Abstract
Astrocytes play multifaceted and vital roles in maintaining neurophysiological function of the central nervous system by regulating homeostasis, increasing synaptic plasticity, and sustaining neuroprotective effects. Astrocytes become activated as a result of inflammatory responses during the progression of pathological changes associated with neurodegenerative disorders. Reactive astrocytes (neurotoxic A1 and neuroprotective A2) are triggered during disease progression and pathogenesis due to neuroinflammation and ischemia. However, only a limited body of literature describes morphological and functional changes of astrocytes during the progression of neurodegenerative diseases. The present review investigated the detrimental and beneficial roles of astrocytes in neurodegenerative diseases reported in recent studies, as these cells have promising therapeutic potential and offer new approaches for treatment of neurodegenerative diseases.
Keywords: A1, A2, astrocytes, neurodegenerative diseases, neuroinflammation, neuron, neuroprotection, neurotoxicity, polarization, reactivity
Introduction
Astrocytes constitute 20–40% of the total number of cells in the mammalian brain (Khakh and Sofroniew, 2015; Liddelow and Barres, 2017a). Astrocytes have been described as passive support cells of neurons for more than 30 years. Recently, astrocytes have been implicated in various physiological functions, such as secretion of trophic molecules, modulation of the brain microenvironment, maintenance of blood-brain barrier (BBB) integrity, and normal function of synapses (Farhy-Tselnicker et al., 2018; Qian et al., 2018; Verkhratsky and Nedergaard, 2018; Walker et al., 2020). In addition, Stadelmann et al. (2019) reported astrocytic glycogen utilization in the presence of high neuronal activity, and Guillamon-Vivancos et al. (2015) reviewed roles of astrocytes in the regulation of blood flow, lipoprotein secretion, and neurogenesis. Universal involvement of reactive astrocytes in neurodegenerative diseases has been described. Astrocytes exhibit wide and varied spectra of reactivity, heterogeneous morphology, and molecular expression (Escartin et al., 2019). Astrocyte reactivity is triggered by chemicals, toxins, oxidative stress, or pathogens (Jensen et al., 2013; Angelova and Abramov, 2016). Moreover, astrocytes change their morphology in response to different pathological conditions, such as epilepsy, mechanical lesions of the cerebrospinal parenchyma, and tumors (Henneberger et al., 2017; Okada et al., 2018; Cheng et al., 2019; Campbell et al., 2020). Numerous molecular triggers can activate astrocytes, such as transforming growth factor β1 (TGF-β1), leukemia inhibitory factor (LIF), and ciliary neurotrophic factor (Tang et al., 2017; Ongali et al., 2018; Wang et al., 2020). After stimulation, astrocytes secrete pro-inflammatory molecules such as reactive oxygen species (ROS), interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor α (TNF-α); or release protective factors such as vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and nerve growth factor (Ludwin et al., 2016; Rizor et al., 2019). Consequently, reactive astrocytes elicit the release of chemokines such as β-chemokine 2 (CCL2), β-chemokine 20 (CCL20), α-chemokine 10 (CXCL10), and α-chemokine 12 (CXCL12) (Correale et al., 2015), as well as cell adhesion molecules including vascular cell adhesion molecule 1, neural cell adhesion molecule 1, and intercellular adhesion molecule 1 (Liddelow and Hoyer, 2016). Moreover, reactive astrocytes can secrete vasoactive molecules including VEGF and heparin-binding epidermal growth factor (HB-EGF) (Liu et al., 2018; Kushwaha et al., 2019). Therefore, it has been speculated that reactive astrocytes may have distinct polarization in neurodegenerative diseases (Liddelow and Barres, 2017a; Cragnolini et al., 2020). Roles of activated astrocytes in neurodegenerative diseases are illustrated in Figure 1.
Figure 1.
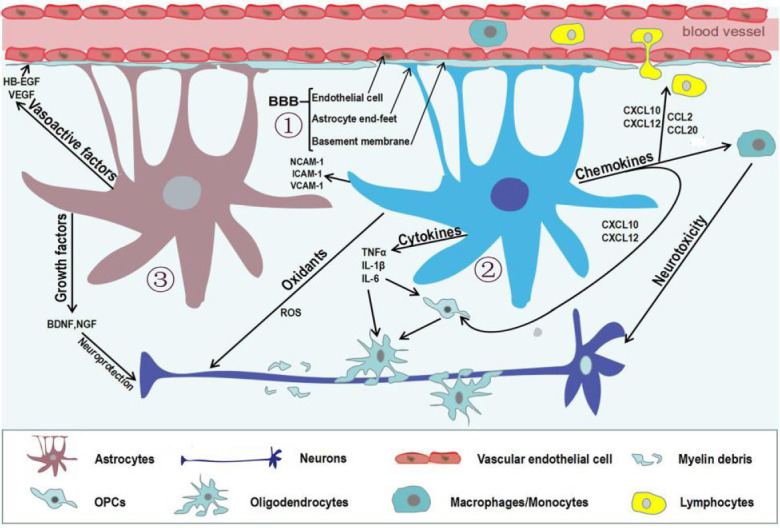
Reactive astrocytes in neurodegenerative diseases.
Astrocytes can be activated by multiple stimuli, particularly cytokines secreted by different cell types in the brain. In turn, activated astrocytes play a positive or negative role in multiple ways. (1) The blood-brain barrier (BBB) established by the end-feet of astrocytes, capillary endothelial cells, and basement membrane maintains homeostasis of the central nervous system. (2) Reactive astrocytes lose end-feet surrounding capillaries, resulting in damage to the BBB. Meanwhile, reactive astrocytes enhance the release of oxidants, cytokines, chemokines, and cell adhesion molecules, which can directly or indirectly damage oligodendrocyte precursor cells, oligodendrocytes, and neurons. (3) Vasoactive factors secreted by activated astrocytes affect normal endothelial cells by regulating junction-related proteins, which preserves the integrity of the BBB. Moreover, reactive astrocytes can promote remyelination and neuronal regeneration by secreting several growth factors.
Increasing evidence describes the dual role of reactive astrocytes in pathophysiological mechanisms underlying neurodegenerative diseases (Oksanen et al., 2019; Cragnolini et al., 2020). However, strategies to evaluate this potential remain scarce. In the present review, we address morphological differentiation and astrocytic phenotypes, and summarize the association of various receptors and molecular signaling pathways of polarized astrocytes. We further elaborate the biphasic roles of activated astrocytes in several neurodegenerative disorders and analyze pathways for potential therapeutic targets in future studies.
Search Strategy and Selection Criteria
We searched PubMed to retrieve publications with the search terms “astrocytes, neurodegenerative diseases, neuroinflammation, A1s, A2s, neurotoxicity, neuroprotection, polarization, reactivity, neuron” and then downloaded and concluded all relative publications in this database.
Switching of Astrocytes from Resting (A0) to Activated Forms (A1 and A2)
Astrocytic polarization has been well elucidated by several studies, as have roles of transcription factors in modulating astrocyte activity in animal models and in vitro models of neurodegenerative diseases. Reactive astrocytes exhibit detrimental or beneficial effects, depending on their reactivity. In lipopolysaccharide-induced and middle cerebral artery occlusion mouse models of infection and stroke, respectively, resting (A0) astrocytes are converted into reactive forms by upregulation or downregulation of certain genes (Zamanian et al., 2012). Based on gene expression profiles, reactive astrocytes exist in two forms: A1 (neurotoxic) and A2 (neuroprotective) (Liddelow and Barres, 2017a).
A1 astrocytes are classically induced by activated neuroinflammatory microglia. Studies have reported that reactive microglia secrete component 1 subcomponent q (C1q), TNF-α, and IL-1α, facilitating the shift of astrocytes toward an A1 phenotype (Liddelow et al., 2017b). A1 astrocytes are highly upregulated by several classical complement cascade genes, resulting in neurotoxicity through secretion of well-characterized neurotoxins (Liddelow and Barres, 2017a). Moreover, A1 astrocytes can cause loss of synaptogenesis, induction of oligodendrocytes, and neuronal death (Liddelow and Barres, 2017a).
A2 astrocytes are considered a neuroprotective astrocytic phenotype following induction by microglia after traumatic brain injury (Shinozaki et al., 2017). Su et al. (2019) described modulation of A2 astrocytes in an acute ischemic spinal cord injury model by silencing of miR-21. Nuclear factor IA (NFIA), a molecular switch that induces human glial competency, causes astrocytes to switch their function towards neuroprotection (A2) in engrafted adult mouse brain (Tchieu et al., 2019). Consequently, A2 astrocytes upregulate several neuroprotective factors that promote synaptic repair, growth, and survival of neurons (Hodgetts and Harvey, 2017; Tome et al., 2017; Lam et al., 2019); in contrast, A1 astrocytes upregulate inflammatory markers (ROS, IL-1β, TNF-α) (Lee et al., 2019). Protective mediators are upregulated in A2 astrocytes, including protein prokineticin-2 (PK2), chitin-like 3, Frizzled class receptor 1 (Liddelow and Barres, 2017a), arginase 1, NF-E2-related factor 2 (Nrf2), pentraxin 3, sphingosine kinase 1, and transmembrane 4 L6 family member 1 (Neal et al., 2018). Therefore, we delineated other mediators that are upregulated or downregulated in these two types of reactive astrocytes (Figure 2).
Figure 2.
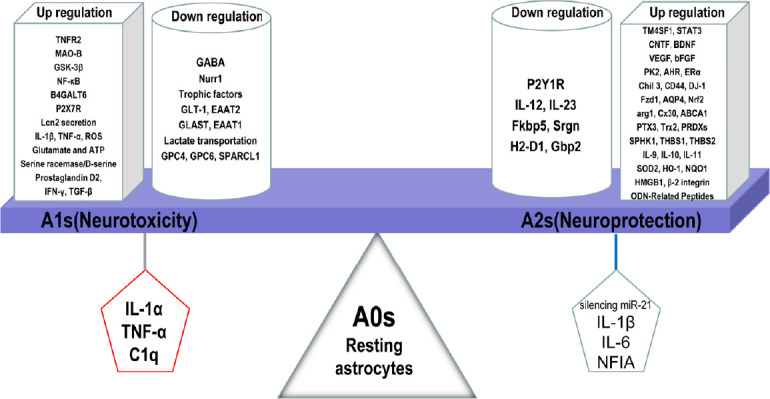
Detrimental and beneficial effects of reactive astrocytes induced by various mediators or up/downregulated genes.
Resting (A0) astrocytes are stimulated to convert into reactive functional phenotypes (A1 and A2). C1q, IL-1α, and TNF-α secreted by pro-inflammatory microglia transform A0 into A1 astrocytes, which is accompanied by up/downregulation of numerous genes leading to neurodegeneration. In contrast, A2 astrocytes are induced from A0 astrocytes by IL-1β, IL-6, NFIA, and silencing of miR-21, potentially leading to neuroprotective effects by up/downregulation of genes in neurodegenerative disorders. C1q: Component 1 subcomponent q; IL-1α: interleukin-1α; IL-1β: interleukin-1β; IL-6: interleukin-6; NFIA: nuclear factor IA; TNF-α: tumor necrosis factor α.
Currently, we have described reactive astrocytes into two states of polarization and their characteristics. However, it has also been postulated that reactive astrocytes exist in several forms, as activated by varied factors and up/downregulation of other genes in response to disease. Further research is required to confirm and strengthen the hypothesis of astrocyte switching.
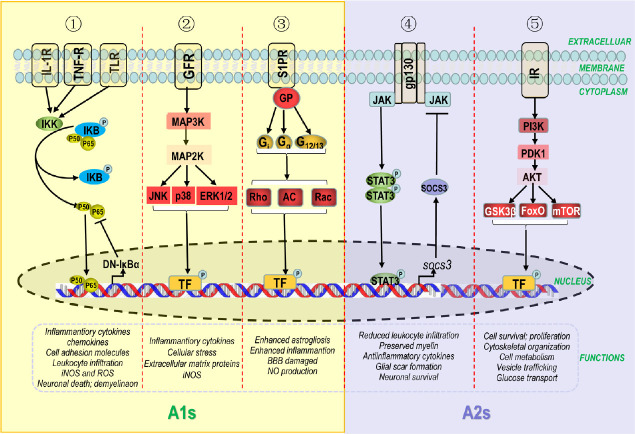
Signaling Pathways for Astrocyte Reactivity: A1/A2 Phenotypes and Their Roles in Neurodegenerative Diseases
Activation of intracellular signaling pathways polarizes astrocytes into an A1 or A2 phenotype during pathological changes after central nervous system (CNS) injury (Liddelow and Barres, 2017a). Astrocyte reactivity is primarily controlled by nuclear factor kappa-B (NF-κB), Toll-like receptor (TLRs), mitogen-activated protein kinase (MAPK), sphingosine 1-phosphate receptor (S1PR), Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), and phosphatidylinositol-3-kinases/protein kinase B (PI3K/AKT) pathways. However, mechanisms determining the fate of astrocytic switching are still poorly understood. Therefore, it is imperative to delineate the neurotoxic and neuroprotective effects of astrocytic signaling pathways in neurodegenerative diseases.
The NF-κB signaling pathway is associated with neuroinflammatory responses of astrocytes, leading to their activation during the progression of neurodegenerative diseases. In addition, NF-κB signaling controls the production of inflammatory cytokines, chemokines, cell adhesion molecules, inducible nitric oxide synthase (iNOS), and ROS, and plays crucial roles in leukocyte infiltration, neuronal death, and demyelination (Colombo and Farina, 2016; Li et al., 2018b). The NF-κB pathway was activated in glial fibrillary acidic protein (GFAP)-positive astrocytes in an animal model of Alzheimer’s disease (AD) induced by amyloid β1–42 (Aβ1–42) oligomers, consequently modulating the expression of downstream genes IL-1β and cyclooxygenase 2 (Carrero et al., 2012). Patients with amyotrophic lateral sclerosis (ALS) showed a similar trend of increased NF-κB activity in spinal cord astrocytes (Ouali Alami et al., 2018). Dominant-negative inhibitor of κBα (DN-IκBα), which is driven by the GFAP promoter, inhibits cytokine expression, protects neurons, and promotes functional recovery after spinal cord or optic nerve injury (Dresselhaus and Meffert, 2019). Conversely, activation of the NF-κB pathway in A1 astrocytes may exert harmful effects and contribute to the pathophysiological progression of neurodegenerative diseases in mouse models. Activation of TLR ligands in astrocytes also promotes inflammatory responses in the CNS (Azam et al., 2019). For example, TLR4 expression increases astrocytic sensitivity to ligands and facilitates conversion of astrocytes to an A1 phenotype, as demonstrated in both a rat model of cerebral ischemia and primary astroglia cell cultures (Leitner et al., 2019).
The S1PR signaling pathway in astrocytes enhances astrogliosis, inflammation, and nitric oxide production, and damages the BBB (Healy and Antel, 2016; Dusaban et al., 2017; Rothhammer et al., 2017). S1PR expression was extensively upregulated in activated astrocytes of mouse models of AD (McManus et al., 2017) and multiple sclerosis (MS) (Van Doorn et al., 2010). Notably, S1PR1 deletion in astrocytes improved disease severity in a myelin oligodendrocyte glycoprotein (MOG35–55)-induced mouse model of MS, suggesting its therapeutic potential to enhance neuroprotection and CNS repair (Healy and Antel, 2016). Unfortunately, the mechanism underlying this effect has not been elucidated.
The MAPK pathway is also activated in astrocytes during neurodegenerative disease, whereby it orchestrates production of inflammatory cytokines and extracellular matrix proteins, and upregulates iNOS expression (Perez-Nievas and Serrano-Pozo, 2018). Active forms of c-jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and P38 mitogen-activated protein kinase (p38 MAPK) are reportedly expressed in activated astrocytes of mouse models and patients with ALS. Similarly, activation of p38 MAPK and JNK in reactive astrocytes is commonly observed in the CNS of patients with multifarious tauopathies (Ben Haim et al., 2015). In the brains of patients with AD, phosphorylated p38 MAPK expression in astrocytes was increased around amyloid plaques (Hensley et al., 1999). Notably, blockade of the p38 MAPK pathway elicited anti-inflammatory effects in TNF-α/IL-1α-induced astrocytes (Da Silva et al., 1997). More recently, a postmortem pathological study demonstrated that these pathways are activated in reactive astrocytes during the early stage of AD (Perez-Nievas and Serrano-Pozo, 2018). Therefore, blockade of MAPK signaling in astrocytes may be a new avenue of research to alleviate the progression of neurodegenerative diseases.
Upregulation of certain signaling pathways in A2 astrocytes is beneficial for neurodegenerative diseases. The JAK/STAT3 pathway regulates astrocytic functions such as reduction of leukocyte infiltration, preservation of myelin preservation, secretion of anti-inflammatory cytokines, formation of glial scars, and promotion of neuronal survival (Colombo and Farina, 2016). In addition, Liddelow and Barres (2017a) suggested that the JAK/STAT3 pathway mediates astrocytes activation. Use of a GFAP or nestin promoter to ablate STAT3 in astrocytes reduced their migration and hypertrophy, leading to widespread infiltration of pro-inflammatory cells (Okada et al., 2006). In mouse models of spinal cord injury, nestin promoter-driven ablation of suppressors of cytokine signaling 3 (SOCS3) inhibited STAT3 signaling, enhanced lesion contraction, increased astrocytic migration, and restored physiological function (Okada et al., 2006).
PI3K/AKT pathway activation occurs in response to a variety of extracellular signals (Jia et al., 2017), and regulates the balance of inflammatory molecules to protect the CNS (Schabbauer et al., 2004; Zhao et al., 2015). AKT2, one isoform of the AKT family, is primarily expressed in astrocytes (Levenga et al., 2017). Activation of AKT regulates cellular functions such as survival, metabolism, skeletal organization, proliferation, and glucose and vesicular transport (Gabbouj et al., 2019). Recently, milk fat globule epidermal growth factor 8 was found to regulate A1/A2 polarization, leading to promotion of neuronal and synaptic growth (resulting in increased survival of neurons) through upregulation of the PI3K/AKT pathway (Xu et al., 2018). Interestingly, enhancement of PI3K/AKT signaling in astrocytes could ameliorate deficits in cognitive function of mouse models and patients with AD (Gabbouj et al., 2019). However, the specific mechanism of this signaling pathway has not been well defined.
Taking these important findings as a whole, we outlined the complex intracellular signaling pathways for astrocytic transformation (A1/A2) and the roles of these two phenotypes in neurodegenerative diseases (Figure 3). In addition, signaling pathways important for the functional roles of each reactive astrocyte phenotype are illustrated, as they provide novel directions for further studies to prevent and treat neurodegenerative diseases.
Figure 3.
Intracellular signaling pathways for astrocytic transformation (A1/A2) and roles in neurodegenerative disease.
Dysfunctional cells (reactive microglia and neurons) secrete molecules recognized by specific receptors in the astrocytic cytomembrane. These extracellular signals activate intracellular signaling pathways including NF-κB, MAPK, S1PR, JAK/STAT3, and PI3K/AKT. (1) The NF-κB pathway is triggered by inflammatory mediators. Receptor-bound protein kinases activate the IKK complex, resulting in phosphorylation of IκBα, the stable inhibitor of NF-κB. p50 and p65, two subunits of NF-κB, translocate to the nucleus and activate transcription of A1-related genes. DN-IκBα inhibits p50 and p65. (2) The MAPK pathway is partly initiated by cytokines and growth factors, which initiate a cascade amplification of phosphorylation. After triggering, JNK, p38, and ERK1/2 upregulate gene transcription of A1 astrocytes by initiating a large number of transcription factors (TF). (3) The S1PR pathway is activated by extracellular sphingosine-1-phosphate (S1P). S1PR binds to heterotrimeric G-proteins (GPs), such as Gi, Gq and G12/13, which in turn affect downstream proteins such as phospholipase adenylate cyclase (AC), Ras homolog (Rho), Ras-related C3 botulinum toxin substrate (Rac), which translocate to the nucleus to activate expression of A1-related genes. (4) The JAK/STAT3 pathway is triggered by cytokines. Upon cytokine coupling, JAK kinase is initiated and STAT3 is gathered to gp130 (intracellular receptor). Consequently, STAT3 is phosphorylated and translocates to the nucleus, whereby it upregulates gene transcription of A2-related genes. STAT3 also promotes SOCS3 expression, which inhibits the JAK/STAT3 pathway. (5) The PI3K/AKT pathway is activated by binding of insulin to insulin receptor (IR), which activates phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K). AKT is activated upon its interaction with phosphoinositide dependent protein kinase 1 (PDK1), which is indirectly phosphorylated by PI3K. Subsequently, AKT modulates transcription factors, such as glycogen synthase kinase 3 (GSK3β), forkhead box (FOX), and mechanistic target for rapamycin (mTOR), which are associated with several functions of A2 astrocytes.
Roles of Reactive (A1/A2) Astrocytes in Neurodegenerative Diseases
Astrocytes are known to alter neurotransmitter synthesis, ultimately modulating their release from glia (Wu et al., 2014; Trujillo-Estrada et al., 2019). Further, the release fates of metabolites (Morita et al., 2019), cytokines, chemokines, and neurotrophic factors (Chou et al., 2008; Li et al., 2019; Yin et al., 2020) are controlled by activated astrocytes. In addition, reactive astrocytes can enhance the process of phagocytosis (Gomez-Arboledas et al., 2018), and production or clearance of ROS in disease models (Ye et al., 2015). Indeed, these cellular functions can be strengthened or weakened by astrocyte activation.
Astrocytes in AD
The role of astrocytes in AD pathogenesis was first reported decades ago in clinical and animal studies. Astrocytes influence cognitive function by regulating the expression of genes encoding extracellular matrix proteins that surround neurons (Dallérac and Rouach, 2016). Astrocytes acquire toxic properties and lose their neuroprotective characteristics during the progression of AD (Acosta et al., 2017). Neuroinflammatory molecules trigger the accumulation of amyloid beta (Aβ) and phosphorylated tau neurofibrillary tangles (Gao et al., 2019). Deposition of Aβ plaque prompts A1 astrocytes to produce more inflammatory mediators, including chemokines, pro-inflammatory cytokines, and ROS, resulting in neuronal damage (Cai et al., 2017). These pro-inflammatory signals provide feedback to astrocytes that accelerates their activation. This vicious cycle provides a chronic inflammatory environment for astrocytes, leading to the pathogenesis and progression of AD.
Reactive astrocytes exert biphasic effects (detrimental or beneficial) in AD (Zhao et al., 2020). A1 astrocytes surround amyloid plaques and secrete pro-inflammatory cytokines (TNF-α, IL-1 and IL-6), leading to AD pathogenesis and progression (Kaur et al., 2019). In addition, A1 astrocytes were activated by ApoE4 in a tauopathy mouse model (Shi et al., 2017b). Activated astrocytes engulf plaque-associated dystrophic neurites and Aβ in vitro and in vivo, indicating their protective role in the attenuation of neurodegenerative processes associated with AD (Liu et al., 2016; Gomez-Arboledas et al., 2018).
Excitotoxicity and neurodegeneration resulting from extracellular glutamate are reduced by synthesis of glutamine and glutamate transporters in reactive astrocytes (Shi et al., 2017a; Mahmoud et al., 2019). Mitigation of astrocyte reactivity by depletion of two intermediate filament proteins, vimentin and GFAP, resulted in the proliferation of plaque-associated malnutritional neurites, consequently reducing activated astrocytes in double-transgenic AD mice (Kraft et al., 2013). Glial cell derived neurotrophic factor (GDNF) secreted from astrocytes can protect neuronal function and cognitive performance in 3xTg-AD mice and MC65 cells (Revilla et al., 2014).
Astrocytes induced by Aβ deposition increased the release of ROS and exhibited harmful effects on cortical neurons (Allaman et al., 2010). However, antioxidants, such as glutathione (GSH) generated by reactive astrocytes, can protect neurons from toxic substances (McGann and Mandel, 2018). A0 astrocytes maintain a balance between ROS production and antioxidant generation under physiological conditions. Reactive astrocytes exert neuroprotective functions when expressing low levels of ROS (Wang et al., 2006), suggesting that ROS production in astrocytes is critical for normal neuronal functions.
Astrocytes in PD
Reactive astrocytes are commonly observed in animal models of Parkinson’s disease (PD), whereby they play a dual role in disease development (Rizor et al., 2019). A1 astrocytes secrete toxic molecules and induce dopaminergic neuronal death in PD mouse models. In mice overexpressing α-synuclein (α-Syn), accumulation of α-Syn induces astrocytes to release chemokines (CCL2, CCL20, CXCL1, and CX3CL1) and pro-inflammatory cytokines (TNF-α, IL-1, IL-6), suggesting that reactive astrocytes induced by α-Syn are injurious in PD (Lee et al., 2010).
Reactive astrocytes have dual functions of generation and removal of ROS in PD. Oxidative stress-induced damage in brain regions is specific to PD (Raza et al., 2019). Studies have elucidated roles for dopamine catalysis, mitochondrial failure to generate ROS, and oxidative stress in astrocytes and PD pathogenesis. Baillet et al. (2010) reported that astrocytes limit synthesis and release of GSH in the substantia nigra of patients, while activated astrocytes upregulate release of antioxidants [superoxide dismutase (SOD), GSH catalase, and peroxidase]. Astrocytic oxidation promotes PD pathogenesis, but A2 astrocytes may regulate detoxification and mitigation of ROS. Therefore, balancing chemical communication between astrocytes and neurons is important for neuronal protection against ROS (Belanger and Magistretti, 2009).
Astrocytic dysfunction in patients with PD has been directly linked to PD proteins such as DJ-1, a parkin-associated protein controlled by the Parkinson disease 7 gene (PARK7). In an animal model of PD, DJ-1 in astrocytes exhibited a neuroprotective effect (De Miranda et al., 2018). Moreover, DJ-1 upregulation in astrocytes protected neurons against rotenone-induced PD, while DJ-1 downregulation enhanced neuronal susceptibility to toxins during PD pathogenesis (Rizor et al., 2019). Another astrocytic gene, parkin, is expressed only under pathogenic conditions and not basal conditions, providing evidence of selectivity for astrocytes (Ledesma et al., 2002). Knockout of parkin induced astrocytic dysfunction and exacerbated neuronal death in an animal model of PD (Singh et al., 2018). Astrocytic Nrf2 also plays a vital role in PD. Nrf2 overexpression in reactive astrocytes was shown to protect dopaminergic neurons in 6-hydroxydopamine, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and A53T mouse models of PD (Jakel et al., 2007; Chen et al., 2009; Gan et al., 2012). Accumulating evidence indicates that astrocytes are a major contributor to the regulation of neuroinflammation in PD. Recently, connexin 30, an astrocytic gap junction protein, was shown to exhibit a critical role in astrocytic neuroprotection in an MPTP-induced PD model (Fujita et al., 2018).
A few studies have found that the phagocytic activities of astrocytes are beneficial due to their recruitment process and clearance of α-Syn fibrils in PD (Loria et al., 2017), although few are accumulated intracellularly (Tremblay et al., 2019). However, transfer of misfolded protein from pathological areas to healthy sites of neurons results in the progression of PD pathology (Rostami et al., 2017). Therefore, astrocytic phagocytic function might act as a dual-edged sword in the progression of PD pathology.
Astrocytes in MS
Astrocytic roles have been implicated in the development of MS. Pro-inflammatory cytokines and chemokines secreted by activated T cells and microglia can activate astrocytes as a consequence of inflammatory responses, oxidative stress, and/or BBB disruption, leading to abnormal signal transduction and antigen presentation in MS (Brosnan and Raine, 2013; Ponath et al., 2018b; Waisman and Johann, 2018; Brambilla, 2019; Wheeler and Quintana, 2019; Yi et al., 2019). TGF-α increases the number of astrocytes by activating the NF-κB pathway in experimental autoimmune encephalomyelitis (EAE) and upregulating lymphocyte-recruiting gene expression in astrocytes of patients with MS (Rothhammer et al., 2018; Ponath et al., 2018a). A1 astrocytes activate and accumulate along with microglia in the lesion site by secreting lymphotoxin-α, IL-6, and lactosylceramide (Yi et al., 2019).
In EAE, A0 astrocytes are activated early at the lesion site due to infiltration of peripheral immune cells into the CNS (Yi et al., 2019); in addition, phagocytosis of myelin debris by astrocytes initiates infiltration of leukocytes during the early stage of pathology (Ponath et al., 2017). However, studies have shown that reactive astrocytes regulate the clearance of myelin debris to initiate remyelination. In cuprizone-treated mice, genetic ablation of astrocytes impaired remyelination, delayed clearance of damaged myelin, and reduced differentiation and maturation of oligodendrocyte precursor cells by preventing microglial migration to the site of demyelination (Skripuletz et al., 2013). Therefore, understanding the correlation between phagocytosis and A1/A2 astrocyte phenotypes is a potential area for therapeutic intervention.
A2 astrocytes may play a vital role in neuroprotection in MS, and it is well known that A1 astrocytes exacerbate inflammation and inhibit regeneration. Activated astrocytes upregulate the expression of retinaldehyde dehydrogenase 2 and control the production of retinoic acid in MS lesions (Mizee et al., 2014). Additionally, Arellano et al. (2015) reported that interferon-γ (IFN-γ) produced by astrocytes has neuroprotective effects during the late stage of EAE. Astrocytes directly interact with oligodendrocyte precursor cells and produce soluble factors. Notably, oligodendrocyte precursor cell proliferation and myelination is impaired by mutation of GFAP in astrocytes (Li et al., 2018a). Therefore, early polarization of astrocytes is a promising therapeutic target for the prevention of inflammation in MS.
A2 astrocytes produce BDNF in the brain. Knockout of BDNF in astrocytes causes axonal loss in EAE (Lee et al., 2012), whereas increased BDNF release by astrocytes enhanced remyelination in a cuprizone-induced model (Yin et al., 2020). Reactive astrocytes act as the dominant cells in CNS defenses against oxidative stress by enhancing the production of antioxidative enzymes in acute and chronic lesions (Yi et al., 2019). Activated astrocytes execute multifaceted functions in MS, mainly depending on the microenvironment, location, and stimuli. However, profound understanding the dual function of reactive astrocytes in MS is important to realize their therapeutic potential and pathogenic mechanisms.
Astrocytes in amyotrophic lateral sclerosis
The role of A1 and A2 astrocytes in neuronal metabolic function is worth emphasizing in the context of ALS. Inflammatory mediators and pro-inflammatory cytokines are closely associated with astrocyte-triggered toxicities in ALS. Astrocytes expressing mutant SOD1 secreted IFN-γ and prostaglandin D2 to induce motor neuron degeneration (Yamanaka and Komine, 2018). A1 astrocytes induce protein aggregation by releasing TGF-β1. Collectively, these findings reveal that A1 astrocytes are an important contributor to ALS pathology. Mitochondrial dysfunction in reactive astrocytes caused motor neuron death due to high ROS levels in an ALS mouse model. In patients with ALS, elevated levels of ROS production, iNOS, and NADPH oxidase in astrocytes aggravated autonomous damage of motor neurons (Marchetto et al., 2008).
A1 astrocytes exert adverse effects on neuroprotection during ALS, while A2 astrocytes may provide a beneficial environment for neurons. Focal transplantation of glial-restricted precursors (GRPs) in SOD1-mutant rats increased their survival time and reduced motor neuron death (Lepore et al., 2008), indicating that transplantation-based astrocyte replacement improved the microenvironment of motor neurons in ALS. Hence, astrocytes play a positive role in ALS and provide a promising therapeutic strategy for patients with ALS.
Inhibition of A1 and Promotion of A2 Astrocytes as Potential Targets in Neurodegenerative Diseases
Neurodegenerative diseases involve complex pathology (Ibrahim and Gabr, 2019; Shefa et al., 2019). Accordingly, it is extremely challenging to completely cure symptoms and pathology with current strategies, including cell transplantation and gene therapies (Lo Furno et al., 2018; Sudhakar and Richardson, 2019). As multifunctional glia in the CNS, astrocytes are a novel treatment target. Astrocytes play dual roles by orchestrating cellular functions to promote desirable physiological effects and mitigate adverse effects. Thus, ameliorating astrocytic dysfunction is a promising therapeutic approach for neurological disorders.
Pro-inflammatory molecules transform astrocytes from an A0 phenotype to neurotoxic A1 phenotype in several neurodegenerative disorders. Glucagon-like peptide-1 receptor (for which NLY01 is potent agonist under clinical investigation) can elicit a protective effect by intervening in microglia-mediated transformation of astrocytes to an A1 phenotype (Yun et al., 2018). Mesenchymal stem cell-derived exosomes suppress A1 activation by downregulating phosphorylation of the NF-κB p65 subunit in spinal cord injury (Wang et al., 2018; Liu et al., 2019). Similarly, fluoxetine exhibits neuroprotective functions by inhibiting A1 activation, lowering Aβ deposition, ameliorating neurotoxicity, and improving behavioral performance (Qiao et al., 2016). Furthermore, inhibition of A1 signaling with adeno-associated viral vectors and selective suppression of astrocytic γ-aminobutyric acid (GABA) synthesis improved cognitive function, including learning and memory abilities, of a mouse model (Furman et al., 2012; Jo et al., 2014). Hoffmann et al. (2015) intensively investigated the S1PR modulator FTY720 in MS, and its active metabolite FTY720-phosphate was shown to produce neurotrophic mediators (HB-EGF and LIF) in human primary astrocytes. Excitation of certain signaling pathways in A2 astrocytes can improve cognitive impairment; for example, insulin therapy was shown to improve the cognitive competence of patients with AD as a result of insulin receptor activation on A2 astrocytes (Finsterwald et al., 2015; Gabbouj et al., 2019).
Increased expression of PK2, which regulates polarization of the astrocytic A2 phenotype, has been observed in inflammatory regions of the brain. The chemokine-like signaling PK2 agonist IS20 induces A2 astrocytes to increase glutamate uptake and decrease inflammatory factors (Neal et al., 2018). Serotonin 1A (5-HT1A) receptors accelerated expression of antioxidative molecules by A2 astrocytes in a PD mouse model, and the 5-HT1A agonist 8-OH-DPAT can protect dopaminergic neurons (Miyazaki and Asanuma, 2016). A previous study demonstrated that modified astrocytes stem cells from glial-restricted precursor cells (astrocytes derived from GBP cells by exposure to bone morphogenetic protein) induced secretion of neurotrophic factors, including BDNF and GDNF, contributing to behavioral recovery and neuronal axon regeneration in a rat model of PD (Proschel et al., 2014).
Collectively, these findings suggest that shifting astrocytes from an A1 to A2 phenotype has potential for the treatment of neurodegenerative diseases.
Conclusions
We described the multidimensional functions of reactive astrocytes in various neurodegenerative disorders, with a focus on prevention of neurodegeneration and enhancement of neuroprotection. These effects partly rely on the various molecules secreted by reactive astrocytes into the microenvironment surrounding neurons. However, understanding the complete role of reactive astrocytes is still in its infancy. Available studies cannot distinguish between the causes and effects of the described phenomena associated with activated astrocytes. Consequently, potential phenotypic characteristics of A1 and A2 astrocytes have been identified depending on the initiation of injury. A1 astrocytes are found predominantly in acute injuries, whereby they enhance neurotoxicity and decrease the survival of neurons and oligodendrocytes. In contrast, A2 astrocytes are present in remyelinating regions, whereby they promote tissue repair and reduce neuronal death. Differential gene expression between A1 and A2 astrocytes may depend on the activation of various signaling pathways by pathophysiology-triggering events. Existing agonists, antagonists, or chemicals that dampen A1 activation or promote A2 activation have therapeutic potential, but the detailed mechanisms have not been investigated. After adapting to the inflammatory microenvironment present in the early stage of neurodegenerative diseases, astrocytes prevent inflammation, facilitate repair of injured tissue, initiate recovery from disease, and enhance physiological phenomena; for these reasons, astrocytes represent an alternative therapeutic strategy for neurodegenerative diseases. In view of the critical and multifactorial functions of astrocytes, inhibiting A1 astrocytes or inducing A2 astrocytes might be a promising and feasible strategy against neurodegenerative diseases of the CNS.
Additional file: Open peer review report 1 (81.7KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported partially by the National Natural Science Foundation of China, No. 81473577 (to CGM), a grant from the Department of Science and Technology of Shanxi Province, China, No. 201803D421073 (to YQY) and No. 201805D111009 (to CGM), a grant from Shanxi Applied Basic Research Project, No. 201901D211538 (to LJS), Datong Bureau of Science and Technology of China, No. 2019198 (to CGM), and Research Project Funds from Shanxi Scholarship Council of China, No. 2014-7 (to CGM).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
P-Reviewer: Li R; C-Editor: Zhao M; S-Editor: Li JY; L-Editors: Deusen AN, Song LP; T-Editor: Jia Y Review
Open peer reviewer: Rongwen Li, Henry Ford Health System Neurology Research, USA.
Funding: This work was supported partially by the National Natural Science Foundation of China, No. 81473577 (to CGM), a grant from the Department of Science and Technology of Shanxi Province, China, No. 201803D421073 (to YQY) and No. 201805D111009 (to CGM), a grant from Shanxi Applied Basic Research Project, No. 201901D211538 (to LJS), Datong Bureau of Science and Technology of China, No. 2019198 (to CGM), and Research Project Funds from Shanxi Scholarship Council of China, No. 2014-7 (to CGM).
References
- 1.Acosta C, Anderson HD, Anderson CM. Astrocyte dysfunction in Alzheimer disease. J Neurosci Res. 2017;95:2430–2447. doi: 10.1002/jnr.24075. [DOI] [PubMed] [Google Scholar]
- 2.Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA, Magistretti PJ. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: Impact on neuronal viability. J Neurosci. 2010;30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelova PR, Abramov AY. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic Biol Med. 2016;100:81–85. doi: 10.1016/j.freeradbiomed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Arellano G, Ottum PA, Reyes LI, Burgos PI, Naves R. Stage-specific role of interferon-Gamma in experimental autoimmune encephalomyelitis and multiple sclerosis. Front Immunol. 2015;6:492. doi: 10.3389/fimmu.2015.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azam S, Jakaria M, Kim IS, Kim J, Haque ME, Choi DK. Regulation of Toll-Like Receptor (TLR) Signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front Immunol. 2019;10:1000. doi: 10.3389/fimmu.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baillet A, Chanteperdrix V, Trocme C, Casez P, Garrel C, Besson G. The role of oxidative stress in amyotrophic lateral sclerosis and Parkinson’s disease. Neurochem Res. 2010;35:1530–1537. doi: 10.1007/s11064-010-0212-5. [DOI] [PubMed] [Google Scholar]
- 7.Belanger M, Magistretti PJ. The role of astroglia in neuroprotection. Dialogues Clin Neurosci. 2009;11:281–295. doi: 10.31887/DCNS.2009.11.3/mbelanger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Haim L, Carrillo-de Sauvage MA, Ceyzériat K, Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015;9:278. doi: 10.3389/fncel.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brambilla R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019;137:757–783. doi: 10.1007/s00401-019-01980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosnan CF, Raine CS. The astrocyte in multiple sclerosis revisited. Glia. 2013;61:453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- 11.Cai Z, Wan CQ, Liu Z. Astrocyte and Alzheimer’s disease. J Neurol. 2017;264:2068–2074. doi: 10.1007/s00415-017-8593-x. [DOI] [PubMed] [Google Scholar]
- 12.Campbell SC, Muñoz-Ballester C, Chaunsali L, Mills WA, Yang JH, Sontheimer H, Robel S. Potassium and glutamate transport is impaired in scar-forming tumor-associated astrocytes. Neurochem Int. 2020;133:104628. doi: 10.1016/j.neuint.2019.104628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrero I, Gonzalo MR, Martin B, Sanz-Anquela JM, Arevalo-Serrano J, Gonzalo-Ruiz A. Oligomers of beta-amyloid protein (Abeta1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1beta, tumour necrosis factor-alpha, and a nuclear factor kappa-B mechanism in the rat brain. Exp Neurol. 2012;236:215–227. doi: 10.1016/j.expneurol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng X, Wang J, Sun X, Shao L, Guo Z, Li Y. Morphological and functional alterations of astrocytes responding to traumatic brain injury. Integr Neurosci. 2019;18:203–215. doi: 10.31083/j.jin.2019.02.110. [DOI] [PubMed] [Google Scholar]
- 16.Chou SY, Weng JY, Lai HL, Liao F, Sun SH, Tu PH, Dickson DW, Chern Y. Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J Neurosci. 2008;28:3277–3290. doi: 10.1523/JNEUROSCI.0116-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo E, Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Correale J, Farez MF. The role of astrocytes in multiple sclerosis progression. Front Neurol. 2015;6:180. doi: 10.3389/fneur.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cragnolini AB, Lampitella G, Virtuoso A, Viscovo I, Panetsos F, Papa M, Cirillo G. Regional brain susceptibility to neurodegeneration: what is the role of glial cells. Neural Regen Res. 2020;15:838–842. doi: 10.4103/1673-5374.268897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da Silva J, Pierrat B, Mary JL, Lesslauer W. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J Biol Chem. 1997;272:28373–28380. doi: 10.1074/jbc.272.45.28373. [DOI] [PubMed] [Google Scholar]
- 21.Dallérac G, Rouach N. Astrocytes as new targets to improve cognitive functions. Prog Neurobiol. 2016;144:48–67. doi: 10.1016/j.pneurobio.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 22.De Miranda BR, Roch EM, Bai Q, El Ayadi A, Hinkle D, Burton EA, Timothy Greenamyre J. Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson’s disease. Neurobiol Dis. 2018;115:101–114. doi: 10.1016/j.nbd.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dresselhaus EC, Meffert MK. Cellular specificity of NF-kappaB function in the nervous system. Front Immunol. 2019;10:1043. doi: 10.3389/fimmu.2019.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dusaban SS, Chun J, Rosen H, Purcell NH, Brown JH. Sphingosine 1-phosphate receptor 3 and RhoA signaling mediate inflammatory gene expression in astrocytes. J Neuroinflammation. 2017;14:111. doi: 10.1186/s12974-017-0882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escartin C, Guillemaud O, Carrillo-de Sauvage MA. Questions and (some) answers on reactive astrocytes. Glia. 2019;67:2221–2247. doi: 10.1002/glia.23687. [DOI] [PubMed] [Google Scholar]
- 26.Farhy-Tselnicker I, Allen NJ. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev. 2018;13:7. doi: 10.1186/s13064-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finsterwald C, Magistretti PJ, Lengacher S. Astrocytes: New targets for the treatment of neurodegenerative diseases. Curr Pharm Des. 2015;21:3570–3581. doi: 10.2174/1381612821666150710144502. [DOI] [PubMed] [Google Scholar]
- 28.Fujita A, Yamaguchi H, Yamasaki R, Cui Y, Matsuoka Y, Yamada KI, Kira JI. Connexin 30 deficiency attenuates A2 astrocyte responses and induces severe neurodegeneration in a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine hydrochloride Parkinson’s disease animal model. J Neuroinflammation. 2018;15:227. doi: 10.1186/s12974-018-1251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabbouj S, Ryhänen S, Marttinen M, Wittrahm R, Takalo M, Kemppainen S, Martiskainen H, Tanila H, Haapasalo A, Hiltunen M, Natunen T. Altered insulin signaling in Alzheimer’s disease brain-special emphasis on PI3K-Akt pathway. Front Neurosci. 2019;13:629. doi: 10.3389/fnins.2019.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan L, Vargas M R, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci. 2012;32:17775–17787. doi: 10.1523/JNEUROSCI.3049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Yan Y, Fang Q, Zhang N, Kumar G, Zhang J, Song LJ, Yu J, Zhao L, Zhang HT, Ma CG. The Rho kinase inhibitor fasudil attenuates Aβ1-42-induced apoptosis via the ASK1/JNK signal pathway in primary cultures of hippocampal neurons. Metab Brain Dis. 2019;34:1787–1801. doi: 10.1007/s11011-019-00487-0. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Arboledas A, Davila JC, Sanchez-Mejias E, Navarro V, Nunez-Diaz C, Sanchez-Varo R, Sanchez-Mico MV, Trujillo-Estrada L, Fernandez-Valenzuela JJ, Vizuete M, Comella JX, Galea E, Vitorica J, Gutierrez A. Phagocytic clearance of presynaptic dystrophies by reactive astrocytes in Alzheimer’s disease. Glia. 2018;66:637–653. doi: 10.1002/glia.23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillamon-Vivancos T, Gomez-Pinedo U, Matias-Guiu J. Astrocytes in neurodegenerative diseases (I): function and molecular description. Neurologia. 2015;30:119–129. doi: 10.1016/j.nrl.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Healy LM, Antel JP. Sphingosine-1-phosphate receptors in the central nervous and immune systems. Curr Drug Targets. 2016;17:1841–1850. doi: 10.2174/1389450116666151001112710. [DOI] [PubMed] [Google Scholar]
- 36.Henneberger C. Does rapid and physiological astrocyte-neuron signalling amplify epileptic activity. J Physiol. 2017;595:1917–1927. doi: 10.1113/JP271958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye QN, Stewart CA, Geddes J, Markesbery WR, Patel E, Johnson GV, Bing G. p38 kinase is activated in the Alzheimer’s disease brain. J Neurochem. 1999;72:2053–2058. doi: 10.1046/j.1471-4159.1999.0722053.x. [DOI] [PubMed] [Google Scholar]
- 38.Hodgetts SI, Harvey AR. Neurotrophic factors used to treat spinal cord injury. Vitam Horm. 2017;104:405–457. doi: 10.1016/bs.vh.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann FS, Hofereiter J, Rübsamen H, Melms J, Schwarz S, Faber H, Weber P, Pütz B, Loleit V, Weber F, Hohlfeld R, Meinl E, Krumbholz M. Fingolimod induces neuroprotective factors in human astrocytes. J Neuroinflammation. 2015;12:184. doi: 10.1186/s12974-015-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibrahim MM, Gabr MT. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen Res. 2019;14:437–440. doi: 10.4103/1673-5374.245463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen CJ, Massie A, De Keyser J. Immune players in the CNS: the astrocyte. J Neuroimmune Pharmacol. 2013;8:824–839. doi: 10.1007/s11481-013-9480-6. [DOI] [PubMed] [Google Scholar]
- 43.Jia D, Zhu Q, Liu H, Zuo C, He Y, Chen G, Lu A. Osteoprotegerin disruption attenuates HySu-induced pulmonary hypertension through integrin alphavbeta3/FAK/AKT pathway suppression. Circ Cardiovasc Genet. 2017;10:e001591. doi: 10.1161/CIRCGENETICS.116.001591. [DOI] [PubMed] [Google Scholar]
- 44.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, Park HJ, Lee DY, Hong J, Kim HY, Oh SJ, Park SJ, Lee H, Yoon BE, Kim Y, Jeong Y, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27:663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- 46.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, Gil SC, Brown J, Wilhelmsson U, Restivo JL, Cirrito JR, Holtzman DM, Kim J, Pekny M, Lee JM. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 2013;27:187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kushwaha R, Mishra J, Gupta AP, Gupta K, Vishwakarma J, Chattopadhyay N, Gayen JR, Kamthan M, Bandyopadhyay S. Rosiglitazone up-regulates glial fibrillary acidic protein via HB-EGF secreted from astrocytes and neurons through PPARgamma pathway and reduces apoptosis in high-fat diet-fed mice. J Neurochem. 2019;149:679–698. doi: 10.1111/jnc.14610. [DOI] [PubMed] [Google Scholar]
- 49.Lam KYC, Wu QY, Hu WH, Yao P, Wang HY, Dong TTX, Tsim KWK. Asarones from Acori Tatarinowii Rhizoma stimulate expression and secretion of neurotrophic factors in cultured astrocytes. Neurosci Lett. 2019;707:134308. doi: 10.1016/j.neulet.2019.134308. [DOI] [PubMed] [Google Scholar]
- 50.Ledesma MD, Galvan C, Hellias B, Dotti C, Jensen PH. Astrocytic but not neuronal increased expression and redistribution of parkin during unfolded protein stress. J Neurochem. 2002;83:1431–1440. doi: 10.1046/j.1471-4159.2002.01253.x. [DOI] [PubMed] [Google Scholar]
- 51.Lee DH, Geyer E, Flach AC, Jung K, Gold R, Flügel A, Linker RA, Lühder F. Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol. 2012;123:247–258. doi: 10.1007/s00401-011-0890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee Y, Lee S, Chang SC, Lee J. Significant roles of neuroinflammation in Parkinson’s disease: therapeutic targets for PD prevention. Arch Pharm Res. 2019;42:416–425. doi: 10.1007/s12272-019-01133-0. [DOI] [PubMed] [Google Scholar]
- 54.Leitner GR, Wenzel TJ, Marshall N, Gates EJ, Klegeris A. Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opin Ther Targets. 2019;23:865–882. doi: 10.1080/14728222.2019.1676416. [DOI] [PubMed] [Google Scholar]
- 55.Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levenga J, Wong H, Milstead RA, Keller BN, LaPlante LE, Hoeffer CA. AKT isoforms have distinct hippocampal expression and roles in synaptic plasticity. Elife. 2017;6:e30640. doi: 10.7554/eLife.30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li K, Li J, Zheng J, Qin S. Reactive astrocytes in neurodegenerative diseases. Aging Dis. 2019;10:664–675. doi: 10.14336/AD.2018.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L, Tian E, Chen X, Chao J, Klein J, Qu Q, Sun G, Sun G, Huang Y, Warden CD, Ye P, Feng L, Li X, Cui Q, Sultan A, Douvaras P, Fossati V, Sanjana NE, Riggs AD, Shi Y. GFAP mutations in astrocytes impair oligodendrocyte progenitor proliferation and myelination in an hiPSC model of Alexander disease. Cell Stem Cell. 2018a;23:239–251e6. doi: 10.1016/j.stem.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li YX, Sibon OCM, Dijkers PF. Inhibition of NF-kappaB in astrocytes is sufficient to delay neurodegeneration induced by proteotoxicity in neurons. J Neuroinflammation. 2018b;15:261. doi: 10.1186/s12974-018-1278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liddelow S, Hoyer D. Astrocytes: adhesion molecules and immunomodulation. Curr Drug Targets. 2016;17:1871–1881. doi: 10.2174/1389450117666160101120703. [DOI] [PubMed] [Google Scholar]
- 61.Liddelow SA, Barres BA. Reactive astrocytes: Production, function, and therapeutic potential. Immunity. 2017a;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017b;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu M, Wu Y, Liu Y, Chen Z, He S, Zhang H, Wu L, Tu F, Zhao Y, Liu C, Chen X. Basic fibroblast growth factor protects astrocytes against ischemia/reperfusion injury by upregulating the caveolin-1/VEGF signaling pathway. J Mol Neurosci. 2018;64:211–223. doi: 10.1007/s12031-017-1023-9. [DOI] [PubMed] [Google Scholar]
- 64.Liu RX, Huang C, Bennett DA, Li H, Wang R. The characteristics of astrocyte on Abeta clearance altered in Alzheimer’s disease were reversed by anti-inflammatory agent (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate. Am J Transl Res. 2016;8:4082–4094. [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, Zhou Z, Zhou Z, Xu T, Jiang T, Yang S, Yin G, Chen J, Fan J, Cai W. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36:469–484. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- 66.Lo Furno D, Mannino G, Giuffrida R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J Cell Physiol. 2018;233:3982–3999. doi: 10.1002/jcp.26192. [DOI] [PubMed] [Google Scholar]
- 67.Loria F, Vargas JY, Bousset L, Syan S, Salles A, Melki R, Zurzolo C. α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. 2017;134:789–808. doi: 10.1007/s00401-017-1746-2. [DOI] [PubMed] [Google Scholar]
- 68.Ludwin SK, Rao VTs, Moore CS, Antel JP. Astrocytes in multiple sclerosis. Mult Scler. 2016;22:1114–1124. doi: 10.1177/1352458516643396. [DOI] [PubMed] [Google Scholar]
- 69.Mahmoud S, Gharagozloo M, Simard C, Gris D. Homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 2019;8:E184. doi: 10.3390/cells8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 71.McGann JC, Mandel G. Neuronal activity induces glutathione metabolism gene expression in astrocytes. Glia. 2018;66:2024–2039. doi: 10.1002/glia.23455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McManus RM, Finucane OM, Wilk MM, Mills KHG, Lynch MA. FTY720 Attenuates infection-induced enhancement of Abeta accumulation in APP/PS1 mice by modulating astrocytic activation. J Neuroimmune Pharmacol. 2017;12:670–681. doi: 10.1007/s11481-017-9753-6. [DOI] [PubMed] [Google Scholar]
- 73.Miyazaki I, Asanuma M. Serotonin 1A receptors on astrocytes as a potential target for the treatment of Parkinson’s disease. Curr Med Chem. 2016;23:686–700. doi: 10.2174/0929867323666160122115057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mizee MR, Nijland PG, van der Pol SM, Drexhage JA, van Het Hof B, Mebius R, Van der Valk P, Van Horssen J, Reijerkerk A, de Vries HE. Astrocyte-derived retinoic acid: a novel regulator of blood-brain barrier function in multiple sclerosis. Acta Neuropathol. 2014;128:691–703. doi: 10.1007/s00401-014-1335-6. [DOI] [PubMed] [Google Scholar]
- 75.Morita M, Ikeshima-Kataoka H, Kreft M, Vardjan N, Zorec R, Noda M. Metabolic plasticity of astrocytes and aging of the brain. Int J Mol Sci. 2019;20:E941. doi: 10.3390/ijms20040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neal M, Luo J, Harischandra DS, Gordon R, Sarkar S, Jin H, Anantharam V, Désaubry L, Kanthasamy A, Kanthasamy A. Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes. Glia. 2018;66:2137–2157. doi: 10.1002/glia.23467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res. 2018;126:39–43. doi: 10.1016/j.neures.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 79.Oksanen M, Lehtonen S, Jaronen M, Goldsteins G, Hämäläinen RH, Koistinaho J. Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell Mol Life Sci. 2019;76:2739–2760. doi: 10.1007/s00018-019-03111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ongali B, Nicolakakis N, Tong XK, Lecrux C, Imboden H, Hamel E. Transforming growth factor-β1 induces cerebrovascular dysfunction and astrogliosis through angiotensin II type 1 receptor-mediated signaling pathways. Can J Physiol Pharmacol. 2018;96:527–534. doi: 10.1139/cjpp-2017-0640. [DOI] [PubMed] [Google Scholar]
- 81.Ouali Alami N, Schurr C, Olde Heuvel F, Tang L, Li Q, Tasdogan A, Kimbara A, Nettekoven M, Ottaviani G, Raposo C, Röver S, Rogers-Evans M, Rothenhäusler B, Ullmer C, Fingerle J, Grether U, Knuesel I, Boeckers TM, Ludolph A, Wirth T, et al. NF-kappaB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J. 2018;37:e98697. doi: 10.15252/embj.201798697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Nievas BG, Serrano-Pozo A. Deciphering the astrocyte reaction in Alzheimer’s disease. Front Aging Neurosci. 2018;10:114. doi: 10.3389/fnagi.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ponath G, Lincoln MR, Levine-Ritterman M, Park C, Dahlawi S, Mubarak M, Sumida T, Airas L, Zhang S, Isitan C, Nguyen TD, Raine CS, Hafler DA, Pitt D. Enhanced astrocyte responses are driven by a genetic risk allele associated with multiple sclerosis. Nat Commun. 2018a;9:5337. doi: 10.1038/s41467-018-07785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ponath G, Park C, Pitt D. The role of astrocytes in multiple sclerosis. Front Immunol. 2018b;9:217. doi: 10.3389/fimmu.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ponath G, Ramanan S, Mubarak M, Housley W, Lee S, Sahinkaya FR, Vortmeyer A, Raine CS, Pitt D. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain. 2017;140:399–413. doi: 10.1093/brain/aww298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Proschel C, Stripay JL, Shih CH, Munger JC, Noble MD. Delayed transplantation of precursor cell-derived astrocytes provides multiple benefits in a rat model of Parkinsons. EMBO Mol Med. 2014;6:504–518. doi: 10.1002/emmm.201302878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qian C, Tan D, Wang X, Li L, Wen J, Pan M, Li Y, Wu W, Guo J. Peripheral nerve injury-induced astrocyte activation in spinal ventral horn contributes to nerve regeneration. Neural Plast. 2018;2018:8561704. doi: 10.1155/2018/8561704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiao J, Wang J, Wang H, Zhang Y, Zhu S, Adilijiang A, Guo H, Zhang R, Guo W, Luo G, Qiu Y, Xu H, Kong J, Huang Q, Li XM. Regulation of astrocyte pathology by fluoxetine prevents the deterioration of Alzheimer phenotypes in an APP/PS1 mouse model. Glia. 2016;64:240–254. doi: 10.1002/glia.22926. [DOI] [PubMed] [Google Scholar]
- 89.Raza C, Anjum R, Shakeel NUA. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019;226:77–90. doi: 10.1016/j.lfs.2019.03.057. [DOI] [PubMed] [Google Scholar]
- 90.Revilla S, Ursulet S, Alvarez-Lopez MJ, Castro-Freire M, Perpina U, Garcia-Mesa Y, Bortolozzi A, Giménez-Llort L, Kaliman P, Cristòfol R, Sarkis C, Sanfeliu C. Lenti-GDNF gene therapy protects against Alzheimer’s disease-like neuropathology in 3xTg-AD mice and MC65 cells. CNS Neurosci Ther. 2014;20:961–972. doi: 10.1111/cns.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rizor A, Pajarillo E, Johnson J, Aschner M, Lee E. Astrocytic oxidative/nitrosative stress contributes to Parkinson’s disease pathogenesis: The dual role of reactive astrocytes. Antioxidants (Basel) 2019;8:E265. doi: 10.3390/antiox8080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rostami J, Holmqvist S, Lindström V, Sigvardson J, Westermark GT, Ingelsson M, Bergström J, Roybon L, Erlandsson A. Human astrocytes transfer aggregated Alpha-synuclein via tunneling nanotubes. J Neurosci. 2017;37:11835–11853. doi: 10.1523/JNEUROSCI.0983-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, Blain M, Healy L, Neziraj T, Borio M, Wheeler M, Dragin LL, Laplaud DA, Antel J, Alvarez JI, Prinz M, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, Chao CC, Wilz A, Blain M, Healy L, Antel J, Quintana FJ. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci U S A. 2017;114:2012–2017. doi: 10.1073/pnas.1615413114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 96.Shefa U, Jeong NY, Song IO, Chung HJ, Kim D, Jung J, Huh Y. Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res. 2019;14:749–756. doi: 10.4103/1673-5374.249218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi X, Wang B, Liu Y, Zhang J, Huang Y, Cao P, Shen Y, Lyu J. Carnosine modulates glutamine synthetase expression in senescent astrocytes exposed to oxygen-glucose deprivation/recovery. Brain Res Bull. 2017a;130:138–145. doi: 10.1016/j.brainresbull.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 98.Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo G, Wang K, Roh J, Robinson G, Finn MB, Jiang H, Sullivan PM, Baufeld C, Wood MW, Sutphen C, McCue L, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017b;549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shinozaki Y, Shibata K, Yoshida K, Shigetomi E, Gachet C, Ikenaka K, Tanaka KF, Koizumi S. Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 2017;19:1151–1164. doi: 10.1016/j.celrep.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 100.Singh K, Han K, Tilve S, Wu K, Geller HM, Sack MN. Parkin targets NOD2 to regulate astrocyte endoplasmic reticulum stress and inflammation. Glia. 2018;66:2427–2437. doi: 10.1002/glia.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, Berger K, Kipp M, Baumgärtner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain. 2013;136:147–167. doi: 10.1093/brain/aws262. [DOI] [PubMed] [Google Scholar]
- 102.Stadelmann C, Timmler S, Barrantes-Freer A, Simons M. Myelin in the central nervous system: structure, function, and pathology. Physiol Rev. 2019;99:1381–1431. doi: 10.1152/physrev.00031.2018. [DOI] [PubMed] [Google Scholar]
- 103.Su Y, Chen Z, Du H, Liu R, Wang W, Li H, Ning B. Silencing miR-21 induces polarization of astrocytes to the A2 phenotype and improves the formation of synapses by targeting glypican 6 via the signal transducer and activator of transcription-3 pathway after acute ischemic spinal cord injury. FASEB J. 2019;33:10859–10871. doi: 10.1096/fj.201900743R. [DOI] [PubMed] [Google Scholar]
- 104.Sudhakar V, Richardson RM. Gene therapy for neurodegenerative diseases. Neurotherapeutics. 2019;16:166–175. doi: 10.1007/s13311-018-00694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang T, Zhong JH, Luo JK, Yang AL, Zhang QM, Zhou JH, Zhang Q. Leukemia inhibitory factor contributes to reactive astrogliosis via activation of signal transducer and activator of transcription 3 signaling after intracerebral hemorrhage in rats. J Neurotrauma. 2017;34:1658–1665. doi: 10.1089/neu.2016.4711. [DOI] [PubMed] [Google Scholar]
- 106.Tchieu J, Calder EL, Guttikonda SR, Gutzwiller EM, Aromolaran KA, Steinbeck JA, Goldstein PA, Studer L. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat Biotechnol. 2019;37:267–275. doi: 10.1038/s41587-019-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tome D, Fonseca CP, Campos FL, Baltazar G. Role of neurotrophic factors in Parkinson’s disease. Curr Pharm Des. 2017;23:809–838. doi: 10.2174/1381612822666161208120422. [DOI] [PubMed] [Google Scholar]
- 108.Tremblay ME, Cookson MR, Civiero L. Glial phagocytic clearance in Parkinson’s disease. Mol Neurodegener. 2019;14:16. doi: 10.1186/s13024-019-0314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trujillo-Estrada L, Gomez-Arboledas A, Forner S, Martini AC, Gutierrez A, Baglietto-Vargas D, LaFerla FM. Astrocytes: From the physiology to the disease. Curr Alzheimer Res. 2019;16:675–698. doi: 10.2174/1567205016666190830110152. [DOI] [PubMed] [Google Scholar]
- 110.Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, Lakeman K, Dijkstra CD, Van Der Valk P, Reijerkerk A, Alewijnse AE, Peters SL, De Vries HE. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58:1465–1476. doi: 10.1002/glia.21021. [DOI] [PubMed] [Google Scholar]
- 111.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Waisman A, Johann L. Antigen-presenting cell diversity for T cell reactivation in central nervous system autoimmunity. J Mol Med (Berl) 2018;96:1279–1292. doi: 10.1007/s00109-018-1709-7. [DOI] [PubMed] [Google Scholar]
- 113.Walker CD, Risher WC, Risher ML. Regulation of synaptic development by astrocyte signaling factors and their emerging roles in substance abuse. Cells. 2020;9:E297. doi: 10.3390/cells9020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang H, Yuan G, Prabhakar NR, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from PC12 cells in response to oxidative stress requires autocrine dopamine signaling. J Neurochem. 2006;96:694–705. doi: 10.1111/j.1471-4159.2005.03572.x. [DOI] [PubMed] [Google Scholar]
- 115.Wang J, Sui RX, Miao Q, Wang Q, Song LJ, Yu JZ, Li YH, Xiao BG, Ma CG. Effect of Fasudil on remyelination following cuprizone-induced demyelination. CNS Neurosci Ther. 2020;26:76–89. doi: 10.1111/cns.13154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Wang L, Pei S, Han L, Guo B, Li Y, Duan R, Yao Y, Xue B, Chen X, Jia Y. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFkappaB P65 subunit in spinal cord injury. Cell Physiol Biochem. 2018;50:1535–1559. doi: 10.1159/000494652. [DOI] [PubMed] [Google Scholar]
- 117.Wheeler MA, Quintana FJ. Regulation of astrocyte functions in multiple sclerosis. Cold Spring Harb Perspect Med. 2019;9:a029009. doi: 10.1101/cshperspect.a029009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu Z, Guo Z, Gearing M, Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s disease model. Nat Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu X, Zhang A, Zhu Y, He W, Di W, Fang Y, Shi X. MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-kappaB and PI3K-Akt pathways. J Cell Physiol. 2018;234:904–914. doi: 10.1002/jcp.26918. [DOI] [PubMed] [Google Scholar]
- 120.Yamanaka K, Komine O. The multi-dimensional roles of astrocytes in ALS. Neurosci Res. 2018;126:31–38. doi: 10.1016/j.neures.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 121.Ye B, Shen H, Zhang J, Zhu YG, Ransom BR, Chen XC, Ye ZC. Dual pathways mediate beta-amyloid stimulated glutathione release from astrocytes. Glia. 2015;63:2208–2219. doi: 10.1002/glia.22886. [DOI] [PubMed] [Google Scholar]
- 122.Yi W, Schluter D, Wang X. Astrocytes in multiple sclerosis and experimental autoimmune encephalomyelitis: Star-shaped cells illuminating the darkness of CNS autoimmunity. Brain Behav Immun. 2019;80:10–24. doi: 10.1016/j.bbi.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 123.Yin JJ, He Y, An J, Miao Q, Sui RX, Wang Q, Yu JZ, Xiao BG, Ma CG. Dynamic balance of microglia and astrocytes involved in the remyelinating effect of Ginkgolide B. Front Cell Neurosci. 2020;13:572. doi: 10.3389/fncel.2019.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao H, Wang SP, Lin JW. Insight into white matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocyte. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:2120–2125. [Google Scholar]
- 127.Zhao Y, Zhang Q, Xi J, Xiao B, Li Y, Ma C. Neuroprotective effect of fasudil on inflammation through PI3K/Akt and Wnt/β-catenin dependent pathways in a mice model of Parkinson’s disease. Int J Clin Exp Pathol. 2015;8:2354–2364. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.