Keywords: brain-derived neurotrophic factor, conditioned medium, dental pulp stem cell, glial cell line-derived nerve growth factor, neurite outgrowth, neurotrophic factor, neurotrophin-3, phaeochromocytoma PC12 cell
Abstract
Dental pulp stem cells (DPSCs) secrete neurotrophic factors which may play an important therapeutic role in neural development, maintenance and repair. To test this hypothesis, DPSCs-conditioned medium (DPSCs-CM) was collected from 72 hours serum-free DPSCs cultures. The impact of DPSCs-derived factors on PC12 survival, growth, migration and differentiation was investigated. PC12 cells were treated with nerve growth factor (NGF), DPSCs-CM or co-cultured with DPSCs using Transwell inserts for 8 days. The number of surviving cells with neurite outgrowths and the length of neurites were measured by image analysis. Immunocytochemical staining was used to evaluate the expression of neuronal markers NeuN, microtubule associated protein 2 (MAP-2) and cytoskeletal marker βIII-tubulin. Gene expression levels of axonal growth-associated protein 43 and synaptic protein Synapsin-I, NeuN, MAP-2 and βIII-tubulin were analysed by quantitative polymerase chain reaction (qRT-PCR). DPSCs-CM was analysed for the neurotrophic factors (NGF, brain-derived neurotrophic factor [BDNF], neurotrophin-3, and glial cell-derived neurotrophic factor [GDNF]) by specific ELISAs. Specific neutralizing antibodies against the detected neurotrophic factors were used to study their exact role on PC12 neuronal survival and neurite outgrowth extension. DPSCs-CM significantly promoted cell survival and induced the neurite outgrowth confirmed by NeuN, MAP-2 and βIII-tubulin immunostaining. Furthermore, DPSCs-CM was significantly more effective in stimulating PC12 neurite outgrowths than live DPSCs/PC12 co-cultures over the time studied. The morphology of induced PC12 cells in DPSCs-CM was similar to NGF positive controls; however, DPSCs-CM stimulation of cell survival was significantly higher than what was seen in NGF-treated cultures. The number of surviving PC12 cells treated with DPSCs-CM was markedly reduced by the addition of anti-GDNF, whilst PC12 neurite outgrowth was significantly attenuated by anti-NGF, anti-GDNF and anti-BDNF antibodies. These findings demonstrated that DPSCs were able to promote PC12 survival and differentiation. DPSCs-derived NGF, BDNF and GDNF were involved in the stimulatory action on neurite outgrowth, whereas GDNF also had a significant role in promoting PC12 survival. DPSCs-derived factors may be harnessed as a cell-free therapy for peripheral nerve repair. All experiments were conducted on dead animals that were not sacrificed for the purpose of the study. All the methods were carried out in accordance with Birmingham University guidelines and regulations and the ethical approval is not needed.
Chinese Library Classification No. R459.9; R364; R622
Introduction
Peripheral nerve injury may occur as a result of acute compression, trauma, iatrogenic during surgical procedures, diabetes and other health conditions and may end with the loss of sensory function, motor function, or both (Aguayo et al., 1973). Regenerative capacity of the injured adult nervous system is limited due to an insufficient pool of precursor cells. Nerve regeneration is also constrained by inhibitory factors and barrier tissues in the injured microenvironment (Luo et al., 2018). Schwann cells (SCs) transplants reported to provide a promising therapeutic strategy for the treatment of peripheral nerve injuries. SCs are peripheral glial cells responsible for clearing out debris from the site of injury. Moreover, they release growth factors to stimulate myelination and axonal regeneration (Frostick et al., 1998).
Both primary SCs (Guenard et al., 1992) and genetically modified SCs (Li et al., 2006) enhance nerve regeneration in animal models; however, SCs’ sourcing and availability are limited and the only method to obtain primary cells is by sacrificing a healthy nerve. Stem cell-based strategies in combination with novel technologies (e.g., hydrogels) have heralded potential new therapeutic approaches for addressing nerve regeneration and repair (Luo et al., 2018). Bone marrow-derived stem cells (Tohill et al., 2004), adipose-derived stem cells (ADSCs) (di Summa et al., 2010) and dental pulp-derived stem cells (DPSCs) have gained increasing interest as alternative sources for peripheral nervous system regeneration therapy (Luo et al., 2018).
In particular, DPSCs have recently gathered much attention in the field of neuroscience research because of their neural crest origin and their apparent superior ability to secrete substantial levels of neurotrophic factors effective for neuronal survival and axonal regeneration (Mead et al., 2013, 2014a, b, 2017).
In nerve repair as in other fields, numerous models have been established to test the efficacy of compounds pre-clinically (Rayner et al., 2018). Pheochromocytoma (PC12) cell line is a well-known and tested neuronal cell model, originated from a tumor of adrenal gland, have been used extensively as a model to study neuronal differentiation, but never had been tested with DPSCs. PC12 cells are dependent on nerve growth factor (NGF) and respond to this neurotrophin by undergoing neural cell differentiation exhibiting a typical neuronal phenotype with neurite outgrowths (Fujita et al., 1989; Spillane et al., 2013). NGF is a neurotrophic factor crucial for the survival and maintenance of sympathetic and sensory neurons, and it binds to the high-affinity tyrosine kinase receptor, TrkA, leading to its phosphorylation and the subsequent activation of phosphatidylinositol-3-kinase (PI3K/Akt) pathways. This, in turn, facilitates the cytoskeletal rearrangements necessary for neurite outgrowth (Chao, 2003; Alberghina and Colangelo, 2006; Sierra-Fonseca et al., 2014).
The primary objective of this study was to evaluate PC12 cells as a model for studying the neurotrophic and neurogenic impact of DPSCs secreted factors. The secondary one was to compare the efficiency of collected and pre-stored DPSCs-CM as a cell-free therapy with live DPSCs/PC12 co-cultures as a cell-based therapy. In addition, this study explored the role of specific neurotrophins (NTFs) in the survival and neurite outgrowth of DPSCs-stimulated PC12 cells.
Materials and Methods
Isolation, culture and characterization of DPSCs
DPSCs were isolated from 4–6 weeks old male Wister-Hann rat (University of Aston, Pharmaceutical Sciences Animal House, Birmingham, UK) via enzymatic digestion as described previously (Davies et al., 2014, 2015). All experiments were conducted on dead animals that were not sacrificed for the purpose of the study. All the methods were carried out in accordance with Birmingham University guidelines and regulations and the ethical approval is not needed. Isolated cells were cultured in alpha-minimum essential medium (α-MEM; Biosera, Heathfield, East Sussex, UK) supplemented with 100 μg/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine and 10% fetal bovine serum (FBS) and incubated at 37°C with 5% CO2 environment. At 70–80% confluence, the cells were sub-cultured using 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) for 10 minutes at 37°C and passaged to new culture flasks or used in experiments. DPSCs at passages 2 to 5 were used in this study.
DPSCs at passage 3 were characterized by semi-quantitative reverse transcription-polymerase chain reaction (qRT-PCR) (sqRT-PCR) using mesenchymal stem cell (MSCs) markers CD90, CD105, CD29, CD14 and CD45, neuronal marker Nestin and pluripotent markers Nanog and SOX2. Briefly, total RNA was prepared from confluent DPSCs cultures by using the RNeasy Mini Kit (Qiagen, Manchester, UK) consisting of RLT lysis buffer and washing buffers (RW1 and RPE). Extracted RNA was used to generate cDNA using a Tetro cDNA synthesis kit (Bioline, London, UK). The cDNA was amplified for sqRT-PCR by using REDTaq ready reaction mix (Sigma-Aldrich, Dorset, UK). The intensity was normalized to expression level of the endogenous reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of the rattus novergicus primers are illustrated in Table 1.
Table 1.
Specific primer sequences used in sqRT-PCR
| Genes | Primer sequences (5′–3′) | Product Size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| CD105 | TTC AGC TTT CTC CTC CGT GT | TGT GGT TGG TAC TGC TGC TC | 324 |
| CD90 | AGC TCT TTG ATC TGC CGT GT | CTG CAG GCA ATC CAA TTT TT | 385 |
| CD29 | ATC ATG CAG GTT GCA GTT TG | CGT GGA AAA CAC CAG CAG T | 385 |
| CD14 | GTT GGG CGA GAA AGG ACT GA | GCT CCA GCC CAG TGA AAG AT | 245 |
| CD45 | AGC TAC CCC TCA AAC GAA GC | TGT GAG TCC CTG GTG GTA CA | 251 |
| Nestin | CAT TTA GAT GCT CCC CAG GA | AAT CCC CAT CTA CCC CAC TC | 285 |
| Nanog | TAT CGT TTT GAG GGG TGA GG | CAG CTG GCA CTG GTT TAT CA | 350 |
| SOX2 | TCC AGT CAA GCC CCA CAT C | TCC GAG TCA CCC TTC CCA | 423 |
| GAPDH | CCC ATC ACC ATC TTC CAG GAG C | CCA GTG AGC TTC CCG TTC AGC | 473 |
Collection of DPSCs-CM
DPSCs at 70–80% confluence were used for preparation and collection of CM: The growth medium was removed, cells were washed twice with PBS, and the culture medium was replaced with serum-free α-MEM. After 72 hours incubation, the culture medium was collected and centrifuged for 5 minutes at 226 × g, 4°C. The supernatant was collected and centrifuged for another 3 minutes at 906 × g, 4°C. The resulting supernatants (denoted as DPSCs-CM) were filtered through a 0.22-μm filter unit (Merck Life Science UK Ltd, Dorset, UK) and stored in at –80°C for further experiments (Inoue et al., 2013).
Quantification of secreted neurotrophic factors
DPSCs culture supernatant was collected after 72 hours under serum-free condition and the total protein content was quantified by bicinchoninic acid assay (BCA) Kit (The Thermo Scientific™ Pierce™ BCA, Thermo Fisher Scientific, Gloucester, UK). Enzyme-linked immunosorbent assays (ELISAs) were performed to determine the concentration of NGF and brain-derived neurotrophic factor (BDNF; R&D Systems, Biotechne, UK), glial cell-derived neurotrophic factor (GDNF; Boster Picokine™, Pleasanton, CA, USA), neurotrophin-3 (NT-3; Fine Test, Rockville, MD, USA), and ciliary neurotrophic factor (CNTF; Thermo Scientific, Gloucester, UK).
PC12 cell culture
PC12 pheochromocytoma cells obtained from the American Type Culture Collection (ATCC) were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich, Dorset, UK) supplemented with 10% horse serum (HS) (Sigma-Aldrich, Dorset, UK), 5% FBS, and penicillin/streptomycin. Before each experiment, PC12 cells were plated on a poly-L-lysine coated plates (Corning Biocoat, Flintshire, UK) or coverslips (Electron Microscopy Science, CN Technical Services, Ltd, Cambridge, UK). PC12 cells were maintained at 37°C in a 95% humidified incubator with 5% CO2 before the experiments. For differentiation experiments, the cells were seeded at low density (5 × 103 cells/cm2) in RPMI 1640 medium supplemented with 10% HS, 5% FBS and penicillin/streptomycin. After 24 hours of plating, the medium was replaced with serum-free RPMI 1640 medium containing either; 50 ng/mL NGF (Cat# N6009, Sigma, St. Louis, MO, USA), 50% DPSCs-CM or co-cultured with DPSCs which had been seeded previously on Transwell culture inserts.
Cells treated with serum-free culture medium alone were used as controls. Culture medium was replaced every 2–3 days. After 8 days of incubation, morphometric analysis was done using ImageJ software (National Institutes of Health, Bethesda, MD, USA) for the average neurite length and the number of cells bearing neurites.
Co-culture experiments
DPSCs were seeded at 105 cells/200 µL into polyester Transwell insert of 24-well plates with 1 µm pore size (Merck Millipore, Life Science UK Ltd, Dorset, UK). DPSCs were seeded on top of the membrane in α-MEM with 10% FBS. After 24 hours, the culture medium was replaced with serum-free α-MEM and inserts were placed over the lower chamber containing the neuronal cell cultures and incubated for 8 days.
Assessment of PC12 survival using live and dead cell assay
To distinguish between live and dead cells, cultures were incubated with calcein-AM and ethidium homodimer-1 (EthD-1) according to live/dead assay kit manufacturer instructions (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). The fluorescence in experimental and control cell samples were measured by Spark® Multimode Microplate Reader (TECAN, Männedorf, Switzerland) using excitation and emission filters (calcein-AM=494/517 nm & EthD-1=528/617).
Assessment of PC12 proliferation and differentiation using immunocytochemistry
PC12 cell cultures were fixed with 4% paraformaldehyde (Alfa Aesar, Tewksbury, MA, USA) for 10 minutes and were permeabilized with 0.2% Triton X-100 for 10 minutes at room temperature. After blocking unspecific binding sites with 3% bovine serum albumin (BSA) (Sigma, Dorset, UK) and 10% goat serum at room temperature for 1 hour, cells were incubated overnight at 4oC with primary antibodies against; polyclonal anti-rabbit Ki-67 (proliferation marker; Cat# ab 66155 at a dilution 1:1000; Abcam, Cambridge, UK), polyclonal anti-mouse βIII-tubulin (cytoskeletal marker; Cat# ab 7751 at a 1:1000 dilution; Abcam), polyclonal anti-rabbit NeuN (mature neuronal marker; Cat# ab 128886 at a 1:1000 dilution, Abcam), monoclonal anti-mouse microtubule associated protein 2 (MAP-2) (mature neuronal marker; Cat# ab 11267 at a 1:500 dilution, Abcam). Immune reactivity was visualized with secondary conjugate Alexa flour 488 (Cat# ab 150113 anti-mouse & Cat# ab 6717 anti-rabbit at a 1:200 dilution, Abcam) and Alexa flour 555 (Cat# ab 150078 anti-rabbit & Cat# ab 6786 anti-mouse at a 1:200 dilution; Abcam) for 1 hour at room temperature. DAPI was used as a nuclear counterstaining. Images were captured using laser confocal microscopy (ZEISS LSM 700, Biomedical Core Facility (BCF), Haifa, Israel).
Neurite outgrowth assessments
Analysis was based on imaging of cells using phase-contrast microscopy (Primo Vert, ZEISS, Cambridge, UK) followed by manual tracing of neurite length using simple neurite tracer plugin and counting the number of cells bearing neurites using cell counter plugin in the ImageJ software (Pemberton et al., 2018). Images of two fields per well were taken and the neurite length was measured from the base of the soma to the end of neurite and the number of differentiated cells was determined by counting the cells that had at least one neurite with a length equal to or longer than cell body diameter. The data was obtained from 3 independent experiments with 10–15 replicates for each group/experiment.
Real time qRT-PCR
Total RNA was extracted from PC12 cultures by using the RNeasy Mini Kit (Qiagen, Manchester, UK) consisting of RLT lysis buffer and washing buffers (RW1 and RPE). Extracted RNA was converted to cDNA using Tetro cDNA synthesis kit (Bioline, London, UK). The cDNA template was used in real-time PCR reaction by using SYBER green reagents (Roche Diagnostics, Burgess Hill, UK). The specific primers for mature neuronal markers; NeuN: F-CAT GAC CCT CTA CAC GCC, R-TGG AGT TGC TGG CTA TCT GT and MAP-2: F-GAT CAA CGG AGA GCT GAC CT, R-TTG GGC CTC CTT CTC TTG TT. Cytoskeletal marker βIII-tubulin: F-ATG AGG GAG ATC GTG CAC A, R-CAC GAC ATC CAG GAC TGA GT. Synaptogenesis and axogenesis markers; GAP-43: F-GTT GAA AAG AAT GAT GAG GAC CA, R-TGC ATC ACC CTT CTT CTC GT and Synapsin-I: F-CCC AGA TGG TTC GAC TAC AC, R-GGG TAT GTT GTG CTG CTG AG are shown in Table 2. Expression levels were obtained from Cp values for each sample by employing the Fit Points method as computed by the Light Cycler 480 software (Roche Diagnostics, Burgess Hill, UK) using hypoxanthine phosphoribosyl transferase (HPRT1: F-CCC AGC GTC GTG ATT AGT GAT G, R-TTC AGT CCT GTC CAT AAT CAGT C) as housekeeper control gene.
Table 2.
Specific primer sequences used in qRT-PCR
| Gene | Primer sequences (5′–3′) | Product Size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| NeuN | CAT GAC CCT CTA CAC GCC | TGG AGT TGC TGG CTA TCT GT | 132 |
| MAP-2 | GAT CAA CGG AGA GCT GAC CT | TTG GGC CTC CTT CTC TTG TT | 124 |
| βIII-tubulin | ATG AGG GAG ATC GTG CAC A | CAC GAC ATC CAG GAC TGA GT | 140 |
| GAP-43 | GTT GAA AAG AAT GAT GAG GAC CA | GCA TCA CCC TTC TTC TCG T | 147 |
| Synapsin-I | CCC AGA TGG TTC GAC TAC AC | GGG TAT GTT GTG CTG CTG AG | 106 |
| HPRT1 | CCC AGC GTC GTG ATT AGT GAT G | TTC AGT CCT GTC CAT AAT CAG TC | 126 |
GAP-43: Growth associated protein 43; HPRT1: hypoxanthine phosphoribosyl transferase; MAP-2: microtubule associated protein 2; qRT-PCR: quantitative reverse transcription-polymerase chain reaction.
Transwell migration assay
Tissue culture inserts (Thin-cert 8 µm pore size, Greiner Bio-One, Stonehouse, UK) were loaded with PC12 cells in serum-free RPMI 1640. The bottom chamber contained either; DPSCs-CM or 10% FBS, 0% FBS which served as a positive and negative controls, respectively. After 24 hours, the cultures were stained with calcein-AM fluorescent stain. The number of calcein-stained migrated cells was counted with ImageJ software and the fluorescent intensity of the migrated cells was measured using a Spark® Multimode Microplate Reader.
Neutralization experiments
Neutralization experiments using specific blocking antibodies were conducted to determine which neurotrophic factors were involved in mediating PC12 cell survival and neurite outgrowth. PC12 was cultured with 50% DPSCs-CM in the presence of anti-NGF antibody (0.25 µg/mL; Cat# MAB556, R&D Systems), anti-GDNF antibody (1 µg/mL; Cat# AF212, R&D Systems), anti-BDNF antibody (2 µg/mL; Cat# AF1494, R&D Systems), anti-NT-3 antibody (2 µg/mL; Cat# AF1404, R&D Systems) or in the presence of mixture of all antibodies using the concentrations stated earlier.
At the beginning of each experiment, cells were washed twice with PBS and all experiments were performed in absence of serum. The cultures were maintained under the same conditions as previous experiments for 8 days, followed by quantification of neurite outgrowth and the number of survived neurons.
Statistical analysis
The difference between mean values was determined by one-way analysis of variance with Tukey’s post hoc test using SPSS Statistics version 26 (IBM, Armonk, NY, USA). The statistical significance was set at P < 0.05.
Results
DPSCs characterization using sqRT-PCR
DPSCs exhibited adhesion capacity to plastic; spindle shaped morphology, and 80% confluence reached in approximately 3–4 days. DPSCs have revealed positive expression for CD90, CD29 and CD105 markers; and negative expression for CD14 and CD45. Nanog and SOX2 expression was positive, expected for MSCs profile (Figure 1).
Figure 1.
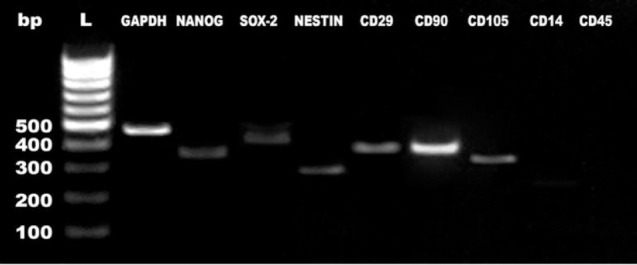
DPSCs characterization by semi-quantitative polymerase chain reaction.
Gene expression profiles of Nanog, Sox2, Nestin, CD29, CD90, CD105, CD14 and CD45 in DPSCs in relation to the expression of a housekeeping gene, GAPDH. bp: Primer band size; DPSC: dental pulp stem cell; L: ladder.
DPSCs promote PC12 survival and proliferation
Preliminary experiments tested different DPSCs-CM concentrations on PC12 cell viability using the MTT assay. The results indicated that DPSCs-CM promoted cell viability which appeared optimal at 50% concentration; while higher concentrations led to a significant reduction in cell viability (data are not shown). Consequently, 50% DPSCs-CM was used in subsequent experiments.
Live/dead cell assay confirmed that DPSCs-CM significantly increased the number of viable PC12 cells in comparison with serum-free (P < 0.001) and NGF (P < 0.05) treated cultures (Figure 2). In the serum-free control group, more than 60% of the culture was showing cell death while only 20% of cell death was detected in DPSCs-CM indicating that DPSCs cultures promoted PC12 cell protection. Interestingly, there was no statistically significant difference in live cell percentage between CM treated group and DPSCs/PC12 co-cultures (P = 0.65; Figure 2A).
Figure 2.
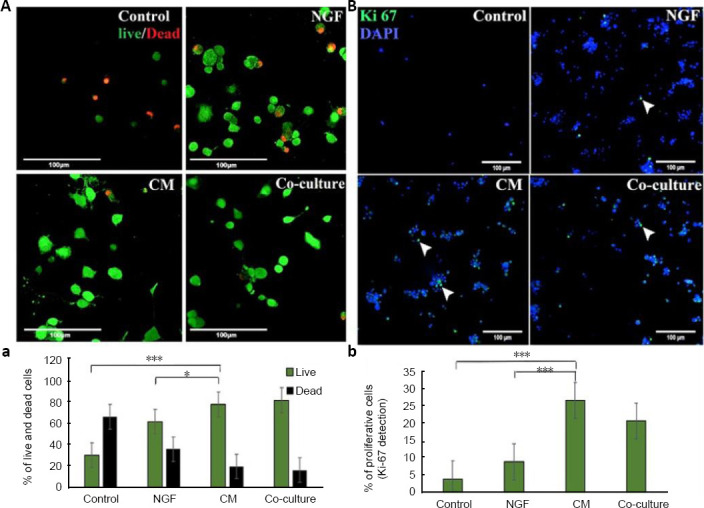
DPSCs mediate PC12 survival and proliferation.
PC12 cells were cultured in serum-free RPMI 1640 (control), 50 ng/mL nerve growth factor (NGF), 50% DPSCs-CM or DPSCs co-cultured with PC12 cells for 8 days. (A) photomicrographs showing live/dead assay where; live cells stained green with calcein-AM and dead cells stained red with EthD-1. (a) Quantitative analysis obtained from microplate reader. (B) Ki-67 immunostaining photomicrographs showing the proliferation marker positive expression. (b) The percentage of proliferative cells (Ki-67-positive cells) counted by using ImageJ cell counter. Scale bars: 100 µm. Data are presented as mean ± SEM from three independent experiments with 10 replicates for each group/experiment. *P < 0.05, ***P < 0.001 (one-way analysis of variance with Tukey’s post hoc test). CM: Conditioned medium; DPSCs: dental pulp stem cells.
Figure 2B presents the results of all tested culture media on the expression of cell proliferation marker Ki-67. PC12 cells treated with DPSCs-CM displayed 25% Ki-67 immunopositivity while serum-free and NGF treated cultures displayed 5% and 10% Ki-67 staining, respectively. There was no significant difference in PC12 cell proliferation between DPSCs-CM and co-culture treated cultures (P = 0.62).
DPSCs stimulate PC12 neuronal differentiation
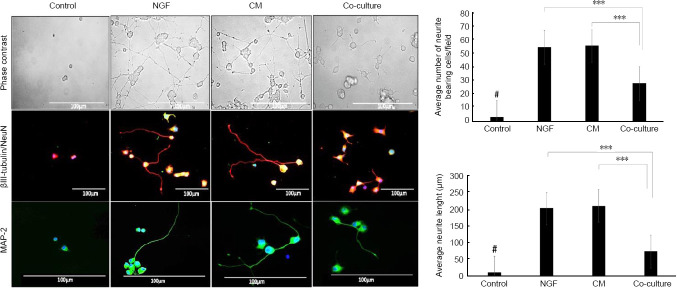
Morphometric analysis of PC12 cells under serum-free control condition revealed that the number of cells per field was greatly reduced with no neurite extensions compared with DPSCs-CM treated culture that revealed a significantly high number of cells with extensive neurite outgrowths (P < 0.001; Figure 3).
Figure 3.
DPSCs mediate PC12 differentiation.
Phase-contrast microscopic images of PC12 cells cultured on poly-L-lysine coated plates for 8 days in serum-free RPMI 1640 (control), 50 ng/mL NGF, 50% DPSCs-CM or DPSCs/PC12 co-cultures. DPSCs-CM and NGF prominently induced outgrowth of neurites from PC12 cells. Cytoskeletal marker βIII-tubulin (red) and mature neuronal marker MAP-2 (green) were used to outline the differences in the neurite length between different treated groups. DAPI was used as a counterstain for nuclei. Scale bar is 100 µm. Bar charts quantitative analysis of the average neurite length and the average number of neurites bearing cells/field using ImageJ analysis. Data are presented as mean ± SEM from three independent experiments with 15 replicates for each group/experiment. ***P < 0.001. #P < 0.001, vs. other three groups. CM: Conditioned medium; DPSCs: dental pulp stem cells; NGF: nerve growth factor.
βIII-tubulin/NeuN and MAP-2 immunocytochemistry was used to further highlight and outline the difference in the neurite lengths between the treated groups. The immunostaining revealed that the serum-free control group were devoid of any neurite extensions, whilst DPSCs-CM showed extensive neurite outgrowth stained with the mature neuronal marker MAP-2 and cytoskeletal marker βIII-tubulin. These immunostainings appeared more prominent than that seen in the co-culture treated group but appeared to be very similar to NGF treated cultures.
qRT-PCR analysis indicated that NeuN and βIII-tubulin were highly expressed in DPSCs-CM and NGF treated culture; however, MAP-2 expression was not significantly altered in the treated groups and this is unlike the immunocytochemical protein expression of the same marker as the molecular information tends to be available only as a snapshot at the end point.
Synapsin-I is an indicator of neuronal synapse formation and GAP-43 is an important marker for neurite sprouting and regeneration after nerve injury. Our data indicated that Synapsin-I and GAP-43 were significantly upregulated after DPSCs-CM and NGF treatments, even more than observed in DPSCs/PC12 co-cultures (Figure 4).
Figure 4.
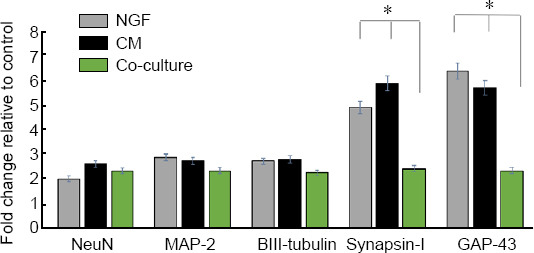
Gene expression analyses of PC12 cells after 8 days of culture with 50 ng/mL NGF, 50% DPSCs-CM and DPSCs/PC12 co-cultures.
Expression levels were obtained from Cp values for each sample by employing the fit points method as computed by the light cycler 480 software. The fold change in the expression was relative to control, HPRT1 was a housekeeping control gene. Data are presented as mean ± SEM from three independent experiments with three replicates for each group/experiment. *P < 0.05 (one-way analysis of variance with Tukey’s post hoc test). CM: Conditioned medium; DPSCs: dental pulp stem cells; NGF: nerve growth factor.
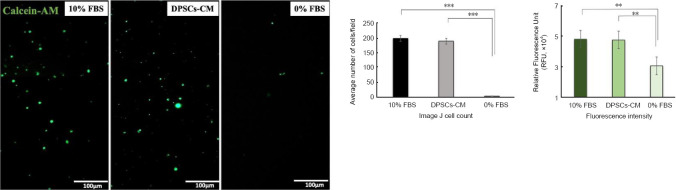
DPSCs-CM stimulate PC12 cell migration
Transwell migration assay was performed to evaluate the chemoattractive potential of DPSCs-CM on PC12 cells. Calcein-AM was used as marker to stain the migrated cells after 24 hours of exposure to DPSCs-CM, 10% FBS and 0% FBS. PC12 migration was significantly enhanced by DPSCs-CM while exposure to serum-free culture had no significant effect on cell migration (P < 0.001; Figure 5).
Figure 5.
DPSCs-CM promotes PC12 cell migration.
Calcein-AM staining of migrated cells after 24-hour exposure to 10% FBS, 0% FBS and DPSCs-CM revealed that CM had a significant chemoattractant effect on PC12 cell line migration. Data are presented as mean ± SEM from three independent experiments with 10 replicates for each group/experiment. **P < 0.01, ***P < 0.001 (one-way analysis of variance with Tukey’s post hoc test). CM: Conditioned medium; DPSCs: dental pulp stem cells; FBS: fetal bovine serum.
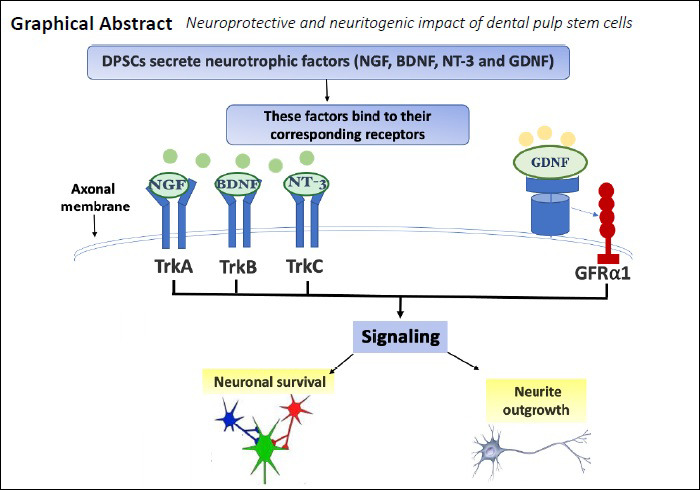
Secreted neurotrophic factors in DPSC-CM
The total protein concentration in the pooled collected serum-free DPSCs-CM was 1800 µg/ml. Neurotrophic factors NGF, BDNF, GDNF and NT-3 were detected in the DPSCs-CM as assessed by ELISA. NGF and NT-3 were predominately detected (191 and 161 pg/mL, respectively) and BDNF and GDNF amounted to 85.5 and 90 pg/mL, respectively. Only a trace amount of CNTF could be detected.
The role of neurotrophic factors in PC12 neuronal differentiation
Neutralizing antibodies were used to block the action of specific neurotrophic factors. Neutralization experiments were performed on PC12 cells incubated under the same conditions as previous experiments.
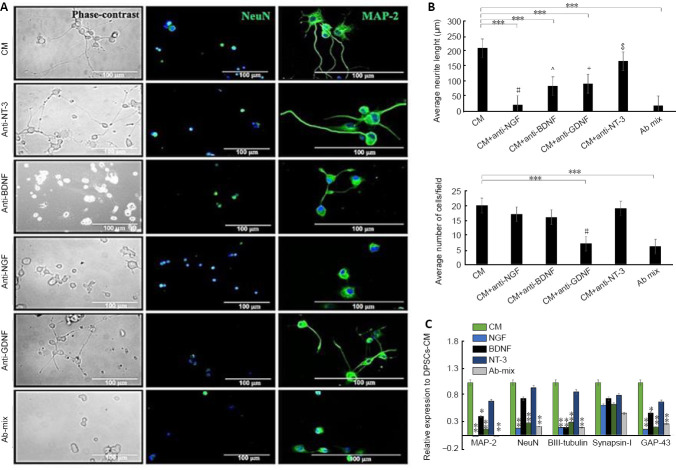
The results from image analysis of NeuN-positive cells indicated that NGF blocking did not significantly affect the number of surviving neurons in DPSCs-CM treated cultures (P = 0.99), however, neutralization of GDNF did significantly reduce the number of surviving PC12 cells (P < 0.001). Further, using a mixture of neutralizing antibodies (Ab mix) did not result in further reduction in the number of neuronal cells (P < 0.001) suggesting that GDNF was a major contributing factor to protect PC12 neuronal cell culture.
Neutralization of NGF, BDNF and GDNF respectively, significantly reduced neurite outgrowth stimulated by DPSCs-CM (P < 0.001). However, blocking of NT-3 did not significantly affect neurite outgrowth in PC12 cells (P = 0.135). These findings suggest that NGF, BDNF and GDNF were important for neurite outgrowth promoted by DPSCs-CM. Gene expression analysis using qRT-PCR indicated that neutralization of NGF and GDNF resulted in significant downregulation of NeuN, MAP-2, GAP-43 and βIII-tubulin underscoring the impact of these neurotrophic factors in the gene expression of neuronal markers (Figure 6).
Figure 6.
Inhibition studies evaluating the role of DPSCs-derived neurotrophic factors after treatment with specific neutralizing antibodies on PC12 cells.
(A) Immunolocalization of NeuN and MAP-2 showing the difference in the number of surviving neurons and the neurite length. DAPI (blue) was used as a nuclear staining. Treatment with anti-NGF antibody completely blocked neurite outgrowth evoked by DPSCs-CM while the addition of anti-GDNF antibody showed significant impact on neuronal survival. Scale bars: 100 µm. (B) Bar charts show quantification of average neurite length and average number of surviving neurons under the effect of specific neutralizing antibodies using ImageJ analysis. # Means significance compared to all groups except Ab mix (P> 0.05). ^ Means significance to all groups except anti-GDNF (P= 0.95). + Means significant to all groups except anti-BDNF (P= 0.95). $ Means significant to all groups except CM (P= 0.135). (C) qRT-PCR data showing that neutralization of NGF and GDNF resulted in significant downregulation of MAP-2, NeuN, βIII-tubulin and GAP-43. Data are presented as mean ± SEM from three independent experiments with 10 replicates for each group/experiment except for qRT-PCR with three replicates for each group/experiment. *P < 0.05, **P < 0.01, ***P < 0.001 in regard to DPSCs-CM (one-way analysis of variance with Tukey’s post hoc test). CM: Conditioned medium; DPSCs: dental pulp stem cells; GDNF: glial cell-derived neurotrophic factor; MAP-2: microtubule associated protein 2; NGF: nerve growth factor; qRT-PCR: quantitative polymerase chain reaction.
Discussion
Neuronal differentiation is dependent on a single or collective effects of extracellular signalling neurotrophic factors which are endogenously to regulate the nervous system development, regeneration and function (Mead et al., 2014a, b). PC12 cells have been widely used as a convenient model system for neurobiological and neurotoxicological studies and neuronal cell differentiation. PC12 cells are rapidly dividing, suspended cells growing in clusters and are highly responsive to NGF; upon exposure to this neurotrophin, cell proliferation is ceased followed by a dramatic change in phenotype with the cells acquiring typical neurite extensions together with a number of properties characteristic of sympathetic neurons as chemical messenger secretions such as acetylcholine or noradrenaline (Greene and Tischler, 1982; Chao, 2003; Alberghina and Colangelo, 2006; Spillane et al., 2013). NGF has a well-known multifunctional role in nociceptive processing. The mediators released from inflammatory cells such as bradykinin, histamine, ATP and serotonin has been shown to be increased with NGF application. These mediators are released from ruptured cells during inflammation or injury and are able to stimulate receptors and ion channels located on the peripheral terminal of the nociceptor, leading to neuronal depolarization and sensitization that manifests as pain hypersensitivity (Barker et al., 2020).
In recent years, DPSCs have been reported to demonstrate ability to secrete a variety of cytokines, growth and angiogenic factors such as vascular endothelial growth factors (VEGF), platelet derived growth factor (PDGF), and fibroblast growth factor (FGF) which have a beneficial effect on various diseases (Tran-Hung et al., 2008; Mita et al., 2015; Yamaguchi et al., 2015). In particular, DPSCs are increasingly gaining attention in the field of stem cell research and therapy for neurodegenerative disease or nerve injuries because of their neural crest origin and significant neurogenic and neurotrophic properties (Kolar et al., 2017). In accordance with the studies from Mead et al. (2013, 2014a, b, 2017), who illustrated that neurotrophic and neuroprotective effects of DPSCs were attributed to the plethora of secreted factors through paracrine mediated mechanism, DPSCs were shown here to be able to induce PC12 differentiation and survival. The neuroprotective effect, i.e cell survival action, of DPSCs-CM was much more prominent as compared to NGF treatment. However, the neurotrophic effect of DPSCs-CM on neurite outgrowths was very similar to that obtained following NGF treatment underlining the importance of NGF for PC12 differentiation. The neuroprotective advantages of CM over NGF treatment could be due to the presence of other soluble factors within CM like GDNF, BDNF, NT-3, and CNTF which have been proven to promote neuronal cell survival in vitro (Price et al., 2005; Schwieger et al., 2015; Palomares et al., 2018). This was in the line with a study done by Palmores et al. 2018, who used adipose stem cells conditioned medium (ADSCs-CM) and the SH-SY5Y neuronal cells and concluded that BDNF, an exogenous neurotrophic growth factor, was able to recover oxidized neuronal cells, but the effect was less pronounced than that obtained with ADSCs-CM. In contrast, Gervois et al. (2017), used DPSCs-CM to promote neurite outgrowth in SH-SY5Y cell line and concluded that exogenous BDNF was able to induce significantly longer neurites in SH-SY5Y than DPSCs-CM.
The present study using the PC12 cell model for the first time with DPSCs, suggested that DPSCs were able to secrete supportive neurotrophic factors of greater neuroprotective action than the widely used PC12 inducer, NGF. This was further confirmed by our finding that neutralizing antibodies against NGF, GDNF and BDNF, significantly attenuated the CM-mediated neurite outgrowth and produced a significant loss of morphological features associated with differentiation as assessed by MAP-2 immunostaining images. Indeed, GDNF and Abs mixture inhibition resulted in significant reduction in the number of survived cells and this was not markedly observed with NGF and BDNF inhibition. These novel results underline that GDNF was critical for PC12 cell survival, whereas NGF, GDNF and BDNF detected in DPSCs-CM were essential for neurite outgrowth. This was in accordance with de Vicente et al. (2002), who confirmed the cytoprotective role of GDNF in the knocked-out mice and concluded that GDNF inhibits cell death induced by serum deprivation.
Blocking of NT-3 did not result in any observed influence on DPSCs-CM stimulated PC12 cell survival or neurite outgrowth. A study done by Willerth and Sakiyama-Elbert (2009), concluded that low NT-3 concentrations provided an initial starting point for neurite outgrowth but did not support complete differentiation of neuronal cell lines. This could possibly give an explanation of our finding that NT-3 concentration in DPSCs-CM may not be enough to sustain PC12 neuronal survival and complete differentiation. It would therefore be interesting to further evaluate the potential impact of different neurotrophic factors, including GDNF, NT-3 and BDNF independently or synergistically on PC12 survival and neuronal differentiation, and their associated signalling downstream over time.
The co-culture system used in this study prevents direct cell-cell contact by using a semi-porous membrane allowing the infusion of soluble molecules from DPSCs to the cultured cells. It was found that there was no remarkable difference in the neuroprotective effects of DPSCs-CM and DPSCs co-cultures as both promoted cell survival and prevented cell death to the same extent. This finding is in the line with Hao et al. (2014), who failed to detect any difference in cortical neuronal survival between ADSCs-CM and ADSCs co-cultures. Another elegant study compared the capacity of ADSCs-CM and ADSCs co-cultures to recover SH-SY5Y cell viability and concluded that both had the same capacity to recover cell viability (Palomares et al., 2018). However, here we demonstrated that DPSCs-CM promoted longer neurite outgrowths from PC12 cells than that observed in DPSCs/PC12 co-cultures. The neurotrophic advantages of CM over co-cultures on PC12 neurite outgrowth length could be attributed to the rapid and concerted action of the neurotrophic factors detected in DPSCs-CM, but in the co-culture group, the time studied probably was not sufficient for the live cells to induce full terminal differentiation of PC12 cells. Further studies are required to analyse the dynamics of secretome production by live DPSCs over extended period of time.
DPSCs-CM was also able to significantly promote PC12 cell migration highlighting the chemoattractive potential of DPSCs which may be important for the attraction of endogenous stem cells to the injury site to promote nerve regeneration. These findings are consistent with other studies describing stimulation of endothelial (Bronckaers et al., 2013; Hilkens et al., 2014) and neuronal cell migration by DPSCs in vitro (Gervois et al., 2017).
In this study, under the influence of DPSCs-CM a dramatic upregulation of neuronal markers NeuN, MAP-2, βIII-tubulin in addition to Synapsin-I and GAP-43 was detected. GAP-43, an important partner in neuronal differentiation and Synapsin-I, an indicator of synapse transmission, were particularly expressed in growing neurons coinciding with the beginning of neurite outgrowth (Mead et al., 2014a, b). These data suggest that soluble secreted factors from DPSCs were able to stimulate relevant neural-associated gene expression in PC12 cells, increasing the expression of neuronal markers and axonal regeneration underlining the significant impact of CM on PC12 neuronal differentiation and synapse formation. The enhanced expression of MAP-2, NeuN and GAP-43 under the influence of DPSCs-CM was significantly reduced upon NGF and GDNF inhibition suggesting that neurotrophic factors NGF and GDNF in the CM mediated PC12 neuronal differentiation and axon formation.
Although interesting findings, but this study had potential limitations; the differentiation effect of DPSCs-CM on PC12 neuronal model was based on morphologically measuring the average length of neurite extension and the number of differentiated cells/field. Although the differentiated cells were positively expressed NeuN and MAP-2, which are mature neuronal markers, but the functionality of these differentiated neurons was not tested via electrophysiology, so it was difficult to confirm whether these differentiated neurons were electrically excitable or not.
Conclusions
To the best of our knowledge, this is the first study addressing the paracrine effects of DPSCs in the well-established neuronal PC12 cell model. Thus, this study demonstrated that CM derived from DPSCs profoundly enhanced the viability, proliferation, migration, and differentiation of PC12 cells in vitro. DPSCs-derived GDNF proved in particularly to be critical for PC12 viability and survival. DPSCs-CM appeared more effective than DPSCs/PC12 co-cultures in promoting PC12 survival and differentiation underscoring that the live cells co-cultures had a delayed lag time in producing effective amounts of trophic factors. These new findings highlight the useful application of the PC12 cell line for exploring the role of specific signalling factors present in DPSCs/MSC secretomes.
Acknowledgments:
This project was done at the University of Birmingham, Dental School, UK under supervision of Dr Ben Scheven.
Footnotes
Conflicts of interest: The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this manuscript.
Financial support: The study was funded by Egyptian Cultural and Educational Bureau in London, Egyptian mission sector and ministry of higher education in Egypt with grant number GAM2649.
Institutional review board statement: All experiments were conducted on dead animals that were not sacrificed for the purpose of the study. All the methods were carried out in accordance with Birmingham University guidelines and regulations and the ethical approval is not needed.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: The study was funded by Egyptian Cultural and Educational Bureau in London, Egyptian mission sector and ministry of higher education in Egypt (grant No. GAM2649).
C-Editors: Zhao M, Li CH; T-Editor: Jia Y
References
- 1.Aguayo AJ, Peyronnard JM, Bray GM. A quantitative ultrastructural study of regeneration from isolated proximal stumps of transected unmyelinated nerves. J Neuropathol Exp Neurol. 1973;32:256–270. doi: 10.1097/00005072-197304000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Alberghina L, Colangelo AM. The modular systems biology approach to investigate the control of apoptosis in Alzheimer’s disease neurodegeneration. BMC Neurosci. 2006;7(Suppl 1(Suppl 1)):S2. doi: 10.1186/1471-2202-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker PA, Mantyh P, Arendt-Nielsen L, Viktrup L, Tive L. Nerve growth factor signaling and its contribution to pain. J Pain Res. 2020;13:1223–1241. doi: 10.2147/JPR.S247472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronckaers A, Hilkens P, Fanton Y, Struys T, Gervois P, Politis C, Martens W, Lambrichts I. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8:e71104. doi: 10.1371/journal.pone.0071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 6.Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. 2015;33:371–382. doi: 10.1007/s00774-014-0601-y. [DOI] [PubMed] [Google Scholar]
- 7.Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA. The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology. 2014;69:342–347. doi: 10.1016/j.cryobiol.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.de Vicente JC, Cabo R, Ciriaco E, Laurà R, Naves FJ, Silos-Santiago I, Vega JA. Impaired dental cytodifferentiation in glial cell-line derived growth factor (GDNF) deficient mice. Ann Anat. 2002;184:85–92. doi: 10.1016/S0940-9602(02)80041-3. [DOI] [PubMed] [Google Scholar]
- 9.di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63:1544–1552. doi: 10.1016/j.bjps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gervois P, Wolfs E, Dillen Y, Hilkens P, Ratajczak J, Driesen RB, Vangansewinkel T, Bronckaers A, Brône B, Struys T, Lambrichts I. Paracrine maturation and migration of SH-SY5Y cells by dental pulp stem cells. J Dent Res. 2017;96:654–662. doi: 10.1177/0022034517690491. [DOI] [PubMed] [Google Scholar]
- 13.Greene LA, Tischler AS. PC12 pheochromocytoma cultures in neurobiological research. Adv Cell Neurobiol. 1982;3:373–414. [Google Scholar]
- 14.Guénard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12:3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao P, Liang Z, Piao H, Ji X, Wang Y, Liu Y, Liu R, Liu J. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab Brain Dis. 2014;29:193–205. doi: 10.1007/s11011-014-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilkens P, Fanton Y, Martens W, Gervois P, Struys T, Politis C, Lambrichts I, Bronckaers A. Pro-angiogenic impact of dental stem cells in vitro and in vivo. Stem Cell Res. 2014;12:778–790. doi: 10.1016/j.scr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Sugiyama M, Hattori H, Wakita H, Wakabayashi T, Ueda M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2013;19(1-2):24–29. doi: 10.1089/ten.tea.2011.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolar MK, Itte VN, Kingham PJ, Novikov LN, Wiberg M, Kelk P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep. 2017;7:12605. doi: 10.1038/s41598-017-12969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Ping P, Jiang H, Liu K. Nerve conduit filled with GDNF gene-modified Schwann cells enhances regeneration of the peripheral nerve. Microsurgery. 2006;26:116–121. doi: 10.1002/micr.20192. [DOI] [PubMed] [Google Scholar]
- 20.Luo L, He Y, Wang X, Key B, Lee BH, Li H, Ye Q. Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells Int. 2018;2018:1731289. doi: 10.1155/2018/1731289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Dental pulp stem cells, a paracrine-mediated therapy for the retina. Neural Regen Res. 2014a;9:577–578. doi: 10.4103/1673-5374.130089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- 23.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014b;9:e109305. doi: 10.1371/journal.pone.0109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. Stem Cells. 2017;35:61–67. doi: 10.1002/stem.2398. [DOI] [PubMed] [Google Scholar]
- 25.Mita T, Furukawa-Hibi Y, Takeuchi H, Hattori H, Yamada K, Hibi H, Ueda M, Yamamoto A. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav Brain Res. 2015;293:189–197. doi: 10.1016/j.bbr.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Palomares T, Cordero M, Bruzos-Cidon C, Torrecilla M, Ugedo L, Alonso-Varona A. The neuroprotective effect of conditioned medium from human adipose-derived mesenchymal stem cells is impaired by N-acetyl cysteine supplementation. Mol Neurobiol. 2018;55:13–25. doi: 10.1007/s12035-017-0714-0. [DOI] [PubMed] [Google Scholar]
- 27.Pemberton K, Mersman B, Xu F. Using ImageJ to assess neurite outgrowth in mammalian cell cultures: research data quantification exercises in undergraduate neuroscience lab. J Undergrad Neurosci Educ. 2018;16:A186–194. [PMC free article] [PubMed] [Google Scholar]
- 28.Price TJ, Louria MD, Candelario-Soto D, Dussor GO, Jeske NA, Patwardhan AM, Diogenes A, Trott AA, Hargreaves KM, Flores CM. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6:4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rayner MLD, Laranjeira S, Evans RE, Shipley RJ, Healy J, Phillips JB. Developing an in vitro model to screen drugs for nerve regeneration. Anat Rec (Hoboken) 2018;301:1628–1637. doi: 10.1002/ar.23918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwieger J, Warnecke A, Lenarz T, Esser KH, Scheper V. Neuronal survival, morphology and outgrowth of spiral ganglion neurons using a defined growth factor combination. PLoS One. 2015;10:e0133680. doi: 10.1371/journal.pone.0133680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sierra-Fonseca JA, Najera O, Martinez-Jurado J, Walker EM, Varela-Ramirez A, Khan AM, Miranda M, Lamango NS, Roychowdhury S. Nerve growth factor induces neurite outgrowth of PC12 cells by promoting Gβγ-microtubule interaction. BMC Neurosci. 2014;15:132. doi: 10.1186/s12868-014-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–203. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 34.Tran-Hung L, Laurent P, Camps J, About I. Quantification of angiogenic growth factors released by human dental cells after injury. Arch Oral Biol. 2008;53:9–13. doi: 10.1016/j.archoralbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Willerth SM, Sakiyama-Elbert SE. Kinetic analysis of neurotrophin-3-mediated differentiation of embryonic stem cells into neurons. Tissue Eng Part A. 2009;15:307–318. doi: 10.1089/ten.tea.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi S, Shibata R, Yamamoto N, Nishikawa M, Hibi H, Tanigawa T, Ueda M, Murohara T, Yamamoto A. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci Rep. 2015;5:16295. doi: 10.1038/srep16295. [DOI] [PMC free article] [PubMed] [Google Scholar]