Keywords: chemokines, cytokines, fibroblast growth factor, gene therapy, growth factors, peripheral nerve system, sciatic nerve regeneration, vascular endothelial growth factor
Abstract
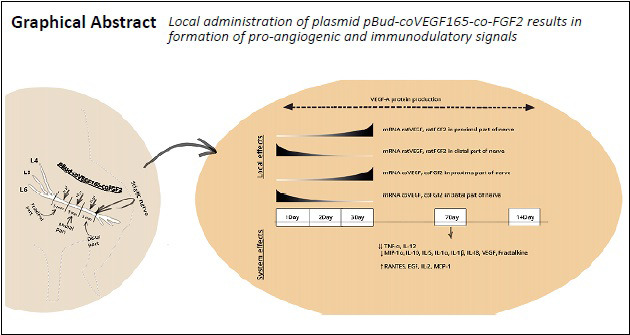
Vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF2) are well-known growth factors involved in the regeneration of various tissues and organs, including peripheral nerve system. In the present study, we elucidated the local and systemic effects of plasmid construct рBud-coVEGF165-coFGF2 injected into the epineurium of intact rat sciatic nerve. Results of histological examination of sciatic nerve and multiplex immunoassays of serum showed the absence of immunogenicity and biosafety of plasmid рBud-coVEGF165-coFGF2. Moreover, local administration of plasmid DNA construct resulted in significantly decreased levels of pro-inflammatory cytokines in the peripheral blood, including tumor necrosis factor α (TNFα) and interleukin-12, and significantly increased levels of cytokines and chemokines including Regulated upon Activation, Normal T Cell Expressed and Presumably Secrete (RANTES), epidermal growth factor, interleukin-2, and monocyte chemoattractant protein 1. These changes in the peripheral blood on day 7 after injection of plasmid construct рBud-coVEGF165-coFGF2 show that the plasmid construct has systemic effects and may modulate immune response. At the same time, reverse transcription-polymerase chain reaction revealed transient expression of coFGF2, coVEGF165, ratFGF2 and ratVEGFA with direct transport of transcripts from distal part to proximal part of the sciatic nerve. Immunohistochemical staining revealed prolonged presence of VEGFA in sciatic nerve till 14 days post-injection. These findings suggest that local administration of plasmid construct рBud-coVEGF165-coFGF2 at a concentration of 30 ng/µL results in the formation of pro-angiogenic stimuli and, and the plasmid construct, used as a drug for gene therapy, might potentially facilitate regeneration of the sciatic nerve. The study was approved by the Animal Ethics Committee of Kazan Federal University, procedures were approved by the Local Ethics Committee (approval No. 5) on May 27, 2014.
Chinese Library Classification No. R459.9; R364; R741
Introduction
Injuries of the peripheral nervous system (PNS) are followed by activation of numerous relative processes in neurons and their axons, Schwann cells (SCs), fibroblasts, vascular endothelial cells, and immune cells. After traumatic injuries of axon, neuron soma produces and transfers to periphrey all necessary factors required for regeneration and transfers to periphery. Therefore, after trauma, the proximal part of nerve is considered to contain more bioactive molecules involved in tissue regeneration than the distal part (Wang et al., 2017; Masgutov et al., 2019). The proximal nerve undergoes Wallerian degeneration (Bastien and Lacroix, 2014), which involves destruction of axons and their myelin sheaths, dedifferentiation of SCs and activation of immune cells (Gaudet et al., 2011; Conforti et al., 2014; Cattin et al., 2015; Masgutova et al., 2017). Dedifferentiation of SCs is accompanied by changes in gene expression, secretion of various growth factors (GFs), compounds of extracellular matrix, cytokines and chemokines (Lee et al., 2009; Chen et al., 2014; Masgutova et al., 2017). In addition to promoting the growth of new axons and their remyelination, factors secreted by SCs are essential for immune cells recruitment and induction of angiogenesis (Wang et al., 2017; Stratton et al., 2018). Immune cells, which are recruited to the site of injury of the PNS, include neutrophils, macrophages, and Т-cells (Selders et al., 2017). Neutrophils are observed in tissue within 6–12 hours post-injury while macrophages are recruited on days 2-3 post-injury with the peak on day 7 post-injury (Gaudet et al., 2011; Bastien and Lacroix, 2014; Van Steenwinckel et al., 2015). Macrophages represent the most numerous population among the activated immune cells (Gaudet et al., 2011; Liu et al., 2019).
SCs, in turn, produce a broad range of compounds, which are essential for recruitment of macrophages. Macrophages also secrete necessary substances for proliferation and dedifferentiation SCs, which are beneficial for axon regeneration, myelination, and angiogenesis (Gaudet et al., 2011; Liu et al., 2019). The synthesized substances include extracellular matrix proteins, proteases, growth factors, cytokines, and chemokines (Martini et al., 2008; Bastien and Lacroix, 2014). The most important growth factors secreted by macrophages include vascular endothelial growth factor-A and -B (VEGFA and VEGF-B), fibroblast growth factor 2 (FGF2), bone morphogenetic protein-2 (BMP2), and other angiogenic factors, which also contribute to the migration of SCs (Cattin et al., 2015; Stratton et al., 2018).
After nerve injury, in the traumatic region, degenerative mechanisms are activated: pro- and anti-inflammatory cytokines are released, resulting in fibroblast activation and collagen hyperplasia (Masgutova et al., 2017; Stratton et al., 2018). Progressive fibrosis and scars compromise nerve regeneration while the influence of microenvironment is a critical criterion for successful regeneration of an injured tissue. To ensure appropriate microenvironment, cells must maintain balanced ratio of nerve growth factors, pro-angiogenic factors, oxidative stress factors and pro-inflammatory cytokines and simultaneously avoid activation of fibroblasts by maintaining the balance of collagen synthesis and degradation (Wakatsuki et al., 2015; Fukui, 2016). Otherwise, intrinsic ability for repair is compromised. The use of external inducing stimuli thereby could serve as a promising therapeutic strategy (Hoyng et al., 2015).
Critical role of growth factors in peripheral nerve regeneration is demonstrated on wide-gamut models. Particularly it has been shown that gene therapy allows achieving the synthesis of biologically active molecules with modified cells of the organism itself. This strategy is based on delivery of therapeutic genes in target cells, which results in production of protein factors contributing to stimulation of regeneration in traumatic areas (Hoyng et al., 2015). The most investigated and frequently mentioned angiogenic factors include vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) produced by naïve endothelial cells and fibroblasts (Spanholtz et al., 2011; Shibuya, 2013; Dumpich and Theiss, 2015). These factors have been successfully applied for stimulation of post-traumatic regeneration of the central nervous system (CNS) and PNS (Muratori et al., 2018).
VEGF acts as a key regulator of angiogenesis and vasculogenesis; it is known to induce proliferation, activation and differentiation of endothelial cells and tube formation, resulting in further development of new vessels (Moccia et al., 2019). VEGF plays a key role in increasing the survival of endothelial cells, as it induces the expression of anti-apoptotic protein (Muratori et al., 2018). VEGF165 has been shown to enhancethe migration of SCs and promotes axon growth (Rosenstein et al., 2010).
FGF2 and its high affinity receptors are expressed in intact PNS and are released after nerve damage or chronic inflammation (Ucuzian et al., 2010). It has been shown that FGF2 contributes to neuron survival and stimulates axon growth both in vitro and in vivo (Woodbury and Ikezu, 2014). The participation of high molecular weight FGF2 isoforms in sensory recovery was demonstrated in a rat model of sciatic nerve injury, which indicates their significant role in peripheral nerve regeneration (Spanholtz et al., 2011). Thus, the delivery of genes of neurotrophic factors to the damaged area could be considered as one of the most promising approaches for stimulating neuroregeneration but this requires comprehensive study.
To date, codon optimization is a widely used method for modification of the nucleotide sequence to increase the efficacy of protein biosynthesis, as well as to change the immunogenicity of a protein product (Frelin et al., 2004). During codon optimization, the most frequently used codons are selected, and this allows synthesis of a functional product (Mauro and Chappell, 2014). In addition, there is the possibility of optimization based on preferred codons without changing the amino acid sequence of the corresponding proteins (Gao et al., 2015). In this work, to achieve sufficient expression of VEGF and FGF, two-cassette plasmid construct pBud-coVEGF165-coFGF2 was applied.
In this study, we suggest that intraneural administration of therapeutic recombinant constructions will promote a local stimulating effect. Taking into account results of our previous study (Masgutova et al., 2017) that revealed comprehension of cellular and molecular interactions between immune system and peripheral nerves, we also tested formation of systemic inflammatory response signaling cascades stimulating regeneration processes in the peripheral nerve.
Materials and Methods
Codon optimized recombinant constructs
In this work, we used the modified two-cassette plasmid construct pBud-coVEGF165-coFGF2 created based on the commercially available plasmid pBudCE4.1 (4595 bp, Cat# V532-20, Invitrogen, MA, USA) by subcloning cDNA of the vegf (isoform 165) and fgf2 genes under the control of the elongation factor-1α (EF-1α) and cytomegalovirus (CMV) promoters, respectively, the tag sequences V5-His and myc- were removed. Expression plasmid vector pBudCE 4.1 (Invitrogen, Waltham, MA, USA); compliation of codon-optimized human genes sequence have been described earlier (Salafutdinov et al., 2010; Solovyeva et al., 2020).
De novo synthesis of vegf165 and fgf2 cDNA nucleotide sequences and development of recombinant constructs were performed by GenScript (Piscataway, NJ, USA). The design allows simultaneous and independent codon expression of optimized sequences of human vegf165 and fgf2.
The concentration of the resulting plasmid DNA was determined spectrophotometrically using NanoDrop 2000 (Thermo Scientific, MA, USA). Purified DNA was dissolved in sterile PBS to a concentration of 30 ng/µL. Optimal plasmid concentration was determined based on our previous study (Masgutov et al., 2011) and publications of other groups (Fu et al., 2007; Pereira Lopes et al., 2013; Boldyreva Mcapital et al., 2018).
Animal experiments
Experiments were carried out using 25 4–6-month-old male Wistar rats weighing 200–300 g (Limited Liability Company “Nursery of the Russian Academy of Medical and Technical Sciences”, Moscow, Russia). All animals were acclimatized for 2 weeks before the start of the experiment. Animals were kept under standard vivarium conditions with the 12-hour day/night schedule, with free access to food and water. Animals were kept and used for experimental procedures in accordance with the rules accepted by the Animal Ethics Committee of Kazan Federal University, and procedures were approved by the Local Ethics Committee (approval No. 5, approved on May 27, 2014). These animals were also used in accordance with international bioethical standards defined by the international guiding principles for biomedical research involving animals issued by Council for International Organizations of Medical Sciences (CIOMS) (2012), the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes and the 3Rs principles (Replace, Reduce, Refine).
Rats were anesthetized by intraperitoneal administration of chloral hydrate (400 mg/kg in 6.4% NaCl) (AppliChem, Darmstadt, Germany), after anesthesia, they had undergone operation to the left and right sciatic nerves. Plasmid pBud-coVEGF165-coFGF2 containing the sequence of vegf165 and fgf2 genes was administered intraneurally into the epineurium of the left sciatic nerve via three points (10 μL/point, 30 ng/μL) in distal, medial and proximal segments, separately with a Hamilton’s syringe. Each sciatic nerve received an injection of 30 μL of solution. An amount of 30 μL of PBS (control) was intraneurally administered into the right sciatic nerve in the similar way. The experiment was conducted without disruption of the integrity of the axons.
Sciatic nerve sampling was performed on days 1, 2, 3, 7, and 14 after the injection of plasmid pBud-coVEGF165-coFGF2. For each study period (1, 2, 3, 7, and 14 days post-injection), five animals were used (n = 25). The left (experimental) and right (intact) sciatic nerves were transversely dissected into three fragments: distal (5 mm), medial (3 mm) and proximal (5 mm).
The distal and proximal samples were frozen in liquid nitrogen, then transferred to –80°C and stored for RNA isolation and real-time reverse transcription-polymerase chain reaction (RT-PCR). Medial sciatic nerve samples were transferred into a 10% buffered formalin solution for morphological analysis and immunohistochemical analysis with antibodies against VEGF-A and FGF2.
To assess the immune status in experimental animals compared with local administration of recombinant pBud-coVEGF165-coFGF2 constructs, multiplex immunoanalysis was performed. Plasmid construct was introduced into the left sciatic nerve of intact rats (n = 10), and PBS (n = 10) was similarly administered in the control group. Venous blood (0.5–0.7 mL) was collected by catheterization of the caudal vein 2 hours prior to the surgery and then 7 days after the introduction of the plasmid. After coagulation for 20 minutes, the blood was centrifuged at 1750 × g for 5 minutes. Blood serum was aseptically collected and frozen at –80°C until the time of the analysis.
RT-PCR
Isolation of total RNA was performed using TRI Reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the standard protocol of the manufacturer. For reverse transcription, 100 ng of RNA template, 100 pmol of random hexametric primer and deionized water were mixed in a 200 μL tube. The mixture was heated for 5 minutes at 65°C, after which it was sharply cooled. Next, 5× reaction buffer (Thermo Fisher Scientific, Waltham, MA, USA), 20 U RNase inhibitor, 100 U reverse transcriptase RevertAid Reverse Transcriptase (Thermo Scientific, MA, USA) were added to the mixture. The synthesis was carried out in the following mode: 2°С 10 minutes, 42°С 60 minutes, and transcription termination at 70°С 10 minutes. The obtained cDNA was used for qPCR.
Quantitative analysis of gene expression of 18S rRNA, fgf2, and vegf isoforms was performed by RT-PCR using CFX384 Real-Time PCR Detection system equipment (BioRad, Hercules, CA, USA).
For PCR reaction in a volume of 10 μL, we used: 1× reaction mixture qPCRmix-HS SYBR (KCl, Tris-HCl (pH 8.8), 6.25 mM MgCl2 , HS Taq DNA polymerase, 0.8 mM deoxynucleotide triphosphates, 900 nM direct and reverse primers, water (Synthol) and 100 ng DNA template (cDNA). As an endogenous control, a set of primers were used to analyze the level of expression of 18S rRNA gene. The prepared 9.9 μL reaction mixture was digested into 384-well plates (Axygen, 384-well Skirted PCR Microplates, CA, USA), and 0.1 μL of cDNA was added to each well. The wells were covered with an optically transparent film (Axygen, Sealing Film, Real Time PCR) and whether PCR amplification at the following temperature conditions: 95°C 30 seconds (calibration), 95°C 5 minutes (preheating), and then 50 cycles: 95°C 30 seconds, 55°C 30 seconds and 72°C 30 seconds.
Evaluation of ectopic expression of human vegf165 and fgf2 mRNA, and the transcriptional activity of similar rat genes was also evaluated. Primers for evolution of the target gene expression are shown in Table 1. Recombinant genes are depicted as coVEGF165 and coFGF2, whereas rat equivalent genes are rVEGF164 and rFGF2. For each target gene, the results were obtained in two independent experiments in triplicate. The amount of RNA was normalized by the amount of cDNA of the “housekeeping” 18S rRNA gene (Chintalgattu et al., 2003). Serial dilution of plasmid DNA with a known number of copies was used to construct a standard curve and to determine the expression level of the studied genes. The expression level of the studied genes in the right sciatic nerve was taken as 100%.
Table 1.
Primers used to evaluate the target gene expression
| Primer | Sequence 5′–3′ |
|---|---|
| rFGF2 | F: GCT GCT GGC TTC TAA GTG TG |
| rFGF2 | R: GTG CCA CAT ACC AAC TGG AG |
| rVEGF164 | F: TAT ATC TTC AAG CCG TCC TGT G |
| rVEGF164 | R: TCT CCT ATG TGC TGG CTT TG |
| rmh18S | F: GCC GCT AGA GGT GAA ATT CTT G |
| rmh18S | R: CAT TCT TGG CAA ATG CTT TCG |
| coVEGF165 | F: GCG GAG AAA GCA CAA GAT CCG |
| coVEGF165 | R: CCT CGG CTT GTC ACA TCT GC |
| coFGF2 | F: GCG GGT TCT TTC TGA GGA T |
| coFGF2 | R: ATA GCC AGA TAT CGG TTG GC |
F: Forward; R: reverse.
Immunohistochemistry
Medial parts of the sciatic nerves were fixed in 10% neutral buffered formalin (pH 7.4) for 24 hours at room temperature, and then embedded in paraffin using standard protocol. 4-μm-thick paraffin cross-sections were dried overnight at 45°C. Immunohistochemistry was performed using Novolink™ Polymer Detection System (Leica Biosystems, Wetzlar, Germany) with antibodies against VEGF (1:100, V-16, sc-7269, mouse monoclonal, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and FGF2 (1:200, N-19, sc-1390, goat polyclonal, Santa Cruz Biotechnology) according to manufacturer’s protocol. Detection was accomplished with 3-amino-9-ethylcarbazole (AEC). Nuclei were additionally stained for 1 minute with Mayer’s hematoxylin. Imaging was performed on the Aperio CS2 slide scanner (Leica Biosystems, Wetzlar, Germany).
Morphological analysis
Medial nerve specimens were cut into continuous cross-sections at 4-μm thickness and the specimens were stained using hematoxylin and eosin (H&E) to observe nerve morphology. For H&E staining, deparaffinized and rehydrated sections were immersed into Mayer’s hematoxylin for 1 minute, followed by incubation for 5 minutes in tap water. Then slides were placed into water-soluble eosin for 30 seconds and washed in distilled water for 5 minutes. Imaging was performed on the Aperio CS2 slide scanner (Leica Biosystems).
Multiplex immunoassay
Rat Cytokine/Chemokine Magnetic Bead Panel (Millipore, Billerica, MA, USA) was used to analyze serum samples according to the manufacturer’s recommendations. Rat cytokine panel includes granulocyte colony-stimulating factor (G-CSF), etaxin, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-1α, leptin, macrophage inflammatory protein (MIP) 1α, IL-4, IL-1β, IL-2, IL-6, epidermal growth factor (EGF), IL-13, IL-10, IL-12p75, interferon-γ (IFNγ), IL-5, IL-17A, and IL-18, Monocyte chemoattractant protein 1 (MCP1), interferon gamma-induced protein 10 (IP-10), growth-regulated oncogene (GRO), VEGF, fractalkine, LIX (C-X-C motif chemokine 5), MIP-2, tumor necrosis factor-α (TNFα), and Regulated upon Activation, Normal T Cell Expressed and Presumably Secrete (RANTES). In each case, serum was collected from 0.5–0.7 mL of peripheral blood. Then 50 µL of serum was used for multiplex immunoassay. Muliplex immunoassay was performed in triplicate for each test serum sample.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). A one-way analysis of variance (ANOVA) with post hoc Tukey’s test was used for multiple comparisons between all experimental groups. All analyses were performed in a blinded manner with respect to the treatment group. A value of P < 0.05 was considered statistically significant. Data were analyzed using the Origin 7.0 SR0 Software (OriginLab, Northampton, MA, USA).
Results
mRNA expression of vegf165 and fgf2 genes
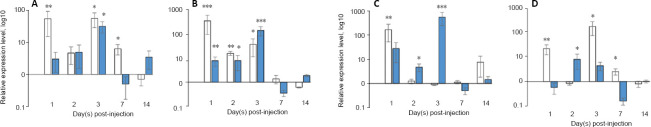
mRNA expression of vegf165 and fgf2 genes was evaluated by RT-PCR in the distal and proximal areas of rat sciatic nerve at 1, 2, 3, 7, and 14 days after administration of the pBud-coVEGF165-coFGF2 recombinant construct. According to our data, 1 day after injection, a multiple increase in mRNA expression of recombinant genes was noted in the distal nerve section (P = 0.003 and P = 0.0008 for recombinant vegf and fgf2, respectively; Figure 1). On the following days, downward trend was observed. In addition, on the first 2 days, transcripts of recombinant vegf165 and fgf2 show an increase mainly in the distal part of the nerve (1 day: P = 0.003 and P = 0.0008; 2 days: P = 0.003 and P = 0.009 for vegf and fgf2 mRNA, respectively). On the 3rd day, on the contrary, expression in the proximal region significantly increased: 31.36 (P = 0.034) for vegf mRNA and 133.28 (P = 0.00000001) for fgf2 mRNA, which was almost four times higher than the expression level of fgf2 in the distal nerve fragment (P = 0.03). On days 7 and 14, there was no significant increase in ectopic expression of vegf and fgf2 mRNA.
Figure 1.
mRNA expression of vegf and fgf2 genes in rat sciatic nerve samples.
(А) coVEGF expression, (B) coFGF2 expression, (C) rat VEGF expression, (D) rat FGF2 expression. White columns represent expression in distal nerve parts, blue columns represents expression in proximal nerve parts. X-line axis indicates time points after administration of рBud-coVEGF165-coFGF2, Y-axis indicates mRNA relative expression. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, vs. the control (one-way analysis of variance with post hoc Tukey’s test). The expression level of the studied genes in the right sciatic nerve was taken as 100%. The analysis was carried out in triplicate. n = 5 animals per group.
In response to administration of the plasmid construct pBud-coVEGF165-coFGF2, the number of transcripts of rat vegf and fgf2 mRNAs significantly changed (Figure 1). The level of expression of the gene encoding VEGF164 in the distal fragment of the nerve was significantly different from the control value (P = 0.024). The transcriptional activity of the vegf164 gene in the proximal fragment gradually increased and reached its peak on the third day (P = 0.00005). As for the distal fragment, by the third day, the number of recombinant vegf164 transcripts in this segment almost equaled the expression level in control samples. The relative expression of rat fgf2 reached its maximum value on the first day after an injection in the distal fragment (P = 0.0024), then the expression level gradually decreased and on the third day, a significant change was observed only in the proximal sciatic nerve (P = 0.034). As in the case of ectopic expression of recombinant factors, on the 7th and 14th days, the transcriptional activity of the genes does not differ from that in the control samples; the values became almost equal to the control values in the distal and proximal fragments of the sciatic nerve.
Morphological changes in rat sciatic nerve
At 1, 2, 3, 7, and 14 days after intraneural administration of the plasmid construct pBud-coVEGF165-coFGF2, no significant changes in the structure of the sciatic nerve were observed. Visually, the first day post-operation was characterized by moderate swelling of the epineurium caused by mechanical damage to the epineurium. Swelling occurred in both groups indicating that it was not due to the vegf165 and fgf2 expression. An inflammatory reaction in the sciatic nerve in response to the introduction of the plasmid pBud-coVEGF165-coFGF2 was not detected during the studied periods (Figure 2).
Figure 2.
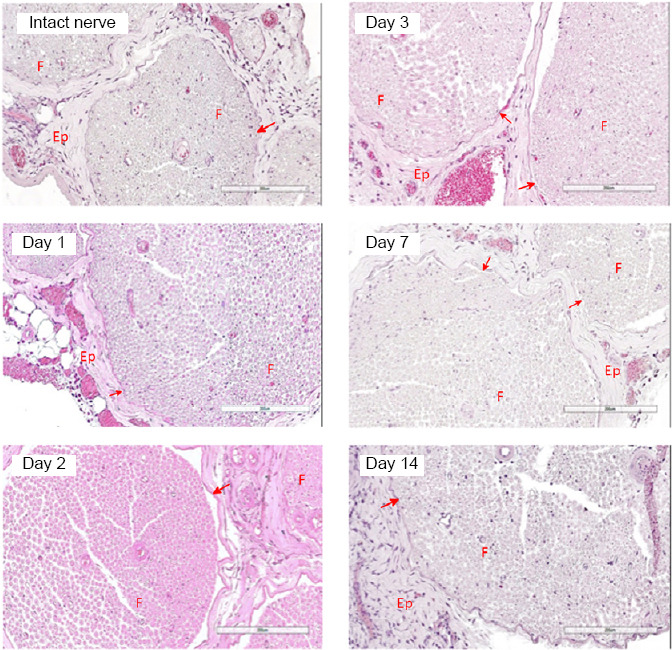
Cross sections of rat sciatic nerve 1, 2, 3, 7, and 14 days after intraneural administration of the plasmid pBud-coVEGF165-coFGF2.
Hematoxylin and eosin staining. No inflammatory or degenerative features observed. Ep: Epineurium; F: fascicle. Red arrows correspond to perineurium. Scale bars: 200 μm. Intact nerve refers to nerve injected with phosphate buffer saline.
VEGF and FGF2 proteins in rat sciatic nerve
On the first day after injection of the plasmid pBud-coVEGF165-coFGF2 in the experimental group, a bright positive staining for VEGF-A was detected in the vascular lumen (Figure 3), which lasted up to 14 days. FGF2 in the lumen of blood vessels was detected only in 3 days, and blood vessels positive for FGF2 were also observed in the control group injected with PBS (Figure 3). On day 14, in the control group, positive staining for VEGF-A was completely absent, which was retained in a small number of vessels in experimental samples (Figure 3). Positive staining for FGF2 on day 14 after injection of plasmid pBud-coVEGF165-coFGF2 was absent in both groups (Figure 3).
Figure 3.
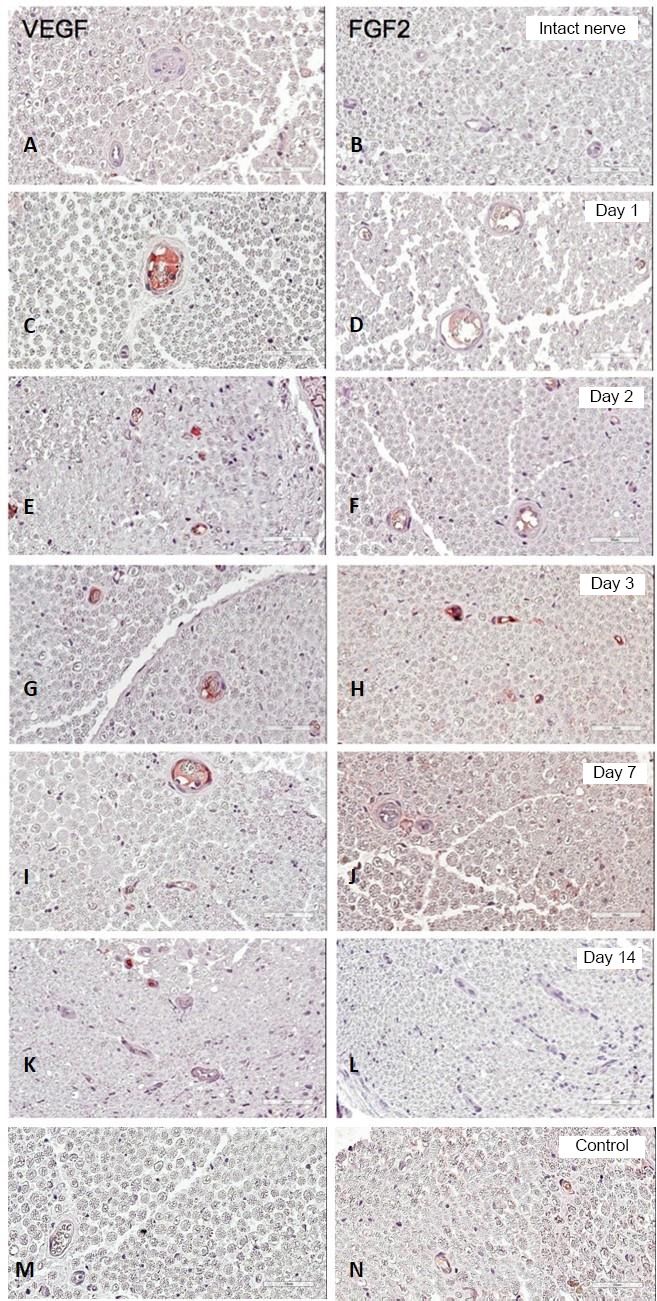
Immunohistochemical staining of VEGF (left column) and FGF2 immunoreactivities (right column).
1, 2, 3, 7, and 14 days after the introduction of plasmid pBud-coVEGF165-coFGF2. Control: Sciatic nerve on the 3rd day post-injection with phosphate buffer saline. Cross sections of the sciatic nerve of a rat. Red staining corresponds to a positive reaction with antibodies against VEGF and FGF2. Scale bars: 50 μm. FGF2: Fibroblast growth factor 2; VEGF: vascular endothelial growth factor
Cytokines/chemokines in the peripheral blood serum of rats
On day 7 post-injection, among 33 proteins stained, 14 were secreted significantly in the blood serum (Table 2). In particular, serum levels of MIP-1α (P < 0.05), IL-10 (P < 0.01), IL-12p75 (P < 0.01), IL-5 (P < 0.01), TNF-α (P < 0.01), IL-1α (P < 0.01), IL-1β (P < 0.01), IL 18 (P < 0.01), VEGF (P < 0.01), Fractalkine (P < 0.01) were decreased. Among these, TNF-α level decreased the most (P = 0.0008) and IL-12 level also decreased significantly (P = 000004).
Table 2.
Levels of cytokines/chemokines (relative units/mL) in the peripheral blood serum of rats after intraneural administration of the plasmid pBud-coVEGF165-coFGF2 or the introduction of PBS
| Cytokine/chemokine | 7 days after PBS administration | 7 days after plasmid DNA administration |
|---|---|---|
| MIP-1α | 226.53±78.47 | 16.58±2.81* |
| IL-10 | 640.88±203.54 | 82.98±155.71** |
| IL-12p75 | 251.94±82.24 | 12.20±0** |
| IL-5 | 45.21±15.4 | 4.90±0** |
| TNF-α | 119.31±29.57 | 2.40±0** |
| IL-1α | 1744.27±798.40 | 470.15±170.16** |
| IL-1β | 369.17±125.96 | 109.28±60.95** |
| IL-18 | 1356.42±367.33 | 737.05±196.84** |
| VEGF | 382.54±61.08 | 70.06±4.67** |
| Fractalkine | 328.64±78.36 | 27.50±5.95** |
| RANTES | 2085.34±465.66 | 4065.98±456.40* |
| EGF | 154.60±46.78 | 201.61±50.16* |
| IL-2 | 11.79±2.36 | 39.07±32.88* |
| MCP-1 | 923.39±300.15 | 1138.39±161.96* |
| Eotaxin | 0.89±0.28 | 1.06±0.74 |
| G-CSF | 4.90±0 | 4.90±0 |
| GM-CSF | 12.20±0 | 12.20±0 |
| Leptin | 11041.29±2188.76 | 13464.47±4308.26 |
| IL-4 | 4.90±0 | 4.90±0 |
| IL-6 | 73.20±0 | 73.20±0 |
| IL-13 | 4.90±0 | 4.90±0 |
| IFNγ | 14.6±0 | 14.6±0 |
| IL-17A | 7.30±0 | 7.30±0 |
| IP-10 | 339.78±73.65 | 345.84±34.77 |
| GRO | 14.60±0 | 14.60±0 |
| LIX | 2686.88±688.60 | 2231.09±301.99 |
| MIP-2 | 24.40±0 | 25.46±2.13 |
*P < 0.05, **P < 0.01, vs. 7 days after PBS administration. EGF: Epidermal growth factor; G-CSF: granulocyte colony-stimulating factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; GRO: growth-regulated oncogene; IFNγ: interferon-γ; IL: interleukin; LIX: MCP-1: monocyte chemoattractant protein 1; MIP-1α: macrophage inflammatory protein; MIP- 2; macrophage inflammatory protein 2; RANTES: Regulated upon Activation, Normal T Cell Expressed and Presumably Secrete; TNFα: tumor necrosis factor alpha; VEGF: vascular endothelial growth factor.
At the same time, the expression of RANTES (P < 0.05), EGF (P < 0.05), IL-2 (P < 0.05), and MCP-1 (P < 0.05) was upregulated (Table 2).
Discussion
For regulation of angiogenesis, it is essential to locally maintain the balance of pro-angiogenic factors. Induction of angiogenesis leads to a rapid excessive accumulation of pro-angiogenic factors (Tahergorabi and Khazaei, 2012). After sprouting completion, the mechanisms underlying the inhibition of angiogenic factors and active synthesis of angiostatins are activated to restore the initial balance (Bisht et al., 2010). Administration of plasmid DNA рBud-coVEGF165-coFGF2 containing human codon-optimized genes of VEGF-A and FGF2 induced angiogenesis, affecting transcriptional activity of vegfa and fgf2 genes in vivo. Moreover, direct transport of mRNA transcripts in distal part and then to the proximal part of the nerve have been observed.
First 3 days were characterized by prominent active secretion of recombinant proteins and translocation to the distal part of the sciatic nerve appeared within the first 3 days after injection of plasmid рBud-coVEGF165-coFGF2, which is beneficial for regeneration and directed axon growth.
By day 7, expression of recombinant vegf and fgf2 in the sciatic nerve ceased. Despite this fact, accumulation of recombinant VEGF-A was demonstrated in the lumen of blood vessels of the medial part of the nerve, which was observed from 1 day to 14 days after injection of plasmid рBud-coVEGF165-coFGF2. Upregulation of FGF-2 expression was observed only at 3 days after injury.
In addition to local effects, based on the results of our multiplex analysis, injection of plasmid pBud-coVEGF165-coFGF2 resulted in changes at the system level. Concentrations of numerous cytokines and chemokines changed. Most of cytokines may regulate angiogenesis and tissue reparative potential. It is worth mentioning that these significant changes in the synthesis of cytokines had been observed on the 7th day, when mRNA of recombinant vegf and fgf2 at the site of administration of plasmid pBud-coVEGF165-coFGF2 already corresponded to the minimum (native) level. However, this fact suggests that this delayed effect might be due to the action of pre-synthesized recombinant proteins.
At 7 days after injection of plasmid pBud-coVEGF165-coFGF2, the levels of pro-angiogenic factors MIP-1α, IL-10, IL-12p75, IL-5, TNFα, IL-1α, IL-1β, IL-18, VEGF, and Fractalkine in blood serum decreased. At the same time, serum levels of RANTES, EGF, IL-2, andMCP-1 were increased. Fibroblasts and SCs are known to secrete interleukins, such as IL-1β, IL-6, chemokines (including MCP and RANTES), GM-CSF and TGFβ (Ozaki et al., 2008). At the very beginning of the article, we focused on the critical role of immune cells in the regeneration of peripheral nerves. Their recruitment and activation is mediated by the factors secreted by SCs and fibroblasts, which play a direct role in post-traumatic nerve regeneration. There is clear evidence that monocytes are involved in this extremely vital process. They differentiate into macrophages under the influence of tissue microenvironment and various colony-stimulating factors, including GM-CSF and G-CSF (Brown et al., 2012). It is also known that MCP-1 production is increased after peripheral nerve injury and MCP-1 promotes the transition of macrophages to the M2 phenotype in the area of injury (Schreiber et al., 2001; Sierra-Filardi et al., 2014; Masgutova et al., 2017). Moreover, MCP-1 and CCR2 were shown to lead to the progression of neuropathic pain (Abbadie et al., 2009; Liou et al., 2013). It is worth emphasizing that the introduction of the plasmid reduces the levels of pro- and anti-inflammatory cytokines, we confidently prove this fact by the absence of a significant difference in the group with PBS administration compared to the multiplex analysis of peripheral blood serum collected from these animals 2 hours before conducting an operation.
Among all of the analyzed cytokines, the levels of pro-inflammatory cytokines changed the most. Hence, the level of TNF decreased by 40 times. It is known that TNF can act on endothelial cells both directly through the induction of cell differentiation and indirectly through stimulation of the secretion of angiogenic factors (Ucuzian et al., 2010). However, prolonged enhanced synthesis of TNF can contribute to demyelination, apoptosis of neurons, expression of endothelial cell adhesion molecules, and affect the activation of immune cellsand the phagocytosis by macrophages (Allan and Rothwell, 2001; Shamash et al., 2002; Chen et al., 2011).
The levels of IL-1α and IL-1β also decreased. These cytokines contribute to remyelination, production of cytokines by endothelial and immune cells and destabilization of the endothelial layer of the vessels, and, together with TNF, they can contribute to death of neurons and activation of monocytes/macrophages (Pineau et al., 2010; Sato et al., 2012; Ye et al., 2013, 2015). IL-1β, when combined with other cytokines, can activate the synthesis of FGF2 by endothelial cells (Lee et al., 2004). In turn, FGF2 enhances the expression of a number of chemokines involved in the recruitment of various immune cells, including monocytes/macrophages, natural killer cells and T lymphocytes (Presta et al., 2009).
An increase in the number of pro-inflammatory cytokines and chemokines occurs due to neutrophils almost immediately after stress exposure (Naldini and Carraro, 2005). Despite their positive effect on nerve regeneration, the action of all cytokines and chemokines is dualistic; this manifests itself in the fact that with prolonged increased secretion, they begin to have a detrimental effect on tissue, primarily due to an inadequate increase in the aggressiveness of immune cells. In this case, axon demyelination and the death of Schwann cells and neurons occur (Pineau et al., 2010; Sato et al., 2012; Ye et al., 2013). Obtained results have shown that on the 7th day after injection of the plasmid, the level of pro-inflammatory cytokines decreases. This is due to the fact that the peak of pro-inflammatory factors has already passed, and since the integrity of the nerve was not compromised, the action of the plasmid by itself was not enough to maintain a long-term increase in the secretion of pro-inflammatory cytokines. As a result, their synthesis was suppressed to restore the original balance and prevent the inadequate response of the immune system.
However, the obtained results cannot be interpreted specifically, since multiplex analysis of peripheral blood serum is not considered to be a standard for understanding the processes of regeneration in the sciatic nerve, but the data obtained may become the pioneer for understanding the participation of a systemic inflammatory reaction in the post-traumatic process. Moreover, we have to concede that evaluation of the lysates from peripheral nerve may clarify numerous questions concerning upregulated expression of VEGF and FGF2 on production of anti- and pro-inflammatory cytokines and evidence on possible local intraneural changes.
Thus, injection of pBud-coVEGF165-coFGF2 results in increased expression of genes encoding the pro-angiogenic factors VEGF-A and FGF2. Together with ectopic expression, an increased transcriptional activity of the studied rat genes occurs. Moreover, upregulated expression is quite transient. This may be due to the fact that the introduced plasmid pBud-coVEGF165-coFGF2 encodes recombinant human proteins. Moreover, significant changes in levels of cytokines and chemokines were observed in 7 days after intraneural injection. First of all, a significant decreased level of secretion of pro-inflammatory cytokines and chemokines takes place. Upregulation of secretion activity is a normal occurrence in the induction of peripheral nerve regeneration and angiogenesis and is usually activated immediately after stress exposure (Chen et al., 2011). Cytokines and chemokines in the PNS are constitutively expressed and play significant roles in activating the proliferation of SCs, regulation of axon growth, remyelination, destabilization of endothelial layer of vessels, and extracellular matrix remodeling of endothelial cells.
In summary, the action of all cytokines and chemokines is dualistic, which manifests itself in the fact that with prolonged increased secretion, cytokines and chemokines begin to produce detrimental effects on tissues, primarily due to an inadequate increase in the aggressiveness of immune cells. Our results are consistent with the normal scenario of angiogenesis and regeneration of the peripheral nerve and indicate that the plasmid construct pBud-coVEGF165-coFGF2 is not immunogenic, and when administered locally, it promotes the activation of angiogenesis and regeneration in rat sciatic nerve.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: This study was supported by the Russian Government Program of Competitive Growth of Kazan Federal University; state assignment 20.5175.2017/6.7 of the Ministry of Education and Science of Russian Federation and Grant of the President of the Russian Federation for state support of the leading scientific schools of the Russian Federation. The work was also supported by the Russian Government Program of Competitive Growth of the Kazan Federal University (to AR); the Russian Foundation for Basic Research grant 18-54-45023 Ind_a (to IS and GM).
Institutional review board statement: The study was approved by the Animal Ethics Committee of Kazan Federal University, procedures were approved by the Local Ethics Committee (approval No. 5) on May 27, 2014.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the Russian Government Program of Competitive Growth of Kazan Federal University; state assignment 20.5175.2017/6.7 of the Ministry of Education and Science of Russian Federation and Grant of the President of the Russian Federation for state support of the leading scientific schools of the Russian Federation. The work was also supported by the Russian Government Program of Competitive Growth of the Kazan Federal University (to AR); the Russian Foundation for Basic Research grant 18-54-45023 Ind_a (to IS and GM).
C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 3.Bastien D, Lacroix S. Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury. Exp Neurol. 2014;258:62–77. doi: 10.1016/j.expneurol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Bisht M, Dhasmana DC, Bist SS. Angiogenesis: future of pharmacological modulation. Indian J Pharmacol. 2010;42:2–8. doi: 10.4103/0253-7613.62395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boldyreva MА, Bondar IV, Stafeev IS, Makarevich PI, Beloglazova IB, Zubkova ES, Shevchenko EK, Molokotina YD, Karagyaur MN, Rаtner ЕI, Parfyonova YV. Plasmid-based gene therapy with hepatocyte growth factor stimulates peripheral nerve regeneration after traumatic injury. Biomed Pharmacother. 2018;101:682–690. doi: 10.1016/j.biopha.2018.02.138. [DOI] [PubMed] [Google Scholar]
- 6.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen KB, Uchida K, Nakajima H, Yayama T, Hirai T, Watanabe S, Guerrero AR, Kobayashi S, Ma WY, Liu SY, Baba H. Tumor necrosis factor-alpha antagonist reduces apoptosis of neurons and oligodendroglia in rat spinal cord injury. Spine (Phila Pa 1976) 2011;36:1350–1358. doi: 10.1097/BRS.0b013e3181f014ec. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, Cescon M, Megighian A, Bonaldo P. Collagen VI regulates peripheral nerve myelination and function. FASEB J. 2014;28:1145–1156. doi: 10.1096/fj.13-239533. [DOI] [PubMed] [Google Scholar]
- 10.Chintalgattu V, Nair DM, Katwa LC. Cardiac myofibroblasts: a novel source of vascular endothelial growth factor (VEGF) and its receptors Flt-1 and KDR. J Mol Cell Cardiol. 2003;35:277–286. doi: 10.1016/s0022-2828(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 11.Conforti L, Gilley J, Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- 12.Dumpich M, Theiss C. VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine. Neural Regen Res. 2015;10:1725–1726. doi: 10.4103/1673-5374.170287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frelin L, Ahlen G, Alheim M, Weiland O, Barnfield C, Liljestrom P, Sallberg M. Codon optimization and mRNA amplification effectively enhances the immunogenicity of the hepatitis C virus nonstructural 3/4A gene. Gene Ther. 2004;11:522–533. doi: 10.1038/sj.gt.3302184. [DOI] [PubMed] [Google Scholar]
- 14.Fu C, Hong G, Wang F. Favorable effect of local VEGF gene injection on axonal regeneration in the rat sciatic nerve. J Huazhong Univ Sci Technolog Med Sci. 2007;27:186–189. doi: 10.1007/s11596-007-0221-z. [DOI] [PubMed] [Google Scholar]
- 15.Fukui K. Reactive oxygen species induce neurite degeneration before induction of cell death. J Clin Biochem Nutr. 2016;59:155–159. doi: 10.3164/jcbn.16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao CY, Xu TT, Zhao QJ, Li CL. Codon optimization enhances the expression of porcine beta-defensin-2 in Escherichia coli. Genet Mol Res. 2015;14:4978–4988. doi: 10.4238/2015.May.12.1. [DOI] [PubMed] [Google Scholar]
- 17.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyng SA, de Winter F, Tannemaat MR, Blits B, Malessy MJ, Verhaagen J. Gene therapy and peripheral nerve repair: a perspective. Front Mol Neurosci. 2015;8:32. doi: 10.3389/fnmol.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HK, Shin YK, Jung J, Seo SY, Baek SY, Park HT. Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia. 2009;57:1825–1834. doi: 10.1002/glia.20894. [DOI] [PubMed] [Google Scholar]
- 20.Lee HT, Lee JG, Na M, Kay EP. FGF-2 induced by interleukin-1 beta through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J Biol Chem. 2004;279:32325–32332. doi: 10.1074/jbc.M405208200. [DOI] [PubMed] [Google Scholar]
- 21.Liou JT, Lee CM, Day YJ. The immune aspect in neuropathic pain: role of chemokines. Acta Anaesthesiol Taiwan. 2013;51:127–132. doi: 10.1016/j.aat.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Peng J, Han GH, Ding X, Wei S, Gao G, Huang K, Chang F, Wang Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen Res. 2019;14:1335–1342. doi: 10.4103/1673-5374.253510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martini R, Fischer S, Lopez-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008;56:1566–1577. doi: 10.1002/glia.20766. [DOI] [PubMed] [Google Scholar]
- 24.Masgutov R, Salafutdinov I, Bogov A, Trofimova A, Khannanova I, Mullin R, Islamov R, Chelyshev Y, Rizvanov A. Stimulation of rat’s sciatic nerve post-traumatic regeneration using plasmids expressing vascular endothelial growth factor and basic fibroblast growth factor. Cell Transplant Tissue Eng. 2011;6:67–70. [Google Scholar]
- 25.Masgutov R, Masgutova G, Mullakhmetova A, Zhuravleva M, Shulman A, Rogozhin A, Syromiatnikova V, Andreeva D, Zeinalova A, Idrisova K, Allegrucci C, Kiyasov A, Rizvanov A. Adipose-derived mesenchymal stem cells applied in fibrin glue stimulate peripheral nerve regeneration. Front Med (Lausanne) 2019;6:68. doi: 10.3389/fmed.2019.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masgutova G, Martynova E, Masgutov R, Mukhametova L, Mullakhmetova A, Kadyrova G, Khaiboullina S, Rizvanov A. Multiplex analysis of the activation of the immune system after transection of the rat’s sciatic Nerve. Bionanoscience. 2017;7:170–176. [Google Scholar]
- 27.Mauro VP, Chappell SA. A critical analysis of codon optimization in human therapeutics. Trends Mol Med. 2014;20:604–613. doi: 10.1016/j.molmed.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moccia F, Negri S, Shekha M, Faris P, Guerra G. Endothelial Ca(2+) signaling, angiogenesis and vasculogenesis: just what it takes to make a blood vessel. Int J Mol Sci. 2019;20:3962. doi: 10.3390/ijms20163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muratori L, Gnavi S, Fregnan F, Mancardi A, Raimondo S, Perroteau I, Geuna S. Evaluation of vascular endothelial growth factor (VEGF) and its family member expression after peripheral nerve regeneration and denervation. Anat Rec (Hoboken) 2018;301:1646–1656. doi: 10.1002/ar.23842. [DOI] [PubMed] [Google Scholar]
- 30.Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005;4:3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki A, Nagai A, Lee YB, Myong NH, Kim SU. Expression of cytokines and cytokine receptors in human Schwann cells. Neuroreport. 2008;19:31–35. doi: 10.1097/WNR.0b013e3282f27e60. [DOI] [PubMed] [Google Scholar]
- 32.Pereira Lopes FR, Martin PK, Frattini F, Biancalana A, Almeida FM, Tomaz MA, Melo PA, Borojevic R, Han SW, Martinez AM. Double gene therapy with granulocyte colony-stimulating factor and vascular endothelial growth factor acts synergistically to improve nerve regeneration and functional outcome after sciatic nerve injury in mice. Neuroscience. 2013;230:184–197. doi: 10.1016/j.neuroscience.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Presta M, Andres G, Leali D, Dell’Era P, Ronca R. Inflammatory cells and chemokines sustain FGF2-induced angiogenesis. Eur Cytokine Netw. 2009;20:39–50. doi: 10.1684/ecn.2009.0155. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salafutdinov II, Shafigullina AK, Yalvac ME, Kudryashova NV, Lagarkova MA, Shutova MV, Kiselev SL, Masgutov RF, Zhdanov RI, Kiyasov AP, Islamov RR, Rizvanov AA. Effect of simultaneous expression of various isoforms of vascular endothelial growth factor VEGF and fibroblast growth factor FGF2 on proliferation of human umbilical cord blood cells HUVEC. Cell Transplant Tissue Eng. 2010;5:62–67. [Google Scholar]
- 37.Sato A, Ohtaki H, Tsumuraya T, Song D, Ohara K, Asano M, Iwakura Y, Atsumi T, Shioda S. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J Neuroinflammation. 2012;9:65. doi: 10.1186/1742-2094-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber RC, Krivacic K, Kirby B, Vaccariello SA, Wei T, Ransohoff RM, Zigmond RE. Monocyte chemoattractant protein (MCP)-1 is rapidly expressed by sympathetic ganglion neurons following axonal injury. Neuroreport. 2001;12:601–606. doi: 10.1097/00001756-200103050-00034. [DOI] [PubMed] [Google Scholar]
- 39.Selders GS, Fetz AE, Radic MZ, Bowlin GL. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater. 2017;4:55–68. doi: 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-α, interleukin-1α, and interleukin-1β. J Neurosci. 2002;22:3052. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sierra-Filardi E, Nieto C, Dominguez-Soto A, Barroso R, Sanchez-Mateos P, Puig-Kroger A, Lopez-Bravo M, Joven J, Ardavin C, Rodriguez-Fernandez JL, Sanchez-Torres C, Mellado M, Corbi AL. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol. 2014;192:3858–3867. doi: 10.4049/jimmunol.1302821. [DOI] [PubMed] [Google Scholar]
- 43.Solovyeva VV, Chulpanova DS, Tazetdinova LG, Salafutdinov, Bozo IY, Isaev AA, Deev RV, Rizvanov AA. In vitro angiogenic properties of plasmid DNA encoding SDF-1alpha and VEGF165 genes. Appl Biochem Biotechnol. 2020;190:773–788. doi: 10.1007/s12010-019-03128-5. [DOI] [PubMed] [Google Scholar]
- 44.Spanholtz TA, Theodorou P, Holzbach T, Wutzler S, Giunta RE, Machens HG. Vascular endothelial growth factor (VEGF165) plus basic fibroblast growth factor (bFGF) producing cells induce a mature and stable vascular network--a future therapy for ischemically challenged tissue. J Surg Res. 2011;171:329–338. doi: 10.1016/j.jss.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 45.Stratton JA, Holmes A, Rosin NL, Sinha S, Vohra M, Burma NE, Trang T, Midha R, Biernaskie J. Macrophages regulate Schwann cell maturation after nerve injury. Cell Rep. 2018;24:2561–2572. doi: 10.1016/j.celrep.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Tahergorabi Z, Khazaei M. A review on angiogenesis and its assays. Iran J Basic Med Sci. 2012;15:1110–1126. [PMC free article] [PubMed] [Google Scholar]
- 47.Ucuzian AA, Gassman AA, East AT, Greisler HP. Molecular mediators of angiogenesis. J Burn Care Res. 2010;31:158–175. doi: 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Steenwinckel J, Auvynet C, Sapienza A, Reaux-Le Goazigo A, Combadiere C, Melik Parsadaniantz S. Stromal cell-derived CCL2 drives neuropathic pain states through myeloid cell infiltration in injured nerve. Brain Behav Immun. 2015;45:198–210. doi: 10.1016/j.bbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 49.Wakatsuki S, Furuno A, Ohshima M, Araki T. Oxidative stress-dependent phosphorylation activates ZNRF1 to induce neuronal/axonal degeneration. J Cell Biol. 2015;211:881–896. doi: 10.1083/jcb.201506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Zhu H, Guo Q, Qian T, Zhang P, Li S, Xue C, Gu X. Overlapping mechanisms of peripheral nerve regeneration and angiogenesis following sciatic nerve transection. Front Cell Neurosci. 2017;11:323. doi: 10.3389/fncel.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodbury ME, Ikezu T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. J Neuroimmune Pharmacol. 2014;9:92–101. doi: 10.1007/s11481-013-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem. 2013;125:897–908. doi: 10.1111/jnc.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]