apoptosis mechanisms (Masoudi et al., 2009). Overall, these data founded the rationale for delivering exogenous (mature) NGF in patients affected by diseases characterized by putative or preclinically-demonstrated unbalance in proNGF/mNGF and TrkA/p75NTR ratios, with the purpose of taking advantage from the selective neurotrophic activity of NGF, based on its extremely high affinity for the TrkA receptor (Barker, 2007).
It is worth mentioning that, besides its neuroprotective and neuro-regenerative actions, NGF has also been indicated as a potent anti-inflammatory factor (Bracci Laudiero and Manni, 2013). Indeed its regulatory effects on cytokine expression (Minnone et al., 2017a) and the evidence that it induces a switch toward the expression of anti-inflammatory macrophage phenotype (Prencipe et al., 2014), extends the rationale for its use in brain diseases characterized by abnormal activation of central inflammatory states. Notably, the activation of p75NTR by proNGF has been pointed as a possible etiological factor in chronic inflammatory diseases (Minnone et al., 2017b).
The pioneering work of Kromer et al. (1987) established that nerve growth factor prevents neuronal death after brain injury in rats. In a seminal clinical study by Olson et al. (1991), the investigators delivered mouse NGF by intraventricular infusion in a single patient with Parkinson’s disease to support medullary adrenal autografts. Many subsequent studies have yielded promising results for diseases of the peripheral nervous system, such as HIV-associated sensory neuropathy (McArthur et al., 2000; Schifitto et al., 2001) and diabetic polyneuropathy (Apfel, 2002).
However, large-scale clinical trials for central nervous system disorders have failed (Thoenen and Sendtner, 2002). These failures can most likely be attributed not to poor drug efficacy, but rather to insufficient delivery due to poor penetrance of the blood-brain barrier. Indeed, NGF does not efficiently enter the brain after systemic administration due to limited blood-brain barrier penetrance and a poor pharmacokinetic profile (Thorne and Frey, 2001). Moreover, adverse side effects of systemic NGF treatment have been reported in clinical trials, including neuropathic pain (Petty et al., 1994). Intracerebral administration, for example intraparenchymal, intraventricular, or intrathecal injection, although effective, is not clinically practical due to its invasiveness and the risk of infection (Bottros and Christo, 2014). Cell-based NGF delivery has been the subject of clinical trials on patients with Alzheimer’s disease, that were subject to ex vivo gene therapy with genetically-engineered autologous grafted fibroblasts secreting NGF (Tuszynski et al., 2005), gene therapy with human NGF genetically-engineered into adeno-associated virus vector, stereotactically injected into the nucleus basalis of Meynert (Mandel, 2010) and human NGF genetically-engineered in human retinal cells encapsulated in polymeric implant stereotactically injected to the basal forebrain (Eriksdotter-Jonhagen et al., 2012). In general, no NGF- or cell-related adverse effects were reported, indicating a satisfactory level of safety and tolerability for this kind of delivery approach. In this context, the easily achieved topical intranasal and ocular delivery of NGF could be an effective alternative to systemic or intracerebral delivery of NGF, as these former modalities bypass the blood-brain barrier (Frey et al., 1997; Thorne and Frey, 2001; Aloe et al., 2014) and target the central nervous system directly, with minimal systemic exposure (Figure 2).
Figure 2.
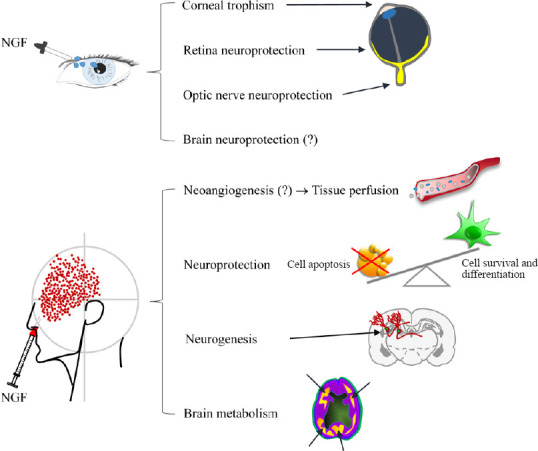
Schematic representation of NGF effects when topically delivered to eye and olfactory epithelia.
(?): Demonstrated in animal models. Adapted from Li et al. (2018) and Tirassa et al. (2018). NGF: Nerve growth factor.
Interestingly, great expectation has been also put in the possible clinical use of BDNF, the major NGF genetically related neurotrophin, since its role in neuronal development, maturation, and health (Siegel and Chauhan, 2000; Autry and Monteggia, 2012). Similarly to NGF, both subcutaneous and intracerebral delivery of BDNF has been attempted, to face the outcomes of major neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease), while its use has been hypothesized also for the treatment of mood disorders and psychosis (Deng et al., 2016; Pollock et al., 2016). Even for BDNF, the limiting issue is the difficulty in finding a proper delivery strategy that could overcome the poor permeability of the blood-brain barrier and also the relatively short half-life of the purified molecule (Deng et al., 2016). Notably, the intranasal delivery approach, applied to mesenchymal stem cells engineered to produce BDNF, seems among the most promising to achieve an effective therapeutic delivery of BDNF to the brain (Ma et al., 2016). In contrast to NGF, whose specific effects on brain neurons is limited to cells of the cholinergic system (Aloe et al., 2012), the potential effects of BDNF appear to be extended to several neuronal phenotypes (Siegel and Chauhan, 2000; Autry and Monteggia, 2012; Deng et al., 2016). On the other hand, NGF administered intranasally induced a generalized enhancement in perfusion and general metabolism (Chiaretti et al., 2017), which in turn could improve the functionality of different brain cell types (Figure 3).
Figure 3.
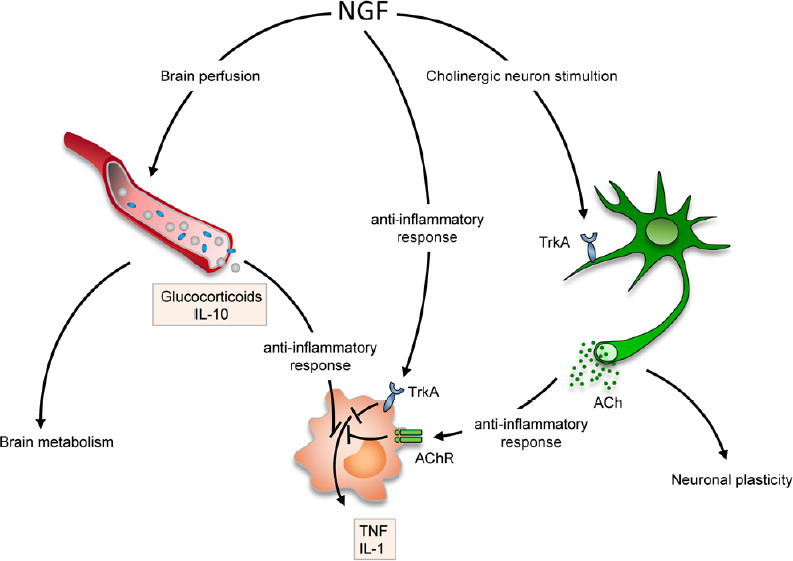
Reported mechanisms of neuroprotection/neuro-repair activated in different pathologies after topical administration of purified NGF.
Besides its selective effect on cholinergic neurons (affected in Alzheimer’s disease), NGF also triggers neuroprotection-neuro-repair by increasing tissue perfusion/metabolism (brain injury, posterior segment of the eye), and/or eliciting anti-inflammatory response, both directly, via the TrkA activation, and indirectly, by enhancing acetylcholine uptake in monocytes/macrophages. IL: Interleukin; NGF: nerve growth factor; TNF: tumor necrosis factor.
The present review is intended to assess current evidence for the intranasal and ocular delivery of NGF to the brain, as potential treatments for neurodegenerative disease.
Search Strategy and Selection Criteria
We performed a PubMed literature search of articles with search terms including “nerve growth factor”, “neurotrophins”, “proNGF”, “Cenegermin”, “intranasal delivery”, “eye drops”, “traumatic brain injury”, “acquired brain injury”, “non-trauamtic brain injury”, “stroke”, “Alzheimer’s disease”, “frontotemporal dementia”, “group B streptococcus meningitis”, “neurotrophic keratitis”, “dry eye disease”, “optic neuropathy”, “glaucoma”, “glioma”, “demyelinating diseases”. Selection criteria were based on the most recent articles (2010-2020) concerning the topical administration of nerve growth factor for the treatment of ocular and brain disorders. In addition, we cited some older references (before 2010) to report the studies that have been made utilizing topical routes of administration for these pathologies.
Cerebral Nerve Growth Factor Delivery via Ocular Administration
NGF binds the TrkA and p75NTR receptors expressed in the anterior segment of the eye, including the iris, ciliary body, lens, cornea, and conjunctiva (Roberti et al., 2014). NGF and its receptors are not limited to the cornea, but are in fact highly expressed throughout the visual system, from the retina to the visual cortex (Wang et al., 2014). In the retina, retinal ganglion cells not only express NGF and its receptors, but also transport NGF along their axons in both retrograde and anterograde manners (Carmignoto et al., 1991), suggesting an important role for NGF in the maintenance of cellular physiology (Roberti et al., 2014).
Animal studies of NGF were initially focused on the biological and pharmacological effects of topical NGF application to the ocular surface (Lambiase et al., 1998). However, absorption studies identified that the effects of topical ocular NGF application were not limited to local action, as NGF applied topically to the ocular surface reaches the retina, optic nerve, and brain (Lambiase et al., 2005, 2007b; Di Fausto et al., 2007). The regulatory mechanisms of protein transport from the ocular surface to the retina, optic nerve, and brain are incompletely understood. Intraocular transit of NGF to the posterior segment from the ocular surface could occur via several routes (Maurice, 2002; Koevary, 2003): (1) Corneal, in which topically delivered NGF travels through the anterior chamber, lens, pupil, iris, or iris root; (2) Conjunctival, in which topically delivered NGF is transported directly across the sclera, choroid, choriocapillaris, and retinal pigment epithelium; or (3) Conjunctival, in which NGF is transported indirectly into the retrobulbar space and optic nerve head. Diffusion of NGF administered to the ocular surface through the vitreous to the retina, with the protein crossing through the choroidal vasculature to the optic nerve head, has also been suggested (Koevary, 2003). Finally, an alternative pathway of anterograde NGF transport to the brain along the axons of RCGs has also been proposed (Carmignoto et al., 1991).
The diffusion of NGF topically administered to the eye has been investigated in animal models. For example, Lambiase et al. (2005) administered radiolabeled NGF eye drops to the conjunctiva, detecting radiolabeled protein in the sclera, optic nerve, and retina, but not in the cornea. NGF delivery to the retina after topical administration can potentially be explained by direct passage of the protein through the conjunctiva and sclera (Lambiase et al., 2005). However, the rapid and consistent uptake of radiolabeled NGF by the optic nerve also suggests the involvement of indirect passage from the retrobulbar space, as well as systemic absorption (Lambiase et al., 2005, 2007b). Moreover, NGF in the retrobulbar space can reach the optic nerves, which are bathed in cerebrospinal fluid, allowing the spread of NGF throughout the cerebrospinal fluid and ultimately the brain (Lambiase et al., 2007b).
Cerebrospinal fluid enters the optic nerve via a glymphatic pathway (Mathieu et al., 2017), and human visual pathway structures directly communicate with the subarachnoid space (Jacobsen et al., 2019). Therefore, the possible roles of ocular paravascular pathways (Wostyn et al., 2016, 2018) in ocular, cerebrospinal fluid, and brain NGF transport require further investigation.
Cerebral Nerve Growth Factor Delivery via Intranasal Administration
Some intranasally delivered molecules are absorbed and released into the central nervous system and systemic circulation through multiple routes (Erdő et al., 2018). The nasal cavity can be divided into three regions, the vestibular, respiratory (or turbinate), and olfactory. Absorption of drugs in the vestibular region is limited due to its small surface area and abundance of squamous epithelial cells, with few, if any, ciliated cells (Grassin-Delyle et al., 2012). The respiratory region includes the lateral walls of the nasal cavities, including the turbinates. Many ciliated and columnar cells are present in the respiratory region, which possess numerous microvilli and cilia, further increasing surface area. The respiratory region is the largest region of the nasal cavity and also the most vascularized region; it is therefore the principal site for systemic absorption of intranasally delivered drugs (Grassin-Delyle et al., 2012).
The respiratory region of the nasal cavity is innervated by the ophthalmic and maxillary branches of the trigeminal nerve. These branches travel to the trigeminal ganglion and enter the brain at the pons, terminating in the spinal trigeminal nuclei of the brainstem (Dhuria et al., 2010). The trigeminal nerve thus represents a possible delivery target for transporting drugs to the central nervous system in addition to the olfactory pathway (Thorne et al., 2004; Crowe et al., 2018).
The third region of the nasal cavity is the olfactory region, located at the apex of the nasal cavity and lined with the olfactory epithelium. Olfactory sensory neurons are bipolar neurons that convey sensory signals to the olfactory bulb, which connects the external environment and the brain, making it a useful pathway for drug delivery (Aderibigbe, 2018). Transport through the olfactory neurons occurs via two pathways, an intracellular and an extracellular pathway. In the intracellular pathway, molecules internalized by the olfactory neurons are delivered to the olfactory bulb as well as directly to the central nervous system through axonal transport (Erdő et al., 2018). In the extracellular pathway, molecules cross the paracellular spaces of the olfactory mucosa, are absorbed by the lamina propria, and are subsequently transported through the perineural space to the subarachnoid space, allowing delivery to brain tissue (Erdő et al., 2018).
Intranasal drugs can be administered by dripping or atomization. The first technique required only a syringe containing the drug, but a compliant child is necessary. However in the last years, the mucosal atomizer device is the most used intranasal delivery device that allows the administration of smaller and well-absorbed particles, with less drug overflow in the oropharynx, with improved clinical efficacy than drops and significantly less opponent behavior in smaller children. It also reduces sneezing and coughing compared to other devices. Deposition efficacies of about 90% can be achieved with 30° administration angles and plume angles of 30° (Tong X, et al., 2016).
Intranasal NGF delivery by mucosal atomizer device is the most commonly used (Chiaretti et al. 2017); it has several advantages over oral or intravenous administration, including non-invasiveness, self-administration, shorter onset of effect, higher bioavailability due to avoidance of hepatic first-pass metabolism inherent to systemic delivery, and minimal side effects. The intranasal pathway represents a potential non-invasive administration route of therapeutic agents for local, systemic, and central nervous system delivery (Erdő et al., 2018).
Clinical Trials on Topical Nerve Growth Factor Administration in Ophthalmology
The potential therapeutic application of NGF has been investigated in ocular pathologies as diverse as corneal ulcers, glaucoma, retinitis pigmentosa, age-related macular degeneration, and dry eye disease (Table 1). We will focus on more recent data, as older trials have been previously reviewed (Aloe et al., 2012; Manni et al., 2013).
Table 1.
Clinical trials with topical NGF administration in ophthalmology
| Disease | Study type | NGF type and dosage | Delivery route | Outcome | Side effects | References |
|---|---|---|---|---|---|---|
| Neurotrophic keratitis | Twelve patients case report | Mouse NGF. Several daily applications of a 200 μg/mL solution for 6 weeks | Topical eye | Healing of all of the ulcers, improved corneal sensitivity and integrity. Improved visual acuity | Not reported | Lambiase et al. (1998) |
| Prospective non-comparative interventional case series; 43 patients | Mouse NGF. Several daily applications of a 200 μg/mL solution until ulcer healing | Topical eye | Complete healing of the epithelia defect. Improved corneal sensitivity and visual activity | Hyperemia and ocular/periocular pain | Bonini et al. (2000) | |
| Observational study on 11 patients | Mouse NGF. Several daily applications of a 200 μg/mL solution until ulcer healing | Topical eye | Ulcer healing between 9 and 43 days after initiation of treatment. No development of systemic anti- NGFab | Mild and transient hyperemia and photophobi | Lambiase et al. (2007a) | |
| Phase II randomized multiple-doses, double masked; 156 patients | Human recombinant NGF 10 μg/mL or 20 μg/mL for 8 weeks | Topical eye | Complete healing of the epithelial defects. Improved corneal defects. Improved corneal | Mild and transient hyperemia | Bonini et al. (2018) | |
| Phase II randomized multicenter, double masked; 48 patients | Human recombinant NGF 20 μg/mL for 8 weeks | Topical eye | Complete healing of the epithelial defects. Improved corneal sensitivity and visual activity | Mild and transient hyperemia | Pflugfelder et al. (2020) | |
| Glaucoma | Three patients case report | Mouse NGF. Four daily applications of a 200 μg/mL solution for 3 months | Topical eye | Improvement in functionality of the inner retinal layer, in the parameters of post-retinal conduction and in visual activity | Local burning during the first week in a single patient | Lambiase et al. (2009a) |
| Bilateral age-related macular degeneration (retinopathy) | Single case study | Mouse NGF. Thrice-daily applications of 200 μg/mL solution 1 year or 5 years in the right eye | Topical eye | Improvement in visual acuity and in the amplitude of the ERG | Slight burning after eye drop application in the first month | Lambiase et al. (2009b) |
| Retinitis pigmentosa | Eight patients case report | Mouse NGF. 1 mg total over 10 days, thrice-daily application | Topical eye | Improvement of visual performance in a minority of patients | Mild corneal irritation | Falsini et al. (2016b) |
| Dry eye syndrome | Phase IIa, prospective open label, multipledose trial, 40 patients | rhNGF 20 μg/mL or at 4 μg/mL twice a day for 28 days | Topical eye | Significant improvement of symptoms | Mild adverse effects | Sacchetti et al. (2020) |
ERG: Electroretinogram; NGF: nerve growth factor; rhNGF: recombinant human nerve growth factor.
Neurotrophic keratitis
The cornea is the most densely innervated tissue in the body. The cornea is innervated by the ophthalmic branch of the trigeminal nerve and autonomic nerves (Shaheen et al., 2014). The corneal epithelium produces and releases neurotrophic factors to support nerve trophism and healing, while corneal nerves produce trophic neuromediators to facilitate the survival, trophism, and healing of the corneal epithelium (Mastropasqua et al., 2017; Sacchetti and Lambiase, 2017; Dua et al., 2018).
Corneal ulcers are caused by diverse endogenous and exogenous insults, and can lead to blindness. The most common causes of corneal nerve damage are herpetic keratitis, chemical burns, physical injuries, corneal surgery, long-term contact lens use, and prolonged use of topical medications (Semeraro et al., 2014). Thus far, over 200 patients have been successfully treated for corneal ulcers of different etiologies using topical NGF administration (Lambiase et al., 2012). Neuropathic pain associated with systemic delivery was nearly absent, and circulating anti-NGF antibodies did not develop (Lambiase et al., 2007a). After 30 years of clinical trials (Bonini et al., 2018), Cenegermin (Oxervate™; Dompè Farmaceutici SpA, Milan, Italy), a bacteria-produced recombinant human NGF (rhNGF), was ultimately approved for treatment of neurotrophic keratitis.
In registered trials, the majority of adults with moderate to severe neurotrophic keratitis experienced complete corneal healing after up to 8 weeks of topical Cenegermin therapy (Bonini et al., 2018; Deeks and Lamb, 2020; Pflugfelder et al., 2020).
Dry eye disease
Dry eye disease is a common condition affecting millions of people worldwide. Dry eye disease occurs when naturally produced tears are unable to provide adequate lubrication to the eyes. Aging and female sex are the greatest risk factors for dry eye disease (O’Neil et al., 2019; Kojima et al., 2020). The prevalence of dry eye disease is expected to continue increasing as the population ages. The corneal surface irregularity caused by dry eye disease compromises visual function by decreasing contrast sensitivity and functional visual acuity. In a study by Sacchetti et al. (2020), 40 consecutive patients with moderate to severe dry eye disease received rhNGF eye drops (20 or 4 µg/mL) in a phase IIa, prospective, open label, multiple-dose clinical trial. Participation was good, with 39/40 patients completing the trial. Both rhNGF eye drop concentrations tested were safe and well-tolerated. Twenty-nine patients experienced at least one adverse effect. All adverse effects were classified as mild, except one instance of bacterial conjunctivitis, which was classified as moderate. Both rhNGF dosages decreased the frequency and severity of dry eye disease symptoms and ocular surface damage, while only the 20 µg/mL dosage improved tear function. Further clinical trials investigating the use of rhNGF for treatment of dry eye disease are ongoing.
Optic neuropathy
Optic neuropathy is the major cause of vision loss worldwide, and effective treatments are still lacking. In addition to glaucomatous etiology, which is the most common cause of optic neuropathy, many other conditions, such as ischemic, demyelinating, inflammatory, genetic, traumatic, toxic, and nutritional etiologies, can induce optic neuropathy, ultimately leading to axonal loss and retrograde retinal ganglion cells death (Biousse and Newman, 2016).
A recent study (Guo et al., 2020) in an animal model of optic nerve injury (Levkovitch-Verbin et al., 2003) demonstrated that topical rhNGF administration via eye drops significantly decreases retinal ganglion cell apoptosis, as visualized by detection of apoptosing retinal cells imaging in vivo (Cordeiro et al., 2017) and quantified by histological analyses. Moreover, rhNGF eye drop administration in the rat model resulted in increased retinal ganglion cell density in the inferior retina, improved axonal survival, and inhibition of astrocyte activity in the optic nerve (Guo et al., 2020). Guo et al. (2020) suggest that neuroprotection on retinal neurons is induced by topical rhNGF through the prevention of secondary degeneration. This mechanism not only explains the visual functional improvements observed in the few human studies (Table 1), but also supports the rationale of large-scale clinical trials in patients with glaucomatous optic neuropathy. Moreover, in patients with glaucoma, detection of apoptosing retinal cells imaging enables visualization of neuronal apoptosis at single-cell resolution in the human retina and correlates with disease progression (Cordeiro et al., 2017). Therefore, this technique could allow investigators to evaluate the neuroprotective effects of rhNGF in patients.
Optic pathway glioma
Optic pathway gliomas are brain tumors involving the optic nerve and optic chiasm, characterized by slow progression and visual loss. Optic pathway glioma affects approximately 20% of patients with type 1 Neurofibromatosis, with onset occurring primarily during childhood (Cassina et al., 2019). No currently available therapeutics prevent visual loss in glioma. NGF eye drop delivery has been investigated to potentially restore and prevent visual loss in patients affected by optic glioma (Chiaretti et al., 2011; Falsini et al., 2011, 2016a). A prospective, randomized, double-blind phase II clinical trial was conducted in 18 optic pathway glioma patients, aged from 2 to 23 years, with stable disease and severe visual loss (Falsini et al., 2016a). Ten patients were randomly assigned to receive a single 10-day course of murine NGF eye drops, while eight patients received placebo. Electrophysiological parameters (electroretinographic photopic negative response amplitude at 180 days and visual-evoked potentials at 30 days) were significantly improved in the NGF-treated group compared with the placebo-treated group. Further, visual field worsening during the follow-up period was detected only in the placebo group. Contrastingly, the majority of NGF-treated subjects experienced significant visual field enlargement.
Intranasal Nerve Growth Factor Administration for Treatment of Brain Disorders
The therapeutic efficacy of intranasal NGF administration has been investigated in many brain diseases, as summarized in Table 2.
Table 2.
Clinical trials with topical NGF administration in brain disorders
| Disease | Study type | NGF type and dosage | Delivery route | Outcome | Side effects | References |
|---|---|---|---|---|---|---|
| Tumors: Optic glioma | Five patients case study | Mouse NGF. 1 mg total over 10 days in daily applications | Topica (eye) | Improvement in visual evoked potentials (VEP) | Not reported | Falsini et al. (2011) |
| Single patient case study | Mouse NGF. 1mg total over 10 days in daily applications | Topica (eye) | Reversible improvements of visual function and electrophysiological measurements | Not reported | Chiaretti et al. (2011) | |
| Prospective randomized double-blind phase II clinical trial (18 patients) | Mouse NGF. 0.5 mg total over 10 days in daily applications | Topica (eye) | Improvements of electrophysiological parameters. Improvement of visual field | Not reported | Falsini et al. (2016a) | |
| Acquired traumatic brain injury, TBI | Single patient case report | Mouse NGF. 0.1 mg/kg over 10 days, twice-daily administration. Four cycles repeated at monthly intervals | Intranasal (MAD) | Improved functional assessment PET/CT, SPECT/ CT, MRI, EEG, VEP. Improved clinical parameters | Not reported | Chiaretti et al. (2017) |
| Acquired traumatic brain injury, TBI | Phase II, randomized, double blind, placebo controlled (106 patients) | Mouse NGF 20 μg /day for two weeks | Intranasal (spray) | Not reported | Completed | Liu (2017) |
| Acquired nontraumatic brain injury, nTBI (GBS meningitis) | Single patient case report | Human recombinant NGF. 0.1 mg/kg over 7 days, thrice-daily administration. Five cycles repeated at monthly intervals | Intranasal (MAD) | Improved functional assessment PET/CT, SPECT/CT. Improved clinical parameters | Not reported | Chiaretti et al. (2020) |
| Acute Ischemic Stroke | Phase IV, randomized, double blind, placebo controlled (106 patients) | Mouse NGF 20 μg/day for two weeks | Intranasal (spray) | Not reported | Ongoing | Liu (2020) |
| Frontotemporal dementia with corticobasal syndrome | Two patients case report | Mouse NGF 2 μg/day for one year | Intranasal (spray) | Improvement in the rate of cognitive decline | Absence of adverse effects | de Bellis et al. (2018) |
CT: Computed tomography; EEG: electroencephalography; MAD: Mucosal Atomization Device; MRI: magnetic resonance imaging; NGF: nerve growth factor; PET: positron emission tomography; SPECT: single photon emission computed tomography.
Alzheimer’s disease
Alzheimer’s disease (AD) is an insidious disorder characterized by a progressive decline in cognitive function. The progressive memory loss of AD is associated with degeneration of the central cholinergic neurons of the basal forebrain, from which the cholinergic neurons project to the cerebral cortex and hippocampus. Decreased levels of NGF and NGF receptors in the basal forebrain have been reported in patients with AD (Mufson et al., 2003). Contrastingly, hippocampal and cortex NGF is unchanged or increases in AD. This supports the hypothesis that physiological NGF transport and signaling are impaired in AD (Williams et al., 2006). Moreover, brain proNGF levels are increased in AD patients due to concomitant impaired conversion of the NGF precursor molecule to its mature form and increased degradation of mNGF (Fahnestock and Shekari, 2019). Additionally, the TrkA/p75NTR ratio is decreased in AD brains, which, together with increased proNGF, activates apoptotic signaling (Fahnestock and Shekari, 2019; Mufson et al., 2019). Increased brain proNGF levels are not only pro-apoptotic, but also affect mNGF receptor binding and axonal trafficking, resulting in retrograde atrophy of cholinergic neurons in the basal forebrain (Ioannou and Fahnestock, 2017; Allard et al., 2018; Fahnestock and Shekari, 2019). In a rat model of AD, altered NGF metabolism in AD results in loss of cortical synapses and atrophy of basal forebrain cholinergic neurons (Cuello et al., 2019; Mufson et al., 2019).
Following these findings, the therapeutic potential of exogenous mNGF has been intensely investigated in AD. The first clinical trials to evaluate the efficacy of mNGF in AD used intraventricular mNGF delivery (Olson et al., 1992; Eriksdotter Jönhagen et al., 1998). Chronic Intraventricular NGF infusion conferred partial therapeutic effects in AD, such as transient increases in [11C]-nicotine uptake and binding in the frontal and temporal cortex, persistently increased cortical blood flow, progressively decreased slow wave electroencephalography activity, and improved verbal episodic memory tests. However, the invasiveness of Intraventricular administration and side effects such as back pain and reversible weight loss were considered to outweigh the positive outcomes, leading to the discontinuation of intraventricular mNGF trials in AD patients (Fahnestock and Shekari, 2019).
Alternative delivery approaches have been investigated, such as genetically engineered NGF secreting human retinal cells encapsulated in implantable cell biodelivery devices (Eriksdotter-Jönhagen et al., 2012; Wahlberg et al., 2012; Eyjolfsdottir et al., 2016), gene therapies with human NGF genetically engineered into autologous grafted fibroblasts (Tuszynski et al., 2005), or delivery using an adeno-associated virus vector (CERE-110) (Rafii et al., 2018). However, a multicenter, randomized clinical trial for virus vector treatment of AD found that the therapeutic did not affect clinical outcomes, and the drug manufacturer, Sangamo, terminated development of CERE-110. However, post-mortem analyses of patients enrolled in the trial revealed that NGF did not directly reach cholinergic neurons at any of the investigated 15 injection sites due to limited diffusion of NGF and inaccurate stereotactic targeting (Castle et al., 2020). Therefore, the potential efficacy of NGF gene therapy in AD has not been refuted (Castle et al., 2020).
In any case, these experimental approaches entail surgical risks, high cost, and safety concerns, suggesting that less invasive strategies for cerebral NGF delivery should be considered. Intranasal delivery has been proposed to deliver NGF to the brain for treatment of AD (Cattaneo et al., 2008; Alexander and Saraf, 2018). The efficacy of intranasal NGF administration has been demonstrated in the AD11 anti-NGF transgenic mouse model, in which intranasal NGF administration alleviates cognitive loss in AD11 mice (De Rosa et al., 2005). A prior study comparing ocular versus intranasal NGF delivery in anti-NGF transgenic mice suggests that intranasal NGF administration is significantly more effective than ocular NGF administration in alleviating the neurodegenerative phenotypic hallmarks of AD11 mice (Capsoni et al., 2009).
Despite these promising preclinical studies, neither intranasal nor ocular topical administration of NGF has been investigated in AD patients yet.
Frontotemporal dementia
Frontotemporal dementia, previously known as Pick’s disease, is a group of neurodegenerative disorders with varying clinical phenotypes that share a natural tendency to target the frontal and temporal lobes (Olney et al., 2017). The atrophy and progressive loss of neurons in these brain regions lead to a gradual and progressive decline in behavioral and/or linguistic functioning (Sivasathiaseelan et al., 2019). Frontotemporal dementia constitutes an important form of young-onset dementia, as it is common in patients younger than 65 years (Bang et al., 2015). About 55% of patients have frontotemporal dementia with ubiquitin-positive inclusions, while 45% have frontotemporal dementia with tau-positive inclusions (Cardarelli et al., 2010).
There are three subtypes or variants of frontotemporal dementia (de Bellis et al., 2018): (1) Behavioral variant of frontotemporal dementia; (2) primary progressive aphasia, including the nonfluent/agrammatic variant, semantic variant, and logopenic variant; and (3) motor-associated disorder of frontotemporal dementia, including corticobasal syndrome, progressive supranuclear palsy, and frontotemporal dementia with motor neuron disease.
Corticobasal syndrome is characterized by progressive deterioration of neurological functions that may involve the motor system, cognition, or both. Corticobasal syndrome generally presents as a movement disorder, with unilateral scarcity of movements and muscle rigidity, sometimes accompanied by tremors (Constantinides et al., 2019).
Intranasal administration of murine NGF has been tested in two corticobasal syndrome patients at different stages of disease (de Bellis et al., 2018). NGF was administered in each nostril once daily for 1 year (range, 12–18 months). No significant side effects were reported, except for rhinitis, rigidity, and moderate psychomotor agitation. Any adverse effects dissipated within 3–4 hours of administration, and no side effects were observed in an 18–24 month follow-up period after the final dose of NGF was administered. Clinical and Positron emission tomography results demonstrated that intranasal NGF administration both attenuated neurodegenerative progression and improved cognitive abilities in these corticobasal syndrome patients.
NGF and demyelinating diseases
NGF involvement in degenerative phenomena of myelin and its trophic role for cells expressing NGF receptor have been demonstrated for many years. NGF implication in multiple sclerosis (MS) become suitable, considering the wide diffusion of this neurotrophin in the central nervous system and the role played by the NGF in immune response and inflammatory phenomena. Many experimental studies suggest an alteration of serum and Cerebrospinal Fluid levels of NGF in patients with MS, related to the acute manifestations of the disease. In MS patients and rats with Experimental Autoimmune Encephalomyelitis have been identified cells able to produce NGF and BDNF and expressing the high affinity receptor for these two molecules (Hammarberg et al, 2000). The functional significance of these cells is to carry out a neuroimmuno-protective action in cerebral inflammation areas (Valdo et al, 2002). The basal levels of NGF in the cerebrospinal fluid of patients with MS increase in the acute phase of the disease and decrease during the remission phase. Moreover, studies on rats affected by experimental autoimmune encephalomyelitis, the most used experimental model of demyelination, highlight the significant changes in synthesis and release of NGF in various areas of the central nervous system, such as the thalamus, the medulla oblongata and the spinal cord. The levels of NGF are different in the various stages of the disease. Furthermore, during the same phase, areas in close proximity show a different NGF content (Oderfeld-Nowak et al, 2003). This detail suggests a different responsiveness to NGF by the affected cells and a different capability to produce NGF itself. Furthermore, NGF produced during the acute and chronic phase of the disease, may be involved in the proliferation and differentiation of oligodendrocytes. It has been hypothesized that altered NGF levels may influence the potential reserve of stem cells in the adult brain, regulating both migratory and differentiation phenomena and providing neuronal survival (Parvaneh Tafreshi, 2006). An experimental finding, supporting the hypothesis of a protective role of NGF, comes from the observation that the endogenous presence of anti-NGF antibodies in rats with experimental autoimmune encephalomyelitis causes more severe symptoms and that the local production of NGF, by T-lymphocytes in rats with optic neuritis, induces a mild clinical and neuropathological picture compared to control rats. The role of NGF in demyelination phenomena was also confirmed by a recent study investigating the usefulness of NGF pretreatment in reducing lidocaine induced myelin damage via the upregulation of BDNF and inhibition of p38 mitogen activated protein kinase in intravertebral anesthesia (Zhao et al, 2017). Based on these experimental studies, NGF is currently considered a promising treatment option for MS and other demyelinating diseases for its direct effects on repairing immune system mediated myelin damage (Acosta et al, 2013).
Traumatic brain injury
The term acquired brain injury was first used in 1996 by the Commission on Accreditation of Rehabilitation Facilities (CARF). In its Glossary, (CARF, 2012) acquired brain injury is defined as “an insult to the brain that affects its structure or function, resulting in impairments of cognition, communication, physical function, or psychosocial behavior. Acquired brain injury includes both traumatic and non-traumatic brain injury (TBI)…”
Globally, TBI is the leading cause of morbidity and mortality among children and young adults (Popescu et al., 2015). Further, children with TBI are more likely to develop behavioral problems linked to an increased propensity for criminal activity (Menon and Bryant, 2019). Therefore, it is of utmost importance to identify and treat TBI in its initial phases not only for individual welfare, but also for public safety. Severe TBI has an exceptionally high mortality rate: 50% of severe TBI patients do not survive, and the majority of survivors are left with varying degrees of disability (Stocchetti and Zanier, 2016).
Clinical outcomes in TBI patients depend on both the primary injury and secondary brain damage (Mckee and Daneshvar, 2015). The primary injury involves tissue damage and disruption caused by the mechanical force of impact. Secondary brain injuries begin to occur within several minutes of primary injury, and persist for days to years following the primary injury (Mckee and Daneshvar, 2015). Secondary TBI brain damage expands progressively and centrifugally, culminating in neuronal cell death. Typically, neurons damaged in the primary injury undergo necrotic cell death, while neurons that succumb to secondary injury undergo apoptotic cell death. The traumatic penumbra surrounds the primary lesion and represents the portion of damaged brain tissue that can potentially be regenerated (Regner et al., 2017).
Indeed, the traumatic penumbra is a crucial therapeutic target for many forms of brain injury. Glial cells are first activated to protect neurons in the penumbra, proliferating and secreting anti-apoptotic molecules such as the neurotrophins NGF and neurotrophin-3 upon activation (Lee et al., 1998; Gottlieb and Matute, 1999; Chen et al., 2006).
mNGF supports neuronal growth, survival, and differentiation (Levi-Montalcini, 1987), restoring the function of injured neurons (Mattson and Scheff, 1994), and has therefore been investigated as a novel treatment strategy for TBI patients.
mNGF has been tested in experimental animal models of TBI-induced brain damage and alleviates TBI-induced neurological deficits. Specifically, intraventricular NGF administration protects cholinergic neurons and prevents their loss of phenotype after severe TBI by increasing choline acetyltransferase synthesis and preventing basal forebrain cholinergic neuron atrophy (Garofalo and Cuello, 1994; Holtzman et al., 1996). Intranasal administration of mNGF to rats with TBI alleviates brain oedema and improves motor function (Tian et al., 2012; Young et al., 2015).
The therapeutic effects of NGF have been evaluated in the pediatric field by Chiaretti’s group (Chiaretti et al., 2002, 2005, 2008a, b, c, 2011, 2017, 2020; Falsini et al., 2011, 2016a; Fantacci et al., 2013). In the first studies, murine NGF was delivered by intraventricular infusion in patients with hypoxic-ischemic brain injury (Chiaretti et al., 2005, 2008a; Fantacci et al., 2013). Intraventricular NGF treatment improved comatose status and cognitive functioning as well as electroencephalography and positron emission tomography parameters. Further, malacic areas were reduced, and cortical perfusion was increased. In addition, doublecortin, a protein expressed by newly formed neurons, was up-regulated in the cerebrospinal fluid of NGF-treated patients (Chiaretti et al., 2008b). In spite of the encouraging results for NGF treatment of neurodegenerative disease, the principal challenge is the invasiveness of intraventricular administration.
Therefore, following these promising results, intranasal delivery of NGF to the brain has been investigated. In a child with severe TBI, intranasal murine NGF delivery improved cognitive and motor functioning, together with increased brain metabolic rate and perfusion in the cortical and subcortical regions (Chiaretti et al., 2017). A brain Magnetic Resonance Imaging also revealed decreased parenchymal lesion size and ventricular dilatation. A clinical trial evaluating the therapeutic efficacy of intranasal NGF in adult patients with TBI has been completed, but the results have not yet been reported (Liu, 2017).
NGF delivered intranasally reaches the spinal cord neurons in rat models of spinal cord injury (SCI), and increases the concentration of NGF and its receptors, improving motor function (de Bellis and Aloe, 2016). This suggests the therapeutic potential of intranasal NGF administration for neuroprotection in acute spinal cord injury, or as a growth factor for stem cell treatment of chronic Spinal Cord Injury.
Non-TBIs
Stroke
While TBIs are the leading cause of brain injury in younger people, stroke is the leading cause of brain injury and disability worldwide. The incidence of new stroke onset in developed countries among all age groups is approximately 160–200 per 100,000 persons per year (Truelsen et al., 2006). Estimates of the prevalence of stroke-related disability are relatively rare and range from 173–623 per 100,000 (Truelsen et al., 2006). Following ischemic stroke, the brain tissue’s blood and oxygen supplies are depleted, leading to neuronal death. Neuroinflammation, excitotoxicity, microglial overactivation, and ionic imbalance contribute to neurodegeneration in ischemic stroke, in addition to other mechanisms (Su, 2017).
The biological mechanisms of non-traumatic brain injuries (nTBI) are similar to those reported above for TBI, although every specific cause of nTBI induces characteristic patterns of damage.
The therapeutic potential of intranasal NGF for stroke was first investigated in animal models, which revealed increased striatal neurogenesis in NGF-treated adult rats after induction of brain ischemia (Zhu et al., 2011). In addition, the NGF dipeptide mimetic molecule GK-2 has also been found to stimulate neurogenesis and synaptic formation in experimental cerebral ischemia (Gudasheva et al., 2019).
Microvessel density is significantly increased in the penumbra of brain infarcts after ischemic injury (Su, 2017). This increased vascular density is a result of reparative angiogenesis and is associated not only with improved functional outcomes in animal models and stroke patients, but also with increased survival (Su, 2017). NGF was initially discovered as a neurotrophic factor, but mounting evidence suggests that NGF is a significant contributor to both physiological and pathological angiogenesis (Turrini et al., 2002; Diao et al., 2016). Indeed, by upregulating pro-angiogenic factors such as Vascular Endotelial Growth Factor and mobilizing endothelial progenitor cells, NGF promotes angiogenesis in the ischemic penumbra, decreasing infarct volume in a rat model of cerebral ischemia (Li et al., 2018).
The combined neurotrophic and angiogenic effects of NGF could contribute to neurological functional recovery following ischemic stroke (Figure 3). Presently, an ongoing Phase IV clinical trial is investigating the therapeutic effects of intranasal NGF in acute ischemic stroke (Liu, 2020). The study is estimated to be complete by December 2020 and is anticipated to identify the therapeutic effects, if any, of intranasal NGF in ischemic stroke.
Group B streptococcus meningitis
Group B streptococcus is the most common causative organism of neonatal sepsis and meningitis, causing more than 40% of all early-onset infections, in spite of implementing the use of maternal intrapartum prophylaxis (Ku et al., 2015). Bacterial meningitis is a serious and potentially life-threatening central nervous system infection and is associated with a variety of neurologic complications, including seizures, subdural effusion, ventriculomegaly, subdural empyema hydrocephalus, ventriculitis, periventricular leukomalacia, and encephalomalacia (Hsu et al., 2018)
In a pilot study, an infant suffering from severe neurological impairment due to Streptococcus agalactiae meningitis was treated with intranasal hrNGF (Chiaretti et al., 2020). Five cycles of intranasal hrNGF treatment (0.1 mg/kg, Cenegermin, Oxervate™ ; Dompè Farmaceutici S.p.A, Milan, Italy) were repeated at monthly intervals. NGF treatment was followed by significant improvement of functional (Positron Emission Tomography and Magnetic Resonance Imaging) and Electroencephalogram parameters, concomitant with improvement of the patient’s clinical and neurological condition (Chiaretti et al., 2020).
Conclusions
Preclinical and clinical studies evaluating the neuroprotective effects of NGF have revealed that both the intranasal and ocular delivery of this neurotrophin are very promising and safe strategies for managing different ocular and brain diseases. Moreover, these routes of administration are easily employed by the physicians and accepted by the patients, especially the pediatric ones. The noticeable reported clinical effects inspire plans for new clinical trials. However, the number of subjects recruited in the majority of the previous trials is very small. Therefore, in order to achieve a better understanding of the neuroprotective mechanisms of NGF, further multicenter, controlled randomized, double blind studies are needed. Novel NGF delivery approaches such as gels, nano drug carrier systems, colloidal carriers, mucoadhesive devices, and controlled delivery systems are under investigation (Aderibigbe, 2018; Agrawal et al., 2018). Additionally, novel approaches with small NGF peptides are under evaluation (Gudasheva et al., 2019; Mitra et al., 2019; Triaca et al., 2020; Windisch M, 2020). Ongoing research aims to address all these challenges.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Acosta CM, Cortes C, MacPhee H, Namaka MP. Exploring the role of nerve growth factor in multiple sclerosis: implications in myelin repair. CNS Neurol Disord Drug Targets. 2013;12:1242–1256. doi: 10.2174/18715273113129990087. [DOI] [PubMed] [Google Scholar]
- 2.Aderibigbe BA. In situ-based gels for nose to brain delivery for the treatment of neurological diseases. Pharmaceutics. 2018;10:40. doi: 10.3390/pharmaceutics10020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA, Alexander A. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018;281:139–177. doi: 10.1016/j.jconrel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Alexander A, Saraf S. Nose-to-brain drug delivery approach: a key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural Regen Res. 2018;13:2102–2104. doi: 10.4103/1673-5374.241458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allard S, Jacobs ML, Do Carmo S, Cuello AC. Compromise of cortical proNGF maturation causes selective retrograde atrophy in cholinergic nucleus basalis neurons. Neurobiol Aging. 2018;67:10–20. doi: 10.1016/j.neurobiolaging.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Aloe L, Bianchi P, De Bellis A, Soligo M, Rocco ML. Intranasal nerve growth factor bypasses the blood-brain barrier and affects spinal cord neurons in spinal cord injury. Neural Regen Res. 2014;9:1025–1030. doi: 10.4103/1673-5374.133161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aloe L, Rocco ML, Bianchi P, Manni L. Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: What went wrong, what went right, and what does the future hold. Int Rev Neurobiol. 2002;50:393–413. doi: 10.1016/s0074-7742(02)50083-0. [DOI] [PubMed] [Google Scholar]
- 9.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386:1672–1682. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker PA. High affinity not in the vicinity. Neuron. 2007;53:1–4. doi: 10.1016/j.neuron.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biousse V, Newman NJ. Diagnosis and clinical features of common optic neuropathies. Lancet Neurol. 2016;15:1355–1367. doi: 10.1016/S1474-4422(16)30237-X. [DOI] [PubMed] [Google Scholar]
- 14.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107:1347–1351. doi: 10.1016/s0161-6420(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 15.Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, Mantelli F REPARO Study Group. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125:1332–1343. doi: 10.1016/j.ophtha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Bothwell M. NGF, BDNF, NT3, and NT4. Handb Exp Pharmacol. 2014;220:3–15. doi: 10.1007/978-3-642-45106-5_1. [DOI] [PubMed] [Google Scholar]
- 17.Bothwell M. Recent advances in understanding neurotrophin signaling. F1000Res. 2016;5:1885. doi: 10.12688/f1000research.8434.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottros MM, Christo PJ. Current perspectives on intrathecal drug delivery. J Pain Res. 2014;7:615–626. doi: 10.2147/JPR.S37591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracci Laudiero ML, Manni L. NGF and immune regulation. In: Kostrzewa RM, editor. Handbook of Neurotoxicity. New York: Springer Verlag; 2013. pp. 1849–1876. [Google Scholar]
- 20.Cardarelli R, Kertesz A, Knebl JA. Frontotemporal dementia: a review for primary care physicians. Am Fam Physician. 2010;82:1372–1377. [PubMed] [Google Scholar]
- 21.CARF. Medical Rehabilitation Standards manual. Commission on Accreditation of Rehabilitation Facilities Glossary. 2012;317 [Google Scholar]
- 22.Carmignoto G, Comelli MC, Candeo P, Cavicchioli L, Yan Q, Merighi A, Maffei L. Expression of NGF receptor and NGF receptor mRNA in the developing and adult rat retina. Exp Neurol. 1991;111:302–311. doi: 10.1016/0014-4886(91)90097-v. [DOI] [PubMed] [Google Scholar]
- 23.Cassina M, Frizziero L, Opocher E, Parrozzani R, Sorrentino U, Viscardi E, Miglionico G, Midena E, Clementi M, Trevisson E. Optic pathway glioma in type 1 neurofibromatosis: review of its pathogenesis, diagnostic assessment, and treatment recommendations. Cancers. 2019;11:1790. doi: 10.3390/cancers11111790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castle MJ, Baltanás FC, Kovacs I, Nagahara AH, Barba D, Tuszynski MH. Postmortem analysis in a clinical trial of AAV2-NGF gene therapy for Alzheimer’s disease identifies a need for improved vector delivery. Hum Gene Ther. 2020;31:415–422. doi: 10.1089/hum.2019.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattaneo A, Capsoni S, Paoletti F. Towards non invasive nerve growth factor therapies for Alzheimer’s disease. J Alzheimers Dis. 2008;15:255–283. doi: 10.3233/jad-2008-15210. [DOI] [PubMed] [Google Scholar]
- 26.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 27.Chen LW, Zhang JP, Kwok-Yan Shum D, Chan YS. Localization of nerve growth factor, neurotrophin-3, and glial cell line-derived neurotrophic factor in nestin-expressing reactive astrocytes in the caudate-putamen of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-treated C57/Bl mice. J Comp Neurol. 2006;497:898–909. doi: 10.1002/cne.21014. [DOI] [PubMed] [Google Scholar]
- 28.Chiaretti A, Antonelli A, Genovese O, Fernandez E, Giuda D, Mariotti P, Riccardi R. Intraventricular nerve growth factor infusion improves cerebral blood flow and stimulates doublecortin expression in two infants with hypoxic-ischemic brain injury. Neurol Res. 2008a;30:223–228. doi: 10.1179/016164107X247948. [DOI] [PubMed] [Google Scholar]
- 29.Chiaretti A, Antonelli A, Genovese O, Pezzotti P, Rocco CD, Viola L, Riccardi R. Nerve growth factor and doublecortin expression correlates with improved outcome in children with severe traumatic brain injury. J Trauma. 2008b;65:80–85. doi: 10.1097/TA.0b013e31805f7036. [DOI] [PubMed] [Google Scholar]
- 30.Chiaretti A, Antonelli A, Mastrangelo A, Pezzotti P, Tortorolo L, Tosi F, Genovese O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J Neurotrauma. 2008c;25:225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- 31.Chiaretti A, Conti G, Falsini B, Buonsenso D, Crasti M, Manni L, Soligo M, Fantacci C, Genovese O, Calcagni ML, Di Giuda D, Mattoli MV, Cocciolillo F, Ferrara P, Ruggiero A, Staccioli S, Colafati GS, Riccardi R. Intranasal nerve growth factor administration improves cerebral functions in a child with severe traumatic brain injury: a case report. Brain Inj. 2017;31:1538–1547. doi: 10.1080/02699052.2017.1376760. [DOI] [PubMed] [Google Scholar]
- 32.Chiaretti A, Eftimiadi G, Buonsenso D, Rendeli C, Staccioli S, Conti G. Intranasal nerve growth factor administration improves neurological outcome after GBS meningitis. Childs Nerv Syst. 2020;36:2083–2088. doi: 10.1007/s00381-020-04590-x. [DOI] [PubMed] [Google Scholar]
- 33.Chiaretti A, Falsini B, Servidei S, Marangoni D, Pierri F, Riccardi R. Nerve growth factor eye drop administration improves visual function in a patient with optic glioma. Neurorehabil Neural Repair. 2011;25:386–390. doi: 10.1177/1545968310395601. [DOI] [PubMed] [Google Scholar]
- 34.Chiaretti A, Genovese O, Riccardi R, Di Rocco C, Di Giuda D, Mariotti P, Pulitanò S, Piastra M, Polidori G, Colafati GS, Aloe L. Intraventricular nerve growth factor infusion: a possible treatment for neurological deficits following hypoxic-ischemic brain injury in infants. Neurol Res. 2005;27:741–746. doi: 10.1179/016164105X35611. [DOI] [PubMed] [Google Scholar]
- 35.Chiaretti A, Piastra M, Caresta E, Nanni L, Aloe L. Improving ischaemic skin revascularisation by nerve growth factor in a child with crush syndrome. Arch Dis Child. 2002;87:446–448. doi: 10.1136/adc.87.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constantinides VC, Paraskevas GP, Paraskevas PG, Stefanis L, Kapaki E. Corticobasal degeneration and corticobasal syndrome: a review. Clin Park Relat Disord. 2019;1:66–71. doi: 10.1016/j.prdoa.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordeiro MF, Normando EM, Cardoso MJ, Miodragovic S, Jeylani S, Davis BM, Guo L, Ourselin S, A’Hern R, Bloom PA. Real-time imaging of single neuronal cell apoptosis in patients with glaucoma. Brain. 2017;140:1757–1767. doi: 10.1093/brain/awx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi: 10.1016/j.lfs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 39.Cuello AC, Pentz R, Hall H. The brain NGF metabolic pathway in health and in Alzheimer’s pathology. Front Neurosci. 2019;13:62. doi: 10.3389/fnins.2019.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bellis A, Aloe L. New non-invasive way to rescue neurons in spinal cord injury via intranasal administration of nerve growth factor: pilot study 8:37-44. 2016 [Google Scholar]
- 41.de Bellis A, de Bellis M, Aloe L. Long-term non-invasive treatment via intranasal administration of nerve growth factor protects the human brain in frontotemporal dementia associated with corticobasal syndrome: a pilot study. J Alzheimers Dis Rep. 2018;2:67–77. doi: 10.3233/ADR-180055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci USA. 2005;102:3811–3816. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeks ED, Lamb YN. Cenegermin: a review in neurotrophic keratitis. Drugs. 2020;80:489–494. doi: 10.1007/s40265-020-01289-w. [DOI] [PubMed] [Google Scholar]
- 44.Deng P, Anderson JD, Yu AS, Annett G, Fink KD, Nolta JA. Engineered BDNF producing cells as a potential treatment for neurologic disease. Expert Opin Biol Ther. 2016;16:1025–1033. doi: 10.1080/14712598.2016.1183641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhuria SV, Hanson LR, Frey WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 46.Di Fausto V, Fiore M, Tirassa P, Lambiase A, Aloe L. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur J Neurosci. 2007;26:2473–2480. doi: 10.1111/j.1460-9568.2007.05883.x. [DOI] [PubMed] [Google Scholar]
- 47.Diao YP, Cui FK, Yan S, Chen ZG, Lian LS, Guo LL, Li YJ. Nerve growth factor promotes angiogenesis and skeletal muscle fiber remodeling in a murine model of hindlimb ischemia. Chin Med J (Engl) 2016;129:313–319. doi: 10.4103/0366-6999.174496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dua HS, Said DG, Messmer EM, Rolando M, Benitez-Del-Castillo JM, Hossain PN, Shortt AJ, Geerling G, Nubile M, Figueiredo FC, Rauz S, Mastropasqua L, Rama P, Baudouin C. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–131. doi: 10.1016/j.preteyeres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–170. doi: 10.1016/j.brainresbull.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Eriksdotter Jönhagen M, Nordberg A, Amberla K, Bäckman L, Ebendal T, Meyerson B, Olson L, Seiger null, Shigeta M, Theodorsson E, Viitanen M, Winblad B, Wahlund LO. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:246–257. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- 51.Eriksdotter-Jonhagen M, Linderoth B, Lind G, Aladellie L, Almkvist O, Andreasen N, Blennow K, Bogdanovic N, Jelic V, Kadir A, Nordberg A, Sundstrom E, Wahlund LO, Wall A, Wiberg M, Winblad B, Seiger A, Almqvist P, Wahlberg L. Encapsulated cell biodelivery of nerve growth factor to the Basal forebrain in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33:18–28. doi: 10.1159/000336051. [DOI] [PubMed] [Google Scholar]
- 52.Eyjolfsdottir H, Eriksdotter M, Linderoth B, Lind G, Juliusson B, Kusk P, Almkvist O, Andreasen N, Blennow K, Ferreira D, Westman E, Nennesmo I, Karami A, Darreh-Shori T, Kadir A, Nordberg A, Sundström E, Wahlund LO, Wall A, Wiberg M, et al. Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: application of a second-generation encapsulated cell biodelivery device. Alzheimers Res Ther. 2016;8:30. doi: 10.1186/s13195-016-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- 54.Fahnestock M, Shekari A. ProNGF and neurodegeneration in Alzheimer’s disease. Front Neurosci. 2019;13:129. doi: 10.3389/fnins.2019.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falsini B, Chiaretti A, Barone G, Piccardi M, Pierri F, Colosimo C, Lazzareschi I, Ruggiero A, Parisi V, Fadda A, Balestrazzi E, Riccardi R. Topical nerve growth factor as a visual rescue strategy in pediatric optic gliomas: a pilot study including electrophysiology. Neurorehabil Neural Repair. 2011;25:512–520. doi: 10.1177/1545968310397201. [DOI] [PubMed] [Google Scholar]
- 56.Falsini B, Chiaretti A, Rizzo D, Piccardi M, Ruggiero A, Manni L, Soligo M, Dickmann A, Federici M, Salerni A, Timelli L, Guglielmi G, Lazzareschi I, Caldarelli M, Galli-Resta L, Colosimo C, Riccardi R. Nerve growth factor improves visual loss in childhood optic gliomas: a randomized, double-blind, phase II clinical trial. Brain. 2016a;139:404–414. doi: 10.1093/brain/awv366. [DOI] [PubMed] [Google Scholar]
- 57.Falsini B, Iarossi G, Chiaretti A, Ruggiero A, Manni L, Galli-Resta L, Corbo G, Abed E. NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J Transl Med. 2016b;14:8. doi: 10.1186/s12967-015-0750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fantacci C, Capozzi D, Ferrara P, Chiaretti A. Neuroprotective role of nerve growth factor in hypoxic-ischemic brain injury. Brain Sci. 2013;3:1013–1022. doi: 10.3390/brainsci3031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frey IW, Liu J, Chen XQ, Thorne RG, Fawcett JR, Ala TA, Rahman YE. Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv. 1997;4:87–92. [Google Scholar]
- 60.Friedman WJ, Greene LA. Neurotrophin signaling via Trks and p75. Exp Cell Res. 1999;253:131–142. doi: 10.1006/excr.1999.4705. [DOI] [PubMed] [Google Scholar]
- 61.Garofalo L, Cuello AC. Nerve growth factor and the monosialoganglioside GM1: analogous and different in vivo effects on biochemical, morphological, and behavioral parameters of adult cortically lesioned rats. Exp Neurol. 1994;125:195–217. doi: 10.1006/exnr.1994.1024. [DOI] [PubMed] [Google Scholar]
- 62.Gottlieb M, Matute C. Expression of nerve growth factor in astrocytes of the hippocampal CA1 area following transient forebrain ischemia. Neuroscience. 1999;91:1027–1034. doi: 10.1016/s0306-4522(98)00612-5. [DOI] [PubMed] [Google Scholar]
- 63.Grassin-Delyle S, Buenestado A, Naline E, Faisy C, Blouquit-Laye S, Couderc L-J, Le Guen M, Fischler M, Devillier P. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol Ther. 2012;134:366–379. doi: 10.1016/j.pharmthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Gudasheva TA, Povarnina PYu, Volkova AA, Kruglov SV, Antipova TA, Seredenin SB. A nerve growth factor dipeptide mimetic stimulates neurogenesis and synaptogenesis in the hippocampus and striatum of adult rats with focal cerebral ischemia. Acta Naturae. 2019;11:31–37. doi: 10.32607/20758251-2019-11-3-31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo L, Davis BM, Ravindran N, Galvao J, Kapoor N, Haamedi N, Shamsher E, Luong V, Fico E, Cordeiro MF. Topical recombinant human nerve growth factor (rh-NGF) is neuroprotective to retinal ganglion cells by targeting secondary degeneration. Sci Rep. 2020;10:3375. doi: 10.1038/s41598-020-60427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Linda H, van Der Meide PH, Cullheim S, Olsson T, Piehl F. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283–5291. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hempstead BL. Deciphering proneurotrophin actions. Handb Exp Pharmacol. 2014;220:17–32. doi: 10.1007/978-3-642-45106-5_2. [DOI] [PubMed] [Google Scholar]
- 69.Hock C, Heese K, Müller-Spahn F, Hulette C, Rosenberg C, Otten U. Decreased trkA neurotrophin receptor expression in the parietal cortex of patients with Alzheimer’s disease. Neurosci Lett. 1998;241:151–154. doi: 10.1016/s0304-3940(98)00019-6. [DOI] [PubMed] [Google Scholar]
- 70.Holtzman DM, Sheldon RA, Jaffe W, Cheng Y, Ferriero DM. Nerve growth factor protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 1996;39:114–122. doi: 10.1002/ana.410390117. [DOI] [PubMed] [Google Scholar]
- 71.Hsu MH, Hsu JF, Kuo HC, Lai MY, Chiang MC, Lin YJ, Huang HR, Chu SM, Tsai MH. Neurological complications in young infants with acute bacterial meningitis. Front Neurol. 2018;9:903. doi: 10.3389/fneur.2018.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 73.Ioannou MS, Fahnestock M. ProNGF, but not NGF, switches from neurotrophic to apoptotic activity in response to reductions in TrkA receptor levels. Int J Mol Sci. 2017;18:599. doi: 10.3390/ijms18030599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobsen HH, Ringstad G, Jørstad ØK, Moe MC, Sandell T, Eide PK. The human visual pathway communicates directly with the subarachnoid space. Invest Ophthalmol Vis Sci. 2019;60:2773–2780. doi: 10.1167/iovs.19-26997. [DOI] [PubMed] [Google Scholar]
- 75.Klesse LJ, Parada LF. Trks: signal transduction and intracellular pathways. Microsc Res Tech. 1999;45:210–216. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<210::AID-JEMT4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 76.Koevary SB. Pharmacokinetics of topical ocular drug delivery: potential uses for the treatment of diseases of the posterior segment and beyond. Curr Drug Metab. 2003;4:213–222. doi: 10.2174/1389200033489488. [DOI] [PubMed] [Google Scholar]
- 77.Koevary SB, Lam V, Patsiopoulos G, Lake S. Accumulation of porcine insulin in the rat brain and cerebrospinal fluid following ocular application. J Ocul Pharmacol Ther. 2003;19:377–384. doi: 10.1089/108076803322279435. [DOI] [PubMed] [Google Scholar]
- 78.Kojima T, Dogru M, Kawashima M, Nakamura S, Tsubota K. Advances in the diagnosis and treatment of dry eye. Prog Retin Eye Res. 2020 doi: 10.1016/j.preteyeres.2020.100842. doi: 101016/jpreteyeres2020100842. [DOI] [PubMed] [Google Scholar]
- 79.Kromer LF. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987;235:214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- 80.Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in the infant. Clin Perinatol. 2015;42:29–45. doi: 10.1016/j.clp.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lambiase A, Aloe L, Centofanti M, Parisi V, Mantelli F, Colafrancesco V, Manni GL, Massimo Gilberto Bucci MG, Bonini S, Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc Natl Acad Sci U S A. 2009a;106:13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lambiase A, Coassin M, Sposato V, Micera A, Sacchetti M, Bonini S, Aloe L. NGF topical application in patients with corneal ulcer does not generate circulating NGF antibodies. Pharmacol Res. 2007a;56:65–69. doi: 10.1016/j.phrs.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Lambiase A, Coassin M, Tirassa P, Mantelli F, Aloe L. Nerve growth factor eye drops improve visual acuity and electrofunctional activity in age-related macular degeneration: a case report. Ann Ist Super Sanità. 2009b;45:439–442. doi: 10.1590/s0021-25712009000400014. [DOI] [PubMed] [Google Scholar]
- 84.Lambiase A, Pagani L, Di Fausto V, Sposato V, Coassin M, Bonini S, Aloe L. Nerve growth factor eye drop administrated on the ocular surface of rodents affects the nucleus basalis and septum: biochemical and structural evidence. Brain Res. 2007b;1127:45–51. doi: 10.1016/j.brainres.2006.09.102. [DOI] [PubMed] [Google Scholar]
- 85.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 86.Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol. 2012;23:296–302. doi: 10.1097/ICU.0b013e3283543b61. [DOI] [PubMed] [Google Scholar]
- 87.Lambiase A, Tirassa P, Micera A, Aloe L, Bonini S. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Invest Ophthalmol Vis Sci. 2005;46:3800–3806. doi: 10.1167/iovs.05-0301. [DOI] [PubMed] [Google Scholar]
- 88.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 89.Lee TH, Kato H, Chen ST, Kogure K, Itoyama Y. Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke. 1998;29:1687–1697. doi: 10.1161/01.str.29.8.1687. [DOI] [PubMed] [Google Scholar]
- 90.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 91.Levkovitch-Verbin H, Quigley HA, Martin KRG, Zack DJ, Pease ME, Valenta DF. A model to study differences between primary and secondary degeneration of retinal ganglion cells in rats by partial optic nerve transection. Invest Ophthalmol Vis Sci. 2003;44:3388–3393. doi: 10.1167/iovs.02-0646. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Li F, Ling L, Li C, Zhong Y. Intranasal administration of nerve growth factor promotes angiogenesis via activation of PI3K/Akt signaling following cerebral infarction in rats. Am J Transl Res. 2018;10:3481–3492. [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X. Effects of intranasal nerve growth factor for traumatic brain injury - ClinicalTrials.gov NCT01212679. 2017. [Accessed April 28, 2020]. Available at: https://clinicaltrials.gov/ct2/show/NCT01212679 .
- 94.Liu X. Effects of intranasal nerve growth factor for acute ischemic stroke - - ClinicalTrials.gov NCT03686163. 2020. [Accessed April 25, 2020]. Available at: https://clinicaltrials.gov/ct2/show/NCT03686163 .
- 95.Ma X, Liu P, Zhang X, Jiang W, Jia M, Wang C, Dong Y, Dang Y, Gao C. Intranasal delivery of recombinant AAV containing BDNF fused with HA2TAT: a potential promising therapy strategy for major depressive disorder. Sci Rep. 2016;6:22404. doi: 10.1038/srep22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mandel RJ. CERE-110, an adeno-associated virus-based gene delivery vector expressing human nerve growth factor for the treatment of Alzheimer’s disease. Curr Opin Mol Ther. 2010;12:240–247. [PubMed] [Google Scholar]
- 97.Manni L, Rocco ML, Bianchi P, Soligo M, Guaragna M, Barbaro SP, Aloe L. Nerve growth factor: basic studies and possible therapeutic applications. Growth Factors. 2013;31:115–122. doi: 10.3109/08977194.2013.804073. [DOI] [PubMed] [Google Scholar]
- 98.Masoudi R, Ioannou MS, Coughlin MD, Pagadala P, Neet KE, Clewes O, Allen SJ, Dawbarn D, Fahnestock M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J Biol Chem. 2009;284:18424–18433. doi: 10.1074/jbc.M109.007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232:717–724. doi: 10.1002/jcp.25623. [DOI] [PubMed] [Google Scholar]
- 100.Mathieu E, Gupta N, Ahari A, Zhou X, Hanna J, Yücel YH. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Invest Ophthalmol Vis Sci. 2017;58:4784–4791. doi: 10.1167/iovs.17-22290. [DOI] [PubMed] [Google Scholar]
- 101.Mattson MP, Scheff SW. Endogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapy. J Neurotrauma. 1994;11:3–33. doi: 10.1089/neu.1994.11.3. [DOI] [PubMed] [Google Scholar]
- 102.Maurice DM. Drug delivery to the posterior segment from drops. Surv Ophthalmol. 2002;47:S41–52. doi: 10.1016/s0039-6257(02)00326-0. [DOI] [PubMed] [Google Scholar]
- 103.McArthur JC, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, Marra C, Rubin M, Cohen BA, Tucker T, Navia BA, Schifitto G, Katzenstein D, Rask C, Zaborski L, Smith ME, Shriver S, Millar L, Clifford DB, Karalnik IJ. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology. 2000;54:1080–1088. doi: 10.1212/wnl.54.5.1080. [DOI] [PubMed] [Google Scholar]
- 104.Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menon DK, Bryant C. Time for change in acquired brain injury. Lancet Neurol. 2019;18:28. doi: 10.1016/S1474-4422(18)30463-0. [DOI] [PubMed] [Google Scholar]
- 106.Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Minnone G, De Benedetti F, Bracci-Laudiero L. NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci. 2017a;18:1028. doi: 10.3390/ijms18051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Minnone G, Soligo M, Caiello I, Prencipe G, Manni L, Marafon DP, Magni-Manzoni S, Manzo A, Benedetti F, Bracci-Laudiero L. ProNGF-p75NTR axis plays a proinflammatory role in inflamed joints: a novel pathogenic mechanism in chronic arthritis. RMD Open. 2017b;3:e000441. doi: 10.1136/rmdopen-2017-000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitra S, Behbahani H, Eriksdotter M. Innovative therapy for Alzheimer’s disease-with focus on biodelivery of NGF. Front Neurosci. 2019;13:38. doi: 10.3389/fnins.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mufson EJ, Counts SE, Ginsberg SD, Mahady L, Perez SE, Massa SM, Longo FM, Ikonomovic MD. Nerve growth factor pathobiology during the progression of Alzheimer’s disease. Front Neurosci. 2019;13:533. doi: 10.3389/fnins.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mufson EJ, Ikonomovic MD, Styren SD, Counts SE, Wuu J, Leurgans S, Bennett DA, Cochran EJ, DeKosky ST. Preservation of brain nerve growth factor in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2003;60:1143–1148. doi: 10.1001/archneur.60.8.1143. [DOI] [PubMed] [Google Scholar]
- 112.Mufson EJ, Lavine N, Jaffar S, Kordower JH, Quirion R, Saragovi HU. Reduction in p140-TrkA receptor protein within the nucleus basalis and cortex in Alzheimer’s disease. Exp Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- 113.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 114.O’Neil E, Henderson M, Massaro-Giordano M, Bunya V. Advances in dry eye disease treatment. Curr Opin Ophthalmol. 2019;30:166–178. doi: 10.1097/ICU.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oderfeld-Nowak B, Zaremba M, Kwiatkowska-Patzer B, Triaca V, Aloe L. High-affinity NGF receptor in the rat spinal cord during acute and chronic phases of experimental autoimmune encephalomyelitis: a possible functional significance. Arch Ital Biol. 2003;141:103–116. [PubMed] [Google Scholar]
- 116.Olney NT, Spina S, Miller BL. Frontotemporal dementia. Neurol Clin. 2017;35:339–374. doi: 10.1016/j.ncl.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olson L, Backlund EO, Ebendal T, Freedman R, Hamberger B, Hansson P, Hoffer B, Lindblom U, Meyerson B, Strömberg I, Sydow O, Seiger Aå. Intraputaminal infusion of nerve growth factor to support adrenal medullary autografts in Parkinson’s disease: one-year follow-up of first clinical trial. Arch Neurol. 1991;48:373–381. doi: 10.1001/archneur.1991.00530160037011. [DOI] [PubMed] [Google Scholar]
- 118.Olson L, Nordberg A, von Holst H, Bäckman L, Ebendal T, Alafuzoff I, Amberla K, Hartvig P, Herlitz A, Lilja A, Lundqvist H, Långström B, Meyerson B, Persson A, Viitanen M, Winblad B, Seiger Å. Nerve growth factor affects11C-nicotine binding, blood flow, EEG, and verbal episodic memory in an Alzheimer patient (Case Report) J Neural Transm Gen Sect. 1992;4:79–95. doi: 10.1007/BF02257624. [DOI] [PubMed] [Google Scholar]
- 119.Park JA, Lee JY, Sato TA, Koh JY. Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat. J Neurosci. 2000;20:9096–9103. doi: 10.1523/JNEUROSCI.20-24-09096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parvaneh Tafreshi A. Nerve growth factor prevents demyelination, cell death and progression of the disease in experimental allergic encephalomyelitis. Iran J Allergy Asthma Immunol. 2006;5:177–181. [PubMed] [Google Scholar]
- 121.Petty BG, Cornblath DR, Adornato BT, Chaudhry V, Flexner C, Wachsman M, Sinicropi D, Burton LE, Peroutka SJ. The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol. 1994;36:244–246. doi: 10.1002/ana.410360221. [DOI] [PubMed] [Google Scholar]
- 122.Pflugfelder SC, Massaro-Giordano M, Perez VL, Hamrah P, Deng SX, Espandar L, Foster CS, Affeldt J, Seedor JA, Afshari NA, Chao W, Allegretti M, Mantelli F, Dana R. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127:14–26. doi: 10.1016/j.ophtha.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 123.Pollock K, Dahlenburg H, Nelson H, Fink KD, Cary W, Hendrix K, Annett G, Torrest A, Deng P, Gutierrez J, Nacey C, Pepper K, Kalomoiris S, D Anderson J, McGee J, Gruenloh W, Fury B, Bauer G, Duffy A, Tempkin T, et al. Human mesenchymal stem cells genetically engineered to overexpress brain-derived neurotrophic factor improve outcomes in Huntington’s disease mouse models. Mol Ther. 2016;24:965–977. doi: 10.1038/mt.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Popescu C, Anghelescu A, Daia C, Onose G. Actual data on epidemiological evolution and prevention endeavours regarding traumatic brain injury. J Med Life. 2015;8:272–277. [PMC free article] [PubMed] [Google Scholar]
- 125.Prencipe G, Minnone G, Strippoli R, De Pasquale L, Petrini S, Caiello I, Manni L, De Benedetti F, Bracci-Laudiero L. Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J Immunol. 2014;192:3345–3354. doi: 10.4049/jimmunol.1300825. [DOI] [PubMed] [Google Scholar]
- 126.Rafii MS, Tuszynski MH, Thomas RG, Barba D, Brewer JB, Rissman RA, Siffert J, Aisen PS. Adeno-associated viral vector (serotype 2)–nerve growth factor for patients with Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2018;75:834–841. doi: 10.1001/jamaneurol.2018.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Regner A, Meirelles L da S, Simon D. Traumatic penumbra: opportunities for neuroprotective and neurorestorative processes. In: Gorbunov NV, Long JB, editors. Traumatic brain injury - pathobiology, advanced diagnostics and acute management. London: IntechOpen; 2017. pp. 49–83. [Google Scholar]
- 128.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roberti G, Mantelli F, Macchi I, Massaro-Giordano M, Centofanti M. Nerve growth factor modulation of retinal ganglion cell physiology. J Cell Physiol. 2014;229:1130–1133. doi: 10.1002/jcp.24573. [DOI] [PubMed] [Google Scholar]
- 130.Rocco ML, Soligo M, Manni L, Aloe L. Nerve growth factor: early studies and recent clinical trials. Curr Neuropharmacol. 2018;16:1455–1465. doi: 10.2174/1570159X16666180412092859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sacchetti M, Lambiase A. Neurotrophic factors and corneal nerve regeneration. Neural Regen Res. 2017;12:1220–1224. doi: 10.4103/1673-5374.213534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sacchetti M, Lambiase A, Schmidl D, Schmetterer L, Ferrari M, Mantelli F, Allegretti M, Garhoefer G. Effect of recombinant human nerve growth factor eye drops in patients with dry eye: a phase IIa, open label, multiple-dose study. Br J Ophthalmol. 2020;104:127–135. doi: 10.1136/bjophthalmol-2018-312470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schifitto G, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, Marra CM, Rubin M, Cohen BA, Tucker T, Koralnik IJ, Katzenstein D, Haidich B, Smith ME, Shriver S, Millar L, Clifford DB, McArthur JC AIDS Clinical Trials Group Team 291. Long-term treatment with recombinant nerve growth factor for HIV-associated sensory neuropathy. Neurology. 2001;57:1313–1316. doi: 10.1212/wnl.57.7.1313. [DOI] [PubMed] [Google Scholar]
- 135.Schor NF. The p75 neurotrophin receptor in human development and disease. Prog Neurobiol. 2005;77:201–214. doi: 10.1016/j.pneurobio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 136.Semeraro F, Semeraro F, Forbice E, Romano V, Angi M, Romano MR, Filippelli ME, Iorio RD, Costagliola C. Neurotrophic keratitis. OPH. 2014;231:191–197. doi: 10.1159/000354380. [DOI] [PubMed] [Google Scholar]
- 137.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 139.Sivasathiaseelan H, Marshall CR, Agustus JL, Benhamou E, Bond RL, van Leeuwen JEP, Hardy CJD, Rohrer JD, Warren JD. Frontotemporal dementia: a clinical review. Semin Neurol. 2019;39:251–263. doi: 10.1055/s-0039-1683379. [DOI] [PubMed] [Google Scholar]
- 140.Skaper SD. The Neurotrophin Family of Neurotrophic Factors: An Overview. In: Skaper SD, editor. Neurotrophic Factors. Totowa, NJ: Humana Press; 2012. pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 141.Skaper SD. Nerve growth factor: a neuroimmune crosstalk mediator for all seasons. Immunology. 2017;151:1–15. doi: 10.1111/imm.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20:148. doi: 10.1186/s13054-016-1318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Su H. Inflammation and genetic factors in stroke pathogenesis. Neuroimmunol Neuroinflamm. 2017;4:260–262. doi: 10.20517/2347-8659.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci. 2002;5:1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 145.Thorne RG, Frey WH. Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 146.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 147.Tian L, Guo R, Yue X, Lv Q, Ye X, Wang Z, Chen Z, Wu B, Xu G, Liu X. Intranasal administration of nerve growth factor ameliorate β-amyloid deposition after traumatic brain injury in rats. Brain Res. 2012;1440:47–55. doi: 10.1016/j.brainres.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 148.Tong X, Dong J, Shang Y, Inthavong K, Tu J. Effects of nasal drug delivery device and its orientation on sprayed particle deposition in a realistic human nasal cavity. Comput Biol Med. 2016;1:40–48. doi: 10.1016/j.compbiomed.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 149.Triaca V, Fico E, Sposato V, Caioli S, Ciotti MT, Zona C, Mercanti D, La Mendola D, Satriano C, Rizzarelli E, Tirassa P, Calissano P. hNGF peptides elicit the NGF-TrkA signalling pathway in cholinergic neurons and retain full neurotrophic activity in the DRG assay. Biomolecules. 2020;10:216. doi: 10.3390/biom10020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Truelsen T, Begg S, Mathers C. The global burden of cerebrovascular disease. WHO, Geneva, Switzerland: 2006. [Google Scholar]
- 151.Turrini P, Gaetano C, Antonelli A, Capogrossi MC, Aloe L. Nerve growth factor induces angiogenic activity in a mouse model of hindlimb ischemia. Neurosci Lett. 2002;323:109–112. doi: 10.1016/s0304-3940(02)00090-3. [DOI] [PubMed] [Google Scholar]
- 152.Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 153.Valdo P, Stegagno C, Mazzucco S, Zuliani E, Zanusso G, Moretto G, Raine CS, Sonetti B. Enhanced expression of NGF receptors in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2002;61:91–8. doi: 10.1093/jnen/61.1.91. [DOI] [PubMed] [Google Scholar]
- 154.Wahlberg LU, Lind G, Almqvist PM, Kusk P, Tornøe J, Juliusson B, Söderman M, Selldén E, Seiger Å, Eriksdotter-Jönhagen M, Linderoth B. Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: a technology platform for restorative neurosurgery. J Neurosurg. 2012;117:340–347. doi: 10.3171/2012.2.JNS11714. [DOI] [PubMed] [Google Scholar]
- 155.Wang H, Wang R, Thrimawithana T, Little PJ, Xu J, Feng ZP, Zheng W. The nerve growth factor signaling and its potential as therapeutic target for glaucoma. Biomed Res Int. 2014;2014:759473. doi: 10.1155/2014/759473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.WHO report. Neurological disorders: public health challenges. Switzerland: 2006. [Google Scholar]
- 157.Williams BJ, Eriksdotter-Jonhagen M, Granholm AC. Nerve growth factor in treatment and pathogenesis of Alzheimer’s disease. Prog Neurobiol. 2006;80:114–128. doi: 10.1016/j.pneurobio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 158.Windisch M. Study of LM11A-31-BHS in Mild-moderate AD Patients. ClinicalTrialsgov NCT03069014. 2020. Available at: https://clinicaltrialsgov/ct2/show/NCT03069014 Accessed April 26, 2020 .
- 159.Wostyn P, Groot VD, Dam DV, Audenaert K, Deyn PPD, Killer HE. The glymphatic system: a new player in ocular diseases. Invest Ophthalmol Vis Sci. 2016;57:5426–5427. doi: 10.1167/iovs.16-20262. [DOI] [PubMed] [Google Scholar]
- 160.Wostyn P, Groot VD, Dam DV, Audenaert K, Deyn PPD, Killer HE. The first histologic evidence of a paravascular pathway within the optic nerve. Invest Ophthalmol Vis Sci. 2018;59:1717–1717. doi: 10.1167/iovs.17-23119. [DOI] [PubMed] [Google Scholar]
- 161.Young J, Pionk T, Hiatt I, Geeck K, Smith JS. Environmental enrichment aides in functional recovery following unilateral controlled cortical impact of the forelimb sensorimotor area however intranasal administration of nerve growth factor does not. Brain Res Bull. 2015;115:17–22. doi: 10.1016/j.brainresbull.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 162.Zhao G, Li D, Ding X, Li L. Nerve growth factor pretreatment inhibits lidocaine induced myelin damage via increasing BDNF expression and inhibiting p38 mitogen activation in the rat spinal cord. Mol Med Rep. 2017;16:4678–4684. doi: 10.3892/mmr.2017.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhu W, Cheng S, Xu G, Ma M, Zhou Z, Liu D, Liu X. Intranasal nerve growth factor enhances striatal neurogenesis in adult rats with focal cerebral ischemia. Drug Delivery. 2011;18:338–343. doi: 10.3109/10717544.2011.557785. [DOI] [PubMed] [Google Scholar]


