Axonal regeneration after injuries to the nervous system has been extensively studied due to its implication in motor and sensory functional recovery. Distinct types of regeneration has been identified, such as canonical axonal regeneration, defined as the growth of axons from the transected axonal stump to reinnervate the original target, or regenerative sprouting, in which the growth occurs from a region of the damaged axon either close or far from the injury site (Tuszynski and Steward, 2012). Both types of axonal regeneration depend on cellular and molecular responses of the injured neuron. However, undamaged neurons can also react to an injury-induced environment by extending sprouts along their axons that functionally synapse with denervated targets, a process known as collateral sprouting (Collyer et al., 2014; Bao et al., 2016).
In the peripheral nervous system (PNS), efficient regenerative processes and target reinnervation after damage is associated with two major events, rapid Wallerian degeneration of the distal segment and the activation of a regeneration-associated genetic program in damaged neurons, increasing their intrinsic growth capacity (Navarro et al., 2007; Chandran et al., 2016). In the case of collateral sprouting of undamaged neurons, axonal growth is triggered after exposure to the Wallerian degeneration environment (Collyer et al., 2014; Bao et al., 2016). In the PNS, this active degeneration program provides a growth-permissive environment free of inhibitory proteins and rich in neurotrophic factors, mainly mediated by dedifferentiation of mature Schwann cells associated to injured axons (Navarro et al., 2007). In clinical practice, ligation of the degenerated stump of an injured nerve to the lateral face of an intact nerve, also known as end-to-side anastomosis, triggers collateral axons extension from undamaged axons leading to functional reinnervation (Bao et al., 2016). Therefore, intact neurons have the ability to locally react to external cues and initiate an active growth process. Nevertheless, there are other aspects of the collateral sprouting mechanism that have not been fully understood, for example, if this type of growth is associated to a specific sprouting transcriptional program as in canonical regenerative processes of the PNS.
To date, evidence for a transcriptional program associated to collateral sprouting from undamaged neurons has been exclusively explored in the central nervous system (CNS). After stroke, undamaged cortical neurons extending collateral sprouting, activate a transcriptome different from the observed in sprouting developing neurons (Li et al., 2015), suggesting that external signals produced or activated by the injury, are able to initiate a growth state in undamaged neurons by triggering a unique transcriptomic program.
In order to investigate the collateral sprouting process in the PNS, we have recently generated a mouse model of partial sciatic nerve injury to study the cellular and molecular changes of undamaged sprouting neurons exposed to a Wallerian degeneration environment. The sciatic nerve in mice is formed by the anastomosis of L3 and L4 spinal nerves (SN), with a small contribution of L5. This anatomical characteristic allows a clean transection of L3 SN allowing the degeneration of its axons distal to the injured site, leaving L4 and L5 SN uninjured (Figure 1; Lemaitre et al., 2020). Thereby, L4 and L5 SN-derived axons in the sciatic nerve remain in close contact with a degenerated environment. One of the advantages of this model is that permits the isolation and analysis of injured and intact somas, grouped in L3 and L4 dorsal root ganglia (DRG), respectively. In this model, 7 days after L3 SN injury, a few newly axonal sprouts were found in degenerated regions of the sciatic nerve, increasing progressively in density after 14 and 21 days post-injury (Figure 1). Using a double injury model and anterograde fluorescent tracing we showed that collateral sprouts that invaded degenerated areas were originated from L4 intact SN axons (Lemaitre et al., 2020). This agrees with earlier studies showing that collateral sprouts are attracted to the Wallerian degeneration-induced environment, probably by diffusible factors released from repair Schwann cells (Cobianchi et al., 2014; Bao et al., 2016). Moreover, we demonstrated that intact sensory neurons conditioned in vivo by the Wallerian degeneration milieu, increased their intrinsic growth capacity in vitro (Lemaitre et al., 2020). This experimental approach resembles the conditioning lesion paradigm, in which a peripheral nerve lesion increases the ability of sensory neurons to growth in vitro through the activation of regeneration-associated genes (RAGs) (Fagoe et al., 2015). Expectedly, in Wallerian degeneration-conditioned neurons this growth enhancement was more associated with an increase in neurite branching than neurite elongation, while in sensory neurons conditioned by a direct peripheral nerve injury, significant changes were found in both parameters (Lemaitre et al., 2020). These results suggest that the growth response of intact neurons triggered by Wallerian degeneration depends on transcriptional changes associated to collateral sprouting. Moreover, it was previously described that the processes of axonal branching from the main axonal shaft, characteristic of collateral sprouting, can be mechanistically dissociated from the elongation of the main axon, as observed in canonical axonal regeneration (Gallo, 2011).
Figure 1.
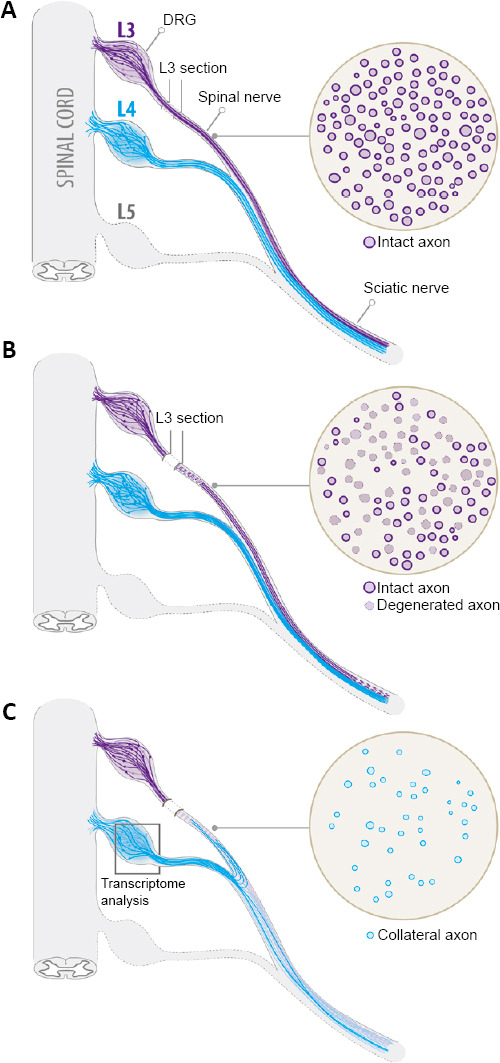
Schematic sequence of the model of partial sciatic nerve injury to study collateral sprouting of sensory neurons.
L3 and L4 DRG and spinal nerves and the sciatic nerve trunk are shown. Before L3 spinal nerve transection, intact L3 spinal nerve axons look rounded and some of them myelinated (A). Few days after injury, damaged L3 spinal nerve axons initiate the Wallerian degeneration program and axons lose their normal structure (B). Fourteen days after transection, collateral sprouts of intact L4 spinal nerve are found in L3 spinal nerve degenerated areas (C). To study the molecular mechanisms of collateral sprouting, after L3 spinal nerve injury, the L4 DRG can easily be removed and used for transcriptomic analysis (C). DRG: Dorsal root ganglion.
To test whether collateral sprouting depends on the activation of unique transcriptional programs, we use the model of partial sciatic nerve injury to study gene expression of intact L4 SN sensory neurons with axons in close contact with the Wallerian degeneration environment and compare it with the regeneration-associated transcriptome of L4 SN sensory neurons triggered by a direct crush injury. Indeed, Wallerian degeneration activates a distinctive transcriptome profile in uninjured neurons, when compared with the injury-induced transcriptome associated with axonal regeneration (Lemaitre et al., 2020). Interestingly, the collateral sprouting transcriptome was associated mainly to the upregulation of collateral sprouting-associated genes (SAGs), while the RAGs included a balanced number of up- and downregulated transcripts. In addition, fold changes in SAGs were order of magnitudes smaller than RAGs (Lemaitre et al., 2020). It is expected that canonical axon regeneration requires the activation of additional genes and pathways in order to regenerate and elongate a whole damaged axon. Also, injured neurons are reacting to direct injury signals that mediate the activation of additional signaling pathways related with cell death, excitability and inflammation (Chandran et al., 2016). A disadvantage of our study is that for molecular analysis of sprouting neurons the entire DRG was examined, including other resident cells such as satellite glial cells, which could also contribute to the dilution of the collateral sprouting-associated changes.
Using deeper bioinformatic analysis we showed that the collateral sprouting transcriptome involved the activation of specific networks, including SAGs previously described in CNS models. After stroke, peri-infarct cortical neurons sprouts allowing reorganization of neuronal connections and functional recovery (Li et al., 2015). It was shown that collateral sprouting and recovery in this model depends on the activation of a unique transcriptome in peri-infarct neurons, induced by the increase expression of the growth and differentiation factor 10 (Gdf10) gene (Li et al., 2015). We found that the collateral sprouting transcriptome in the PNS include upregulation of Gdf10 and downstream transcription factors Smad2/3 in a highly activated network, along with other novel genes such as the rho guanine nucleotide exchange factor 5 (Arhgef5) and the smooth muscle alpha-2 actin (Lemaitre et al., 2020). Moreover, forced overexpression of these sprouting-associated genes in sensory neurons in vitro increased their intrinsic branching capacity in a higher magnitude than classic RAGs, such as activating transcription factor 3, and induced equivalent lineal neurites outgrowth (Fagoe et al., 2015; Lemaitre et al., 2020), evidencing a unique and novel mechanism for axonal growth and branching.
Then, we recently showed that the collateral sprouting mechanism is associated to the regulation of sprouting associated genes in uninjured axons, triggered by exposure to the Wallerian degeneration milieu. However, it still remains unknown how collateral sprouts overcome the inhibitory barrier of surrounded Schwann cells and myelin proteins, which normally are restricting axonal growth in order to maintain axonal function (Bustos et al., 1991; Navarro et al., 2007). Inhibition of RNA synthesis in rat nerves by local administration of Actinomycin D, triggers axonal sprouting and an increase in axonal microtubules (Bustos et al., 1991). It is proposed that in normal conditions, mature Schwann cells are repressing the intrinsic growth capacity of axons and that unleashing this inhibition by RNA synthesis blockage allows the axon to growth. In the case of Wallerian degeneration, the trans-differentiation of Schwann cells into a reparative phenotype will unleash their growth inhibition over axons. In our model, we showed that after a partial sciatic nerve injury Schwann cells associated with undamaged axons of the peri-degenerated area remained morphologically intact (Lemaitre et al., 2020). So, how spared axons bypass this inhibitory barrier and initiate a process of collateral sprouting remains unclear. One possibility is that the regulation of SAGs in uninjured axons, such as Gdf10 or Arhgef5, mediates cellular changes that increase their growth ability in order to overcome the inhibitory cues imposed by Schwann cells. Interestingly, Bustos et al. (1991) reported that the sprouting response was restricted to the nerve region treated with Actinomycin D, suggesting a local mechanism of axonal sprouting. Considering this result, is unlikely that the collateral sprouting mechanism depends only on the activation of a growth transcriptional profile in axons, but also on local changes associated with other resident cells in the nerve, such as Schwann cells. In a model of end-to-side anastomosis, it was demonstrated that collateral axons sprouted from intact myelinated axons at the nodes of Ranvier in nerve regions that were in contact with the degenerated environment, but did not reported evidence of collateral sprouting far from the site of anastomosis (Zhu et al., 2008). In our study, we identify novel genes associated with collateral sprouting and showed the effect of their forced upregulation in the growth capacity of sensory cells cultured alone. However, analysis of the role of these genes in vivo and further molecular analysis of intact Schwann cells exposed to Wallerian degeneration environment are necessary to fully understand the collateral sprouting mechanism.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Wang L; T-Editor: Jia Y
References
- 1.Bao Q, Xiao C, Wang T, Gu Y. Novel atraumatic end-to-side repair model exhibits robust collateral sprouting independent of donor fiber injury. Plast Reconstr Surg. 2016;137:523–533. doi: 10.1097/01.prs.0000475764.76278.eb. [DOI] [PubMed] [Google Scholar]
- 2.Bustos J, Vial JD, Faúndez V, Alvarez J. Axons sprout and microtubules increase after local inhibition of rna synthesis, and microtubules decrease after inhibition of protein synthesis: a morphometric study of rat sural nerves. Eur J Neurosci. 1991;3:1123–1133. doi: 10.1111/j.1460-9568.1991.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 3.Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M, Yekkirala A, Barrett L, Blesch A, Michaelevski I, Davis-Turak J, Gao F, Langfelder P, Horvath S, He Z, Benowitz L, Fainzilber M, Tuszynski M, et al. A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron. 2016;89:956–970. doi: 10.1016/j.neuron.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobianchi S, de Cruz J, Navarro X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp Neurol. 2014;255:1–11. doi: 10.1016/j.expneurol.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Collyer E, Catenaccio A, Lemaitre D, Diaz P, Valenzuela V, Bronfman F, Court FA. Sprouting of axonal collaterals after spinal cord injury is prevented by delayed axonal degeneration. Exp Neurol. 2014;261:451–461. doi: 10.1016/j.expneurol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Fagoe ND, Attwell CL, Kouwenhoven D, Verhaagen J, Mason MR. Overexpression of ATF3 or the combination of ATF3, c-Jun, STAT3 and Smad1 promotes regeneration of the central axon branch of sensory neurons but without synergistic effects. Hum Mol Genet. 2015;24:6788–6800. doi: 10.1093/hmg/ddv383. [DOI] [PubMed] [Google Scholar]
- 7.Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol. 2011;71:201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- 8.Lemaitre D, Hurtado ML, De Gregorio C, Oñate M, Martínez G, Catenaccio A, Wishart TM, Court FA. Collateral sprouting of peripheral sensory neurons exhibits a unique transcriptomic profile. Mol Neurobiol. 2020;57:4232–4249. doi: 10.1007/s12035-020-01986-3. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, Bahjat FR, Stenzel-Poore MP, Kawaguchi R, Coppola G, Carmichael ST. GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci. 2015;18:1737–1745. doi: 10.1038/nn.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu QT, Zhu JK, Chen GY. Location of collateral sprouting of donor nerve following end-to-side neurorrhaphy. Muscle Nerve. 2008;38:1506–1509. doi: 10.1002/mus.21116. [DOI] [PubMed] [Google Scholar]


